Abstract
Context:
The endometrium is a multicellular, steroid-responsive tissue that undergoes dynamic remodeling every menstrual cycle in preparation for implantation and, in absence of pregnancy, menstruation. Androgen receptors are present in the endometrium.
Objective:
The objective of the study was to investigate the impact of androgens on human endometrial stromal cells (hESC).
Design:
Bioinformatics was used to identify an androgen-regulated gene set and processes associated with their function. Regulation of target genes and impact of androgens on cell function were validated using primary hESC.
Setting:
The study was conducted at the University Research Institute.
Patients:
Endometrium was collected from women with regular menses; tissues were used for recovery of cells, total mRNA, or protein and for immunohistochemistry.
Results:
A new endometrial androgen target gene set (n = 15) was identified. Bioinformatics revealed 12 of these genes interacted in one pathway and identified an association with control of cell survival. Dynamic androgen-dependent changes in expression of the gene set were detected in hESC with nine significantly down-regulated at 2 and/or 8 h. Treatment of hESC with dihydrotestosterone reduced staurosporine-induced apoptosis and cell migration/proliferation.
Conclusions:
Rigorous in silico analysis resulted in identification of a group of androgen-regulated genes expressed in human endometrium. Pathway analysis and functional assays suggest androgen-dependent changes in gene expression may have a significant impact on stromal cell proliferation, migration, and survival. These data provide the platform for further studies on the role of circulatory or local androgens in the regulation of endometrial function and identify androgens as candidates in the pathogenesis of common endometrial disorders including polycystic ovarian syndrome, cancer, and endometriosis.
The human endometrium is a multicellular, steroid-target tissue that undergoes dynamic remodeling during every menstrual cycle (1). Cell proliferation, angiogenesis, differentiation (decidualization), and shedding (menstruation) are controlled by cyclical variations in estrogen and progesterone secreted by the ovaries. In normal women the circulating level of androgen is higher than estrogen; the major circulating androgens include dehydroepiandrosterone (DHEA) and DHEA sulfate, androstenedione, and testosterone (T) (2). During the normal menstrual cycle, androgens are secreted by the ovary and adrenal gland with the latter the major source of DHEA and DHEA sulfate (3). Peripheral levels of androstenedione and T peak midcycle, consistent with approximately 50% being derived from ovarian tissue (3). Although circulating concentrations of dihydrotestosterone (DHT) are low, both isoforms of 5α-reductase (types 1 and 2) are expressed in endometrial epithelial cells, consistent with the potential for local metabolism of T to DHT (4). Only T and DHT are capable of binding with high specificity and affinity to the androgen receptor (AR), a member of a superfamily of ligand-activated transcription factors that includes estrogen receptors (ER; ERα, ERβ) and progesterone receptor (http://www.nursa.org/). In the human endometrium, immunoexpression of AR in the functional (upper) layer is most intense in stromal fibroblasts during the estrogen-dominated proliferative phase (1, 4), down-regulated during the secretory phase, but maintained in stromal cells within the basal compartment throughout the cycle (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). Expression in glandular epithelial cells is up-regulated in the functional compartment during the mid-secretory phase and can be detected in both stromal and epithelial cells in first trimester decidua (5).
A number of lines of evidence suggest that endogenous or exogenous androgens can alter endometrial function. For example, higher circulating concentrations of free T and overexpression of endometrial AR (6) have been detected in women with polycystic ovarian syndrome (PCOS), a condition associated with increased rates of miscarriage, endometrial hyperplasia, and endometrial cancer (7). Local intrauterine delivery of the androgenic progestagen levonorgestrel results in decidualization of endometrial stromal cells, atrophy of the glandular and surface epithelium and changes in vascular morphology (8), and administration of androgens to female transsexuals results in endometrial atrophy (9). The mechanisms responsible for these androgen-dependent changes in endometrial cell function remain unexplored.
To date, most studies examining the impact of androgens on gene expression have been conducted using cell lines derived from prostatic cancers. The most robust evidence for direct binding of ligand-activated AR to promoters of androgen-regulated genes being obtained by combining chromatin immunoprecipitation (ChIP) with tiled oligonucleotide microarrays (10–12). The endometrium, like the prostate, has a well-defined stromal compartment and a steroid-dependent secretory epithelium capable of forming hormone-dependent adenocarcinomas. When Nantermet et al. compared data sets obtained from array studies using the ventral prostates (13) and uterus (14) from rats, they found a common target gene set (n = 28) regulated by DHT underlining the existence of common androgen-regulated pathways in these two organs.
In the current study, we screened gene data sets, performed bioinformatics for promoter and pathway analysis, and confirmed androgen-dependent changes in a target set of androgen-regulated genes using primary human endometrial AR-positive stromal cells. These studies revealed a novel role for androgen in endometrial cell survival and highlighted the opportunities to be gained by bioinformatic analysis of genomic data sets from different tissues/species.
Materials and Methods
In silico analysis of endometrial data sets and identification of putative androgen-regulated genes
Microarray, ChIP, and published gene data sets (Supplemental Table 1) were recovered from the Gene Expression Omnibus [GSE4888 (15), GSE7868 (12)] and published data sets (14, 16). The strategy for comparison of data sets is outlined in Supplemental Fig. 2. The GSE4888 data set included raw signal intensities; global normalization to the 75th percentile was undertaken and all subsequent calculations were done using log2-transformed values. Fold change and false discovery rate was calculated using the SAM (Significance Analysis Microarray software developed at Stanford University and available free to academic users by registering at http://www-stat-class.stanford.edu/∼tibs/clickwrap/sam.html.). For the data set generated from rat uterus (14), gene identifiers were mapped on to the human ortholog. Data generated from array analyses of endometrium of normoovulatory women (15) were processed to identify those genes differentially expressed between the proliferative [AR immunoexpression intense in stromal cells (1, 4)] and midsecretory phases; this comparison was not made in the original study (15). The final endometrial androgen target gene set was generated by filtering for entries overlapping between all the data sets derived from the studies in both human and rat endometrium (14–16) and human prostatic cells (11, 17, 18) (Supplemental Fig. 2).
Promoter and pathway analysis
Promoter regions of genes in the endometrial androgen target gene set (Table 1) were screened for transcription factor binding sites using MAPPER (http://bio.chip.org/mapper (19); using sequences 10 kb upstream and 4 kb downstream from the transcription start site of each gene as retrieved from Ensemble 54 (build NCBI36). Gene ontology and biological pathway analyses were performed using MetaCore version 5.4 build 19940 (GeneGo, Inc., St. Joseph, MI).
Table 1.
Endometrium androgen target gene set identified by stringent in silico screening of publicly available data sets
| Gene ID | Name | Predicted change* | Gene ID | Name | Predicted change* |
|---|---|---|---|---|---|
| NM_018677 | ACSS2 | Up | NM_000805 | GAST | Down |
| NM_000610 | CD44 | Up | NM_002425 | MMP10 | Down |
| NM_006079 | CITED2 | Up | NM_006207 | PDGFRL | Down |
| NM_000115 | EDNRB | Up | NM_021127 | PMAIP1 | Down |
| NM_005797 | EVA1/MPZL2 | Up | NM_138818 | PRUNE2 | Down |
| NM_000187 | HGD | Up | NM_024745 | SHCBP1 | Down |
| NM_000240 | MAOA | Up | NM_007117 | TRH | Down |
| NM_003621 | PPFIBP2 | Up |
Patients and tissues
Endometrial tissues were collected by suction curette (n = 14, Pipelle; Laboratoire CCD, Paris, France) or after hysterectomy (n = 10) from women with regular menstrual cycles who had not received hormonal treatments during the 3 months before tissue collection. Samples were staged based on standard histological criteria, the patient's reported last menstrual period, and circulating estradiol (E2) and progesterone levels at the time of collection. First-trimester decidua (n = 4; 8–12 wk gestation) were collected from women undergoing the surgical termination of pregnancy. Written informed consent was obtained from all subjects and ethical approval granted by the Lothian Research Ethics Committee. Tissues were fixed in 4% neutral buffered formalin, immersed in RNAlater (Ambion, Austin, TX), or used for isolation of primary cells.
Endometrial stromal cell culture
Primary human endometrial stromal cells (hESC) were purified from proliferative stage samples as previously described (20). Cells were maintained at 37 C in DM-RPMI 1640 [containing 10% heat inactivated fetal calf serum, penicillin (100 U), streptomycin (0.1 mg/ml), Fungizone (1 μg/ml) (Life Technologies, Inc., Paisley, UK), l-glutamine (2 mm), and gentamicin (0.02 mg/ml)]. For gene expression studies, they were seeded at 2 × 105/well in six-well culture plates and allowed to reach 80% confluence, incubated for 24 h in phenol red-free DM-RPMI 1640 (10% charcoal stripped fetal calf serum and supplements as above) followed by 24 h in phenol red-free, serum-free medium. Treatments were performed using serum-free medium containing vehicle (ethanol) or DHT (10−8 m) for 2, 8, or 24 h (n = 6) for measurement of mRNA or 8, 24, or 48 h (n = 6) for measurement of protein. hESC derived from proliferative phase endometrium express both ERα and ERβ [(21); Collins, F., unpublished observations]; therefore, DHT was chosen for these studies because, unlike T, it is not metabolized to E2. To ensure that concentrations of DHT were maintained throughout the culture period, media were refreshed after 8 h.
Gene expression analysis
Total RNA was purified using TRIzol (Invitrogen, Paisley, UK) according to the manufacturer's instructions; cDNA was prepared using the SuperScript VILO cDNA synthesis kit (Invitrogen) using 400 ng of template RNA. PCR was performed in 10-μl reactions that contained 5.0 μl of TaqMan master mix (Applied Biosystems, Warrington, UK), 200 nm of each primer, 5 nm of probe, and 4.5 μl of template (1:20 dilution of cDNA). Details of primer/probes are given in Supplemental Table 2; PCR was 95 C for 10 min plus 40 cycles of 95 C for 15 sec and 60 C for 60 sec. Target genes were quantified using standard template dilution curves and normalization to endogenous controls (18S, actin).
Immunohistochemistry
Primary antibodies are listed in Supplemental Table 3; negative controls had nonimmune serum. Tissues sections were immunostained using standard protocols with antigen retrieval at pH 6. Primary hESC were fixed in cold methanol and permeabilized in 0.2% IGEPAL (octylphenoxypolyethoxyethanol; Sigma, St. Louis, MO) with 1% BSA and 10% blocking serum. Endogenous peroxidase was blocked using 0.15% hydrogen peroxide in methanol. For visualization of bound primary antibodies, cells were incubated with biotinylated secondary antibodies (goat antimouse Ig, goat antirabbit Ig, or rabbit antigoat Ig; Dako UK Ltd., Ely, UK) diluted to 1:500 for 30 min at room temperature followed by reagents from the ImmPACT diaminobenzidine kit (Vector Laboratories, Burlingame, CA).
Apoptosis assay
Apoptosis was measured using the Apo-ONE homogeneous caspase-3/7 assay kit (Promega Ltd., Hampshire, UK): the amount of fluorescent product generated was proportional to the amount of active caspase-3/7 in the sample. Cells were incubated with staurosporine (30–500 nm) alone (positive control) or in the presence of DHT, E2, or DHT plus E2 all at 10−8 m for 0.5–3 h.
Wound-healing assay
hESC (passage <5) were seeded at 2 × 105 well in six-well plates in DM-RPMI 1640, supplemented as above, and grown until confluent. Forty-eight hours before scratch, medium was replaced with serum/phenol-free RPMI 1640. One hour before the scratch, cells were pretreated for 1 h with 10−5 m flutamide or medium supplemented with vehicle. Each well of cells was scratched with a sterile 200-μl pipette tip and then incubated in the presence of DHT (10−8 m) or vehicle in serum-free media (n = 6 experiments, duplicate wells). For each well, six images were captured along the length of each wound at 0 and 24 h using an Axiovert 200M inverted microscope (Carl Zeiss, Jena, Germany). Images were analyzed using AxioVision release 4.72, and calculations of average distance closed for each sample were based on three measurements at identical positions along the wound at 0 and 24 h.
Statistical analysis
Values presented are averages ± sem. Differences in mean values were evaluated by an unpaired t test and two-way ANOVA analysis using Bonferroni for post hoc comparisons. P < 0.05 was considered to be statistically significant.
Results
Identification of putative androgen target genes expressed in human endometrium
Rigorous in silico comparisons of a gene set extracted from molecular phenotyping of human endometrium from normoovulatory women (15) with data sets of androgen-regulated genes identified in rat uterus, decidualized human cells, and androgen-responsive prostatic cell lines (Supplemental Table 1 and Supplemental Fig. 2) resulted in the identification of only 15 genes, hereafter referred to as the endometrial androgen target gene set (Table 1). Consistent with the inclusion of genes identified by ChIP array (11, 12) in our screening strategy, consensus androgen response elements were identified in the promoter regions of all candidate genes (Supplemental Fig. 3). Sequences with homology to consensus binding sites for other proteins implicated in steroid-dependent changes in gene transcription, including estrogen and progesterone receptors, activator protein-1, and SP-1 were also represented in the same promoter regions (Supplemental Fig. 3).
Validation of expression of androgen target gene set in human endometrium and decidua
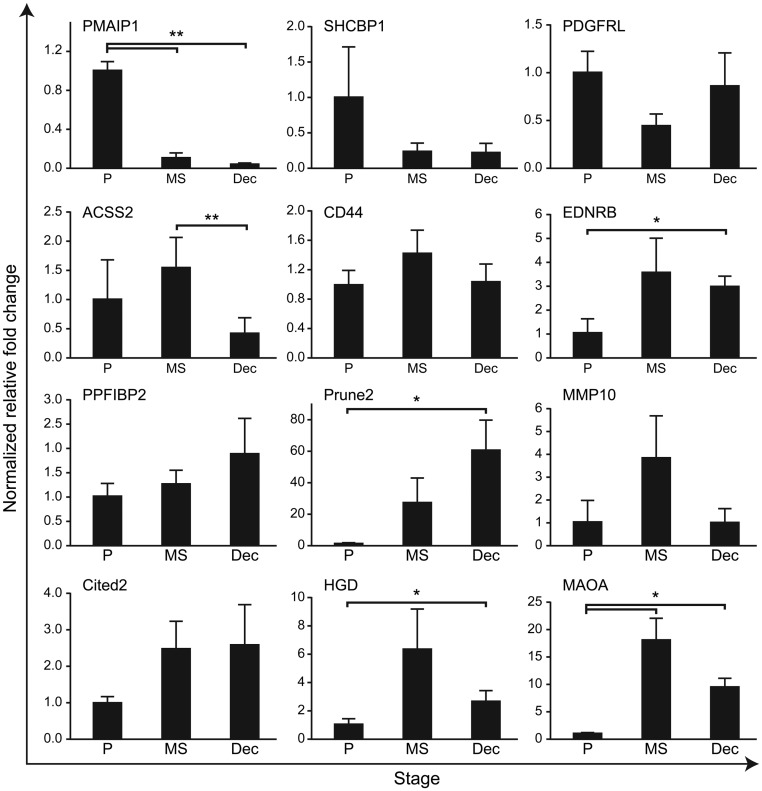
mRNA encoded by all 15 of the androgen target gene set were detected in total tissue homogenates of endometrial tissue obtained during the proliferative phase (Fig. 1 and data not shown) when immunoexpression of AR is restricted to the stromal compartment (Supplemental Fig. 1). Fourteen were also detected in extracts of first-trimester decidua (TRH, not detectable, Fig. 1, and data not shown). Expression of phorbol-12-myristate-13-acetate-induced protein 1 (PMAIP1) mRNA was higher in proliferative compared with midsecretory phase endometrium (P < 0.01), whereas the opposite result was obtained for monoamine oxidase type A (MAOA) (Fig. 1). Using commercially available antibodies, we were able to confirm expression of proteins encoded by PMAIP1, MAOA, cAMP response element-binding protein (CBP)/p300-interacting transactivator 2 (CITED2), prune homolog 2 (PRUNE2/BNIPXL), and CD44 using sections of human endometrium and decidua; all four proteins were expressed in the stromal compartment during the proliferative phase (Supplemental Fig. 4). Subcellular patterns of expression in primary hESC mimicked those in vivo with CITED2 detected in the nucleus, PRUNE2 localized to the cytoplasm, and PMAIP1 in both compartments (Supplemental Fig. 5).
Fig. 1.
Expression of candidate androgen-regulated gene mRNA in total tissue extracts from human endometrium and decidua as determined by quantitative RT-PCR. Samples were homogenized from functional endometrium recovered during the proliferative (P) and midsecretory phases (MS) as well as from first-trimester decidua (Dec). Concentrations displayed relative to those in proliferative phase in each case (n = 4–6 per group).
Regulation of target genes in primary endometrial stromal cells by DHT is time dependent
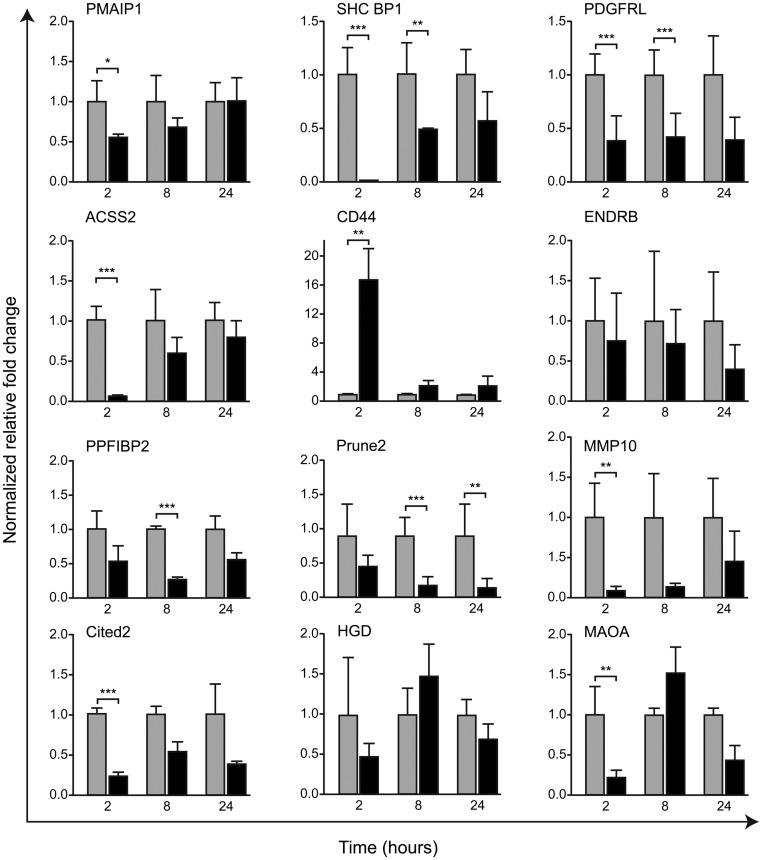
During in vitro incubation the primary hESC retained the phenotype of the stromal cells within the functional layer of the proliferative phase endometrium being immunopositive both CD10 [a stromal cell marker (22)] and AR; the latter was also detected as a full-length protein on Western blots (Supplemental Fig. 5). We confirmed that expression of AR mRNA was maintained up to and including passage 5 but thereafter declined (not shown), and therefore, all subsequent evaluations were undertaken on primary hESC at passages 3–5. Expression of 12 of 15 of the endometrial target gene set was detected in primary hESC (Fig. 2); concentrations of mRNA encoded by three putative AR target genes [EVA1, gastrin (GAST), TRH] were too low to be investigated further. For nine of 15 of the genes [PMAIP1, SHC SH2 binding protein 1 (SHC BP1), platelet-derived growth factor receptor-like (PDGFRL), acyl-CoA synthetase short-chain family member 2 (ACSS2), matrix metallopeptidase 10 (stromelysin 2/MMP10, CITED2, MAOA] incubation of cells with DHT resulted in a significant reduction in their mRNA at one or more time points. The exception was CD44, which was increased at 2 h, returning to control levels at 8/24 h (Fig. 2).
Fig. 2.
Time-dependent changes in androgen-regulated gene expression in primary hESC. Cells were incubated with vehicle (gray bars) or 10−8 m DHT (black bars) for 2, 8, and 24 h (n = 6 each time point). Concentration of mRNA was quantified by quantitative RT-PCR and expressed as fold change compared with time-matched vehicle-treated hESC. Note that with the exception of CD44, incubation of cells with DHT resulted in either a significant reduction in concentration of mRNA or no significant change but with a trend to a reduction. For most genes, changes in mRNA expression were time dependent with the most striking change in total concentrations at 2 h for eight of 12 of the genes. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Pathway analysis identifies apoptosis as an androgen-dependent process
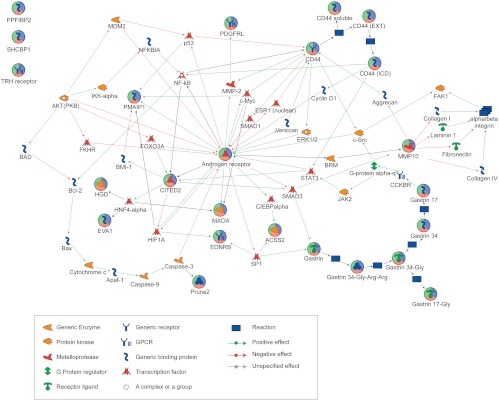
Pathway analyses revealed 12 of 15 of the androgen target gene set interacted, directly or indirectly, on one network associated with androgen-regulated cell function (Fig. 3) and an association with cell death (Table 2). Additional bioinformatic analysis of subnetwork interactions formed between the androgen target gene set highlighted a relationship between AR-CD44-endothelin receptor B and gastrin associated with response to cell stress. A further association was identified between AR-CITED2-CD44 and PMAIP1 in control of apoptosis (Table 3).
Fig. 3.
Pathway analysis of endometrial androgen target gene set. Note 12 of 15 androgen-regulated genes identified by in silico screening (indicated with multicolored circles) were predicted to interact in a single Metacore pathway centered on AR; intermediate molecules are indicated to show putative intermediate signaling molecules. Key shows the functional classification of the target genes and the arrows indicate predicted regulation (red, negative; green, positive).
Table 2.
Gene ontology processes associated with the androgen candidate gene set identified by androgen candidate gene ontology identified using Metacore
| Processes | Target | P value | Z-score |
|---|---|---|---|
| Regulation of biological quality (57.5%), regulation of apoptosis (42.5%), regulation of programmed cell death (42.5%) | 14 | 5.74e-43 | 119.50 |
| Regulation of biological quality (54.5%), embryonic placenta development (13.6%), oxygen homeostasis (9.1%) | 6 | 2.18e-16 | 61.16 |
| Regulation of ion transport (27.0%), positive regulation of hydrolase activity (32.4%), positive regulation of phospholipase activity (24.3%) | 4 | 5.94e-10 | 35.52 |
Note that the term target indicates the number of candidate genes/proteins/compounds (objects) in a data set that are associated with a given network/process. The P value (the probability of a random intersection) indicates a measure of relevance of the intersection between a gene/protein and an entity in a particular ontology. The lower the P value, the higher is the nonrandomness of finding such intersection. The Z-score ranks the networks with regard to the number of objects present in the networks. The higher the Z-score, the higher the number of objects from the data set.
Table 3.
Subnetworks within the candidate gene set and associated processes identified by androgen candidate gene ontology identified using Metacore
| Network | GO processes | P value | Z-score |
|---|---|---|---|
| AR, CD44, EDNRB, CITED2, gastrin | Response to stress, regulation of catalytic activity | 3.00e-32 | 86.27 |
| PDGF-R-β, CITED2, CD44 | Response to hormone stimulus, regulation of locomotion | 7.21e-20 | 56.87 |
| AR, CITED2, CD44, PMAIP1 | Regulation of apoptosis, regulation of programmed cell death, regulation of developmental process | 2.14e-14 | 43.52 |
| ACSS1, gastrin, pyrophosphate cytoplasm, acetyl-CoA cytoplasm | Acetyl-CoA biosynthetic process, IL-8 production | 1.24e-08 | 43.12 |
| EDNRB, HGD, TRH receptor, c-Src, connexin 43 | Dopamine receptor signaling pathway, regulation of nucleotide biosynthetic process | 3.06e-07 | 25.93 |
| CITED2, ACSA, EDNRB, HNF4-α, HIF1A | Developmental process, response to hypoxia | 2.52e-07 | 26.76 |
Note that the term target indicates the number of candidate genes/proteins/compounds (objects) in a data set that are associated with a given network/process. The P value (the probability of a random intersection) indicates a measure of relevance of the intersection between a gene/protein and an entity in a particular ontology. The lower the P value, the higher is the nonrandomness of finding such intersection. The Z-score ranks the networks with regard to the number of objects present in the networks. The higher the Z-score, the higher the number of objects from the data set. GO, Gene ontology.
Functional studies confirm that androgens may protect primary stromal hESC against apoptosis and reduce cell migration
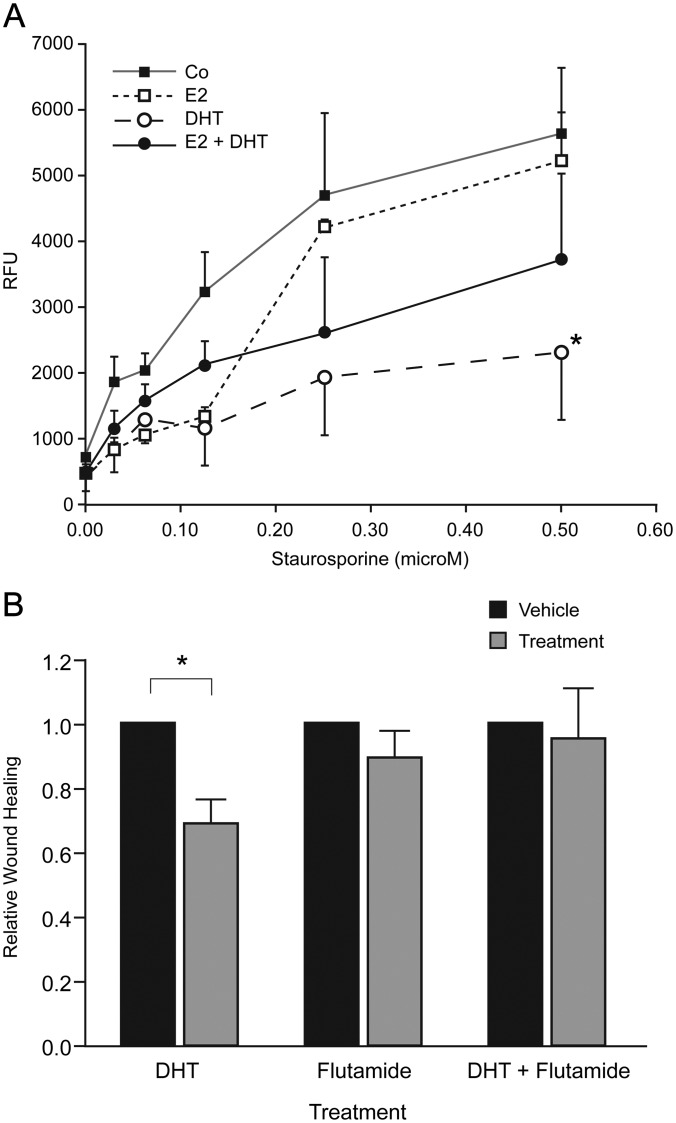
The impact of DHT on apoptosis was investigated using a caspase-3/7 fluorescent assay. Caspase activity was not detected in control hESC cultures or after treatment of cells with DHT alone (not shown). When apoptosis was induced by treatment of cells with staurosporine (30–500 nm), addition of DHT significantly reduced caspase activation. Addition of E2 had no effect and simultaneous addition of E2 and DHT partially abrogated the antiapoptotic impact of the DHT (Fig. 4A).
Fig. 4.
Treatment of hESC with DHT alters apoptosis and proliferation. A, Apoptosis as measured by caspase-3/7 assay in cells treated with staurosporine. Note addition of DHT had a significant impact (*, P < 0.05) on the rate of apoptosis when compared with controls but that the impact of DHT was blunted in the presence of E2 (n = 4). B, Wound-healing assay. Addition of DHT had a significant impact (*, P < 0.05) compared with its vehicle control (ethanol), whereas flutamide alone had no impact compared with vehicle (methanol) and pretreatment of cells with flutamide blocked the DHT-dependent reduction in wound closure. DHT, 10−8 m; flutamide, 10−5 m; n = 6.
To determine whether exposure to androgens could have a direct impact on migration of hESC, a wound-healing assay was performed using cells pretreated with vehicle or flutamide that were subsequently incubated with or without DHT for 24 h (Fig. 4B). A significant reduction in the rate of wound healing was detected when cells were incubated with DHT alone (P < 0.05), whereas addition of flutamide alone had no effect pretreatment of cells with flutamide abrogated the impact of DHT (Fig. 4B).
Discussion
Expression of AR and enzymes capable of local biosynthesis of androgenic ligands has been well documented in human endometrium, but little is known about the direct impacts of androgens on gene expression in this dynamic tissue. In this study, rigorous in silico comparisons of genomic data sets derived from normal human endometrium (15) with those identified as androgen regulated by array analysis of uteri from DHT-exposed rats (14), androgen-treated human decidualized cells (16), and genes identified as containing regions within their promoters capable of binding ligand activated androgen receptors (11, 12) and has enabled us to identify, for the first time, a group of androgen-regulated genes expressed in primary hESC. Our strategy included data sets based on ChIP-on-chip (11, 12) conducted using in prostate cancer-derived epithelial cells treated for short duration with androgen. We included the ChIP data sets because they provided a stringent, unbiased way of focusing on genes that were direct targets of androgen action; unfortunately, ChIP data sets for androgen-treated endometrial cells were not available. Only a small number of genes were identified as common to all of these data sets reflecting differences in tissue/cell context.
One unexpected finding was that treatment of primary hESC with DHT resulted in a transient but significant reduction in expression of most of the mRNA encoded by our androgen target gene set. Consistent with the likely differences in availability of modulators of androgen action (coactivators/corepressors) between the AR-positive cells from endometrium (primary stroma) and those used for the ChIP arrays (transformed AR positive prostatic epithelial cells), four genes (ACSS2, CITED2, MAOA, PPFIBP2) showed DHT-dependent changes in expression that were opposite in the two cell types [Table 1 (11, 17)].
Notably, the regulation of three of our androgen target gene set, namely PRUNE2, PMAIP1, and ACSS2, has never been investigated in the context of endometrial function. Promoter analysis identified multiple binding sites for both AR and Sp1 and pathway analysis (see Fig. 3) supported a role for Sp1 in mediating AR-dependent changes in expression of EDNRB and ACSS2. In this study we focused on the impact of androgens on stromal cells recovered from the functional layer of the endometrium during the proliferative phase at a time when AR is not expressed in epithelial cells (Supplemental Fig. 1). However, we did detect expression of MAOA, CD44, CITED2, PRUNE2, and PMAIP1 in endometrial epithelial cells by immunohistochemistry, and therefore, further studies are required to determine whether androgens also have an impact on their expression in this cell type.
Analysis of the androgen target gene set with Metacore software (GeneGo) revealed a strong association with processes regulating programmed cell death (Table 2). Apoptosis is an important regulator of endometrial function, with reports that it occurs predominantly in the late secretory and menstrual phases associated with alterations in the expression of Bcl-2 and Bax (23). Two members of our target gene set, PRUNE2 (BMCC1) and PMAIP1, encode proapoptotic proteins not previously investigated in the context of endometrial function. PRUNE2 (BMCC1) is highly expressed in the nervous system; although some studies have reported androgen regulation in prostate cancer cells (24), this has been disputed (25). PMAIP1, also known as NOXA (Latin for damage), encodes a Bcl-2 homology 3-only member of the Bcl-2 family of proteins. The protein has been implicated in controlling cell death pathways in several cell types by virtue of competitive binding to proteins including the antiapoptotic proteins Bcl-2 protein A1 Bcl-xL and MCL1 (26). In neuroblastoma cells, gene knockdown of PMAIP1 significantly reduces apoptosis (26). Expression of PMAIP1 can be repressed by glucocorticoids in lymphoblasts of children with acute lymphoblastic leukemia (27), consistent with detection of a putative glucocorticoid response element in our TRANSFAC analysis.
Conversely, introduction of AR into AR-negative prostate cancer cells is reported to up-regulate expression of PMAIP1 (28), highlighting the importance of cell context in the regulation of this gene. Although initial studies reported that PMAIP1 is a primary p53 response gene, it is now apparent that multiple signals can induce its expression (reviewed in Ref. 29). Reduced expression of PRUNE2 and PMAIP1 in the endometrium in response to androgens is consistent with reports that the amount of apoptosis is very low in the endometrium of patients with PCOS (30), a condition associated with elevated circulating androgens, elevated expression of endometrial AR, and infertility (6). CD44, the only gene identified as up-regulated by androgen action in our primary stromal cells, has also been implicated in regulatory pathways involving the tumor suppressor p53. Its overexpression antagonizes the tumor-suppressive apoptotic and growth-inhibitory actions of p53 in mammary epithelial cells (31). Stromal cell decidualization during the secretory phase is associated with reduced expression of AR in the functional layer, but expression in the basal compartment is maintained (Supplemental Fig. 1). These temporal and spatial differences in the pattern of expression of AR could result in variations in expression of androgen-regulated genes in the endometrium and may protect basal stromal cells from apoptosis.
In addition to those genes implicated in control of apoptosis and/or associated with the activities of the tumor suppressor p53 genes, several of the genes we have identified as androgen regulated in endometrial stromal cells have also been implicated in development of cancers. For example, SHCBP1 (SHC SH2 binding protein 1) has been cited as one of a panel of six markers for the diagnosis of early cervical cancer (32). PDGFRL, a gene that encodes a secreted protein with significant sequence homology to the ligand-binding domain of platelet-derived growth factor receptor-β, has been proposed as a tumor suppressor (33). CITED2, a CBP/p300-dependent transcriptional coactivator, was associated with increased expression of matrix metalloproteinase (MMP) 13 and enhanced invasiveness of colon cancer cells (34). All three genes have been detected in uterine tissue. For example, Kim et al. (35) reported that expression of SHCBP1 was down-regulated in endometrium recovered during the proliferative phase from women with PCOS compared with controls, a result consistent with our novel data demonstrating a striking and significant reduction in hESC treated with DHT. Talbi et al. (15) have reported that expression of PDGFL and CITED2 were cycle dependent, and studies in mice identified Cited2 as one of a group of genes up-regulated in the uterus 4 h after progesterone treatment (36). However, we believe the current study is the first to report that these genes might be targets for androgen action within the stromal compartment of the human endometrium. Data herein provide novel insight into the mechanisms that might contribute to the increased risk of endometrial cancer observed in women with PCOS (7).
Endometriosis is a chronic condition characterized by the presence of endometrial tissue outside the uterine cavity and, like PCOS and endometrial hyperplasia, has been associated with a progesterone-resistant phenotype (37). The synthetic androgen, Danazol (17α-ethinyl testosterone) is an effective treatment for pain associated with endometriosis but is no longer used due to unacceptable side effects (38). Although disturbances in androgen-dependent gene expression have not been investigated in the context of this disease, it is notable that both AR and 5α-reductase enzymes have been detected in pelvic endometriosis (39). Altered expression of three of the genes in our androgen target set has also been recorded in women suffering from endometriosis. Cells recovered from menstrual effluent of women with endometriosis are reported to have increased expression of CD44 splice variants. Cells from the endometrium of women with endometriosis are reported to exhibit increased adherence to peritoneal mesothelial cells (40) and reduced expression of MAOA and CITED2 (37). It has been suggested that alterations in expression of MMP in endometriotic lesions would favor disturbances in tissue invasion/remodeling within the endometriotic tissue (41); expression of MMP10 has not been investigated to date and would merit further investigation in these patients.
In conclusion, using rigorous bioinformatics to compare data sets between species and between tissues, we have identified a small cohort of androgen-responsive genes expressed in the human endometrium. Pathway analysis and functional assays suggest androgen-dependent changes in gene expression may have a significant impact on stromal cell migration and survival in this tissue. These novel data provide the platform for further investigations on the role of circulatory or local androgens in the regulation of endometrial function that will require studies on intact tissue in which the dynamics of stromal-epithelial interactions are maintained. These data identify androgens as candidates in the pathogenesis of common endometrial disorders including PCOS, endometrial cancer, and endometriosis.
Supplementary Material
Acknowledgments
We thank Dr. Alistair Williams for histological assessment of endometrial biopsies, research nurses Catherine Murray and Sharon McPherson for patient recruitment, and Karen Tuzi and Arantza Esnal for technical support.
This work was supported by Project Grant 083908 from the Wellcome Trust (principal investigators H.O.D.C. and P.T.K.S.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ACSS2
- Acyl-CoA synthetase short-chain family member 2
- AR
- androgen receptor
- CBP
- cAMP response element-binding protein
- ChIP
- chromatin immunoprecipitation
- CITED2
- CBP/p300-interacting transactivator 2
- DHEA
- dehydroepiandrosterone
- DHT
- dihydrotestosterone
- E2
- estradiol
- ER
- estrogen receptor
- hESC
- human endometrial stromal cell
- MAOA
- monoamine oxidase type A
- MMP
- matrix metalloproteinase
- PCOS
- polycystic ovarian syndrome
- PMAIP1
- phorbol-12-myristate-13-acetate-induced protein 1
- PRUNE2
- prune homolog 2
- T
- testosterone.
References
- 1. Critchley HO, Saunders PT. 2009. Hormone receptor dynamics in a receptive human endometrium. Reprod Sci 16:191–199 [DOI] [PubMed] [Google Scholar]
- 2. Burger HG. 2002. Androgen production in women. Fertil Steril 77(Suppl 4):S3–S5 [DOI] [PubMed] [Google Scholar]
- 3. Abraham GE. 1974. Ovarian and adrenal contribution to peripheral androgens during the menstrual cycle. J Clin Endocrinol Metab 39:340–346 [DOI] [PubMed] [Google Scholar]
- 4. Ito K, Suzuki T, Akahira J, Moriya T, Kaneko C, Utsunomiya H, Yaegashi N, Okamura K, Sasano H. 2002. Expression of androgen receptor and 5α-reductases in the human normal endometrium and its disorders. Int J Cancer 99:652–657 [DOI] [PubMed] [Google Scholar]
- 5. Milne SA, Henderson TA, Kelly RW, Saunders PT, Baird DT, Critchley HO. 2005. Leukocyte populations and steroid receptor expression in human first-trimester decidua: regulation by antiprogestin and prostaglandin E analog. J Clin Endocrinol Metab 90:4315–4321 [DOI] [PubMed] [Google Scholar]
- 6. Apparao KB, Lovely LP, Gui Y, Lininger RA, Lessey BA. 2002. Elevated endometrial androgen receptor expression in women with polycystic ovarian syndrome. Biol Reprod 66:297–304 [DOI] [PubMed] [Google Scholar]
- 7. Giudice LC. 2006. Endometrium in PCOS: implantation and predisposition to endocrine CA. Best Pract Res Clin Endocrinol Metab 20:235–244 [DOI] [PubMed] [Google Scholar]
- 8. Guttinger A, Critchley HO. 2007. Endometrial effects of intrauterine levonorgestrel. Contraception 75:S93–S98 [DOI] [PubMed] [Google Scholar]
- 9. Miller N, Bédard YC, Cooter NB, Shaul DL. 1986. Histological changes in the genital tract in transsexual women following androgen therapy. Histopathology 10:661–669 [DOI] [PubMed] [Google Scholar]
- 10. Chang GT, Blok LJ, Steenbeek M, Veldscholte J, van Weerden WM, van Steenbrugge GJ, Brinkmann AO. 1997. Differentially expressed genes in androgen-dependent and -independent prostate carcinomas. Cancer Res 57:4075–4081 [PubMed] [Google Scholar]
- 11. Massie CE, Adryan B, Barbosa-Morais NL, Lynch AG, Tran MG, Neal DE, Mills IG. 2007. New androgen receptor genomic targets show an interaction with the ETS1 transcription factor. EMBO Rep 8:871–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang Q, Li W, Liu XS, Carroll JS, Jänne OA, Keeton EK, Chinnaiyan AM, Pienta KJ, Brown M. 2007. A hierarchical network of transcription factors governs androgen receptor-dependent prostate cancer growth. Mol Cell 27:380–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nantermet PV, Xu J, Yu Y, Hodor P, Holder D, Adamski S, Gentile MA, Kimmel DB, Harada S, Gerhold D, Freedman LP, Ray WJ. 2004. Identification of genetic pathways activated by the androgen receptor during the induction of proliferation in the ventral prostate gland. J Biol Chem 279:1310–1322 [DOI] [PubMed] [Google Scholar]
- 14. Nantermet PV, Masarachia P, Gentile MA, Pennypacker B, Xu J, Holder D, Gerhold D, Towler D, Schmidt A, Kimmel DB, Freedman LP, Harada S, Ray WJ. 2005. Androgenic induction of growth and differentiation in the rodent uterus involves the modulation of estrogen-regulated genetic pathways. Endocrinology 146:564–578 [DOI] [PubMed] [Google Scholar]
- 15. Talbi S, Hamilton AE, Vo KC, Tulac S, Overgaard MT, Dosiou C, Le Shay N, Nezhat CN, Kempson R, Lessey BA, Nayak NR, Giudice LC. 2006. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology 147:1097–1121 [DOI] [PubMed] [Google Scholar]
- 16. Cloke B, Huhtinen K, Fusi L, Kajihara T, Yliheikkilä M, Ho KK, Teklenburg G, Lavery S, Jones MC, Trew G, Kim JJ, Lam EW, Cartwright JE, Poutanen M, Brosens JJ. 2008. The androgen and progesterone receptors regulate distinct gene networks and cellular functions in decidualizing endometrium. Endocrinology 149:4462–4474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nickols NG, Dervan PB. 2007. Suppression of androgen receptor-mediated gene expression by a sequence-specific DNA-binding polyamide. Proc Natl Acad Sci USA 104:10418–10423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Velasco AM, Gillis KA, Li Y, Brown EL, Sadler TM, Achilleos M, Greenberger LM, Frost P, Bai W, Zhang Y. 2004. Identification and validation of novel androgen-regulated genes in prostate cancer. Endocrinology 145:3913–3924 [DOI] [PubMed] [Google Scholar]
- 19. Marinescu VD, Kohane IS, Riva A. 2005. The MAPPER database: a multi-genome catalog of putative transcription factor binding sites. Nucleic Acids Res 33:D91–D97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kane N, Jones M, Brosens JJ, Saunders PT, Kelly RW, Critchley HO. 2008. Transforming growth factor-β1 attenuates expression of both the progesterone receptor and Dickkopf in differentiated human endometrial stromal cells. Mol Endocrinol 22:716–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bombail V, MacPherson S, Critchley HO, Saunders PT. 2008. Estrogen receptor related β is expressed in human endometrium throughout the normal menstrual cycle. Hum Reprod 23:2782–2790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Toki T, Shimizu M, Takagi Y, Ashida T, Konishi I. 2002. CD10 is a marker for normal and neoplastic endometrial stromal cells. Int J Gynecol Pathol 21:41–47 [DOI] [PubMed] [Google Scholar]
- 23. Vaskivuo TE, Stenbäck F, Karhumaa P, Risteli J, Dunkel L, Tapanainen JS. 2000. Apoptosis and apoptosis-related proteins in human endometrium. Mol Cell Endocrinol 165:75–83 [DOI] [PubMed] [Google Scholar]
- 24. Clarke RA, Zhao Z, Guo AY, Roper K, Teng L, Fang ZM, Samaratunga H, Lavin MF, Gardiner RA. 2009. New genomic structure for prostate cancer specific gene PCA3 within BMCC1: implications for prostate cancer detection and progression. PLoS One 4:e4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Salagierski M, Verhaegh GW, Jannink SA, Smit FP, Hessels D, Schalken JA. 2010. Differential expression of PCA3 and its overlapping PRUNE2 transcript in prostate cancer. Prostate 70:70–78 [DOI] [PubMed] [Google Scholar]
- 26. Hagenbuchner J, Ausserlechner MJ, Porto V, David R, Meister B, Bodner M, Villunger A, Geiger K, Obexer P. 2010. The anti-apoptotic protein BCL2L1/Bcl-xL is neutralized by pro-apoptotic PMAIP1/Noxa in neuroblastoma, thereby determining bortezomib sensitivity independent of prosurvival MCL1 expression. J Biol Chem 285:6904–6912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schmidt S, Rainer J, Riml S, Ploner C, Jesacher S, Achmüller C, Presul E, Skvortsov S, Crazzolara R, Fiegl M, Raivio T, Jänne OA, Geley S, Meister B, Kofler R. 2006. Identification of glucocorticoid-response genes in children with acute lymphoblastic leukemia. Blood 107:2061–2069 [DOI] [PubMed] [Google Scholar]
- 28. Lin Y, Kokontis J, Tang F, Godfrey B, Liao S, Lin A, Chen Y, Xiang J. 2006. Androgen and its receptor promote Bax-mediated apoptosis. Mol Cell Biol 26:1908–1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ploner C, Kofler R, Villunger A. 2008. Noxa: at the tip of the balance between life and death. Oncogene 27(Suppl 1):S84–S92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Villavicencio A, Bacallao K, Gabler F, Fuentes A, Albornoz J, Casals A, Vega M. 2007. Deregulation of tissue homeostasis in endometria from patients with polycystic ovarian syndrome with and without endometrial hyperplasia. Gynecol Oncol 104:290–295 [DOI] [PubMed] [Google Scholar]
- 31. Godar S, Ince TA, Bell GW, Feldser D, Donaher JL, Bergh J, Liu A, Miu K, Watnick RS, Reinhardt F, McAllister SS, Jacks T, Weinberg RA. 2008. Growth-inhibitory and tumor-suppressive functions of p53 depend on its repression of CD44 expression. Cell 134:62–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Steinau M, Rajeevan MS, Lee DR, Ruffin MT, Horowitz IR, Flowers LC, Tadros T, Birdsong G, Husain M, Kmak DC, Longton GM, Vernon SD, Unger ER. 2007. Evaluation of RNA markers for early detection of cervical neoplasia in exfoliated cervical cells. Cancer Epidemiol Biomarkers Prev 16:295–301 [DOI] [PubMed] [Google Scholar]
- 33. Xu M, Kao MC, Nunez-Iglesias J, Nevins JR, West M, Zhou XJ. 2008. An integrative approach to characterize disease-specific pathways and their coordination: a case study in cancer. BMC Genomics 9(Suppl 1):S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bai L, Merchant JL. 2007. A role for CITED2, a CBP/p300 interacting protein, in colon cancer cell invasion. FEBS Lett 581:5904–5910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim JY, Song H, Kim H, Kang HJ, Jun JH, Hong SR, Koong MK, Kim IS. 2009. Transcriptional profiling with a pathway-oriented analysis identifies dysregulated molecular phenotypes in the endometrium of patients with polycystic ovary syndrome. J Clin Endocrinol Metab 94:1416–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jeong JW, Lee KY, Kwak I, White LD, Hilsenbeck SG, Lydon JP, DeMayo FJ. 2005. Identification of murine uterine genes regulated in a ligand-dependent manner by the progesterone receptor. Endocrinology 146:3490–3505 [DOI] [PubMed] [Google Scholar]
- 37. Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR, Lessey BA, Giudice LC. 2007. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology 148:3814–3826 [DOI] [PubMed] [Google Scholar]
- 38. Selak V, Farquhar C, Prentice A, Singla A. 2007. Danazol for pelvic pain associated with endometriosis. Cochrane Database Syst Rev CD000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Carneiro MM, Morsch DM, Camargos AF, Reis FM, Spritzer PM. 2008. Androgen receptor and 5α-reductase are expressed in pelvic endometriosis. BJOG 115:113–117 [DOI] [PubMed] [Google Scholar]
- 40. Griffith JS, Liu YG, Tekmal RR, Binkley PA, Holden AE, Schenken RS. 2010. Menstrual endometrial cells from women with endometriosis demonstrate increased adherence to peritoneal cells and increased expression of CD44 splice variants. Fertil Steril 93:1745–1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhou HE, Nothnick WB. 2005. The relevancy of the matrix metalloproteinase system to the pathophysiology of endometriosis. Front Biosci 10:569–575 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.