Abstract
Background
The “biological susceptibility” model posits that some individuals, by genetic predisposition, are highly sensitive to environmental stimuli. Exposure to adverse stimuli leads to negative outcomes, and better outcomes follow favourable stimuli. Recent studies indicate that individuals carrying the low-activity (short; s) variant of the serotonin transporter (5-HTT)-linked polymorphic region (5-HTTLPR) show an enhanced vulnerability to posttraumatic stress disorder (PTSD). Simultaneously, they respond poorly to exposure therapy, the first-line treatment to enhance fear extinction in individuals with PTSD. Given that s-allele carriers also show improved adaptive responding when exposed to positive stimuli, we hypothesized that this trait could be used to offset impaired fear extinction.
Methods
We explored this hypothesis preclinically using wild-type and 5-HTT knockout (5-HTT−/−) male rats (n = 36) that share behavioural similarities with 5-HTTLPR s-allele carriers. Subsequent to cued fear conditioning, animals were tested for short- (1 and 2 days postconditioning) and long-term (6 days postconditioning) fear extinction in the absence or presence of a secondary “distracting” stimulus predicting the delivery of sucrose pellets.
Results
Introducing a secondary stimulus predicting sucrose pellets that distracts attention away from the fear-predicting stimulus led to a long-lasting improvement of impaired fear extinction in 5-HTT−/− male rats.
Limitations
The context-dependency of the efficacy of the “distraction therapy” was not tested. In addition, it remains to be clarified whether the positive valence of the distracting stimulus is critical for the distraction of attention or whether a neutral and/or novel stimulus can induce similar effects. Finally, although of lesser importance from a therapeutic perspective, underlying mechanisms remain to be elucidated.
Conclusion
These data indicate that positive environmental stimuli can be used to offset heightened responses to negative stimuli, particularly in individuals characterized by inherited 5-HTT downregulation and high sensitivity to environmental stimuli.
Introduction
Posttraumatic stress disorder (PTSD), like many other neuropsychiatric disorders, is shaped by gene × environment interactions. According to the classic “diathesis–stress” model, individuals characterized by high stress sensitivity who encounter adversity are at heightened risk — because of their genetic make-up — for PTSD.1 Others who lack the genetic vulnerability would be resilient to the negative impact of adversity signals. The overarching aim of this field is to uncover the mechanisms through which stress-related genes influence PTSD risk, which may ultimately inform its cure. Yet, despite extensive research, these mechanisms are still unclear, and the current first-line treatment of PTSD consists of exposure. This form of therapy involves exposure to fear-eliciting cues in the absence of the fear itself, leading to fear extinction. Unfortunately, a substantial proportion of patients do not respond to this type of treatment. According to the diathesis–stress model, this may be because of continued exposure to adversity signals among stress-sensitive individuals. The more recently established “biological susceptibility” model posits that some individuals, owing to genetic predisposition, are highly sensitive to environmental stimuli.2 Exposure to adverse stimuli leads to negative outcomes, whereas a favourable environment leads to better outcomes. Individuals characterized by low sensitivity to environmental stimuli are expected to be resilient to the negative influences of adverse stimuli, but they benefit less from favourable ones. This biological susceptibility model changes our view on neuropsychiatric disorders like PTSD, as it emphasizes the importance of the environment. Thus, stress-sensitive individuals may respond more strongly than stress-resilient individuals to favourable environmental stimuli, and this is not only relevant to the prevention of PTSD, but also to its cure when negative behavioural responses have been established.
A genetic factor increasing sensitivity to both negative and positive environmental stimuli, probably owing to an increased state of vigilance,3 is the common serotonin transporter-linked polymorphic region (5-HTTLPR). Its low activity (short; s) allelic variant has been associated with anxiety-related traits and increased risk for stress-related disorders, including PTSD,4,5 as well as improved performance in a variety of cognitive tasks employing positive stimuli. As we hypothesized, the beneficial adaptive responses to positive stimuli may be used to offset the mal-adaptive responses to negative stimuli.3
Bryant and colleagues6 recently showed that 5-HTTLPR s-allele carriers with PTSD displayed more severe PTSD 6 months after exposure therapy than other patients. Based on the biological susceptibility model, we predict that s-allele carriers with PTSD will benefit from a “distraction” therapy, in which the presentation of positive stimuli draws attention away from fear-eliciting stimuli.
In line with 5-HTTLPR findings in humans, serotonin transporter knockout (5-HTT−/−) rats and mice have shown a failure to extinguish fear7,8 as well as improved adaptive responses to positive stimuli.9,10 In the present study, we used 5-HTT−/− rats, which are well accepted as a model for the 5-HTTLPR s-allele,3,11 to test our prediction, and we designed a “distraction” therapy in which the fear-eliciting stimulus was copresented with a sucrose pellet–predicting stimulus during fear extinction.
Methods
Animals
Serotonin transporter knockout rats (Slc6a41Hubr) were generated in a Wistar background by N-ethyl-N-nitrosurea (ENU)-induced mutagenesis;12,13 these rats have been described previously.14 Experimental animals were derived from crossing heterozygous 5-HTT knockout (5-HTT+/−) rats that were outcrossed for at least 10 generations with wild-type Wistar rats obtained from Harlan Laboratories. After weaning at the age of 21 days, ear cuts were taken for genotyping, which was performed by KBioscience. All animals were maintained on a 22-hour food-deprivation schedule (2 h of free food access after each experimental session), with freely available water. A 12-hour light–dark cycle was maintained, with lights on at 8 am. We used 36 5-HTT+/+ and 36 5-HTT−/− male rats in the experiment, with 12 rats per genotype for each experimental group. All experiments were approved by the Committee for Animal Experiments of the Radboud University Nijmegen Medical Centre, Nijmegen, the Netherlands, and all efforts were made to minimize animal suffering and to reduce the number of animals used.
Apparatus
The food conditioning and extinction sessions were conducted in 4 identical operant chambers (context A; TSE Systems GmbH; length/width/height = 27 × 27 × 26 cm) equipped with fan-containing, sound-attenuating cubicles attached to an in-house developed interface that was controlled by a home-written software package developed in C#. Each chamber was made entirely of aluminum, except for the clear Plexiglas front wall and metal floor, and was equipped with a red house light and a speaker that could produce an 85 dB, 2 kHz tone. In addition, a yellow light-emitting diode (LED)–equipped food magazine for 45 mg food pellet delivery was recessed in the bottom centre of the left side wall, which could be accessed through a hinged panel (opening activated a microswitch used to record food magazine visits). The fear conditioning session occurred in an opaque white inset chamber equipped with a white house light and a metal shock grid (context B). During this session, the fan of the sound-attenuating cubicle was turned off to increase context specificity. We videotaped behaviour using a top-view camera.
Behavioural procedure
The experiment encompassed 4 different phases: food conditioning, fear conditioning, therapy and posttherapy phases. In Appendix 1, Figure 1, available at cma.ca/jpn, we provide a schematic overview of the experiment and the experimental groups.
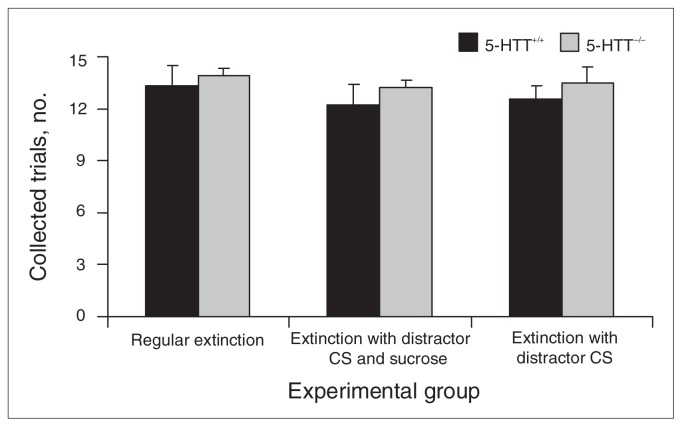
Fig. 1.
Collected trials in 5-HTT+/+ and 5-HTT−/− rats during food conditioning session 3. Data represent the mean (and standard errors of the mean) number of collected trials. CS = conditional stimulus. 5-HTT = serotonin transporter.
Food conditioning
During 3 food-conditioning sessions (day 1–3) in context A, rats of all groups were trained to acquire a Pavlovian association between the illumination of the food magazine LED light (distractor conditional stimulus [CS]; 30-s duration) and a 45 mg sucrose pellet (Bio-Serv), which was delivered coincidentally with the start of the distractor CS. After a habituation phase of 2 minutes, a total of 15 distractor CS were presented at 1-minute intervals. We cleaned the chambers with 70% alcohol (EtOH) after each session.
Fear conditioning
On day 4, all rats were trained in context B to acquire a Pavlovian association between an auditory stimulus (fear CS; 30-s, 2 kHz, 85 dB) and a mild footshock (0.5 mA, 1 s), which terminated coincidentally with the end of the fear CS. Animals were allowed 2 minutes to habituate to the chamber, after which a total of 5 fear CS–footshock pairings were given at intervals of 1 minute. After the last pairing, rats were left in the chamber for another 2 minutes before they were returned to their home cages. We cleaned the chambers with water after each session.
Therapy phase
Extinction sessions on day 5 and 6 took place in context A. During these sessions, rats of the regular extinction (RE) group received only the fear CS, whereas the distractor with sucrose (DS) group received the fear CS and the distractor CS at the same time as well as a 45 mg sucrose pellet delivered coincidentally at the beginning of the distractor CS presentation (Appendix 1, Fig. 1). The distractor only (DO) group received only the combined fear CS/distractor CS (no sucrose pellet). After 2 minutes of habituation, a total of 10 fear CS, fear CS/distractor CS, or fear CS/distractor CS/sucrose pellet combinations were administered at intervals of 2 minutes (± 1 min). We cleaned the chambers with 70% EtOH after each session. Freezing (no visible movement except respiration) was scored using the software package Observer version 5.0 (Noldus Information Technology). We determined baseline freezing levels during the 2 minutes of habituation of the extinction session on day 5; the freezing responses in reaction to the fear CS are presented as a mean for each session.
Posttherapy phase
The extinction session on day 10 was executed identically to the therapy phase except that all groups received only the fear CS (i.e., no distractor CS or sucrose pellets).
Statistical analysis
All analyses were performed using SPSS version 17.0 (SPSS Inc.). We analyzed the accumulated data from trials conducted during the food conditioning phase using a genotype (2 levels) × treatment (3 levels) × session (3 levels) repeated-measures analysis of variance (ANOVA), and the data accumulated for session 3 were analyzed separately using a genotype × treatment ANOVA. Freezing behaviour and magazine visits during the CS in the therapy and posttherapy phases were analyzed using a genotype × treatment ANOVA. When appropriate, we conducted Bonferroni corrected post-hoc tests and Student t tests. We considered results to be significant at p < 0.05.
Results
Food conditioning
During 3 sessions on days 1, 2 and 3, animals acquired a Pavlovian association between the distractor CS and the delivery of a sucrose pellet in the food magazine. We observed no learning differences between groups when the food conditioning phase was statistically analyzed as a whole (interactions with session: genotype × session, F2,132 = 2.612, p = 0.08; treatment × session, F4,132 = 0.418, p = 0.80; genotype × treatment × session, F4, 132 = 0.500, p = 0.74; main effects: genotype F1,66 = 0.552, p = 0.46; treatment, F2,66 = 0.445 p = 0.64; genotype × treatment, F2,66 = 0.021, p = 0.98) or for session 3 (genotype F1,66 = 1.427, p = 0.24; treatment, F2,66 = 0.544, p = 0.58; genotype × treatment, F2,66 = 0.033, p = 0.97; Fig. 1).
Therapy phase
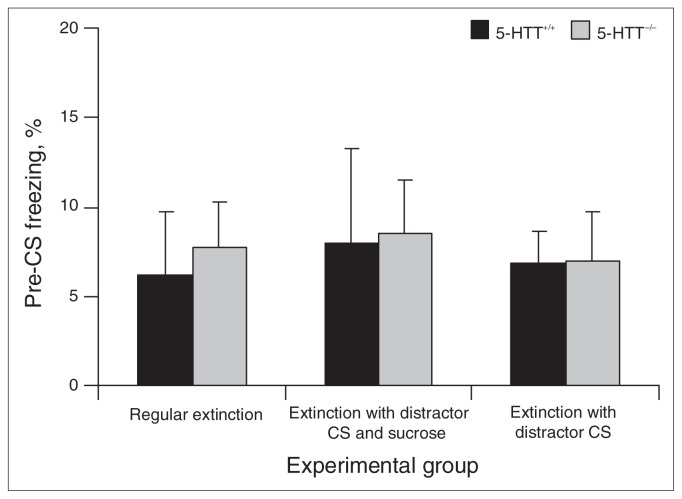
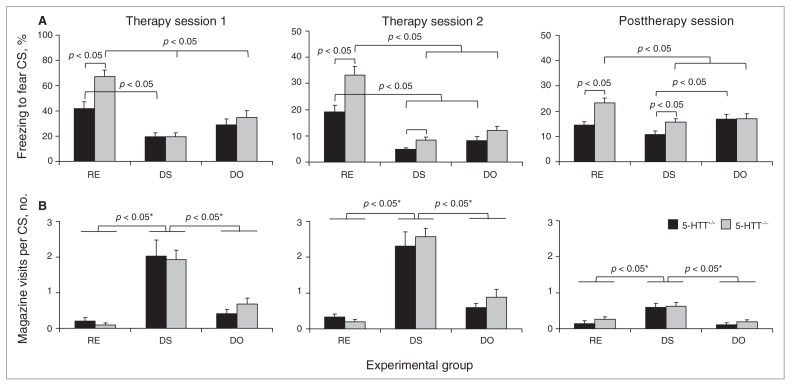
On day 5, 24 hours after the fear-conditioning session on day 4, all groups underwent extinction training of the fear CS–induced fear response. Animals showed no differences in freezing in the 2 minutes before CS exposure (genotype, F1,66 = 0.071, p = 0.79; treatment, F2,66 = 0.098, p = 0.91; genotype × treatment, F2,66 = 0.025, p = 0.98; Fig. 2), indicating that there were no basal differences in freezing. Subsequently, RE animals were exposed to the fear CS only, DS animals to the fear CS/distractor CS compound stimulus with sucrose pellet delivery and DO animals to the fear CS/distractor CS compound stimulus without sucrose pellet delivery. Significant main genotype (F1,66 = 7.768, p = 0.007) and treatment (F2,66 = 30.227, p < 0.001) effects, and a genotype × treatment interaction (F2,66 = 4.164, p = 0.020) were observed for freezing behaviour (Fig. 3A, left panel). Subsequent analysis of the main treatment effect using Bonferroni-corrected post-hoc tests revealed significant differences in freezing between the RE and DS group (p < 0.001), the RE and DO group (p < 0.001) and the DS and DO group (p = 0.023). Furthermore, analysis of the genotype × treatment interaction with Student t tests revealed significant difference between 5-HTT+/+ RE and 5-HTT−/− RE rats (t22 = 3.295, p = 0.003), indicating impaired fear extinction in 5-HTT−/− animals under regular extinction conditions. Additionally, 5-HTT+/+ DS rats showed significantly reduced levels of freezing compared with 5-HTT+/+ RE rats (t22 = 3.483, p = 0.002), an effect not observed in 5-HTT+/+ DO rats (t22 = 1.762, p = 0.09). Compared with 5-HTT−/− RE animals, both 5-HTT−/− DS (t22 = 7.974, p < 0.001) and 5-HTT−/− DO (t22 = 4.384, p < 0.001) rats showed reduced freezing in response to the fear CS. This reduction was largest in the 5-HTT−/− DS animals, as indicated by a direct comparison between 5-HTT−/− DS and 5-HTT−/− DO rats (t22 = 2.552, p = 0.018). Together, these data show that both the DS and DO conditions effectively increased fear extinction in 5-HTT−/−rats, but only DS was effective in 5-HTT+/+ rats.
Fig. 2.
Percent of freezing in 5-HTT+/+ and 5-HTT−/− rats during the 2 minutes before conditional stimulus (CS) exposure of therapy session 1. Data represent the mean (and standard errors of the mean) percentage of freezing for the total pre-CS period. 5-HTT = serotonin transporter.
Fig. 3.
Behaviour of 5-HTT+/+ and 5-HTT−/− rats during therapy and posttherapy sessions. (A) Mean (and standard errors of the mean) percentage of freezing in 5-HTT+/+ and 5-HTT−/− rats during conditional stimulus (CS) presentation for therapy sessions 1 (left panel) and 2 (middle panel) and the post-therapy session (right panel). (B) Mean (and standard errors of the mean) number of magazine visits per CS presentation for therapy sessions 1 (left panel) and 2 (middle panel) and the posttherapy session (right panel). *Bonferroni-corrected. 5-HTT = serotonin transporter; DO = distractor only; DS = distractor with sucrose; RE = regular extinction.
In the second extinction session of the therapy phase, reflecting extinction memory recall, we observed a significant main genotype (F1,66 = 18.742, p < 0.001) and treatment (F2,66 = 52.022, p < 0.001) effect, and genotype × treatment interaction (F2,66 = 4.207, p = 0.019) for freezing behaviour (Fig. 3A, middle panel). Subsequent analysis of the significant main treatment effect using Bonferroni-corrected post-hoc tests revealed significant differences between RE and DS (p < 0.001) and RE and DO (p < 0.001) treatment groups. Additional analysis of the genotype × treatment interaction indicated a significant difference between 5-HTT+/+ RE and 5-HTT−/− RE rats (t22 = 3.282, p = 0.003) and between 5-HTT+/+ DS and 5-HTT−/− DS animals (t22 = 2.735, p = 0.012). Further, freezing in both genotypes of the DS and DO group was significantly reduced compared with the same genotype of the RE group (5-HTT+/+: RE v. DS, t22 = 5.268, p < 0.001, and RE v. DO, t22 = 3.560, p = 0.002; 5-HTT−/−: RE v. DS, t22 = 6.928, p < 0.001, and RE v. DO, t22 = 4.474, p < 0.001). Thus, during the second extinction phase, DS and DO conditions were effective in reducing fear in both 5-HTT−/− and 5-HTT+/+ rats.
Regarding food magazine visits during the extinction sessions, we observed a main treatment effect for extinction session 1 (F2,66 = 34.036, p < 0.001) and extinction session 2 (F2,66 = 54.104, p < 0.001; Fig. 3B, left and middle panels). Bonferroni-corrected post-hoc tests revealed significant differences in food magazine visits between the DS and RE (p < 0.001) and DS and DO (p < 0.001) treatment groups in both sessions, indicating increased visiting in the DS treatment group. No main genotype effects (all F1,66 < 0.597, p = 0.44) genotype × treatment interactions (all F2,66 < 0.576, p = 0.57) were observed, indicating that genotype differences in freezing reduction during the DS and DO therapies are not owing to differences in magazine visits.
Posttherapy phase
To assess whether the DS and DO therapies had long-lasting effects, we analyzed conditioned freezing on day 10 in the absence of the distractor CS. Analysis of the freezing data revealed a significant main genotype (F1,66 = 10.692, p = 0.002), treatment (F2,66 = 5.788, p = 0.005) effect and a genotype × treatment interaction (F2,66 = 3.361, p = 0.041; Fig. 3A, right panel). Subsequent Bonferroni-corrected post-hoc testing of the main treatment effect revealed a significant difference between the RE and DS treatment conditions (p = 0.004). Further analysis of the genotype × treatment interaction indicated a significant genotype difference for the RE (t22 = 3.726, p = 0.001) and DS (t22 = 2.497, p = 0.020) treatment conditions, but also between 5-HTT+/+ DS and 5-HTT+/+ DO rats (t22 = 2.646, p = 0.015). In addition, 5-HTT−/− DS (t22 = 3.185, p = 0.004) and DO (t22 = 2.234, p < 0.036) rats showed reduced freezing compared with 5-HTT−/− RE rats, indicating more effective extinction of the fear CS–induced fear response in 5-HTT−/− animals of the DS and DO groups in the long term. This effect was not observed in wild-type 5-HTT+/+ animals (RE v. DS, t22 = 1.994, p = 0.06; RE v. DO, t22 = 1.033, p = 0.31).
Also during the posttherapy phase, magazine visits did not interfere with the conditioned freezing responses. We observed a main treatment effect for the amount of food magazine visits (F2,66 = 18.109, p < 0.001; Fig. 3B, right panel). Bonferroni-corrected post-hoc analyses revealed significant differences in food magazine visits between the DS and RE (p < 0.001) and DS and DO (p < 0.001) groups, indicating that magazine visits were increased in the DS treatment group. Yet, no main genotype effect (F1,66 = 1.437, p = 0.24) or genotype × treatment interaction (F2,66 = 0.156, p = 0.86) was observed.
Discussion
The present study showed that repeated exposure to a negative stimulus led to negative behavioural outcomes (impaired fear extinction) in stress-sensitive rats, according to the diathesis–stress model. Interestingly, additional exposure to a positive stimulus reduced the negative response to the negative stimulus in stress-sensitive rats, as predicted by the biological susceptibility model. The unique aspect of this finding is that changing environmental stimuli can alter behavioural responses, and our data imply that this can take place within an individual, even when a negative response has been acquired. More specifically, the impaired cue-induced fear extinction in 5-HTT−/− rats was improved by introducing a positive environmental stimulus, a sucrose pellet–predicting CS that distracts attention away from the fear-predicting CS. This improvement was preserved in a posttherapy session 6 days after fear conditioning in which the fear-associated CS was presented alone, indicating long-lasting successful extinction without a spontaneous recovery of the fear response to levels of regular extinction (RE). The fact that 5-HTT−/− rats of both the DS and DO groups showed reduced levels of freezing compared with 5-HTT−/− RE rats reveals that the positive CS alone (i.e., without sucrose reward during the therapy phase) had sufficient efficacy to increase extinction. The 5-HTT+/+ DS and DO rats also showed a potent reduction of the fear response in the therapy phase sessions. However, during the posttherapy phase, 5-HTT+/+ DS and DO rats were indistinguishable from 5-HTT+/+ RE rats, indicating no additional beneficial effect of the distraction therapy in 5-HTT+/+ rats. Magazine visits were treatment-dependent, but not genotype-dependent, indicating that levels of freezing were not directly influenced by the number of magazine visits.
Although the conditioned freezing response during the posttherapy session was reduced by 33% in in 5-HTT−/− DS and 27% in 5-HTT−/− DO rats compared with 5-HTT−/− RE animals, these decreases were lower than those observed during the therapy phase. This could be owing to the fact that (next to 5-HTT+/+ animals) 5-HTT−/− rats showed a time-dependent reduction in freezing under RE conditions (i.e., reduced freezing after multiple fear CS exposure sessions). Nonetheless, freezing levels in 5-HTT−/− DS and DO rats were significantly lower than in 5-HTT−/− RE rats, indicating the beneficial effects of a distraction-based extinction strategy. Moreover, a more substantial freezing reduction during the posttherapy phase in the DS and DO groups may also have been prevented by a floor effect, as levels of freezing during this session approached baseline levels of freezing. Potentially, these issues can be further addressed in future studies by making the fear conditioning phase more aversive (e.g., more conditional stimulus–unconditional stimulus trials), likely resulting in more resistance to extinction throughout multiple exposure sessions.
The finding that 5-HTT−/− rats are particularly sensitive to distraction of attention and behavioural adaptation fits well into the biological susceptibility model and the associated phenomenon of “phenotypic plasticity”: the differential expression of alternative phenotypes from a single genotype depending on environmental conditions.15–17 In the present context, increased plasticity refers to an increased sensitivity to emotionally relevant stimuli, as is seen in 5-HTT−/− rats and 5-HTTLPR s-allele carriers. This increased sensitivity may result in emotional (perseverative) behaviour toward environmental stimuli under unchanging conditions (i.e., when there are no stimuli that draw attention away from stimuli predicting adversity). This is, for instance, expressed by impaired fear extinction in 5-HTT−/− rats in response to a conditioned stimulus. Hence, it might be the case that impaired fear extinction is due to the overwhelming effect of the Pavlovian “fear CS–fear” association at the expense of the extinction “fear CS–no fear” association. It is also possible that the fear CS–no fear association is weaker than the fear CS–fear association, because the former is not based on direct reinforcement (i.e., it had little emotional value for the environment-sensitive 5-HTT knockout rats). Simultaneously, owing to their increased plasticity, 5-HTT−/− rats show flexible adaptation in response to explicit changing in environmental stimuli conditions, as observed during the therapy and posttherapy phases. This suggests that the distractor CS–food reward association carried sufficient emotional strength to compete with and overpower the fear CS–fear association. Interestingly, these phenotypic plasticity effects have also been observed in 5-HTTLPR s-allele carriers. Thus, s-allele carriers had an increased risk for posthurricane PTSD in high-risk environments (e.g., high crime/unemployment rates, low levels of social support), but a decreased risk in low-risk environments.18,19
Serotonin plays a central role in phenotypic plasticity,15,16 potentially by modulating neuroplasticity in corticolimbic regions important for proper stress coping, fear extinction and behavioural flexibility.20–22 For example, 5-HTT−/− rodents show morphologic changes in prefrontal cortical and amygdala pyramidal neurons,8 alterations in neocortical cell density,23 and differential c-Fos (immediate early gene, marker for neuronal activity) immunoreactivity in the amygdala and prefrontal cortex in reaction to extinction of conditioned behaviour.24 Alterations in these corticolimbic circuits, such as a functional uncoupling between the prefrontal cortex and amygdala25,26 and amygdala hyperreactivity in reaction to fearful stimuli (i.e., angry faces)27 have also been described in human s-allele carriers. Intriguingly, a recent study revealed that patients with PTSD had abnormally reduced amygdala 5-HTT expression, as measured using positron emission tomography.28 Hence, the phenotypic plasticity profile associated with 5-HTT genetic downregulation and characteristic of the biological susceptibility model may be related to the neurodevelopmental changes observed in these corticolimbic circuits. More research is needed to investigate this mechanistic account.
Limitations
A limitation of the present study is that we did not investigate to what extent familiarity and/or valence value contributed to the ability of the distractor CS to offset preservative behaviour in 5-HTT−/− rats. It remains to be clarified whether a novel and/or neutral stimulus can induce similar effects. However, as discussed, the emotional valence value of the stimulus may be essential for its ability to offset perseverative responding toward another emotionally valenced stimulus in 5-HTT−/−animals. Hence, a neutral stimulus may lack the strength to distract attention. Another limitation is that we did not address the context dependency of the “distraction therapy.” Fear conditioning took place in a context different from magazine training and the therapy and posttherapy sessions. It has been well established that fear extinction is context-dependent, and that re-exposure to the fear-conditioning context leads to the return of the conditioned freezing response after extinction in another context.29 It therefore remains to be tested whether the present distraction-based cognitive approach is effective when presented in the fear-conditioning context. Nonetheless, our data clearly show that fear extinction is impaired in 5-HTT−/− rats in a context that is distinctive from fear conditioning. In addition, context-induced fear extinction is improved in 5-HTT−/− mice.30 These findings suggest that 5-HTT−/− rodents are particularly sensitive to discrete conditioned stimuli rather than contextual information.
Conclusion
To our knowledge, we show for the first time that sensitivity to positive stimuli can be used to offset impaired fear extinction in response to fear-eliciting cues, particularly in individuals characterized by inherited 5-HTT downregulation and stress sensitivity. As such, our results not only support the biological susceptibility model, but are also relevant in relation to 5-HTT genetic variance.
Acknowledgments
We thank Dick Heeren for his technical assistance. This work was funded by The Netherlands Organization for Scientific Research (NWO), grant #86410003, awarded to J.R. Homberg. The NWO had no further role in the design of the study, in the collection, analysis and interpretation of data, in the writing of the report, and in the decision to submit the paper for publication.
Footnotes
Competing interests: None declared for L.J.P. Nonkes and M. de Pooter. As above for J.R. Homberg.
Contributors: L.J.P. Nonkes and J.R. Homberg designed the study. L.J.P. Nonkes and M. de Pooter acquired and analyzed the data. L.J.P. Nonkes wrote the article, which M. de Pooter and J.R. Homberg reviewed. All authors approved its publication.
References
- 1.Burmeister M, McInnis MG, Zollner S. Psychiatric genetics: progress amid controversy. Nat Rev Genet. 2008;9:527–40. doi: 10.1038/nrg2381. [DOI] [PubMed] [Google Scholar]
- 2.Ellis BJ, Boyce WT, Belsky J, et al. Differential susceptibility to the environment: an evolutionary–neurodevelopmental theory. Dev Psychopathol. 2011;23:7–28. doi: 10.1017/S0954579410000611. [DOI] [PubMed] [Google Scholar]
- 3.Homberg JR, Lesch KP. Looking on the bright side of serotonin transporter gene variation. Biol Psychiatry. 2011;69:513–9. doi: 10.1016/j.biopsych.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 4.Xie P, Kranzler HR, Poling J, et al. Interactive effect of stressful life events and the serotonin transporter 5-HTTLPR genotype on post-traumatic stress disorder diagnosis in 2 independent populations. Arch Gen Psychiatry. 2009;66:1201–9. doi: 10.1001/archgenpsychiatry.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Z, Baker DG, Harrer J, et al. The relationship between combat-related posttraumatic stress disorder and the 5-HTTLPR/rs25531 polymorphism. Depress Anxiety. 2011;28:1067–73. doi: 10.1002/da.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bryant RA, Felmingham KL, Falconer EM, et al. Preliminary evidence of the short allele of the serotonin transporter gene predicting poor response to cognitive behavior therapy in posttraumatic stress disorder. Biol Psychiatry. 2010;67:1217–9. doi: 10.1016/j.biopsych.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 7.Schipper P, Kiliaan AJ, Homberg JR. A mixed polyunsaturated fatty acid diet normalizes hippocampal neurogenesis and reduces anxiety in serotonin transporter knockout rats. Behav Pharmacol. 2011;22:324–34. doi: 10.1097/FBP.0b013e328347881b. [DOI] [PubMed] [Google Scholar]
- 8.Wellman CL, Izquierdo A, Garrett JE, et al. Impaired stress-coping and fear extinction and abnormal corticolimbic morphology in serotonin transporter knock-out mice. J Neurosci. 2007;27:684–91. doi: 10.1523/JNEUROSCI.4595-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brigman JL, Mathur P, Harvey-White J, et al. Pharmacological or genetic inactivation of the serotonin transporter improves reversal learning in mice. Cereb Cortex. 2010;20:1955–63. doi: 10.1093/cercor/bhp266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nonkes LJ, Maes JH, Homberg JR. Improved cognitive flexibility in serotonin transporter knockout rats is unchanged following chronic cocaine self-administration. Addict Biol. 2011 Jul 25; doi: 10.1111/j.1369-1600.2011.00351.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Caspi A, Hariri AR, Holmes A, et al. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am J Psychiatry. 2010;167:509–27. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smits BM, Mudde J, Plasterk RH, et al. Target-selected mutagenesis of the rat. Genomics. 2004;83:332–4. doi: 10.1016/j.ygeno.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Smits BM, Mudde JB, van de Belt J, et al. Generation of gene knockouts and mutant models in the laboratory rat by ENU-driven target-selected mutagenesis. Pharmacogenet Genomics. 2006;16:159–69. doi: 10.1097/01.fpc.0000184960.82903.8f. [DOI] [PubMed] [Google Scholar]
- 14.Homberg JR, Olivier JD, Smits BM, et al. Characterization of the serotonin transporter knockout rat: a selective change in the functioning of the serotonergic system. Neuroscience. 2007;146:1662–76. doi: 10.1016/j.neuroscience.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 15.Anstey ML, Rogers SM, Ott SR, et al. Serotonin mediates behavioral gregarization underlying swarm formation in desert locusts. Science. 2009;323:627–30. doi: 10.1126/science.1165939. [DOI] [PubMed] [Google Scholar]
- 16.Branchi I. The double edged sword of neural plasticity: increasing serotonin levels leads to both greater vulnerability to depression and improved capacity to recover. Psychoneuroendocrinology. 2011;36:339–51. doi: 10.1016/j.psyneuen.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 17.Bagot RC, Meaney MJ. Epigenetics and the biological basis of gene × environment interactions. J Am Acad Child Adolesc Psychiatry. 2010;49:752–71. doi: 10.1016/j.jaac.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Kilpatrick DG, Koenen KC, Ruggiero KJ, et al. The serotonin transporter genotype and social support and moderation of posttraumatic stress disorder and depression in hurricane-exposed adults. Am J Psychiatry. 2007;164:1693–9. doi: 10.1176/appi.ajp.2007.06122007. [DOI] [PubMed] [Google Scholar]
- 19.Koenen KC, Aiello AE, Bakshis E, et al. Modification of the association between serotonin transporter genotype and risk of posttraumatic stress disorder in adults by county-level social environment. Am J Epidemiol. 2009;169:704–11. doi: 10.1093/aje/kwn397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coppens CM, de Boer SF, Koolhaas JM. Coping styles and behavioural flexibility: towards underlying mechanisms. Philos Trans R Soc Lond B Biol Sci. 2010;365:4021–8. doi: 10.1098/rstb.2010.0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dolan RJ. The human amygdala and orbital prefrontal cortex in behavioural regulation. Philos Trans R Soc Lond B Biol Sci. 2007;362:787–99. doi: 10.1098/rstb.2007.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Curr Opin Neurobiol. 2006;16:723–7. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Altamura C, Dell’Acqua ML, Moessner R, et al. Altered neocortical cell density and layer thickness in serotonin transporter knockout mice: a quantitation study. Cereb Cortex. 2007;17:1394–401. doi: 10.1093/cercor/bhl051. [DOI] [PubMed] [Google Scholar]
- 24.Nonkes LJ, Tomson K, Maertin A, et al. Orbitofrontal cortex and amygdalar over-activity is associated with an inability to use the value of expected outcomes to guide behaviour in serotonin transporter knockout rats. Neurobiol Learn Mem. 2010;94:65–72. doi: 10.1016/j.nlm.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Heinz A, Braus DF, Smolka MN, et al. Amygdala-prefrontal coupling depends on a genetic variation of the serotonin transporter. Nat Neurosci. 2005;8:20–1. doi: 10.1038/nn1366. [DOI] [PubMed] [Google Scholar]
- 26.Pezawas L, Meyer-Lindenberg A, Drabant EM, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–34. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- 27.Hariri AR, Mattay VS, Tessitore A, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–3. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- 28.Murrough JW, Huang Y, Hu J, et al. Reduced amygdala serotonin transporter binding in posttraumatic stress disorder. Biol Psychiatry. 2011;70:1033–8. doi: 10.1016/j.biopsych.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bouton M, Woods A, Moody E, et al. Counteracting the context-dependence of extinction: relapse and tests of some relapse prevention methods. In: Hermans D, Vansteenwegen D, editors. Fear and learning: from basic processes to clinical implications. Washington: American Psychological Association; 2006. pp. 175–96. [Google Scholar]
- 30.Muller JM, Morelli E, Ansorge M, et al. Serotonin transporter deficient mice are vulnerable to escape deficits following inescapable shocks. Genes Brain Behav. 2011;10:166–75. doi: 10.1111/j.1601-183X.2010.00652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]