Abstract
Objective:
Lipid accumulation in skeletal muscle and the liver is strongly implicated in the development of insulin resistance and type 2 diabetes, but the mechanisms underpinning fat accrual in these sites remain incompletely understood. Accumulating evidence of muscle mitochondrial dysfunction in insulin-resistant states has fuelled the notion that primary defects in mitochondrial fat oxidation may be a contributory mechanism. The purpose of our study was to determine whether patients with congenital lipodystrophy, a disorder primarily affecting white adipose tissue, manifest impaired mitochondrial oxidative phosphorylation in skeletal muscle.
Research Design and Methods:
Mitochondrial oxidative phosphorylation was assessed in quadriceps muscle using 31P-magnetic resonance spectroscopy measurements of phosphocreatine recovery kinetics after a standardized exercise bout in nondiabetic patients with congenital lipodystrophy and in age-, gender-, body mass index-, and fitness-matched controls.
Results:
The phosphocreatine recovery rate constant (k) was significantly lower in patients with congenital lipodystrophy than in healthy controls (P < 0.001). This substantial (∼35%) defect in mitochondrial oxidative phosphorylation was not associated with significant changes in basal or sleeping metabolic rates.
Conclusions:
Muscle mitochondrial oxidative phosphorylation is impaired in patients with congenital lipodystrophy, a paradigmatic example of primary adipose tissue dysfunction. This finding suggests that changes in mitochondrial oxidative phosphorylation in skeletal muscle could, at least in some circumstances, be a secondary consequence of adipose tissue failure. These data corroborate accumulating evidence that mitochondrial dysfunction can be a consequence of insulin-resistant states rather than a primary defect. Nevertheless, impaired mitochondrial fat oxidation is likely to accelerate ectopic fat accumulation and worsen insulin resistance.
Insulin resistance (IR) precedes and predicts the development of type 2 diabetes mellitus (T2DM) (1). It also underpins the association between obesity and T2DM and accounts for many of the associated abnormalities that constitute the metabolic syndrome. Insulin sensitization implemented by lifestyle intervention, bariatric surgery, or pharmacotherapy is highly effective in reducing the risk of subsequent diabetes. However, the molecular basis of IR remains incompletely understood.
One prevalent hypothesis posits accumulation of toxic lipid species in nonadipose tissues, notably skeletal muscle and liver, as a cause of IR (2). Supporting evidence includes elegant studies in mice genetically engineered to promote or limit fat accumulation in specific nonadipose tissues (reviewed in Ref. 2). These and other studies have elucidated the potential contributions of two categories of mechanism: excess lipid supply or delivery to tissues, and impaired lipid export or oxidation. However, their relative importance remains largely unknown in most patients. Impaired mitochondrial fat oxidation has recently emerged as potentially important (3). An association between mitochondrial dysfunction and IR is found in several, although not all, studies, but whether as a cause or consequence of ectopic lipid accumulation remains unclear (3). Supporting data include morphological, genetic, and biochemical analyses of tissue samples and isolated mitochondria, and noninvasive magnetic resonance spectroscopy (MRS)-based measures of mitochondrial function (Ref. 3 and references therein). Although some studies have shown unchanged or increased mitochondrial mass and/or oxidative function in insulin-resistant states (4, 5), on balance the evidence for an association between IR and reduced mitochondrial oxidative function appears robust; however, the causal direction remains uncertain (reviewed in Ref. 3, 6).
We have shown that muscle mitochondrial oxidative phosphorylation, as measured by postexercise phosphocreatine (PCr) recovery kinetics, is impaired in nondiabetic but severely insulin-resistant patients harboring mutations in the INSR (7). This suggests that it is at least possible for primary congenital IR to lead to a reduction in muscle mitochondrial oxidative phosphorylation in humans. Several mouse studies have reached a similar conclusion (3).
Congenital lipodystrophy is a rare cause of severe IR characterized by extreme dysfunction of white adipose tissue, in which mutations in proteins involved in adipocyte differentiation (LMNA, ZMPSTE24, PPARG, BSCL2, AKT2), fatty acid uptake by adipocytes (CAV1, PTRF), triglyceride synthesis (AGPAT2), and/or lipid droplet formation (CIDEC, PLIN1) lead to a partial or generalized lack of adipose tissue, considerable accumulation of ectopic fat, particularly in the liver and skeletal muscle, and severe IR (8). Lipodystrophy can be a model for other, more common examples of “white fat failure” and ectopic fat deposition, such as obesity-related IR in the setting of a high-fat/high-carbohydrate diet.
Here we ask whether patients with congenital, genetically proven lipodystrophy also manifest impaired skeletal muscle mitochondrial function, as measured by postexercise PCr recovery kinetics. PCr recovery relies purely on oxidative ATP synthesis (9) and can be quantified using 31P-MRS, most conveniently as the PCr recovery rate constant (k), which is proportional to functional mitochondrial oxidative capacity.
Subjects and Methods
Participants
Participants provided written informed consent, and studies were conducted in accordance with the principles of the Declaration of Helsinki. Clinical studies were approved by a National Health Service Research Ethics Committee.
Patients with lipodystrophy were identified as part of a long-standing study of human IR syndromes. We recruited patients without overt diabetes because hyperglycemia is a known cause of mitochondrial dysfunction. Healthy age-, gender-, and body mass index (BMI)-matched control volunteers were recruited by advertisement. All were sedentary nonsmokers without medical disorders likely to affect energy metabolism. Data from 12 of the healthy volunteers were included in a previously published study (7).
Experimental protocol and PCr recovery kinetics after exercise
See Supplemental Data (published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org).
Intramyocellular lipid (IMCL) measurement
IMCL was determined as described previously (10).
Statistical analysis
Statistical analysis was performed in SPSS PASW Statistics 18 (SPSS Inc., Chicago, IL). Quantitative data are presented as mean ± sem. Two-tailed independent samples t test was used to compare group means, with significance defined as P < 0.05.
Results
Characteristics of the participants (Table 1 and Supplemental Table 1)
Table 1.
Characteristics of the healthy volunteers (controls) and patients with lipodystrophy
| Gender | Controls 3 M, 12 F | Lipodystrophy 1 M, 6 F | P value 0.760 |
|---|---|---|---|
| Age (yr) | 29.5 ± 1.7 | 34.8 ± 3.6 | 0.148 |
| Weight (kg) | 69.2 ± 3.6 | 67.9 ± 5.2 | 0.839 |
| BMI (kg/m2) | 24.8 ± 1.1 | 24.4 ± 1.3 | 0.855 |
| Fat mass (kg) | 21.3 ± 2.3 | 11.0 ± 1.7 | 0.009 |
| FFM (kg) | 47.0 ± 2.7 | 57.5 ± 4.6 | 0.048 |
| Glucose (mg/dl) | 79.3 ± 1.8 | 82.9 ± 3.6 | 0.389 |
| Insulin (μU/ml) | 5 ± 1 | 19 ± 4 | 0.021 |
| HOMA-IR | 0.6 ± 0.3 | 2.3 ± 1.3 | <0.0001 |
| Adiponectin (mg/liter) | 5.8 ± 0.7 | 2.2 ± 0.5 | 0.003 |
| Leptin (μg/liter) | 13.9 ± 2.6 | 4.4 ± 1.2 | 0.027 |
| Triglyceride (mg/dl) | 88.5 ± 8.8 | 194.7 ± 35.4 | 0.017 |
| HDL-cholesterol (mg/dl) | 61.8 ± 3.9 | 27.0 ± 3.9 | <0.0001 |
| NEFA (μmol/liter) | 243 ± 22 | 183 ± 26 | 0.118 |
| Exercise weight (kg) | 2.9 ± 0.2 | 3.6 ± 0.5 | 0.118 |
| Muscular strength (Nm/kg FFM) | 1.96 ± 0.09 | 1.97 ± 0.18 | 0.942 |
| k (min−1) | 2.57 ± 0.13 | 1.68 ± 0.10 | <0.001 |
| Predicted VO2max (ml/kg/min) | 38.2 ± 1.5 | 35.5 ± 1.7 | 0.335 |
| Resting pHi | 7.015 ± 0.008 | 7.025 ± 0.010 | 0.480 |
| End of exercise pHi | 7.072 ± 0.006 | 7.058 ± 0.013 | 0.266 |
| PCr depletion (% of resting) | 22.1 ± 1.8 | 24.5 ± 2.6 | 0.455 |
| Resting Pi:PCr | 0.088 ± 0.003 | 0.098 ± 0.006 | 0.099 |
| Resting Pi:ATP | 0.334 ± 0.012 | 0.400 ± 0.019 | 0.007 |
| Resting PCr:ATP | 3.81 ± 0.06 | 4.12 ± 0.10 | 0.013 |
| Resting PDE:ATP | 0.407 ± 0.021 | 0.631 ± 0.074 | 0.023 |
Data are presented as mean ± sem. To convert the values for glucose to millimoles per liter, multiply by 0.0555; to convert insulin to picomoles per liter, multiply by 6.945; to convert triglyceride to millimoles per liter, multiply by 0.00113; to convert HDL-cholesterol to millimoles per liter, multiply by 0.0259. Bold P values are statistically significant. M, Male; F, female; HOMA-IR, homeostatic model assessment-IR; NEFA, nonesterified fatty acid; k, PCr recovery rate constant; VO2max, maximal oxygen consumption; pHi, intracellular pH; Pi, inorganic phosphate; PDE, phosphodiester; FFM, fat-free mass.
Seven patients (P1–P7) with mutations known to be associated with partial or generalized lipodystrophy were recruited. All were unrelated, except P2 and P3 who were nonidentical twins. Six had familial partial lipodystrophy (FPLD), four due to mutations in LMNA (FPLD2), and two to mutations in PPARG (FPLD3). One patient (P6), the only male, had congenital generalized lipodystrophy due to compound heterozygous mutations in BSCL2. Six patients had acanthosis nigricans, a cutaneous marker of severe IR. Two patients (P1 and P7) were taking metformin, but this was stopped 72 h before the study. One patient (P5) was taking fenofibrate and omega-3 fatty acid ethyl esters at the time of the study. Glycated hemoglobin levels were normal in six of the patients and minimally elevated in one. IMCL levels were elevated in five patients (Supplemental Table 1).
Control participants were matched for age, gender, and BMI with the patients. As a group, the patients had a significantly lower fat mass and greater lean mass than controls, higher insulin and triglyceride concentrations, and lower adiponectin, leptin, and high-density lipoprotein (HDL)-cholesterol concentrations. Estimated maximal oxygen consumption (VO2max) and muscle strength were similar in both groups (Table 1).
Assessment of muscle mitochondrial function
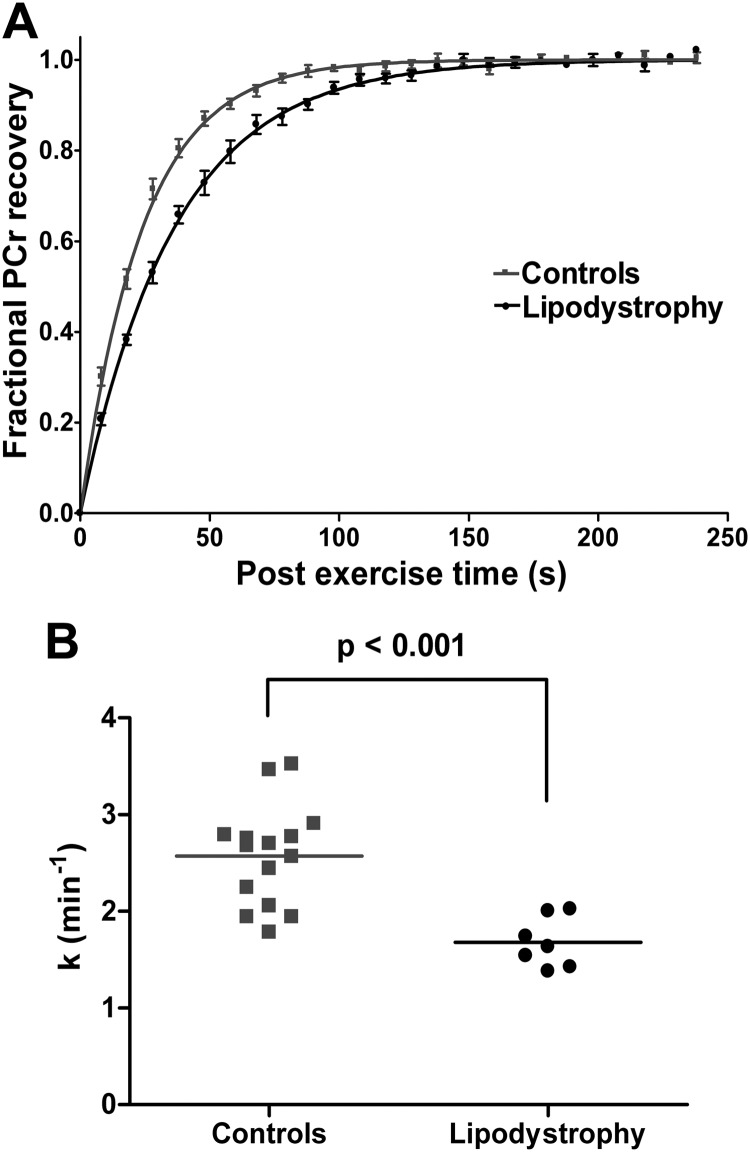
Postexercise PCr recovery was significantly slower in the lipodystrophic patients compared with controls (Fig. 1 and Table 1). Neither the resting nor end-exercise intracellular pH (pHi) differed significantly between the patients and the controls (Table 1), and end-exercise pHi was not significantly lower than resting pHi in either group. The resting phosphate metabolite ratios inorganic phosphate:ATP, PCr:ATP, and phosphodiester:ATP were significantly higher in the lipodystrophy patients (Table 1), whereas inorganic phosphate:PCr did not differ significantly; these findings may reflect a slight reduction in muscle cellular ATP concentrations in the patients, which would, however, require direct measurement in biopsy samples for confirmation.
Fig. 1.
31P-MRS measurements of mitochondrial oxidative phosphorylation. A, Mean fractional PCr recovery curves for controls (gray squares; n = 15) and patients with lipodystrophy (black circles; n = 7). Five spectra were averaged to give a time resolution of 10 sec for clarity in this figure. The monoexponential fit using the mean recovery rate constant is shown for controls (gray line) and patients with lipodystrophy (black line). B, Recovery rate constant (k) as measured from the PCr recovery for controls (gray squares; n = 15) and patients with lipodystrophy (black circles; n = 7). The horizontal line represents the mean for each group.
Impact of mitochondrial dysfunction on metabolic rate
To determine whether IR and/or impaired mitochondrial oxidative phosphorylation might alter metabolic rate, we measured basal metabolic rate and sleeping metabolic rate. Although both were higher in the lipodystrophic group, results were similar in both groups after correcting for fat-free mass (Supplemental Table 2).
Discussion
We have documented slower postexercise PCr resynthesis in seven patients with three distinct subtypes of genetically proven lipodystrophy and severe IR. We selected patients without overt diabetes to minimize the confounding influence of hyperglycemia. This did, however, make recruitment very difficult because most patients with congenital lipodystrophy develop diabetes at a relatively early age. We could not recruit any with lipodystrophy due to PLIN1 or CIDEC mutations because all our patients with these disorders are already diabetic. Nevertheless, our data suggest that mitochondrial oxidative phosphorylation is impaired regardless of the cause of lipodystrophy.
Substantial evidence now suggests that impairment of mitochondrial oxidative function in skeletal muscle is associated with ectopic lipid accumulation, IR, and T2DM (3, 6). However, the direction of causality remains uncertain (3, 6). One line of evidence suggesting that this may be a consequence of reduced insulin action arose from human studies in type 1 diabetes (insulin deprivation), suggesting that reduced insulin signaling can lead to a reduction in muscle mitochondrial ATP production rate and reduced expression of oxidative phosphorylation genes (11). This was supported by rodent studies demonstrating reduced mitochondrial oxidative metabolism in tissues in which expression of either the insulin receptor itself (12) or proximal insulin signaling components (13) were reduced. Additionally, in rodents fed high-fat/high-sucrose diets, IR preceded impairments in mitochondrial oxidative function (14).
We recently reported human evidence to support these findings by demonstrating that primary IR due to loss-of-function mutations in the INSR can lead to slower skeletal muscle PCr recovery after exercise (7). We now corroborate this in another human congenital disorder in which mitochondrial oxidative phosphorylation in skeletal muscle is likely to be a consequence of primary adipose tissue dysfunction. Congenital lipodystrophy is a rare monogenic disorder in which mutations in 10 different genes have been identified, each plausibly linked to defects in adipocyte differentiation or lipid metabolism (8). In a few subtypes (particularly those due to CIDEC and PLIN1 mutations) the proteins are almost exclusively expressed in white adipose tissue, so it is clear that peripheral IR in muscle must be a consequence of primary defects in adipose tissue function. In other cases (such as those associated with mutations in LMNA and PPARG), the proteins involved are more widely expressed so one cannot entirely rule out a primary role for them in myocytes and even in myocyte mitochondrial dysfunction; nevertheless, the dominant clinical defect appears to be impaired white adipose tissue function.
PCr recovery after exercise is a widely accepted measure of mitochondrial function. It is a system property, being an integrated function of muscle mitochondrial numbers; mitochondrial content of enzymes, transporters, and respiratory chain complexes; the activity of relevant cytosolic enzymes; and the cardiorespiratory physiology affecting substrate and oxygen supply to active muscle (15, 16). In many physiological situations where changes in these factors are expected or measured (e.g. by ex vivo measurements), PCr recovery kinetics almost invariably behave in the expected manner. We chose to measure PCr recovery kinetics by 31P-MRS because this is widely accepted as the only noninvasive measure of skeletal muscle mitochondrial oxidative capacity in vivo, and it has the advantage over muscle biopsy of studying a much larger volume of active muscle while retaining good correlations with biopsy measures of mitochondrial oxidative function (17). Our patients had no clinical evidence of cardiovascular or pulmonary disease, and therefore no reason to suppose that the substantial (∼35%) defect we identified lies “upstream” of the mitochondrion; nor is it the consequence of differing aerobic fitness or relative muscular strength, which was similar in both groups. We cannot comment on whether the observed impairment of mitochondrial oxidative function was secondary to a reduction in mitochondrial content as previously suggested (18–20) because muscle biopsies were not provided.
In conclusion, we have documented evidence of impairment of muscle mitochondrial oxidative phosphorylation in human congenital lipodystrophies. These data support emerging evidence that mitochondrial dysfunction can be a consequence, rather than necessarily a cause of IR. Nevertheless, this secondary metabolic defect may compound the problem by exacerbating the mismatch between the need for mitochondrial fat oxidation and the capacity to oxidize fat, and thus accelerate ectopic fat accumulation and progression to overt diabetes. Our data do not negate the possibility that in other states muscle mitochondrial dysfunction could cause IR, but they highlight the need to not simply assume that this is the case.
Supplementary Material
Acknowledgments
We thank all the participants in the study and J. Harris, L. McGrath, and the staff of the Wellcome Trust Clinical Research Facility for assistance with clinical studies.
This work was supported by grants from the Wellcome Trust (to S.O. and D.B.S.), the U.K. National Institute for Health Research Cambridge Biomedical Research Centre, the U.K. Medical Research Council Centre for Obesity and Related Metabolic Diseases, and the Clinical Research Infrastructure Grant.
Authors' Contributions: D.B.S. designed the study. A.Sl., K.T., S.B., T.A.C., and P.R.M. developed the methodology used. A.Sl., A.St., L.W., N.S., and D.B.S. researched the data. A.St., A.G., V.I.C., S.N., and D.B.S. recruited participants and supervised the studies. A.Sl., A.St., S.O., G.J.K., and D.B.S. analyzed the data and wrote the manuscript, which was then edited by all the authors.
Disclosure Summary: The authors have declared that no conflict of interest exists.
Footnotes
- BMI
- Body mass index
- FPLD
- familial partial lipodystrophy
- HDL
- high-density lipoprotein
- IMCL
- intramyocellular lipid
- IR
- insulin resistance
- MRS
- magnetic resonance spectroscopy
- PCr
- phosphocreatine
- pHi
- intracellular pH
- T2DM
- type 2 diabetes mellitus.
References
- 1. Tabák AG, Jokela M, Akbaraly TN, Brunner EJ, Kivimäki M, Witte DR. 2009. Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: an analysis from the Whitehall II study. Lancet 373:2215–2221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Savage DB, Petersen KF, Shulman GI. 2007. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev 87:507–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Patti ME, Corvera S. 2010. The role of mitochondria in the pathogenesis of type 2 diabetes. Endocr Rev 31:364–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hancock CR, Han DH, Chen M, Terada S, Yasuda T, Wright DC, Holloszy JO. 2008. High-fat diets cause insulin resistance despite an increase in muscle mitochondria. Proc Natl Acad Sci USA 105:7815–7820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bajpeyi S, Pasarica M, Moro C, Conley K, Jubrias S, Sereda O, Burk DH, Zhang Z, Gupta A, Kjems L, Smith SR. 2011. Skeletal muscle mitochondrial capacity and insulin resistance in type 2 diabetes. J Clin Endocrinol Metab 96:1160–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schrauwen P, Schrauwen-Hinderling V, Hoeks J, Hesselink MK. 2010. Mitochondrial dysfunction and lipotoxicity. Biochim Biophys Acta 1801:266–271 [DOI] [PubMed] [Google Scholar]
- 7. Sleigh A, Raymond-Barker P, Thackray K, Porter D, Hatunic M, Vottero A, Burren C, Mitchell C, McIntyre M, Brage S, Carpenter TA, Murgatroyd PR, Brindle KM, Kemp GJ, O'Rahilly S, Semple RK, Savage DB. 2011. Mitochondrial dysfunction in patients with primary congenital insulin resistance. J Clin Invest 121:2457–2461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garg A. 2011. Lipodystrophies: genetic and acquired body fat disorders. J Clin Endocrinol Metab 96:3313–3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Taylor DJ, Bore PJ, Styles P, Gadian DG, Radda GK. 1983. Bioenergetics of intact human muscle. A 31P nuclear magnetic resonance study. Mol Biol Med 1:77–94 [PubMed] [Google Scholar]
- 10. Finucane FM, Horton J, Purslow LR, Savage DB, Brage S, Besson H, Horton K, Rolfe Ede L, Sleigh A, Sharp SJ, Martin HJ, Sayer AA, Cooper C, Ekelund U, Griffin SJ, Wareham NJ. 2009. Randomized controlled trial of the efficacy of aerobic exercise in reducing metabolic risk in healthy older people: the Hertfordshire Physical Activity Trial. BMC Endocr Disord 9:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Karakelides H, Asmann YW, Bigelow ML, Short KR, Dhatariya K, Coenen-Schimke J, Kahl J, Mukhopadhyay D, Nair KS. 2007. Effect of insulin deprivation on muscle mitochondrial ATP production and gene transcript levels in type 1 diabetic subjects. Diabetes 56:2683–2689 [DOI] [PubMed] [Google Scholar]
- 12. Boudina S, Bugger H, Sena S, O'Neill BT, Zaha VG, Ilkun O, Wright JJ, Mazumder PK, Palfreyman E, Tidwell TJ, Theobald H, Khalimonchuk O, Wayment B, Sheng X, Rodnick KJ, Centini R, Chen D, Litwin SE, Weimer BE, Abel ED. 2009. Contribution of impaired myocardial insulin signaling to mitochondrial dysfunction and oxidative stress in the heart. Circulation 119:1272–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cheng Z, Guo S, Copps K, Dong X, Kollipara R, Rodgers JT, Depinho RA, Puigserver P, White MF. 2009. Foxo1 integrates insulin signaling with mitochondrial function in the liver. Nat Med 15:1307–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bonnard C, Durand A, Peyrol S, Chanseaume E, Chauvin MA, Morio B, Vidal H, Rieusset J. 2008. Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J Clin Invest 118:789–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Forbes SC, Raymer GH, Kowalchuk JM, Thompson RT, Marsh GD. 2008. Effects of recovery time on phosphocreatine kinetics during repeated bouts of heavy-intensity exercise. Eur J Appl Physiol 103:665–675 [DOI] [PubMed] [Google Scholar]
- 16. McMahon S, Jenkins D. 2002. Factors affecting the rate of phosphocreatine resynthesis following intense exercise. Sports Med 32:761–784 [DOI] [PubMed] [Google Scholar]
- 17. Larson-Meyer DE, Newcomer BR, Hunter GR, Joanisse DR, Weinsier RL, Bamman MM. 2001. Relation between in vivo and in vitro measurements of skeletal muscle oxidative metabolism. Muscle Nerve 24:1665–1676 [DOI] [PubMed] [Google Scholar]
- 18. Ritov VB, Menshikova EV, He J, Ferrell RE, Goodpaster BH, Kelley DE. 2005. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes 54:8–14 [DOI] [PubMed] [Google Scholar]
- 19. Morino K, Petersen KF, Dufour S, Befroy D, Frattini J, Shatzkes N, Neschen S, White MF, Bilz S, Sono S, Pypaert M, Shulman GI. 2005. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest 115:3587–3593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boushel R, Gnaiger E, Schjerling P, Skovbro M, Kraunsøe R, Dela F. 2007. Patients with type 2 diabetes have normal mitochondrial function in skeletal muscle. Diabetologia 50:790–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.