Abstract
Context:
The human endometrium has an exceptional capacity for repeated repair after menses, but its regulation remains undefined. Premenstrually, progesterone levels fall and prostaglandin (PG) F2α synthesis increases, causing spiral arteriole constriction. We hypothesized that progesterone withdrawal, PGF2α, and hypoxia increase vascular endothelial growth factor (VEGF), an endometrial repair factor.
Design and Results:
Endometrial biopsies were collected (n = 47) with ethical approval and consent. VEGF mRNA, quantified by quantitative RT-PCR, was increased during menstruation (P < 0.01).VEGF protein was maximally secreted from proliferative endometrial explants. Treatment of an endometrial epithelial cell line and primary human endometrial stromal cells with 100 nm PGF2α or hypoxia (0.5% O2) resulted in significant increases in VEGF mRNA and protein. VEGF was maximal when cells were cotreated with PGF2α and hypoxia simultaneously (P < 0.05–0.001). Secretory-phase endometrial explants also showed an increase in VEGF with cotreatment (P < 0.05). However, proliferative-phase explants showed no increase in VEGF on treatment with PGF2α and/or hypoxia. Proliferative tissue was induced to increase VEGF mRNA expression when exposed to progesterone and its withdrawal in vitro but only in the presence of hypoxia and PG. Hypoxia-inducible factor-1α (HIF-1α) silencing with RNA interference suppressed hypoxia-induced VEGF expression in endometrial cells but did not alter PGF2α-induced VEGF expression.
Conclusions:
Endometrial VEGF is increased at the time of endometrial repair. Progesterone withdrawal, PGF2α, and hypoxia are necessary for this perimenstrual VEGF expression. Hypoxia acts via HIF-1α to increase VEGF, whereas PGF2α acts in a HIF-1α-independent manner. Hence, two pathways regulate the expression of VEGF during endometrial repair.
The human endometrium has a remarkable capacity for scar-free repair after the inflammatory process of menstruation (1, 2). Currently, our understanding of this process is limited, with the factors involved and their regulation remaining elusive. Delayed repair may result in the common gynecological complaint of prolonged, heavy menstrual bleeding (HMB). One in three women considers their menstruation excessive, rising to one in two as menopause approaches (3). Although medical treatment options are available, a significant proportion of women require surgery due to treatment failure (4). It is essential to fully understand the endometrial repair processes to improve medical treatments and avoid surgical risks. Furthermore, elucidating the mechanisms of efficient endometrial repair may have wider implications, identifying novel treatment targets in tissue sites where persistent inflammation and scarring are problematic.
Angiogenesis is mandatory for efficient repair and remodeling. Vascular endothelial growth factor (VEGF) is a key factor in physiological and pathological angiogenesis (5–7). This secreted protein stimulates endothelial cell proliferation and migration (5, 8, 9). It acts through two receptors, VEGFR1 and VEGFR2, both expressed in the human endometrium (10, 11). In the primate, VEGF is essential for neoangiogenesis during postmenstrual repair (9). Aberrant endometrial expression of VEGF has been demonstrated in women with HMB, with lower levels in these women compared with normal controls (12). However, the regulation of VEGF during endometrial repair remains undefined.
As the corpus luteum regresses at the end of the menstrual cycle, progesterone levels fall. This triggers the release of inflammatory mediators within the endometrium that initiate menstruation (1). Although endometrial repair was traditionally considered to be regulated by estrogen, research in the mouse model of simulated menstruation has suggested it may be an estrogen-independent event (13). We propose that progesterone withdrawal simultaneously initiates menstruation and repair. After progesterone withdrawal, there is an increase in endometrial prostaglandin (PG) synthesis, namely PGE2 and PGF2α (1, 14). PGF2α is a potent vasoconstrictor and, alongside other factors, acts on the spiral arterioles to cause intense vasoconstriction premenstrually (15). The role of PGE2 during endometrial repair has not been fully defined. Spiral arteriole vasoconstriction is likely to cause an episode of transient, local hypoxia in the uppermost endometrial zones. Classic experiments in the rhesus monkey first suggested the presence of perimenstrual hypoxia (15). More recently, use of a marker of cell hypoxia, pimonidazole, in mice has confirmed oxygen levels below 10 mm Hg during menstruation (9). Hypoxia-inducible factor 1 (HIF-1) is a transcription factor known to regulate the cellular response to hypoxia. In hypoxic conditions the two subunits (HIF-1α and HIF-1β) dimerize and initiate transcription of target genes, including factors involved in angiogenesis (16–18). HIF has been identified in the human endometrium during the late secretory and menstrual phases (19).
We hypothesized that 1) VEGF is increased during menstruation when endometrial repair is initiated, and 2) this increase is regulated by progesterone withdrawal, PG synthesis, and hypoxic conditions. Herein we demonstrate endometrial VEGF expression is maximal during the menstrual phase. In vitro endometrial cell studies revealed that hypoxia, PGE2, and PGF2α induce VEGF expression. Ex vivo endometrial explant studies showed that VEGF expression was increased by hypoxia and PG only in tissue from the secretory phase. However, when proliferative explants were exposed to progesterone in vitro followed by progesterone withdrawal in hypoxic conditions, VEGF expression was significantly increased. Furthermore, we show that hypoxia regulates VEGF expression via HIF-1α, whereas PGF2α induces VEGF expression independently of HIF-1α.
Materials and Methods
Human endometrial tissue collection
Endometrial biopsies (n = 47) were collected with a suction curette (Pipelle, Laboratorie CCD, Paris, France) from women (median age 42 yr, range 22–50) attending the gynecological outpatient department. All reported regular menstrual cycles (21–35 d) and no exogenous hormone exposure for 3 months before biopsy. Women with large fibroids (>3 cm) or endometriosis were excluded. Tissue was divided and 1) placed in RNAlater, RNA stabilization solution [Ambion (Europe) Ltd., Warrington, UK], 2) fixed in neutral buffered formalin for wax embedding, and 3) placed in PBS for in vitro culture. Biopsies were consistent for 1) histological dating [criteria of Noyes et al. (20)], 2) reported last menstrual period, and 3) serum progesterone and estradiol concentrations at time of biopsy (Table 1). Seven women providing a biopsy in the proliferative, early/mid-secretory phase returned for an additional biopsy 3–6 months after insertion of the levonorgestrel-releasing intrauterine system (LNG-IUS) for management of heavy menstrual bleeding. Written consent was obtained from participants and ethical approval granted from the Lothian Research Ethics Committee.
Table 1.
Serum estradiol and progesterone levels at the time of endometrial biopsy
| Number | Mean estradiol levels [pmol/liter (range)] | Mean progesterone levels [nmol/liter (range)] | |
|---|---|---|---|
| Menstrual | 8 | 197.25 (55–514) | 3.71 (1.24–10.59)a |
| Proliferative | 14 | 476.18 (79–1105) | 2.68 (0.97–7.1)a |
| Early secretory | 9 | 497.50 (289–841) | 59.60 (23.2–112.91) |
| Mid secretory | 10 | 638.00 (242–1949) | 64.30 (25.47–114.53) |
| Late secretory | 6 | 318.22 (59.09–819) | 8.22 (1.06–16.95)a |
Progesterone levels are significantly lower than in the early-mid secretory phases (P < 0.001).
Immunohistochemistry
The 3-μm paraffin sections were dewaxed and rehydrated. Antigen retrieval was undertaken by microwaving sections in a high-pH heat-induced antigen retrieval buffer (pH 9.0). Endogenous peroxidase activity was blocked by 3% hydrogen peroxide. Sections were sequentially incubated in avidin and biotin (Vector Laboratories, Peterborough, UK) and protein block (Dako, Cambridge, UK). Mouse monoclonal anti-VEGF antibody (12.4 μg/ml; R&D Systems, Abingdon, UK) was applied overnight at 4 C. Negative controls were incubated with an equivalent concentration of mouse IgG2B. Biotinylated rabbit antimouse secondary antibody was used at 1:200. Avidin-biotin-peroxidase complex (ABC-Elite; Vector) was applied for 30 min and liquid diaminobenzidine kit (Zymed Laboratories, San Francisco, CA) used for detection. The reaction was stopped with distilled water and sections counterstained with hematoxylin, dehydrated, and mounted with Pertex (Cellpath plc, Hemel Hempstead, UK).
Culture of endometrial explants
All explants were cultured on sterile capillary bedding just covered with serum-free RPMI 1640 medium to minimize tissue hypoxia. Four experiments were carried out.
1) Proliferative (n = 3) and secretory (n = 3–6) phase biopsies were divided into four equal explants and treated with vehicle or 100 nm PGF2α and placed in normoxic conditions (21% O2, 5% CO2, 37 C) or in a sealed hypoxic chamber (0.5% O2, 5% CO2, 37 C; Coy Laboratory Products, Grass Lake, MI) for 24 h.
2) Proliferative biopsies (n = 4) were divided into eight explants. All were treated with 1 μm medroxyprogesterone acetate (MPA) for 24 h. Explants were then treated for 48 h with 1) 1 μm MPA plus vehicle, 2) 1 μm MPA plus 8.4 μm indomethacin [a cyclooxygenase (COX) inhibitor], 3) 1 μm MPA plus 1 μm mifepristone (RU486, a progesterone receptor antagonist), or 4) 1 μm MPA plus 1 μm mifepristone plus 8.4 μm indomethacin. This 48-h incubation was carried out in both normoxic and hypoxic conditions.
3) Four additional endometrial biopsies were divided into four explants and treated with progesterone (1 μm) for 24 h before washing with PBS followed by treatment with progesterone or vehicle in normoxic and hypoxic conditions for 48 h.
4) Endometrial tissue from across the menstrual cycle (n = 22) was weighed and cultured for 24 h in 1 ml RPMI 1640 at 37 C in normoxia and the culture supernatant analyzed by ELISA.
Culture of endometrial cells
Human Ishikawa endometrial adenocarcinoma cells (European Collection of Cell Cultures, Centre for Applied Microbiology, Wiltshire, UK) were stably transfected with the PGF2α receptor (FPS cells) (21). Primary human endometrial stromal (HES) cells were isolated from endometrial tissue (n = 3) by enzymatic digestion as previously described (22).
To determine the effect of PGF2α and/or hypoxic conditions on VEGF expression, approximately 4 × 105 FPS or HES cells were seeded in six-well plates. The following day, cells were incubated in serum-free culture medium containing 8.4 μm indomethacin (COX enzyme inhibitor) for at least 16 h. Cells were then treated with vehicle or 100 nm PGF2α and placed in normoxia (21% O2, 5% CO2, 37 C) or a sealed hypoxic chamber (0.5% O2, 5% CO2, 37 C) for 2, 4, 8, 24, and 48 h.
Two different 19-nucleotide short hairpin RNA (shRNA) sequences derived from human HIF-1α mRNA (U22431; bp 1470–1489 and bp 2192–2211) and a scrambled control oligonucleotide were used and have been described previously (23, 24). These sequences are termed HIF-1α/shRNA1470 and HIF-1α/shRNA2192. Cells were transiently transfected with the shRNA sequences using a lentiviral vector at MOI 10 for 24 h. Cells were incubated in serum-free medium overnight before placement in the hypoxic chamber for 8 h. Cells were washed with PBS and harvested, and RNA or protein was extracted for PCR or Western blot analysis.
Quantitative RT-PCR
Expression of VEGF in endometrial tissue and cells was determined by quantitative RT-PCR (TaqMan) analysis. Total RNA from cells and endometrial biopsies was extracted using the RNeasy Mini Kit (QIAGEN Ltd., Sussex, UK) according to manufacturer's instructions. Samples were treated for DNA contamination by DNA digestion during RNA purification. RNA samples were reverse transcribed using MgCl2 (5.5 mm), deoxy (d) nucleotide triphosphates (0.5 mm each) random hexamers (2.5 μm), ribonuclease inhibitor (0.4 U/μl), and multiscribe reverse transcriptase (1.25 U/μl; all from PE Biosystems, Warrington, UK); 200–400 ng of RNA was added. A tube with no reverse transcriptase and another tube with water were included as controls. To measure cDNA expression, a reaction mix was prepared containing TaqMan buffer (5.5 mm MgCl2, 200 μm dATP 200 μm dCTP, 200 μm dGTP, 400 μm deoxyuridine triphosphate), ribosomal 18S primers/probe (Applied Biosystems, Warrington, UK) and specific forward and reverse primers and probes: VEGF forward primer 5′-TACCTCCACCATGCCAAGT-3′, reverse primer 5′-TAGCTGCGCTGATAGACAT-3′, and probe 5′-ACTTCGTGATGATTCTGCC-3′; lamin A/C forward primer 5′-AGCAAAGTGCGTGAGGAGTT-3′, reverse primer 5′-AGGTCACCCTCCTTCTTGGT-3′, and Universal probe library no. 17. A 0.4-μl volume of cDNA was added for each PCR. Negative controls (water instead of cDNA) were included in each run. PCR was carried out using ABI Prism 7900 (Applied Biosystems). Samples were analyzed in triplicate using Sequence Detector version 2.3 (PE Biosystems). Expression of target mRNA was normalized to RNA loading for each sample using the 18S rRNA as an internal standard. There was no significant change in 18S rRNA expression between normoxic and hypoxic conditions.
Nuclear protein extraction
Cytoplasmic proteins were extracted from endometrial epithelial (FPS) cells with a cytoplasmic protein lysis buffer [10 mm HEPES (pH 7.8), 10 nm KCl, 2 mm MgCl2, 1 mm dithiothreitol, 0.1 mm EDTA, 10% Nonidet P-40] containing protease inhibitors (Roche Diagnostics Ltd., Lewes, UK). The membrane fraction was pelleted by centrifugation at 13,000 rpm for 1 min at 4 C. Cytoplasmic fraction supernatant was removed and stored at −80 C. The nuclear fraction was extracted with a nuclear protein lysis buffer [50 mm HEPES (pH 7.8), 50 nm KCl, 300 mm NaCl, 0.1 mm EDTA, 1 mm dithiothreitol, 10% glycerol] containing protease inhibitors (Roche) followed by agitation for 20 min at 4 C and centrifugation at 13,000 rpm for 5 min at 4 C. Nuclear fraction supernatant was stored at −80 C. Protein content was determined using protein assay kits (Bio-Rad, Hemel Hempstead, UK).
HIF-1α Western blot analysis
A total of 15 μg nuclear protein from FPS cells was resuspended in a 2:1 ratio with Laemmli buffer [125 mm Tris-HCl (pH 6.8), 4% sodium dodecyl sulfate, 5% 2-mercaptoethanol, 20% glycerol, and 0.05% bromophenol blue] and denatured for 5 min at 90 C. Proteins were separated on 4–12% Bis-Tris gels (NuPAGE Novex; Invitrogen, Carlsbad, CA) and transferred onto polyvinylidene difluoride membranes (Millipore, Watford, UK). Membranes were blocked overnight before incubation with primary antibodies for 2 h at room temperature: mouse monoclonal HIF-1α antibody (BD Biosciences, Oxford, UK) at 1:250 and rabbit polyclonal to β-actin (Abcam, Cambridge, UK) at 1:5000. After washing, the membrane was incubated with horseradish peroxidase-conjugated goat antimouse IgG (Dako) or horseradish peroxidase-conjugated mouse antirabbit IgG (Sigma Aldrich Company Ltd. Dorset, UK) at 1:20000. Chemiluminescent horseradish peroxidase substrate (Immobilon Western; Millipore Corp., Billerica, MA) was used for detection according to manufacturer's instructions.
VEGF ELISA
Levels of VEGF in culture supernatants were determined in triplicate samples using a commercially available ELISA kit (PeproTech, London, UK), according to manufacturer's instructions. Protein values in explant experiments were normalized for tissue weight.
Statistics
For cell and tissue culture, mRNA results are expressed as fold increase, where relative expression of mRNA on treatment was divided by the relative expression after vehicle treatment. Data are presented as mean ± sem, and significance of raw data was determined using one-way ANOVA with Tukey's multiple-comparison test. For endometrium from across the menstrual cycle, mRNA results are expressed as quantity relative to a comparator, a sample of RNA from the liver. Significant differences in mRNA and protein were determined using Kruskal-Wallis nonparametric test with Dunn's multiple-comparison posttest (Prism version 4.02; GraphPad Software, Inc., San Diego, CA).
Results
VEGF levels are increased at menstruation
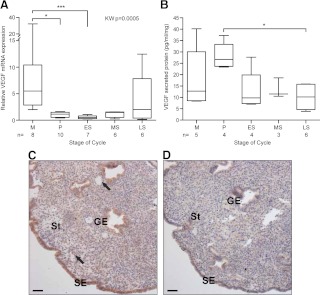
Endometrial VEGF mRNA expression varied significantly across the menstrual cycle (P = 0.0005) (Fig. 1A). Maximal expression was observed in menstrual endometrium, when levels were significantly higher than in the proliferative (P < 0.05) and early secretory (P < 0.01) phases. VEGF protein secreted by endometrial explants from across the menstrual cycle was measured by ELISA. VEGF protein secretion was significantly greater from proliferative explants compared with late secretory endometrium (P < 0.05) (Fig. 1B). Immunolocalization of VEGF protein in menstrual endometrium (Fig. 1C) showed positive cytoplasmic staining in the surface epithelial cells (SE), glandular epithelial cells (GE), stromal compartment (St), and perivascular cells (arrows).
Fig. 1.
Endometrial VEGF is increased during menstruation. A, VEGF mRNA expression in endometrial biopsies from across the menstrual cycle; B, VEGF secreted protein from endometrial explants collected at each menstrual cycle stage; C, immunolocalization of VEGF in menstrual endometrium; D, menstrual negative control tissue. ES, Early-secretory; GE, glandular epithelial cells; KW, Kruskal Wallis; LS, late-secretory; M, menstrual; MS, mid-secretory; P, proliferative; SE, surface epithelial cells; St, stromal cell compartment. *, P < 0.05; ***, P < 0.001. Arrows indicate perivascular cells. Scale bar, 50 μm.
Regulation of VEGF in endometrial epithelial cells
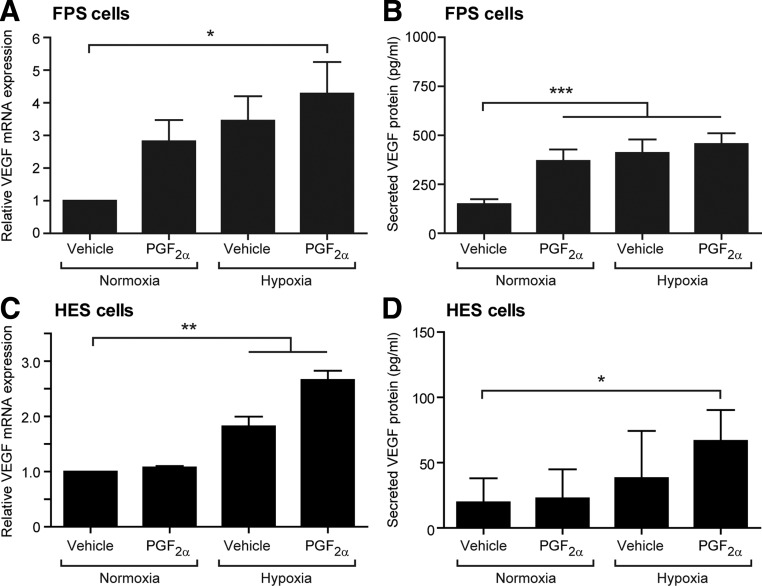
An endometrial epithelial cell line stably transfected with the PGF2α receptor (FPS cells) was used to investigate the regulation of VEGF expression. This cell line was used because primary endometrial epithelial cells have a limited capacity for proliferation in culture but do contain this PG receptor in vivo (25, 26). FPS cells treated with 100 nm PGF2α or hypoxia showed a trend toward increased VEGF mRNA expression; this increase reached significance when both factors were present simultaneously (P < 0.05) (Fig. 2A). VEGF secreted protein levels were significantly increased in the culture supernatants from FPS cells treated for 24 h with PGF2α, hypoxia, or both (P < 0.001) (Fig. 2B).
Fig. 2.
The regulation of VEGF in human endometrial cells. A, VEGF mRNA expression in a human endometrial epithelial cell line (FPS cells) treated with vehicle, 100 nm PGF2α, hypoxia, or both hypoxia and PGF2α for 24 h; B, VEGF secreted protein levels in the culture supernatants from FPS cells; C, VEGF mRNA expression in primary HES cells treated with vehicle, 100 nm PGF2α, hypoxia, or both hypoxia and PGF2α for 24 h; D, VEGF secreted protein levels in the culture supernatants from these HES cells. All results are from three separate experiments; HES cells were isolated from three different women. Normoxia = 21% O2; hypoxia = 0.5% O2. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Regulation of VEGF in primary HES cells
Treatment of HES cells with 100 nm PGF2α in normoxia had no effect on VEGF mRNA expression (Fig. 2C). However, incubation of HES cells in hypoxic conditions for 24 h revealed a significant increase in VEGF (P < 0.01). Simultaneous treatment of HES cells with PGF2 and hypoxia also significantly elevated VEGF mRNA expression (P < 0.01) (Fig. 2C). VEGF secreted protein levels were significantly increased when HES cells were incubated for 24 h with PGF2α and hypoxia vs. vehicle-treated cells in normoxia (P < 0.05) (Fig. 2D).
Regulation of VEGF levels in human endometrial tissue explants
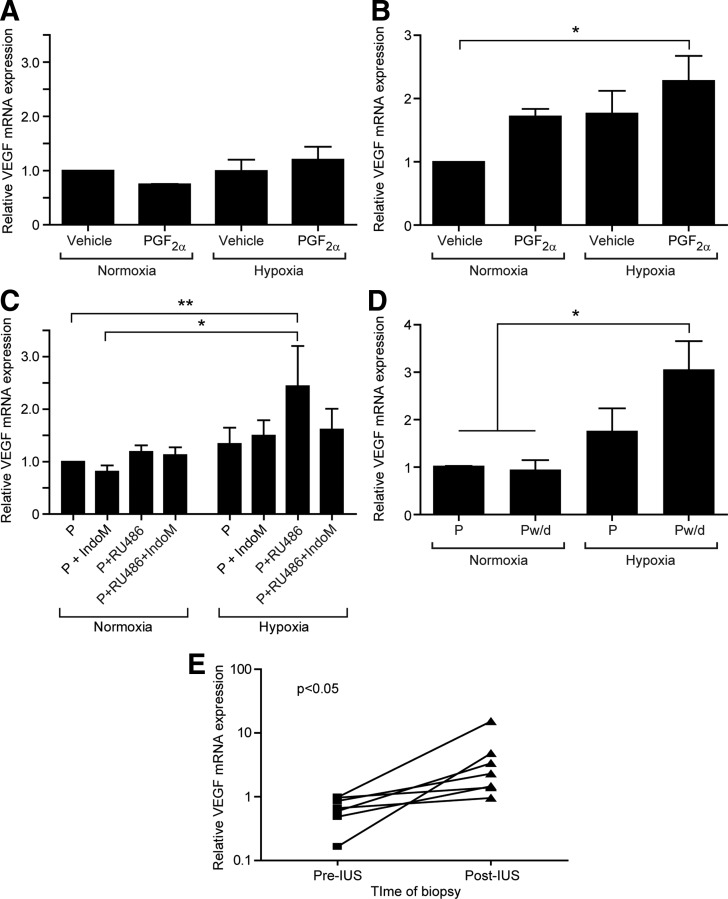
Proliferative endometrial explants treated with 100 nm PGF2α in normoxia or hypoxia showed no significant changes in VEGF mRNA expression (Fig. 3A). In contrast, explants from the secretory phase displayed significant increases in VEGF mRNA expression after cotreatment with PGF2α and hypoxia (P < 0.05) (Fig. 3B). This varied response between endometrium from the proliferative and secretory phase suggests previous progesterone exposure may influence the ability of tissue to respond.
Fig. 3.
The effect of progesterone withdrawal, prostaglandins, and hypoxia on VEGF expression in the endometrium. A, VEGF mRNA expression in endometrial explants from the proliferative phase treated with vehicle, 100 nm PGF2α, hypoxia, or both hypoxia and PGF2α (n = 3) for 24 h. B, VEGF mRNA expression in secretory endometrial explants treated with vehicle, 100 nm PGF2α, hypoxia, or both hypoxia and PGF2α (n = 3) for 24 h. C, Proliferative-phase explants (n = 4) were pretreated with1 μm MPA and then 1) maintained in 1 μm MPA (P), 2) cotreated with 1 μm MPA and 1 μm RU486, a progesterone receptor antagonist (P+RU486), or 3) cotreated with 1 μm MPA, 1 μm RU486, and 8.4 μm indomethacin, a COX enzyme inhibitor (P+RU486+IndoM). Identical treatments were incubated in either normoxia (21% O2) or hypoxia (0.5% O2). D, Endometrial explants pretreated with 1 μm progesterone followed by maintenance in progesterone (P) or incubation with vehicle to induce progesterone withdrawal (Pw/d) in normoxia and hypoxia. E, Insertion of the LNG-IUS (IUS), with consequent local reduction in progesterone receptor expression analogous to local progesterone withdrawal, resulted in a significant increase in VEGF mRNA expression; note logarithmic scale on y-axis. Normoxia = 21% O2; hypoxia = 0.5% O2. *, P < 0.05; **, P < 0.01.
The effect of progesterone, and its withdrawal, on VEGF expression
To test the hypothesis that previous progesterone exposure is necessary for VEGF induction, we used ex vivo models of progesterone withdrawal. Proliferative endometrial biopsies were divided into eight equal explants and pretreated for 24 h with progestin (MPA). Explants were then cotreated with MPA plus 1) vehicle; 2) indomethacin, a COX inhibitor; 3) a progesterone receptor antagonist (RU486); or 4) RU486 and indomethacin for 48 h. Ex vivo progesterone antagonism in normoxia did not show significant up-regulation of VEGF mRNA (Fig. 3C). in vivo, progesterone withdrawal is followed by hypoxia due to spiral arteriole vasoconstriction. When we antagonized progesterone in hypoxic conditions to mimic the in vivo scenario more accurately, there was a significant increase in VEGF mRNA expression (Fig. 3C). This increase was abrogated by cotreatment with indomethacin to suppress PG production, suggesting that both hypoxia and PG are necessary to increase endometrial VEGF expression after progesterone withdrawal. Because synthetic agents such as MPA and RU486 may have off-target effects, additional endometrial biopsies were treated with progesterone for 24 h before treatment with vehicle or maintenance in progesterone. Again, only explants exposed to progesterone withdrawal in hypoxic conditions showed a significant increase in VEGF expression (P < 0.05) (Fig. 3D).
We examined paired endometrial biopsies collected from the same woman before and 3–6 months after LNG-IUS insertion (n = 7). The LNG-IUS is known to down-regulate the endometrial progesterone receptor (27) and may represent an in vivo model of progesterone deprivation. We identified a significant up-regulation of VEGF mRNA expression in endometrium collected after LNG-IUS insertion when compared with preinsertion biopsies (P < 0.05) (Fig. 3D).
The contribution of HIF-1α to the regulation of endometrial VEGF
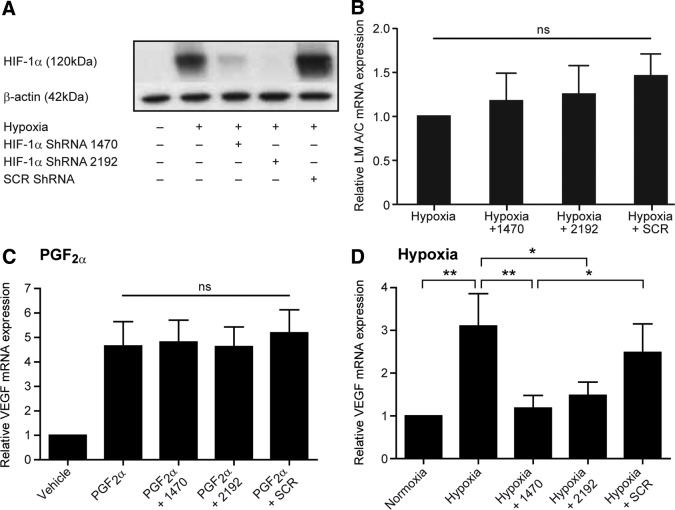
To assess the contribution of the transcription factor HIF-1α to endometrial VEGF expression, we transfected FPS cells with two different shRNA sequences against HIF-1α (HIF-1α/sh1470 and HIF-1α/sh2192) and confirmed HIF-1α silencing by Western blot analysis (Fig. 4A). Transfection with either sequence reduced HIF-1α protein levels in response to hypoxia, whereas a control scrambled sequence had no such effect (Fig. 4A). Specificity of the shRNA sequences was examined by measurement of lamin A/C mRNA levels, which revealed no significant changes upon transfection with any construct (Fig. 4B). HIF-1α silencing had no significant impact on PGF2α-induced VEGF mRNA expression (Fig. 4C). In contrast, hypoxic induction of VEGF was significantly lower in cells where HIF-1α was silenced when compared with untransfected cells or cells transfected with HIF-1α/shSCR (P < 0.05) (Fig. 4D).
Fig. 4.
HIF-1α silencing in FPS cells prevents hypoxic induction of VEGF- but not PGF2α-mediated increases. A, Confirmation of HIF-1α protein knockdown by Western blot with transfection of two short hairpin sequences against HIF-1α (HIF-1/sh1470 and HIF-1α/sh2192), compared with a scrambled sequence (shSCR) or untransfected cells after 8 h in hypoxic conditions (0.5% O2); B, specificity of the knockdown was confirmed by examining lamin A/C mRNA expression, which showed no significant changes with transfection of any construct; C, silencing of HIF-1α had no significant effect on PGF2α-mediated VEGF expression; D, cells transfected with shRNA against HIF-1α had a significantly abrogated response to hypoxic stimulation, with low levels of VEGF expression compared with untransfected cells or those transfected with a scrambled sequence. *, P < 0.05; **, P < 0.01; ns, nonsignificant.
Discussion
The novel data presented herein detail the requirement for progesterone withdrawal, hypoxic conditions, and PG production to increase endometrial VEGF during menstruation. We confirm that hypoxia increases endometrial VEGF production and describe, for the first time, its dependence upon endometrial HIF-1α. In addition, we reveal a novel PGF2α-mediated increase in endometrial VEGF expression that is independent of HIF-1α.
Electron microscopy of the human endometrium has shown that repair commences on d 2 of the menstrual cycle (28). Because repair is initiated at menstruation, the factors involved must be up-regulated during this phase. Herein we have shown significant up-regulation of endometrial VEGF mRNA and protein during menstruation. This is consistent with findings in the rhesus macaque, where heightened expression of VEGF was present in the newly formed surface epithelium and stroma during postmenstrual repair (29). Previous immunohistochemical and in situ hybridization studies have also shown increased VEGF expression in the human endometrium during menstruation (30, 31). VEGF is a potent angiogenic factor that stimulates endothelial cell proliferation and migration (5). A recent study examining the decidualized mouse uterus and rhesus macaque endometrium found that VEGF blockade with VEGF-Trap (a VEGF blocker) completely inhibited neovascularization and reepithelialization during endometrial repair (9). Interestingly, a previous study of the human endometrium found significantly decreased VEGF mRNA and protein levels in women with HMB when compared with controls (12). Therefore, delineation of the regulation of this important repair factor may provide novel therapeutic targets for HMB.
Herein we have shown that progesterone withdrawal, hypoxia, and PG production are required to increase VEGF expression in endometrial explants. An elegant study of ovariectomized, artificially cycling macaques reported an estrogen-independent elevation in stromal VEGF immediately after progesterone withdrawal (29). An estrogen-dependent increase in VEGF occurred subsequently, during the proliferative phase, suggesting estradiol is involved in remodeling but not in the initial phase of endometrial repair. Our ex vivo models further delineate events after progesterone withdrawal in the human endometrium. Progesterone withdrawal appears necessary, but alone is not sufficient, to induce endometrial VEGF. Downstream hypoxia and PG production are also required for endometrial VEGF expression. Previous studies have shown hypoxic induction of VEGF in isolated endometrial stromal and epithelial cells (32), but to our knowledge, this is the first time hypoxia has been shown to be involved in VEGF induction in endometrial tissue. PGs have previously been shown to increase VEGF expression in cancer cells (16, 33), but we demonstrate a potential physiological role in the human endometrium.
We examined endometrial biopsies from women collected before and 3–6 months after LNG-IUS insertion for treatment of HMB. LNG-IUS insertion dramatically down-regulates endometrial progesterone receptors, providing an in vivo model of local endometrial progesterone deprivation (27, 34). In addition, progestogen exposure has been shown to reduce endometrial perfusion and profoundly decrease vasomotion (35). This may lead to endometrial hypoxia. Endometrial samples taken after LNG-IUS insertion showed a significant increase in VEGF mRNA expression. Because normal endometrial architecture is maintained in this model, our findings support the role of progesterone withdrawal and downstream hypoxia in the up-regulation of endometrial VEGF. Furthermore, the LNG-IUS is well established as a treatment for HMB, and this stimulation of VEGF production may contribute to its efficacy.
For the first time, we have demonstrated that hypoxic induction of VEGF in endometrial cells is mediated via HIF-1α. HIF-1 is a heterodimeric factor. The HIF-1α subunit undergoes proteasomal degradation in normoxia, whereas HIF-1β is constitutively expressed. In hypoxic conditions, HIF-1α binds with HIF-1β, translocates to the nucleus, and increases the transcription of target genes, including those involved in angiogenesis (16, 17, 36). HIF-1α has been shown to be induced in late secretory and menstrual endometrium (19). A hypoxic response element is present in the VEGF promoter region, and HIF-1 has been shown to regulate VEGF expression in hypoxic Hep3B cells (37). In contrast, HIF-1-independent hypoxic regulation of VEGF has been described in colon cancer cells (23). Our results suggest HIF-1 is involved in hypoxia-induced increases in endometrial VEGF production at menstruation but that PGF2α acts independently of HIF to increase VEGF. A previous study of endometrial adenocarcinoma explants and cells showed that PGF2α activates the ERK1/2 signaling pathway in an epidermal growth factor receptor-dependent manner to increase VEGF promoter activity (33). Additional studies are required to determine whether a similar mechanism of action is present in normal endometrial tissue.
In conclusion, our data generated from 1) in vitro cell studies, 2) ex vivo explant culture, and 3) an in vivo model of progesterone deprivation indicate that progesterone withdrawal, hypoxia, and PGF2α are involved in production of VEGF for endometrial repair. There is evidence that women with HMB have decreased levels of VEGF during menstruation (12). These women are also known to have aberrant PG production and signaling (38, 39) and may therefore have suboptimal initiation of repair leading to heavy, prolonged bleeding. Additional studies are required to determine whether there is a defective hypoxic response in women with HMB, resulting in less menstrual expression of VEGF. Delineation of the physiological regulation of endometrial repair may lead to novel, efficacious treatments for these women.
Supplementary Material
Acknowledgments
We thank all women who participated in this study and the clinical research nurses Sharon McPherson, Catherine Murray, and Catherine Cairns for their help with recruitment. In addition, we acknowledge the help of Ronnie Grant with illustrations, Sarah McDonald for technical assistance, and Sheila Milne for secretarial support. We thank Professor T. Cramer for kindly providing the shRNA constructs.
Grant support was received from Medical Research Council G0600048.
Disclosure Summary: The authors have no conflicts of interest to declare.
Footnotes
- COX
- Cyclooxygenase
- d
- deoxy
- HES
- human endometrial stromal
- HIF-1
- hypoxia-inducible factor 1
- HMB
- heavy menstrual bleeding
- LNG-IUS
- levonorgestrel-releasing intrauterine system
- MPA
- medroxyprogesterone acetate
- PG
- prostaglandin
- shRNA
- short hairpin RNA
- VEGF
- vascular endothelial growth factor.
References
- 1. Critchley HO, Jones RL, Lea RG, Drudy TA, Kelly RW, Williams AR, Baird DT. 1999. Role of inflammatory mediators in human endometrium during progesterone withdrawal and early pregnancy. J Clin Endocrinol Metab 84:240–248 [DOI] [PubMed] [Google Scholar]
- 2. Maybin J, Critchley H. 2009. Repair and regeneration of the human endometrium. Expert Rev Obstet Gynecol 4:283–298 [Google Scholar]
- 3. Prentice A. 1999. Health care implications of dysfunctional uterine bleeding. Baillieres Best Pract Res Clin Obstet Gynaecol 13:181–188 [DOI] [PubMed] [Google Scholar]
- 4. Cromwell DA, Mahmood TA, Templeton A, van der Meulen JH. 2009. Surgery for menorrhagia within English regions: variation in rates of endometrial ablation and hysterectomy. BJOG 116:1373–1379 [DOI] [PubMed] [Google Scholar]
- 5. Ferrara N. 2004. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev 25:581–611 [DOI] [PubMed] [Google Scholar]
- 6. Carmeliet P. 2005. VEGF as a key mediator of angiogenesis in cancer. Oncology 69(Suppl 3):4–10 [DOI] [PubMed] [Google Scholar]
- 7. Smith SK. 2001. Regulation of angiogenesis in the endometrium. Trends Endocrinol Metab 12:147–151 [DOI] [PubMed] [Google Scholar]
- 8. Blum S, Issbrüker K, Willuweit A, Hehlgans S, Lucerna M, Mechtcheriakova D, Walsh K, von der Ahe D, Hofer E, Clauss M. 2001. An inhibitory role of the phosphatidylinositol 3-kinase-signaling pathway in vascular endothelial growth factor-induced tissue factor expression. J Biol Chem 276:33428–33434 [DOI] [PubMed] [Google Scholar]
- 9. Fan X, Krieg S, Kuo CJ, Wiegand SJ, Rabinovitch M, Druzin ML, Brenner RM, Giudice LC, Nayak NR. 2008. VEGF blockade inhibits angiogenesis and reepithelialization of endometrium. FASEB J 22:3571–3580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nayak NR, Critchley HO, Slayden OD, Menrad A, Chwalisz K, Baird DT, Brenner RM. 2000. Progesterone withdrawal up-regulates vascular endothelial growth factor receptor type 2 in the superficial zone stroma of the human and macaque endometrium: potential relevance to menstruation. J Clin Endocrinol Metab 85:3442–3452 [DOI] [PubMed] [Google Scholar]
- 11. Punyadeera C, Thijssen VL, Tchaikovski S, Kamps R, Delvoux B, Dunselman GA, de Goeij AF, Griffioen AW, Groothuis PG. 2006. Expression and regulation of vascular endothelial growth factor ligands and receptors during menstruation and post-menstrual repair of human endometrium. Mol Hum Reprod 12:367–375 [DOI] [PubMed] [Google Scholar]
- 12. Malik S, Day K, Perrault I, Charnock-Jones DS, Smith SK. 2006. Reduced levels of VEGF-A and MMP-2 and MMP-9 activity and increased TNF-α in menstrual endometrium and effluent in women with menorrhagia. Hum Reprod 21:2158–2166 [DOI] [PubMed] [Google Scholar]
- 13. Kaitu'u-Lino TJ, Morison NB, Salamonsen LA. 2007. Estrogen is not essential for full endometrial restoration after breakdown: lessons from a mouse model. Endocrinology 148:5105–5111 [DOI] [PubMed] [Google Scholar]
- 14. Sugino N, Karube-Harada A, Taketani T, Sakata A, Nakamura Y. 2004. Withdrawal of ovarian steroids stimulates prostaglandin F2α production through nuclear factor-κB activation via oxygen radicals in human endometrial stromal cells: potential relevance to menstruation. J Reprod Dev 50:215–225 [DOI] [PubMed] [Google Scholar]
- 15. Markee JE. 1940. Menstruation in intraocular transplants in the rhesus monkey. Contr Embryol Carnegie Instn 28:219–308 [Google Scholar]
- 16. Fukuda R, Kelly B, Semenza GL. 2003. Vascular endothelial growth factor gene expression in colon cancer cells exposed to prostaglandin E2 is mediated by hypoxia-inducible factor 1. Cancer Res 63:2330–2334 [PubMed] [Google Scholar]
- 17. Higgins DF, Biju MP, Akai Y, Wutz A, Johnson RS, Haase VH. 2004. Hypoxic induction of Ctgf is directly mediated by Hif-1. Am J Physiol Renal Physiol 287:F1223–F1232 [DOI] [PubMed] [Google Scholar]
- 18. Semenza GL. 2000. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol 88:1474–1480 [DOI] [PubMed] [Google Scholar]
- 19. Critchley HO, Osei J, Henderson TA, Boswell L, Sales KJ, Jabbour HN, Hirani N. 2006. Hypoxia-inducible factor-1α expression in human endometrium and its regulation by prostaglandin E-series prostanoid receptor 2 (EP2). Endocrinology 147:744–753 [DOI] [PubMed] [Google Scholar]
- 20. Noyes RW, Hertig AT, Rock J. 1950. Dating the endometrial biopsy. Fertil Steril 1:3–25 [DOI] [PubMed] [Google Scholar]
- 21. Sales KJ, Maudsley S, Jabbour HN. 2004. Elevated prostaglandin EP2 receptor in endometrial adenocarcinoma cells promotes vascular endothelial growth factor expression via cyclic 3′,5′-adenosine monophosphate-mediated transactivation of the epidermal growth factor receptor and extracellular signal-regulated kinase 1/2 signaling pathways. Mol Endocrinol 18:1533–1545 [DOI] [PubMed] [Google Scholar]
- 22. Kane N, Jones M, Brosens JJ, Saunders PT, Kelly RW, Critchley HO. 2008. Transforming growth factor-β1 attenuates expression of both the progesterone receptor and dickkopf in differentiated human endometrial stromal cells. Mol Endocrinol 22:716–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mizukami Y, Li J, Zhang X, Zimmer MA, Iliopoulos O, Chung DC. 2004. Hypoxia-inducible factor-1-independent regulation of vascular endothelial growth factor by hypoxia in colon cancer. Cancer Res 64:1765–1772 [DOI] [PubMed] [Google Scholar]
- 24. Sowter HM, Raval RR, Moore JW, Ratcliffe PJ, Harris AL. 2003. Predominant role of hypoxia-inducible transcription factor (Hif)-1α versus Hif-2α in regulation of the transcriptional response to hypoxia. Cancer Res 63:6130–6134 [PubMed] [Google Scholar]
- 25. Milne SA, Jabbour HN. 2003. Prostaglandin (PG) F2α receptor expression and signaling in human endometrium: role of PGF2α in epithelial cell proliferation. J Clin Endocrinol Metab 88:1825–1832 [DOI] [PubMed] [Google Scholar]
- 26. Milne SA, Perchick GB, Boddy SC, Jabbour HN. 2001. Expression, localization, and signaling of PGE2 and EP2/EP4 receptors in human nonpregnant endometrium across the menstrual cycle. J Clin Endocrinol Metab 86:4453–4459 [DOI] [PubMed] [Google Scholar]
- 27. Critchley HO, Wang H, Kelly RW, Gebbie AE, Glasier AF. 1998. Progestin receptor isoforms and prostaglandin dehydrogenase in the endometrium of women using a levonorgestrel-releasing intrauterine system. Hum Reprod 13:1210–1217 [DOI] [PubMed] [Google Scholar]
- 28. Ludwig H, Spornitz UM. 1991. Microarchitecture of the human endometrium by scanning electron microscopy: menstrual desquamation and remodeling. Ann NY Acad Sci 622:28–46 [DOI] [PubMed] [Google Scholar]
- 29. Nayak NR, Brenner RM. 2002. Vascular proliferation and vascular endothelial growth factor expression in the rhesus macaque endometrium. J Clin Endocrinol Metab 87:1845–1855 [DOI] [PubMed] [Google Scholar]
- 30. Zhang L, Scott PA, Turley H, Leek R, Lewis CE, Gatter KC, Harris AL, Mackenzie IZ, Rees MC, Bicknell R. 1998. Validation of anti-vascular endothelial growth factor (anti-VEGF) antibodies for immunohistochemical localization of VEGF in tissue sections: expression of VEGF in the human endometrium. J Pathol 185:402–408 [DOI] [PubMed] [Google Scholar]
- 31. Charnock-Jones DS, Sharkey AM, Rajput-Williams J, Burch D, Schofield JP, Fountain SA, Boocock CA, Smith SK. 1993. Identification and localization of alternately spliced mRNAs for vascular endothelial growth factor in human uterus and estrogen regulation in endometrial carcinoma cell lines. Biol Reprod 48:1120–1128 [DOI] [PubMed] [Google Scholar]
- 32. Sharkey AM, Day K, McPherson A, Malik S, Licence D, Smith SK, Charnock-Jones DS. 2000. Vascular endothelial growth factor expression in human endometrium is regulated by hypoxia. J Clin Endocrinol Metab 85:402–409 [DOI] [PubMed] [Google Scholar]
- 33. Sales KJ, List T, Boddy SC, Williams AR, Anderson RA, Naor Z, Jabbour HN. 2005. A novel angiogenic role for prostaglandin F2α-FP receptor interaction in human endometrial adenocarcinomas. Cancer Res 65:7707–7716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Critchley HO, Kelly RW, Baird DT, Brenner RM. 2006. Regulation of human endometrial function: mechanisms relevant to uterine bleeding. Reprod Biol Endocrinol 4(Suppl 1):S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hickey M, Carati C, Manconi F, Gannon BJ, Dwarte D, Fraser IS. 2000. The measurement of endometrial perfusion in norplant users: a pilot study. Hum Reprod 15:1086–1091 [DOI] [PubMed] [Google Scholar]
- 36. Hänze J, Weissmann N, Grimminger F, Seeger W, Rose F. 2007. Cellular and molecular mechanisms of hypoxia-inducible factor driven vascular remodeling. Thromb Haemost 97:774–787 [PubMed] [Google Scholar]
- 37. Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. 1996. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 16:4604–4613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Smith SK, Abel MH, Kelly RW, Baird DT. 1981. Prostaglandin synthesis in the endometrium of women with ovular dysfunctional uterine bleeding. Br J Obstet Gynaecol 88:434–442 [DOI] [PubMed] [Google Scholar]
- 39. Smith OP, Jabbour HN, Critchley HO. 2007. Cyclooxygenase enzyme expression and E series prostaglandin receptor signalling are enhanced in heavy menstruation. Hum Reprod 22:1450–1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.