Abstract
Objective
To assess the immediate and longer-term effects of the use of hormonal contraception on the progression of HIV-1 disease in postpartum women.
Design
A prospective cohort study.
Methods
Information on contraceptive use, breastfeeding and intercurrent illnesses was obtained from HIV-infected postpartum Kenyan women monthly in the first year postpartum and quarterly in the second year. Blood was collected for T-cell subset analyses and HIV-1-RNA levels at months 1, 3, 6, 9, 12, 18, and 24 postpartum. The immediate effect of the initiation of oral contraceptive pills (OCP) and depot medroxyprogesterone acetate (DMPA) was assessed by comparing the change in the HIV-1-RNA plasma viral load and CD4 T-cell counts among women remaining off these contraceptive methods with those initiating them. The longer-term effects of OCP and DMPA on disease progression were assessed using Loess curves and linear mixed effects models to compare changes over the first 24 months postpartum in these same disease progression markers.
Results
There were no significant immediate or longer-term effects of the use of OCP or DMPA on HIV-1-RNA plasma viral loads and CD4 T-cell counts in this cohort of HIV-infected postpartum Kenyan women.
Conclusion
Comprehensive contraceptive counselling for HIV-1-infected women requires an understanding of the effects of various contraceptive methods on HIV-1 disease progression. In this study, hormonal contraception reassuringly had no immediate or longer-term effects on the rate of disease progression in chronically HIV-1-infected postpartum women. This highly effective family planning method may provide a useful and safe option for the prevention of mother-to-child transmission of HIV-1.
Keywords: Contraception, depot medroxyprogesterone acetate, HIV-1, oral contraceptive pill, postpartum, progression
Introduction
Several epidemiological studies have assessed the relationship between hormonal contraceptive (HC) use and the acquisition of HIV-1, but little is known about the influence HC use may have on HIV-1 pathogenesis [1,2]. Given that HCs are used by over 100 million women worldwide, and that globally in 2005 approximately 18 million women were living with HIV-1 infection, it is imperative to understand whether HCs affect HIV-1 disease progression [3,4].
One study examining the use of HC at the time of HIV-1 infection showed that the use of depot medroxyprogesterone acetate (DMPA) or oral contraceptive pills (OCP) appeared to increase the risk of acquiring multiple HIV-1 variants, with resultant accelerated HIV-1 disease progression among DMPA users [5,6]. In that case, however, the effect of HCs use on viral load seemed to be caused by the effect of HC on the acquisition of a diverse virus population. Therefore, it remains unclear whether HCs have any direct effect when used during chronic infection. Another study assessing the short-term use of HCs found an increase in cervical shedding of HIV-1 in HC users, but no significant difference in systemic levels of virus [7]. Finally, a large longitudinal study found no difference in the change in HIV-1-RNA plasma viral load over time, and a slight increase in the CD4 cell count over time among HC users compared with women not using HCs [8].
We conducted a prospective cohort study of HIV-1-infected postpartum women in Nairobi, Kenya. The primary aim of this study is to examine correlates of HIV-1 disease progression among postpartum African women. In the present analysis, we describe the relationship between HC use and HIV-1 disease progression in the first 24 months postpartum.
Methods
Study population and procedures
Between October 2000 and June 2005 pregnant HIV-1 seropositive women were referred to the study clinic at Kenyatta National Hospital and enrolled in the prospective cohort study as previously described [9]. After obtaining written informed consent, a standardized questionnaire was used to collect information on history, including contraceptive use. Women received short-course zidovudine to reduce the risk of HIV-1 transmission to the baby [10]. At approximately 32 weeks’ gestation, blood was collected for T-cell subset analyses and HIV-1-RNA levels. At delivery, blood was collected for HIV-1-RNA levels.
Women attended monthly clinic visits in the first year after delivery and quarterly visits in the second year postpartum. At these visits, information on contraceptive use, breastfeeding, and intercurrent illnesses was obtained. Women interested in obtaining hormonal contraception were referred to Nairobi City Council Clinics for contraceptive counselling and management. Blood was collected for T-cell subset analyses and HIV-1-RNA levels at months 1, 3, 6, 9, 12, 18 and 24 postpartum, and women with severe immunosuppression (CD4 cell count < 200 cells/μl) were provided with cotrimoxazole prophylaxis and referred to HIV-1 treatment programmes providing antiretroviral therapy. Women received iron and multivitamin supplementation in the first 6 months postpartum.
Laboratory procedures
Lymphocyte differential counts and CD4 and CD8 cell percentages were conducted using a FACScan flow cytometer (Becton Dickinson, Mountain View, California, USA). Plasma HIV-1 RNA was quantified using the Gen-Probe HIV-1 virus load assay [11].
Statistical analysis
Statistical analyses were performed using SPSS 12.0 for Windows (SPSS, Inc., Chicago, Illinois, USA) or S-PLUS 2000 (Insightful Corporation, Seattle, Washington, USA). Women were censored at the initiation of antiretroviral treatment. Analysis of the immediate effect of HC use was performed using the independent samples t-test, Pearson's chi-square test, and linear regression controlling for importantpotentialconfoundingvariables.Thepatterns of log10 HIV-1 plasma RNA load and CD4 cell count over time were assessed using Loess curves and multivariate linear mixed effects models with an autoregressive 1 correlation structure controlling for baseline levels of the outcome of interest, gravidity, marital status, and breastfeeding status. Women were included in analyses regardless of contraceptive switching. Washout periods to allow for hormone levels to wash out when women switched off of HC were not used because they had very minor effects on the final results.
Results
Of 319 women enrolled in the study, 283 women had viral load, CD4 cell count and contraceptive data after one month postpartum and were included in the analysis of the longer-term effects of HC. Twenty-two women were censored at the time of initiation of antiretroviral treatment (median of 19 months postpartum). The analysis of the immediate effects of HC included 193 women with a visit between one and 6 months postpartum, who did not use HCs or switched from not using HCs to using them. There were no significant differences in the baseline plasma viral load and CD4 cell count between the women excluded from the analysis of immediate effects and the women included.
Immediate effect of hormonal contraceptive use on HIV-1 disease status
To assess the immediate effect of the initiation of HC use on HIV-1 disease status, women were classified into three categories on the basis of their contraceptive use status at the first two visits that were more than one month postpartum (to remove the effects of prenatal zidovudine treatment). These categories were: (i) non-hormonal users: women who reported no HC use at both of these visits (n = 109); (ii) DMPA users: women who reported no HC use at their first visit, and initiated the use of DMPA before their second visit (n = 41); and (iii) OCP users: women who reported no HC use at their first visit, and initiated the use of OCP before their second visit (n = 43). Women who initiated HCs were similar to women in the non-hormonal group in terms of age, education, and gravidity, but tended to be more likely to be married (Table 1).
Table 1.
Characteristics of women initiating hormonal contraceptives and immediate effect of hormonal contraceptives on HIV-1 disease status.
| Variable | NH (N=109) Mean (95% CI) or percentage | OCP (N=41) Mean (95% CI) or percentage | DMPA (N=43) Mean (95% CI) or percentage | P value (OCP versus NH) | P value (DMPA versus NH) |
|---|---|---|---|---|---|
| Demographics | |||||
| Age | 25.51 (24.68, 26.34) | 24.66 (23.58, 25.74) | 25.51 (24.06, 26.97) | 0.3 | 1.0 |
| Education (years) | 9.13 (8.65, 9.60) | 9.80 (8.92, 10.69) | 9.12 (8.36, 9.87) | 0.2 | 1.0 |
| Gravidity | 1.27 (1.03, 1.51) | 0.97 (0.69, 1.26) | 1.21 (0.78, 1.65) | 0.1 | 0.8 |
| Married | 80.7% (88/109) | 92.7% (38/41) | 93.0% (40/43) | 0.08 | 0.06 |
| Breastfed in interval | 66% (70/106) | 73% (29/41) | 65% (28/43) | 0.5 | 0.9 |
| Timing of visits | |||||
| Months between visits | 2.77 (2.40, 3.14) | 2.58 (2.13, 3.04) | 2.46 (1.99, 2.94) | 0.6 | 0.4 |
| Months postpartum at first visita | 1.83(1.60, 2.07) | 1.32 (1.13, 1.51) | 1.42 (1.15, 1.69) | 0.001 | 0.02 |
| Months postpartum at second visita | 4.60 (4.07, 5.13) | 3.90 (3.34, 4.47) | 3.89 (3.19, 4.58) | 0.07 | 0.1 |
| HIV-1 disease status | |||||
| Log10 HIV-1 RNA at first visita | 4.88 (4.70, 5.07) | 4.47 (4.15, 4.80) | 4.83 (4.52, 5.14) | 0.03 | 0.8 |
| Log10 HIV-1 RNA at second visita | 4.92 (4.73, 5.11) | 4.30 (3.92, 4.68) | 4.89 (4.60, 5.18) | 0.002 | 0.9 |
| Change in log10 HIV-1 RNA per quarter | 0.07 (–0.14, 0.28) | –0.18 (–0.42, 0.06) | 0.09 (–0.14, 0.31) | 0.2* | 0.9* |
| CD4 cell count at first visita | 515.10 (457.62, 572.58) | 589.29 (505.54, 673.04) | 551.07 (473.40, 628.74) | 0.2 | 0.5 |
| CD4 cell count at second visita | 495.52 (440.24, 550.81) | 574.29 (493.21, 655.37) | 558.67 (436.61, 680.73) | 0.1 | 0.3 |
| Change in CD4 cell count per quarter | –16.27 (–51.48, 18.94) | –16.34 (–112.02, 79.35) | 30.45 (–101.16, 162.05) | 1.0* | 0.3* |
CI, Confidence interval; DMPA, depot medroxyprogesterone acetate; NH, non-hormonal; OCP, oral contraceptive pill.
At first visit all women were not using hormonal contraceptives. At second visit, OCP and DMPA groups were using hormonal contraceptives.
P>0.05 in multivariate model including breastfeeding status, gravidity, time of first visit, marital status, and CD4 cell count or log10 viral load at first visit.
Women who initiated OCP use had a first visit that was significantly earlier (P = 0.001), and a significantly lower log10 HIV-1-RNA plasma viral load at their first visit (P = 0.03) and at their second visit (P = 0.002) compared with women in the non-hormonal group (Table 1). There was no significant difference in the change in the log10 HIV-1-RNA plasma viral load per quarter of follow-up (P = 0.2), or in the change in CD4 cell count per quarter of follow-up (P = 1.3) between women initiating OCP use and women in the non-hormonal group, indicating no significant immediate effect of OCP initiation on HIV-1 disease status (Table 1). Controlling for potential confounding variables in a multivariate model did not alter these results (Table 1, footnote).
Women who initiated DMPA use were similar to women in the non-hormonal group, with the exception of having a first visit that was significantly earlier (P = 0.02, Table 1). There was no significant difference in the change in the log10 HIV-1-RNA plasma viral load per quarter of follow-up (P = 0.8), or in the change in CD4 cell count per quarter of follow-up (P = 0.7) between women initiating DMPA use and women in the non-hormonal group, indicating no significant immediate effect of DMPA initiation on HIV-1 disease status (Table 1). Controlling for potential confounding variables in a multivariate model did not alter these results (Table 1, footnote) and results for the change over time in viral load and the change over time in CD4 cell count were similar when DMPA and OCP users were combined into one group (data not shown).
Longer-term effect of hormonal contraceptive use on HIV-1 disease status
The median time of the initiation of OCP and DMPA in the cohort was 3.26 months postpartum [interquartile range (IQR) 2.13, 6.11] and 4.20 months postpartum (IQR 2.10, 7.43), respectively. To assess the longer-term effect of HC use on HIV-1 disease status, analyses were limited to visits 3 or more months postpartum to allow for the initiation of contraceptive use. A total of 283 women had visits 3 or more months postpartum, with 73.64 person-years of follow-up of exposure to OCP, 123.32 person-years of exposure to DMPA, and 153.00 person-years of no exposure to hormonal contraception.
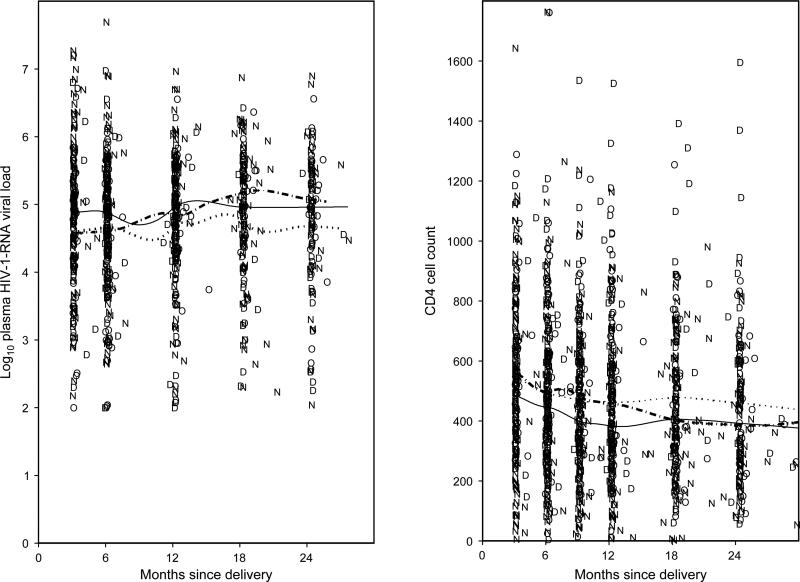
Figure 1 shows the changes in log10 plasma HIV-1-RNA and CD4 T-cell counts over time for OCP, DMPA and non-hormonal use. Using linear mixed effects models, there was no significant difference in the levels of plasma HIV-1 RNA at the baseline visit (approximately 3 months postpartum) in women currently exposed to OCP or DMPA compared with non-hormonal users (P = 0.3 and P = 0.9, respectively). In addition, there was no difference in the change over time up to 24 months postpartum in plasma HIV-1 RNA during DMPA use compared with non-hormonal use (P = 0.7), even after controlling for potential confounding variables (P = 1.0). There was, however, a trend for plasma HIV-1 RNA to increase faster over time up to 24 months postpartum during OCP use compared with non-hormonal use (0.015 versus 0.002 log10 copies per month, P = 0.08). This relationship was still not statistically significant after controlling for potential confounding variables (P = 0.1). Similar models indicated no differences in baseline (approximately 3 months postpartum) levels of CD4 T-cell counts during OCP and DMPA use compared with non-hormonal use (P = 0.2 and P = 0.2), and no differences in the change in CD4 T-cell counts over time during OCP use, after controlling for potential confounding variables (P = 0.9). There was, however, a trend for a slower decrease in CD4 cell counts in DMPA users that was still not statistically significant after controlling for potential confounding variables (–1.69 versus –4.53 cells/month, P = 0.08).
Fig. 1. Longer-term effect of hormonal contraceptive use on HIV-1 disease status.
D, Depot medroxyprogesterone acetate; N,
Depot medroxyprogesterone acetate; N, , non-hormonal; O,
, non-hormonal; O, , oral contraceptive pill.
, oral contraceptive pill.
Discussion
Effective contraceptive choices for HIV-infected women are essential to decrease mother-to-child transmission of HIV-1 through the prevention of unintended pregnancies, and to reduce the incidence of morbidity and mortality associated with pregnancy in HIV-infected women [12]. Comprehensive family planning counselling for HIV-infected women, however, requires knowledge regarding the potential health effects of using various contraceptive methods [13]. This study adds to that knowledge by finding no significant immediate or longer-term effects of OCP or DMPA use on plasma HIV-1-RNA levels or on CD4 T-cell counts in a cohort of HIV-infected postpartum women.
Hormonal contraception could influence HIV-1 disease progression by altering the host immune system and vulnerability to the virus or through direct effects on the virus itself [14–17]. We assessed both of these potential mechanisms by looking at changes in both HIV-1-RNA levels and CD4 T-cell counts, which have been shown to be good surrogate markers of HIV-1 disease progression [18]. This is the first study in Africa to assess the relationship between HC use and disease progression in postpartum women. Our results are similar to those found in both a study of the short-term effects of hormonal contraception conducted in a cohort of HIV-infected Kenyan women, and a cohort study looking at longer-term effects conducted in a general population of HIV-infected women in the United States [7,8].
The strengths of our study include the frequent measurement of exposure to HC and disease progression markers, and the extended follow-up of women, allowing assessment of both immediate and longer-term effects. Limitations include the fact that the study was not randomized so that bias could be induced by women self-selecting contraceptive methods, and the small number of women who initiated HC in the analysis of immediate effects, which may have limited power. In addition, the generalizability of these results may be limited to the population of postnatal HIV-infected women with well-preserved CD4 cell counts.
The prevention of mother-to-child transmission treatment programmes are a key entry point for women in Africa to learn of their HIV infection status, to initiate family planning methods, and to be counselled about condom use and the idea of dual protection to prevent subsequent pregnancies. Effective contraceptive counselling in this large specific group requires an understanding of the effects of contraceptive methods on the health of HIV-infected women. Hormonal contraception, a highly effective family planning method, reassuringly has no immediate or longer-term effects on the rate of disease progression in HIV-infected postpartum women.
Acknowledgments
Sponsorship: This research was supported by National Institutes of Health grants R01 HD23421 and AI27757. P. Otieno was a scholar in the AIDS International Training and Research Program supported by the NIH Fogarty International Center grant D43-TW00007.
The study was approved by the University of Nairobi Ethical Review Committee and the University of Washington Institutional Review Board, and all women provided informed consent. Human experimentation guidelines of the University of Nairobi and University of Washington were followed in the conduct of the clinical research.
References
- 1.Martin HL, Jr, Richardson BA, Mandaliya K, Achola JO, Overbaugh J, Kreiss JK. The early work on hormonal contraceptive use and HIV acquisition. J Acquir Immune Defic Syndr. 2005;38(Suppl. 1):S12–S14. doi: 10.1097/01.qai.0000167027.33525.1c. [DOI] [PubMed] [Google Scholar]
- 2.Morrison CS, Richardson BA, Celentano DD, Chipato T, Mmiro F, Mugerwa R, et al. Prospective clinical trials designed to assess the use of hormonal contraception and risk of HIV acquisition. J Acquire Immune Defic Syndr. 2005;38(Suppl. 1):S17–S18. doi: 10.1097/01.qai.0000167029.41149.ad. [DOI] [PubMed] [Google Scholar]
- 3.Population Reference Bureau . Family planning worldwide 2002 data sheet. Population Reference Bureau; Washington, DC: 2002. [Google Scholar]
- 4.UNAIDS . AIDS epidemic update: December 2005. Joint United Nations Programme on HIV/AIDS (UNAIDS) and World Health Organization; Geneva, Switzerland: 2005. [PubMed] [Google Scholar]
- 5.Lavreys L, Baeten JM, Kreiss JK, Richardson BA, Chohan BH, Hassan W, et al. Injectable contraceptive use and genital ulcer disease during the early phase of HIV-1 infection increase plasma virus load in women. J Infect Dis. 2004;190:2057–2058. doi: 10.1086/380974. [DOI] [PubMed] [Google Scholar]
- 6.Sagar M, Lavreys L, Baeten JM, Richardson BA, Mandaliya K, Ndinya-Achola J, et al. Identification of modifiable factors that affect the genetic diversity of the transmitted HIV-1 population. AIDS. 2004;18:615–619. doi: 10.1097/00002030-200403050-00005. [DOI] [PubMed] [Google Scholar]
- 7.Wang C, McClelland RS, Overbaugh J, Reilly M, Panteleeff DD, Mandaliya K, et al. The effect of hormonal contraception on genital tract shedding of HIV-1. AIDS. 2004;18:205–209. doi: 10.1097/00002030-200401230-00009. [DOI] [PubMed] [Google Scholar]
- 8.Cejtin HE, Jacobson L, Springer G, Watts DH, Levine A, Greenblatt R, et al. Effect of hormonal contraceptive use on plasma HIV-1 RNA levels among HIV-infected women. AIDS. 2003;17:1702–1704. doi: 10.1097/00002030-200307250-00019. [DOI] [PubMed] [Google Scholar]
- 9.Otieno PA, Brown ER, Mbori-Ngacha DA, Nduati RW, Farquhar C, Obimbo EM, et al. HIV-1 disease progression in breast feeding and formula feeding mothers: a prospective 2-year comparison of T-cell subsets, HIV-1 RNA levels, and mortality. J Infect Dis. 2007;195:220–229. doi: 10.1086/510245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaffer N, Chuachoowong R, Mock PA, Bhadrakom C, Siriwasin W, Young NL, et al. Short-course zidovudine for perinatal HIV-1 transmission in Bangkok, Thailand: a randomised controlled trial. Lancet. 1999;353:773–780. doi: 10.1016/s0140-6736(98)10411-7. [DOI] [PubMed] [Google Scholar]
- 11.Emery S, Bodrug S, Richardson BA, Giachetti C, Bott MA, Panteleeff D, et al. Evaluation of the performance of the Gen-Probe human immunodeficiency virus type 1 viral load assay using primary isolates from Kenya. J Clin Microbiol. 2000;38:2688–2695. doi: 10.1128/jcm.38.7.2688-2695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duerr A, Hurst S, Kourtis AP, Rutenberg N, Jamieson DJ. Integrating family planning and prevention of mother-to-child HIV transmission in resource-limited settings. Lancet. 2005;366:261–263. doi: 10.1016/S0140-6736(05)66917-6. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell HS, Stephens E. Contraceptive choice for HIV positive women. Sex Transm Infect. 2004;80:167–173. doi: 10.1136/sti.2003.008441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Auerbach L, Hafner T, Huber JC, Panzer S. Influence of low-dose oral contraception on peripheral blood lymphocyte subsets at particular phases of the hormonal cycle. Fertil Steril. 2002;78:83–89. doi: 10.1016/s0015-0282(02)03173-4. [DOI] [PubMed] [Google Scholar]
- 15.Hunt JS, Miller L, Platt JS. Hormonal regulation of uterine macrophages. Dev Immunol. 1998;6:105–110. doi: 10.1155/1998/87527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prakash M, Kapembwa MS, Gotch F, Patterson S. Oral contraceptive use induces upregulation of the CCR5 chemokine receptor on CD4(+) T cells in the cervical epithelium of healthy women. J Reprod Immunol. 2002;54:117–131. doi: 10.1016/s0165-0378(01)00125-5. [DOI] [PubMed] [Google Scholar]
- 17.Benki S, McClelland RS, Overbaugh J. Risk factors for human immunodeficiency virus type-1 acquisition in women in Africa. J Neurovirol. 2005;11:58–65. [PubMed] [Google Scholar]
- 18.Geskus RB, Miedema FA, Goudsmit J, Reiss P, Schuitemaker H, Coutinho RA. Prediction of residual time to AIDS and death based on markers and cofactors. J Acquir Immune Defic Syndr. 2003;32:514–521. doi: 10.1097/00126334-200304150-00008. [DOI] [PubMed] [Google Scholar]