Abstract
Nanoparticles are a promising technology for delivery of new types of therapeutics. A polymer library approach has allowed engineering of polymeric particles that are particularly effective for the delivery of DNA and siRNA to human cells. Certain chemical structural motifs, degradable linkages, hydrophobicity, and biophysical properties are key for successful intracellular delivery. Small differences to biomaterial structure, and especially the type of degradable linkage in the polymers, can be critical for successful delivery of siRNA vs. DNA. Furthermore, subtle changes to biomaterial structure can facilitate cell-type gene delivery specificity between human brain cancer cells and healthy cells as well as between human retinal endothelial cells and epithelial cells. These polymeric nanoparticles are effective for nucleic acid delivery in a broad range of human cell types and have applications to regenerative medicine, ophthalmology, and cancer among many other biomedical research areas.
GENE THERAPY AND THE NEED FOR NON-VIRAL MATERIALS
Since the discovery of DNA as the substrate for inheritable information,58 there has been interest in manipulating DNA to better understand and engineer biological function. Gene therapy is the use of exogenous nucleic acid as a drug. There is great potential in this approach as virtually all diseases have a genetic component, even certain diseases and target pathways that may be seen as currently undruggable. Moreover, rather than treating the symptoms of a disease, gene therapy could enable clinicians to directly treat its prime cause. This approach could be used for monogenic diseases such as cystic fibrosis, hemophilia, and severe combined immunodeficiency.56 Moreover, gene delivery could be used in the future to treat acquired diseases that also have genetic components. The scope of amenable diseases is broad, and as more information is gleaned from the human genome, these may include cancer, cardiovascular disease, neurological diseases, Crohn’s disease, type 2 diabetes, and other diseases.12 Gene delivery can also lead to successful genetic vaccines. In this approach, rather than deliver a traditional antigen, DNA is delivered and the antigen is encoded in the DNA. This technique can be used to treat infectious diseases or as an immunotherapy to treat other diseases such as cancer.35 Delivery of double stranded RNA rather than DNA offers a complementary approach where target genes can be silenced.14 Together, the potential capabilities of using nucleic acids as drugs for genetic medicine are far-reaching.
The potential of gene therapy has not been realized due to the challenge of safe and effective delivery. In many instances, a clinician or scientist may know which cells need to be targeted in a patient’s body, which gene is mutated, and what sequence of nucleic acid needs to be delivered to repair the genetic defect. However, after more than 1,600 clinical trials,2 safe and effective gene therapy has not yet been demonstrated and there currently is not an FDA-approved treatment. Most of these gene therapy trials (~75%) have used viruses to deliver the genes. The most common vectors utilized have been adenovirus, retrovirus, vaccinia virus, adeno-associated virus, herpes simplex virus, pox virus, and lentivirus. The primary concern with viral gene therapy is safety, as human studies have shown the potential for cancer-causing insertional mutagenesis11 and life-threatening immune response.22 Other limitations with using a viral approach depend on the type of vector and include small cargo-capacity, tropism to only particular cells, manufacturing challenges, and resistance to repeated administrations.53 While improvements to viral gene therapy are ongoing, others believe that non-viral approaches would be best to alleviate the concerns of virus-mediated delivery.
Many promising non-viral approaches use biomaterials and nanotechnology to construct nucleic-acid containing particles that behave like synthetic viruses. These biomaterials are chemical agents such as polymers, lipids, peptides, sugars, or other materials, often cationic, designed to bind to and/or encapsulate nucleic acids to improve their stability and delivery.34, 37, 39 Cationic lipid/liposomal formulations have been the most commonly used biomaterials for non-viral nucleic acid delivery.2 Unfortunately, even with the most advanced lipid materials, most lipoplexes are toxic, interact non-specifically with serum proteins and cells, aggregate, and have other limitations.30, 43
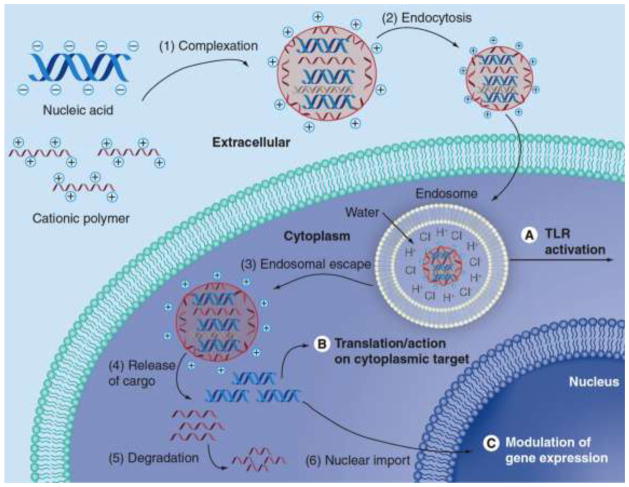
To improve non-viral vector design, it is important to consider the specific mechanistic steps of transport to design biomaterials that can overcome delivery bottlenecks. Recent work in the field has identified several key steps and these are highlighted in Figure 1. The steps in non-viral gene delivery include (1) biomaterial binding/complexation/condensation of nucleic acid,4, 16 (2) cellular uptake/endocytosis,24, 25, 57 (3) endosomal escape,47 (4) release of cargo/biomaterial unbinding,42 (5) biomaterial degradation, and in the case of DNA, (6) nuclear import.13 Following nuclear import, important downstream steps for DNA expression include promoter binding to the DNA, transcription to mRNA, and translation into protein. Although these mechanistic steps have been identified, they are still not well understood and further research is necessary to elucidate how biomaterial structure precisely affects each step in gene delivery.
Figure 1.
Gene delivery mechanism. Reproduced with permission from: Sunshine, Bishop, & Green, Therapeutic Delivery 2011 Volume 2, Issue 4, pp. 493–521.51
POLYMERIC NANOPARTICLES
Cationic polymers are a class of biomaterials especially promising for gene delivery. Polymers are promising because they have large structural diversity and there is flexibility in altering polymer structure to enhance gene delivery. Cationic charge is important to ensure electrostatic attraction between the polymer and the strongly negatively charged nucleic acid. This is important as nucleic acids, large hydrophilic, and negatively charged biomolecules, are generally not able to enter cells efficiently on their own. However, if the nucleic acids bind positively charged molecules like cationic polymers, their charge, which comes from anionic phosphate groups, is neutralized. This charge neutralization allows large nucleic acids, such as DNA, to collapse into smaller shapes and become more compact. Depending on the charge ratio between the polymers and the nucleic acids, the overall complexes can carry a neutral to positive charge, which can help the particles to interact favorably with the negatively charged surface of the cell. Complexation/DNA condensation is also important to compact DNA and reduce its susceptibility to nucleases.
There are many types of polymers used for non-viral gene delivery such as polyethylenimine,9 chitosan,31 cyclodextrins,38 polypeptides,15 and others. One class of polymers of interest for gene delivery are the poly(beta-amino ester)s (PBAEs), a class of polymers first developed by David Lynn and co-workers in the Langer Lab.29 Over two thousand PBAEs were subsequently synthesized and screened for efficacy in COS-7 cells4 and the leading structures were further investigated and found to work in vivo.16, 23 Another class of polymers of interest for gene delivery are poly(amido amine)s (PAAs).28, 41 These polymers can be bioreducible through the use of disulfide-containing monomers in the polymer backbone and have shown high gene delivery efficacy with a range of polymer structures.5, 27 PAAs share many properties with PBAEs, including the ability to bind nucleic acids to form nanoparticles, facilitate cellular uptake, promote endosomal escape, and allow intracellular delivery.
My lab is interested in synthetic polymers with differential structures and structural diversity for use in gene delivery. We synthesize new PBAEs and PAAs utilizing commercially available amine-containing monomers and either diacrylates or diacrylamides and design the polymers to be degradable through hydrolytic and/or bioreducible linkages.48, 50, 55 There are many advantages of these types of materials including their ease in synthesis, diverse biophysical properties, and tunable biological activity. Critically, they have low cytotoxicity due to their degradability. This reduced cytotoxicity can be 100-fold compared to commonly used non-degradable, cationic polymers such as polyethylenimine.17 We have systematically investigated new PBAEs to examine the differential effects of changes to polymer backbone, side-chain, and end-group combinatorially.7, 50 We have also developed new, specialized polymer end-groups and found that these can have a dramatic effect on cell-type specificity45, 49, 54 or can enable triggered release.55
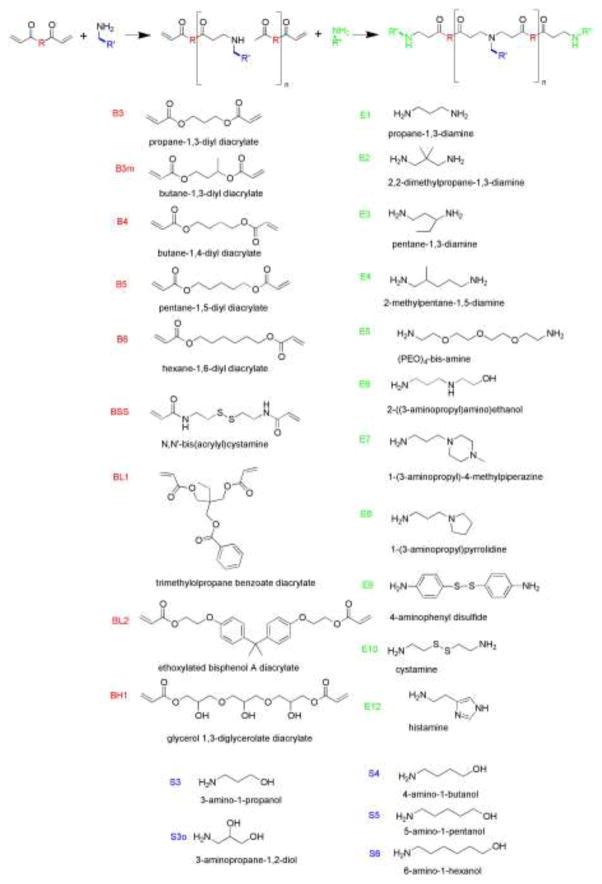
These PBAEs and PAAs are synthesized by a Michael Addition reaction with a monomer containing a primary amine and either a diacrylate monomer or diacrylamide monomer respectively. This step-growth polymerization forms linear polymers with a range of molecular weights from 2,000–48,000 Da depending on synthesis conditions.50 Often these reactions are performed neat or with DMSO as a solvent to minimize the steps required between initial synthesis and cell-based testing. We have found that trace DMSO and trace unreacted monomer do not cause cytotoxicity. To synthesize polymers with various end-group molecules, a two-step procedure is performed. First, an acrylate-terminated or acrylamide-terminated base polymer is synthesized. Second, a molar excess of an amine-containing molecule is added to this base polymer.55 For purified synthesis, the reaction is performed in tetrahydrofuran, and then diethyl ether is used to precipitate the polymer and remove unreacted monomer. Molecular weight and polymer structure are verified by gel permeation chromatography and 1H-NMR. Representative monomers used to synthesize the polymers are shown in Figure 2. The naming convention used for each polymer is composed of the monomer used as the backbone (B), followed by the monomer used as the side chain (S), and finally the monomer used as the end group (E). In most cases, the number after “B” refers to the number of carbons between acrylate groups, and the number after “S” refers to the number of carbons between amine and alcohol groups in the side chain. Other modifiers for polymer naming include “m” for methyl and “o” for hydroxyl. Nanoparticle biophysical properties including particle size, zeta potential, and particle concentration have been characterized by dynamic light scattering, transmission electron microscopy, and nanoparticle tracking analysis.7, 8, 55
Figure 2.
Monomer structures and polymer synthesis. The polymer library consists of polymers synthesized by the combination of 9 backbone monomers “B”, 5 side chain monomers “S”, and 11 end-group monomers “E.”
NANOPARTICLES FOR DNA DELIVERY
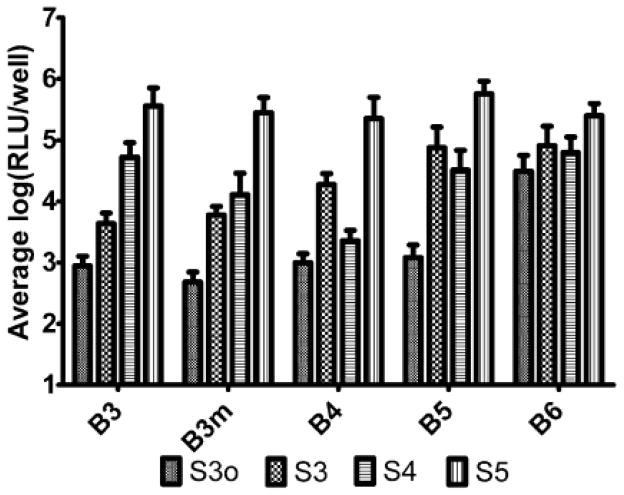
We have found that our polymers work especially well for DNA delivery and that small changes to structural composition make dramatic differences to transfection efficacy. Polymers containing linear hydrocarbon backbones were determined to be the most effective for gene delivery. Hydrophobic modifications (such as BL1 and BL2) caused increased toxicity while hydrophilic modifications (BH1), decreased efficacy.50 With linear stretches of hydrocarbons in the backbone and side chain of the polymers, increased hydrophobicity tended to increase transfection efficacy. The trend of increased hydrophobicity of the backbone causing increased transfection efficacy was most significant when the side chain was less hydrophobic itself (S3o, S3). Similarly, the trend of increased hydrophobicity of the side chain causing increased transfection efficacy was most significant when the backbone was less hydrophobic itself (B3, B3m, B4) (Figure 3). Thus, the overall polymer (backbone + side chain) can reach a hydrophobicity limit, beyond which, further hydrophobicity does not improve efficacy and may even cause cytotoxicity among certain cell types. Interestingly, these increases in hydrophobicity can increase transfection nearly 1,000-fold in side chain cases such as B3-S3 compared to B3-S5 and 100-fold in backbone cases such as B3m-S3o compared to B6-S3o.50
Figure 3.
Increased hydrophobicity improves gene delivery of LUC DNA to COS-7 cells. Average log-scale luminescence post-transfection (mean±s.e) of end-modified polymers with the same base polymer. Reproduced with permission from: Sunshine et al., Biomacromolecules 2011 Volume 12, Issue 10, pp. 3592–600.50
Polymer end group composition also caused significant changes to transfection efficacy, even when polymer molecular weight and nanoparticle properties were kept constant.50, 55 With the same base polymer composition, end-group changes such as E12 to E5 increase efficacy by 10-fold on average and in some cases over 1,000-fold. Performance of polymer end-groups also tended to cluster based on structure with E1 and E3 end-groups (propane diamine-based structures), producing polymers with similar efficacy, as do polymers containing E6, E7, and E8 end-groups (contain one or two secondary/tertiary amines) 50.
While the molecular weight among the polymers we have tested has varied considerably depending on synthesis conditions (2,000–110,000 Da), this difference generally has low correlation to transfection efficacy across polymer structures (linear regression, R2 =0.003, p = 0.7833).50 Although, for a given polymer structure, optimization of monomer molar ratio during synthesis can vary molecular weight and improve transfection.7 Nanoparticle self-assembly conditions including the weight ratio of polymer to DNA, make an important difference to transfection efficacy. Over a range of 30 wt polymer:wt DNA to 150 wt polymer:wt DNA, 60 wt polymer:wt DNA (w/w) was determined to be the most effective.50 Luciferase expression gene delivery studies show that higher w/w ratios can boost transfection efficacy by 10,000-fold compared to lower w/w ratios. In addition, certain polymers, such as those composed of B3-S5 as a base polymer, require a higher weight ratio than 60 w/w, such as 125 w/w, to become effective.
For regenerative medicine applications, such as programming stem cells18, 59 or reprogramming human cells into human induced stem cells (iPSCs),52, 60 methods for safe and effective gene transfer are especially needed. As the delivery of multiple factors is required for reprogramming, co-delivery and co-expression are also needed for this application in particular. We determined that these polymeric nanoparticles contain 30–120 plasmids per particle.8 The cargo capacity varied with polymer structure and also correlated to coexpression. For example, polymer B4-S4-E7, with ~120 plasmids per particle, showed co-expression in ~5 times as many IMR90 human fibroblasts as compared to polymer B3-S5-E7, which had a lower ~30 plasmids per particle loading capacity.8 We have also found that these polymers work well for transfection of mammary epithelial cells and that relative transfection in a 2D monolayer in vitro system correlates well to transfection in a 3D system of organoids.7 Transfection with these polymers is non-cytotoxic and preserves healthy organoid morphology and development over time.
Cancer therapy is a promising area for genetic medicine as cancer is often caused by mutations to tumor suppressors, which if reintroduced, could cause targeted apoptosis in only cancer cells. Cancer is estimated to cause more than 577,000 deaths in 2012 and is the second leading cause of death in the United States.1 Cancer gene therapy can be targeted to cancer cells through nanoparticle size and the enhanced permeation and retention (EPR) effect,3 ligand conjugation36 or coating mediated uptake,21, 44, 61 and transcriptional targeting.46 These methods for targeting and cancer specificity greatly expand the therapeutic window compared to conventional cancer chemotherapies.
Our lab is particularly interested in gene-based treatments for glioblastoma, an aggressive primary brain tumor. Although advancements have been made in treating glioblastoma, median survival of patients with high-grade tumors is a tragically short, at approximately 14 months following surgery, chemotherapy, and radiation-based therapy.10, 32, 33 We are developing nanoparticle formulations to target and treat both the bulk of a glioblastoma tumor as well as brain tumor stem cells (BTSCs).54 Using fluorescently labeled DNA, nanoparticle cellular uptake was found to be high for most end-modified PBAE structures evaluated in GB319 human brain tumor-derived astrocytes. Successful cellular uptake is necessary, but not sufficient, for effective gene delivery, and polymers B4-S4-E7 and B4-S5-E7 had the highest levels of transfection. Across multiple polymers, it was determined that the E7 end-group is particularly effective for transfecting these cells. Nanoparticles formed with polymer B4-S4-E7 transfected 60% of these human brain cancer cells in a single dose while maintaining >80% cell viability.54 Following a single dose, exogenous gene expression peaked at ~1 week and persisted for at least 2 weeks, depending on polymer structure.
For clinical benefit, a nanoparticle treatment must be able to be easily administered by a clinician. The polymeric nanoparticles described above are typically prepared fresh, formed through self-assembly in aqueous buffer during an incubation period and then degraded in water through hydrolysis of ester linkages. The last point is a particular concern as polymers such as B4-S4-E7 degrade fully in water within 24 hrs.55 While this works well to ensure nucleic acid release and minimizes potential cytotoxicity of cationic polymers, it does not enable a viable shelf-life. Thus, we endeavored to formulate these biodegradable nanoparticles in a manner that would extend their shelf-life and clinical ease of use. We found that by lyophilizing these biodegradable nanoparticles in the presence of sucrose, shelf-life could be extended for at least 3 months, while preserving efficacy and viability.54 The presence of sucrose was key to maintaining small particle size (100–200 nm) and high particle concentration following lyophilization. Our on-going studies (unpublished) suggest that formulations of these particles can be stored freeze-dried for over a year, and then simply by adding water, their efficacy is restored to the equivalent level of freshly prepared particles and they are also able to be readily injected once water is added.
NANOPARTICLES FOR SIRNA DELIVERY
Polymers from the same class of materials useful for intracellular DNA delivery may also be useful for intracellular siRNA delivery. However, there are important differences between these two nucleic acid cargos. DNA is a molecule ~200 times larger in size than siRNA and it has a circular, supercoiled structure, whereas siRNA’s structure is similar to a short, stiff rod. siRNA is also active in the cytoplasm, whereas DNA needs to be transported into the nucleus to be effective. Due to these differences, in previous work we found that for successful PBAE-mediated siRNA delivery, the siRNA molecules needed to be pre-assembled together as a particulate scaffold first, before addition of the polymer.26
Recently we have shown that gold-siRNA scaffolds are not necessarily needed for PBAE-mediated gene delivery and that end-modified PBAEs and end-modified PAAs can directly form complexes or nanoparticles for successful siRNA delivery.55 Biophysically, the nanoparticles for DNA delivery and for siRNA formed with the same polymers are similar. Particle size is ~100 nm when formed with a range of polymers for both nucleic acids, although in some cases such as B4-S5-E6 at 60 w/w, siRNA particles are significantly smaller than DNA particles (50 nm vs. 100 nm). Zeta potential of the particles is also similar as all particles are generally neutral to slightly positive (0 mV – 15 mV). They also form a similar concentration of particles of approximately 1010 particles per microgram of nucleic acid.55
In spite of these biophysical similarities, transfection efficacy between DNA and siRNA cargos was not strongly correlated and more than half of the polymers evaluated were not able to successfully deliver siRNA. Moreover, for siRNA delivery, higher w/w ratios of polymer to nucleic acid were necessary to mediate siRNA transfection compared to DNA transfection and this difference was also apparent by evaluating polymer/nucleic acid binding strength by gel electrophoresis. In comparing polymers best for DNA delivery and those best for siRNA delivery, B4-S5-E6 and B5-S3-E7 transfected twice or more as many human umbilical vein endothelial cells (HUVECs) when carrying DNA vs. siRNA, B5-S3-E10 and BSS-S4-E1 transfected five times or more HUVECS when carrying siRNA vs. DNA, and B4-S5-E4 and B4-S4-E7 worked very well for both DNA delivery and siRNA delivery, transfecting 60%–90% of these human primary cells with both types of nucleic acid.55
In evaluating these finding some general trends were evident. Small particle size (50–100 nm) was necessary, but not sufficient, to ensure successful DNA or siRNA delivery. Having a higher concentration of particles formed through self-assembly at a standard nucleic acid dose had a slightly positive correlation to improved transfection efficacy, as did having a more positively charged zeta potential.55 Interestingly, disulfide-containing polymers were much more relatively effective for siRNA delivery rather than DNA delivery. This held true when the disulfide was along the backbone (BSS-S4-E1) or the end-group (B5-S3-E10). Since siRNA delivery needs to occur in the cytoplasm and disulfide containing polymers can promote a triggered release when the polymer moves from the extracellular environment to the cytoplasm due to increased levels of glutathione reductase and other reducing enzymes,6, 20, 40 we hypothesize that this quick release is key for successful siRNA delivery with these materials. In contrast for DNA delivery, a more gradual polymer degradation and nucleic acid release may be key to protect the nucleic acid until the nanoparticle traffics through the cell and reaches the nucleus. Our lab is currently researching these mechanisms. Successful intracellular delivery is dependent on multiple parameters and further study is necessary to elucidate how polymer structure more precisely affects function at each delivery step.
NANOPARTICLES FOR CELL-SPECIFIC DELIVERY
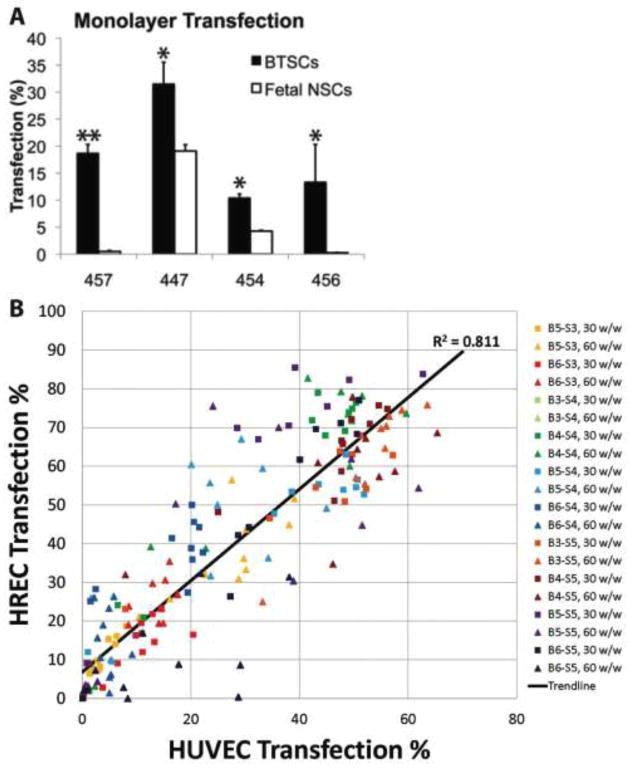
While the cell targeting strategies for cancer gene therapy previously mentioned (ligand targeting, transcriptional targeting, etc.) can facilitate cell-specific delivery, our lab has become increasingly interested in how biomaterial structure itself may also directly mediate cell specificity. For the application to glioblastoma, we found that nanoparticles most effective for transfection of brain tumor stem cells (BTSCs) were not necessarily the same nanoparticles that most successfully transfected BTSC-derived astrocytes. These polymeric nanoparticles also differed in their transfection properties of healthy astrocytes and fetal neural stem cells. Interestingly, we found that certain polymers such as B4-S5-E7 and B4-S5-E6 can significantly transfect BTSCs and do not transfect normal neural stem cells (Figure 4A).54 This differs from other polymers such as B4-S4-E7 that non-specifically transfect BTSCs, neural stem cells, bulk glioblastoma astrocytes, and healthy astrocytes. It is intriguing that such a small difference to chemical structure (5 carbons vs 4 carbons in the hydroxyl-terminated side chain) can make such a significant difference to cell type specify and motivates a more detailed exploration of this biomaterial-mediated phenomenon.
Figure 4.
Biomaterial structure mediates cell-specific delivery. (A) Polymeric nanoparticles preferentially transfect brain tumor stem cells (BTSC) rather than fetal neural stem cells (NSCs). Reproduced with permission from 54. (B) Polymeric nanoparticles highly effective for transfection of macrovasculature (HUVEC) are also highly effective for transfection of microvasculature (HREC). Reproduced with permission from 45. All cells were transfected with GFP DNA and measured by flow cytometry.
We have also observed that with the same base polymer, modification of just the polymer terminal group of a linear PBAE may also correlate to cell-type specificity. We have shown that for a single base polymer, a single end-capping molecule did not create a polymer that was optimal at transfect different types of cells. Instead, each cell type including COS-7, HeLa, HepG2, HUVEC, DC2.4, and hMSC, were transfected optimally by a different end-capped version of the same base polymer.49 Strikingly, end-group structure could cause an order of magnitude change in percent of cells transfected within a cell-type and between cell types.
More recently, we have investigated a broader array of different polymers where the backbone, side chain, and end-capping groups of the polymers have been systematically varied. We have found that with over 100 different polymers, there is a high correlation between polymers that transfect human microvascular endothelial cells and macrovascular endothelial cells (R2 = 0.81) (Figure 4B) and a very low correlation between the polymers that transfect either of these human endothelial cells with polymers that transfect human epithelial cells (R2 = 0.21).45 For an ophthalmic gene delivery application to retinal cells for example, polymers based on B4-S4 or B3-S5 transfect human retinal endothelial cells (HREC) very highly (~80%) while minimally transfecting human retinal pigment epithelial (RPE) cells (0%–5% transfected). Conversely, B6-S5 or B6-S3-based polymers transfect RPE significantly (~25%) while having a lower level of transfection with HRECs. As the nanoparticles themselves have similar properties, we hypothesize that these cell-specific transfection differences are mediated by small chemical differences to the polymer structures. We are currently investigating how this biomaterial-based specificity can be combined with traditional methods of cell targeting3, 21, 36, 44, 46, 61 to further enhance cell-specific intracellular delivery.
FUTURE DIRECTIONS
In this work I have presented work from my lab in synthesizing and evaluating polymeric nanoparticles for intracellular delivery of nucleic acids. Biomaterials are presented that can achieve very high, and in some cases, virus-equivalent,19 non-viral gene delivery efficacy. Importantly, these materials alleviate many of the concerns associated with viruses by having quick biodegradation55 and increased safety, large cargo capacity,8 and ease and stability in manufacture54 among other benefits. However, while a polymer library approach facilitated the selection of the lead polymer structures, a full rational basis for their selection is still not well understood. Amines are important to allow for binding to nucleic acid and for endosomal buffering. Degradable linkages are important for nucleic acid release and reduced cytotoxicity. There needs to be a delicate balance of polymer structural features such as hydrophobicity and side chain length. Small particle size, positive zeta potential, relatively high nanoparticle concentration, and sufficient polymer-nucleic acid binding strength are all parameters that have some influence on transfection efficacy. Future work is needed to identify additional polymer design criteria and determine differential effects of structure on gene delivery transport bottlenecks. Biomaterial-based cell specificity and the possible mechanisms that may mediate it, such as biomaterial interactions with cell membranes, extracellular proteins, and/or intracellular machinery, need to be elucidated. Progress in this field can lead to new translational technologies that can potentially benefit the health of many and is a fascinating area of research within biomedical engineering.
Acknowledgments
This manuscript is based on the 2011 Rita Schaffer Memorial Lecture that was presented in Hartford, CT on October 15, 2011 at the Annual BMES Meeting. I am very grateful to the students in my lab for their critical thinking, dedication, and hard work over the last three years. The Rita Schaffer Young Investigator Award is given in part for a recently published paper and I would like to especially thank Stephany Tzeng, an NSF Graduate Fellow and BME Ph.D. candidate, for her excellent work on gene delivery strategies to treat brain cancer that we published together in Biomaterials.54 I would also like to thank my collaborator, Dr. Alfredo Quiñones-Hinojosa, who is a great colleague and inspires me. I am very thankful to Johns Hopkins for the opportunity to collaborate with such excellent people and conduct research on exciting biomedical engineering problems.
As a young investigator, I would also like to thank the wonderful mentors that I feel privileged to have had guide me through the years. I cannot list them all here, but in particular, I would like to thank Bob Langer who was my advisor at MIT during both my Ph.D. and postdoctoral work. I cannot count the many things that I have learned from Bob, but I can count amongst the most important is a passion for tackling a challenge that can have the potential to improve people’s lives. I would also like to thank my undergraduate research advisor, Todd Przybycien at Carnegie Mellon, who cultivated in me a strong desire for scientific research and introduced me to the fields of biomaterials and gene delivery, fields with which I fell in love.
Finally, I would like to thank the institutions that funded this research. This work was funded in part by the following sources: the Maryland Technology Development Corporation Maryland Stem Cell Research Fund (2009-MSCRFE-0098-00), the National Institutes of Health (R21CA152473), and the Institute for Nanobiotechnology at the Johns Hopkins University.
References
- 1.American Cancer Society. Cancer facts and figures. 2005. Cancer facts and figures 2012: American Cancer Society. [Google Scholar]
- 2.Gene therapy clinical trials worldwide. J Gene Medicine. 2012 [Google Scholar]
- 3.Acharya S, Sahoo SK. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv Drug Delivery Rev. 2011;63(3):170–183. doi: 10.1016/j.addr.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Anderson DG, Lynn DM, Langer R. Semi-automated synthesis and screening of a large library of degradable cationic polymers for gene delivery. Angew Chem Int Ed. 2003;42:3153–3158. doi: 10.1002/anie.200351244. [DOI] [PubMed] [Google Scholar]
- 5.Arote RB, Jiang HL, Kim YK, Cho MH, Choi YJ, Cho CS. Degradable poly(amido amine)s as gene delivery carriers. Expert Opin Drug Deliv. 2011;8(9):1237–1246. doi: 10.1517/17425247.2011.586333. [DOI] [PubMed] [Google Scholar]
- 6.Austin CD, Wen X, Gazzard L, Nelson C, Scheller RH, Scales SJ. Oxidizing potential of endosomes and lysosomes limits intracellular cleavage of disulfide-based antibody-drug conjugates. Proc Natl Acad Sci U S A. 2005;102(50):17987–17992. doi: 10.1073/pnas.0509035102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhise NS, Gray RS, Sunshine JC, Htet S, Ewald AJ, Green JJ. The relationship between terminal functionalization and molecular weight of a gene delivery polymer and transfection efficacy in mammary epithelial 2-D cultures and 3-D organotypic cultures. Biomaterials. 2010;31(31):8088–8096. doi: 10.1016/j.biomaterials.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhise NS, Shmueli RB, Gonzalez J, Green JJ. A novel assay for quantifying the number of plasmids encapsulated by polymer nanoparticles. Small. 2012;8(3):367–373. doi: 10.1002/smll.201101718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci U S A. 1995;92(16):7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buckner JC. Factors influencing survival in high-grade gliomas. Seminars in Oncology. 2003;30:10–14. doi: 10.1053/j.seminoncol.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 11.Check E. Gene therapy put on hold as third child develops cancer. Nature. 2005;433(7026):561–561. doi: 10.1038/433561a. [DOI] [PubMed] [Google Scholar]
- 12.Consortium WTCC. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447(7145):661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dean DA, Strong DD, Zimmer WE. Nuclear entry of nonviral vectors. Gene Ther. 2005;12(11):881–890. doi: 10.1038/sj.gt.3302534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391(6669):806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 15.Gabrielson NP, Lu H, Yin L, Li D, Wang F, Cheng J. Reactive and Bioactive Cationic alpha-Helical Polypeptide Template for Nonviral Gene Delivery. Angew Chem, Int Ed. 2012;51(5):1143–1147. doi: 10.1002/anie.201104262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green JJ, Langer R, Anderson DG. A combinatorial polymer library approach yields insight into nonviral gene delivery. Acc Chem Res. 2008;41(6):749–759. doi: 10.1021/ar7002336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green JJ, Shi J, Chiu E, Leshchiner ES, Langer R, Anderson DG. Biodegradable polymeric vectors for gene delivery to human endothelial cells. Bioconjug Chem. 2006;17:1162–1169. doi: 10.1021/bc0600968. [DOI] [PubMed] [Google Scholar]
- 18.Green JJ, Zhou BY, Mitalipova MM, Beard C, Langer R, Jaenisch R, Anderson DG. Nanoparticles for gene transfer to human embryonic stem cell colonies. Nano Lett. 2008;8(10):3126–3130. doi: 10.1021/nl8012665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Green JJ, Zugates GT, Tedford NC, Huang Y, Griffith LG, Lauffenburger DA, Sawicki JA, Langer R, Anderson DG. Combinatorial modification of degradable polymers enables transfection of human cells comparable to adenovirus. Adv Mater. 2007;19(19):2836–2842. [Google Scholar]
- 20.Griffith OW. Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic Biol Med. 1999;27(9–10):922–935. doi: 10.1016/s0891-5849(99)00176-8. [DOI] [PubMed] [Google Scholar]
- 21.Harris TJ, Green JJ, Fung PW, Langer R, Anderson DG, Bhatia SN. Tissue-specific gene delivery via nanoparticle coating. Biomaterials. 2010;31(5):998–1006. doi: 10.1016/j.biomaterials.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hollon T. Researchers and regulators reflect on first gene therapy death. Nat Med. 2000;6(1):6–6. doi: 10.1038/71545. [DOI] [PubMed] [Google Scholar]
- 23.Huang YH, Zugates GT, Peng W, Holtz D, Dunton C, Green JJ, Hossain N, Chernick MR, Padera RF, Jr, Langer R, Anderson DG, Sawicki JA. Nanoparticle-delivered suicide gene therapy effectively reduces ovarian tumor burden in mice. Cancer Res. 2009;69(15):6184–6191. doi: 10.1158/0008-5472.CAN-09-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khalil IA, Kogure K, Akita H, Harashima H. Uptake pathways and subsequent intracellular trafficking in nonviral gene delivery. Pharmacol Rev. 2006;58(1):32–45. doi: 10.1124/pr.58.1.8. [DOI] [PubMed] [Google Scholar]
- 25.Lai SK, Hida K, Man ST, Chen C, Machamer C, Schroer TA, Hanes J. Privileged delivery of polymer nanoparticles to the perinuclear region of live cells via a non-clathrin, non-degradative pathway. Biomaterials. 2007;28(18):2876–2884. doi: 10.1016/j.biomaterials.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 26.Lee JS, Green JJ, Love KT, Sunshine J, Langer R, Anderson DG. Gold, poly(beta-amino ester) nanoparticles for small interfering RNA delivery. Nano Lett. 2009;9(6):2402–2406. doi: 10.1021/nl9009793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin C, Engbersen JF. Effect of chemical functionalities in poly(amido amine)s for non-viral gene transfection. J Control Release. 2008;132(3):267–272. doi: 10.1016/j.jconrel.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 28.Lin C, Zhong Z, Lok MC, Jiang X, Hennink WE, Feijen J, Engbersen JF. Linear poly(amido amine)s with secondary and tertiary amino groups and variable amounts of disulfide linkages: synthesis and in vitro gene transfer properties. J Control Release. 2006;116(2):130–137. doi: 10.1016/j.jconrel.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 29.Lynn DM, Langer R. Degradable poly(beta-amino esters): Synthesis, characterization, and self-assembly with plasmid DNA. J Am Chem Soc. 2000;122:10761–10768. [Google Scholar]
- 30.Mahato RI. Water insoluble and soluble lipids for gene delivery. Adv Drug Deliv Rev. 2005;57(5):699–712. doi: 10.1016/j.addr.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 31.Mao HQ, Roy K, Troung-Le VL, Janes KA, Lin KY, Wang Y, August JT, Leong KW. Chitosan-DNA nanoparticles as gene carriers: synthesis, characterization and transfection efficiency. J Control Release. 2001;70(3):399–421. doi: 10.1016/s0168-3659(00)00361-8. [DOI] [PubMed] [Google Scholar]
- 32.McGirt MJ, Chaichana KL, Gathinji M, Attenello FJ, Than K, Olivi A, Weingart JD, Brem H, Quinones-Hinojosa AR. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg. 2009;110(1):156–162. doi: 10.3171/2008.4.17536. [DOI] [PubMed] [Google Scholar]
- 33.McGirt MJ, Than KD, Weingart JD, Chaichana KL, Attenello FJ, Olivi A, Laterra J, Kleinberg LR, Grossman SA, Brem H, Quinones-Hinojosa A. Gliadel (BCNU) wafer plus concomitant temozolomide therapy after primary resection of glioblastoma multiforme. J Neurosurg. 2009;110(3):583–588. doi: 10.3171/2008.5.17557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Merdan T, Kopecek J, Kissel T. Prospects for cationic polymers in gene and oligonucleotide therapy against cancer. Adv Drug Delivery Rev. 2002;54(5):715–758. doi: 10.1016/s0169-409x(02)00046-7. [DOI] [PubMed] [Google Scholar]
- 35.Nguyen DN, Green JJ, Chan JM, Langer R, Anderson DG. Polymeric Materials for Gene Delivery and DNA Vaccination. Adv Mater. 2009;21(8):847–867. doi: 10.1002/adma.200801478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ogris M, Walker G, Blessing T, Kircheis R, Wolschek M, Wagner E. Tumor-targeted gene therapy: strategies for the preparation of ligand-polyethylene glycol-polyethylenimine/DNA complexes. J Control Release. 2003;91(1–2):173–181. doi: 10.1016/s0168-3659(03)00230-x. [DOI] [PubMed] [Google Scholar]
- 37.Partridge KA, Oreffo ROC. Gene delivery in bone tissue engineering: Progress and prospects using viral and nonviral strategies. Tissue Eng. 2004;10(1–2):295–307. doi: 10.1089/107632704322791934. [DOI] [PubMed] [Google Scholar]
- 38.Pun SH, Davis ME. Development of a nonviral gene delivery vehicle for systemic application. Bioconjug Chem. 2002;13(3):630–639. doi: 10.1021/bc0155768. [DOI] [PubMed] [Google Scholar]
- 39.Putnam D. Polymers for gene delivery across length scales. Nat Mater. 2006;5(6):439–451. doi: 10.1038/nmat1645. [DOI] [PubMed] [Google Scholar]
- 40.Read ML, Singh S, Ahmed Z, Stevenson M, Briggs SS, Oupicky D, Barrett LB, Spice R, Kendall M, Berry M, Preece JA, Logan A, Seymour LW. A versatile reducible polycation-based system for efficient delivery of a broad range of nucleic acids. Nucleic Acids Res. 2005;33(9):e86. doi: 10.1093/nar/gni085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Richardson SC, Pattrick NG, Man YK, Ferruti P, Duncan R. Poly(amidoamine)s as potential nonviral vectors: ability to form interpolyelectrolyte complexes and to mediate transfection in vitro. Biomacromolecules. 2001;2(3):1023–1028. doi: 10.1021/bm010079f. [DOI] [PubMed] [Google Scholar]
- 42.Schaffer DV, Fidelman NA, Dan N, Lauffenburger DA. Vector unpacking as a potential barrier for receptor-mediated polyplex gene delivery. Biotechnol Bioeng. 2000;67(5):598–606. doi: 10.1002/(sici)1097-0290(20000305)67:5<598::aid-bit10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 43.Segura T, Shea LD. Materials for non-viral gene delivery. Annu Rev Mater Res. 2001;31(1):25–46. [Google Scholar]
- 44.Shmueli RB, Anderson DG, Green JJ. Electrostatic surface modifications to improve gene delivery. Expert Opin Drug Deliv. 2010;7(4):535–550. doi: 10.1517/17425241003603653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shmueli RB, Sunshine JC, Xu Z, Duh EJ, Green JJ. Gene delivery nanoparticles specific for human microvasculature and macrovasculature. Nanomedicine: NBM. 2012 doi: 10.1016/j.nano.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Showalter SL, Huang YH, Witkiewicz A, Costantino CL, Yeo CJ, Green JJ, Langer R, Anderson DG, Sawicki JA, Brody JR. Nanoparticulate delivery of diphtheria toxin DNA effectively kills Mesothelin expressing pancreatic cancer cells. Cancer Biol Ther. 2008;7(10):1584–1590. doi: 10.4161/cbt.7.10.6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sonawane ND, Szoka FC, Verkman AS. Chloride accumulation and swelling in endosomes enhances DNA transfer by polyamine-DNA polyplexes. J Biol Chem. 2003;278(45):44826–44831. doi: 10.1074/jbc.M308643200. [DOI] [PubMed] [Google Scholar]
- 48.Sunshine J, Bhise N, Green JJ. Degradable polymers for gene delivery. Conf Proc IEEE Eng Med Biol Soc. 2009;1:2412–2415. doi: 10.1109/IEMBS.2009.5334767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sunshine J, Green JJ, Mahon K, Yang F, Eltoukhy A, Nguyen DN, Langer R, Anderson DG. Small molecule end group of linear polymer determine cell-type gene delivery efficacy. Adv Mater. 2009;21(48):4947–4951. doi: 10.1002/adma.200901718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sunshine JC, Akanda MI, Li D, Kozielski KL, Green JJ. Effects of base polymer hydrophobicity and end-group modification on polymeric gene delivery. Biomacromolecules. 2011;12(10):3592–3600. doi: 10.1021/bm200807s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sunshine JC, Bishop CJ, Green JJ. Advances in polymeric and inorganic vectors for nonviral nucleic acid delivery. Therapeutic Delivery. 2011;2(4):493–521. doi: 10.4155/tde.11.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 53.Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4(5):346–358. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- 54.Tzeng SY, Guerrero-Cazares H, Martinez EE, Sunshine JC, Quinones-Hinojosa A, Green JJ. Non-viral gene delivery nanoparticles based on poly(beta-amino esters) for treatment of glioblastoma. Biomaterials. 2011;32(23):5402–5410. doi: 10.1016/j.biomaterials.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tzeng SY, Yang PH, Grayson WL, Green JJ. Synthetic poly(ester amine) and poly(amido amine) nanoparticles for efficient DNA and siRNA delivery to human endothelial cells. Int J Nanomed. 2011;6:3309–3322. doi: 10.2147/IJN.S27269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Verma IM, Somia N. Gene therapy -- promises, problems and prospects. Nature. 1997;389(6648):239–242. doi: 10.1038/38410. [DOI] [PubMed] [Google Scholar]
- 57.von Gersdorff K, Sanders NN, Vandenbroucke R, De Smedt SC, Wagner E, Ogris M. The internalization route resulting in successful gene expression depends on both cell line and polyethylenimine polyplex type. Mol Ther. 2006;14(5):745–753. doi: 10.1016/j.ymthe.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 58.Watson JD, Crick FH. Molecular structure of nucleic acids; a structure for deoxyribose nucleic acid. Nature. 1953;171(4356):737–738. doi: 10.1038/171737a0. [DOI] [PubMed] [Google Scholar]
- 59.Yang F, Cho SW, Son SM, Bogatyrev SR, Singh D, Green JJ, Mei Y, Park S, Bhang SH, Kim BS, Langer R, Anderson DG. Genetic engineering of human stem cells for enhanced angiogenesis using biodegradable polymeric nanoparticles. Proc Natl Acad Sci U S A. 2010;107(8):3317–3322. doi: 10.1073/pnas.0905432106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II, Thomson JA. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324(5928):797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou J, Liu J, Cheng CJ, Patel TR, Weller CE, Piepmeier JM, Jiang Z, Saltzman WM. Biodegradable poly(amine-co-ester) terpolymers for targeted gene delivery. Nat Mater. 2012;11(1):82–90. doi: 10.1038/nmat3187. [DOI] [PMC free article] [PubMed] [Google Scholar]