Abstract
Background
Major depressive disorder (MDD) is associated with difficulty disengaging attention from emotionally negative information. Few studies have investigated whether euthymic individuals with a history of depression (remitted MDD [rMDD]) show similar deficits, and little is known about concomitant neurophysiological features of such deficits. To fill these gaps, we investigated cognitive control over emotional stimuli in participants with rMDD and controls without history of depression or psychopathology.
Methods
We collected 128-channel event-related potentials (ERPs) while participants performed a cued emotional conflict task. During the task, a cue instructed the participant to respond to the actual or opposite valence of an upcoming happy or sad face.
Results
We enrolled 15 individuals with rMDD and 18 controls in our study. Event-related potentials showed no group differences in response to the cues, highlighting preserved preparatory processes when anticipating an emotional conflict. However, relative to the control group, the rMDD group responded more slowly and showed reduced N450 amplitudes on trials that required disengaging from negative faces (pressing “happy” in response to a sad face).
Limitations
The sample size was small, and the null finding in the cue-locked N2 analyses may be owing to low power.
Conclusion
Our results suggest a selective deficit in cognitive control over sad stimuli in individuals with rMDD. Additional studies will be required to pinpoint whether the current findings stem from impairments in response conflict, conflict monitoring and/or attentional disengagement in response to sad stimuli. Moreover, future studies are warranted to evaluate whether decreased cognitive control in response to negative information might increase the risk for future depressive episodes.
Introduction
With a lifetime prevalence of 16%, major depressive disorder (MDD) is one of the most common psychiatric disorders.1 Moreover, episodes are recurrent for more than 80% of afflicted individuals.2 Emerging evidence suggests that depression is not only characterized by deficits in the activation of negative cognitions, but also by an impaired ability to inhibit or disengage from negative stimuli3,4 (for reviews, see De Raedt and Koster5 and Gotlib and Joormann6). This implies that depressed individuals have a reduced ability to ignore goal-irrelevant negative cues. Critically, such cognitive deficits have also emerged in samples with remitted major depressive disorder (rMDD)7,8 and in unaffected offspring of depressed individuals.9 Collectively, these data suggest that reduced cognitive control in response to negatively valenced information might represent a trait-like marker of MDD vulnerability.9 However, the specific functional significance and underlying neural correlates of these deficits remain largely unexplored.
In this endeavour, cognitive control, particularly the ability to inhibit prepotent responses or disengage from negative stimuli, has been primarily investigated with emotional Stroop,10,11 affective interference12 or emotional conflict13 tasks. Although these paradigms are valuable, they cannot disentangle effects of depression on preparatory processes versus cognitive control in response to incongruent targets. Given well-known effects of depression on effort-based processes,5,6,14 it is plausible that diminished cognitive control in depressed or rMDD groups might reflect weak recruitment of cognitive resources even before incongruent stimuli are presented. Cognitive recruitment before the presentation of the stimulus is an adaptive process to pursue goal-relevant behaviour. A reduced recruitment of preparatory control could therefore result in slower response time on these trials, and could (partially) explain the increased interference effects in individuals with depression. It is difficult to test this hypothesis using paradigms in which the presence or absence of conflict is not evident until the target stimulus is presented.
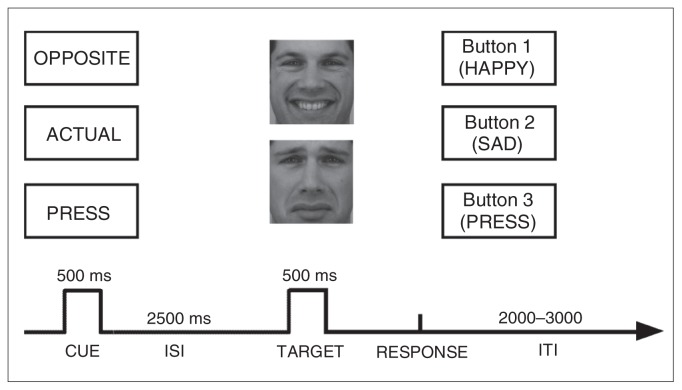
To address this key issue, we developed a new paradigm —the cued emotional conflict task — in which preparatory processes (cued anticipation) and cognitive control can be probed separately (Fig. 1). On individual trials, a preparatory cue is followed by a sad or happy face. The cue consists of a single word that instructs participants to respond in 1 of 3 ways. If the cue reads “actual,” participants have to press a button corresponding to the emotion presented on the face (e.g., press a button labelled “sad” in response to a sad face). If the cue reads “opposite,” participants are instructed to press a button corresponding to the emotion opposite to that presented on the face (e.g., press a button labelled “happy” in response to a sad face). Finally, if the cue reads “press,” participants are prompted to respond with a third button (labelled “press”) to simply indicate that a face has been presented. Critically, in this task a fixed interval of 2500 ms separates the cue and face presentations, which allowed us to separate cued anticipation from target processing. Emotional conflict is assumed to occur in response to faces presented after the “opposite” cue as opposed to the “actual” cue. The need for cognitive control is assumed to be smallest in “press” trials, which are included primarily as a sensory-motor control condition and to probe possible global response time differences between groups.
Fig. 1.
Schematic overview of the cued emotional conflict task. First, a cue is presented in the centre of the screen (“actual,” “opposite” or “press”), followed by a face with an emotional expression (happy or sad). The task allowed for the investigation of (1) preparatory processes (independent of the emotional stimulus) and (2) cognitive control in response to an emotional stimulus. ISI = interstimulus interval; ITI = intertrial interval.
In addition to behavioural measures, event-related potentials (ERPs) were recorded. This technique was applied because of its excellent temporal resolution and the reliable effects of cognitive control manipulations on the amplitudes of 2 frontocentral ERPs, namely the early N2 and the late N450.15–19 Event-related potential analysis focused on the N2 in response to the cues as well as the N2 and N450 components in response to the targets (faces). The N2 is a negative voltage deflection that emerges 200–400 ms poststimulus primarily over anterior scalp sites, and it has been linked to cognitive control and conflict detection.15,16 Larger (more negative) N2 amplitudes have been linked to increased cognitive control in preparation for inhibition of a prepotent response, as well as to incongruent versus congruent trials.17 The N450 is a negative phasic voltage deflection that emerges about 400–500 ms poststimulus primarily over central scalp sites.18,19 This component is assumed to index cognitive processes elicited by conflict detection and conflict monitoring most likely at the response stage.18,19 The focus on these components was also motivated by the observation of reduced N2 and N450 amplitudes on incongruent Stroop trials in adults with MDD.20 Moreover, source localization analyses have identified regions within the anterior cingulate cortex (ACC) as the potential generator of both the N215 and N450,18 a brain area implicated in the pathophysiology of depression.6,20
The aim of our study was to investigate cognitive control over emotional stimuli in participants with rMDD compared with controls without a history of depression or psychopathology. We hypothesized that, relative to the control group, the rMDD group would show cognitive control deficits in response to emotionally negative information. Therefore, we expected greater response time differences on “opposite” versus “actual” trials in the rMDD versus the control group, specifically when the cues were followed by sad faces. For the ERP data, we performed cue- and target-locked analyses. For ERPs time-locked to cues, we expected an increased N2 on “opposite” trials versus “actual” trials in both groups as an indication of greater preparatory processes linked to the upcoming conflict on opposite trials. However, because depression generally impairs effortful processing,14 we hypothesized that this preparatory N2 effect would be reduced in the rMDD versus the control group. For the target ERPs, we expected sad faces presented after the “opposite” cue to elicit smaller N2 and N450 amplitudes in the rMDD group relative to the control group. This result would support the hypothesis of diminished cognitive control in response to sad information in individuals with rMDD. In light of possible group differences in affective ratings of emotional stimuli, we also examined putative effects of affective ratings on behavioural and ERP variables.
Methods
Participants
Internet postings were used to recruit individuals with rMDD and controls. Participants deemed to be eligible after a phone screening were invited for a Structured Clinical Interview for DSM-IV (SCID),21 which was administered by a masters-level, licensed mental health counsellor (N.B.). Exclusion criteria for all participants were current psychopathology; a history of neurologic conditions, including loss of consciousness for more than 5 minutes; substance abuse in the last year; lifetime substance dependence; current psychotropic medication; and a Beck Depression Inventory-II score greater than 13 (cutoff for mild depression).22 Participants in the control group were required to have no current or past Axis I disorders and no family history of mood disorders; participants with rMDD were required to have no current Axis I disorders or past mood disorders other than MDD. Further inclusion criteria for the rMDD group included remission from at least 1 major depressive episode in the past 5 years; remission for at least 6 months before testing; absence of psychotropic medications for at least 16 weeks before testing; and subthreshold level ratings (2 or lower on the SCID, covering the last 2 months) of fewer than 2 MDD symptoms, neither of which was depressed mood or anhedonia.
Written informed consent was obtained before the SCID. Participants who met all inclusion criteria were subsequently invited for a separate ERP session. The protocol was approved by the Harvard Committee on the Use of Human Subjects.
Material
Cued emotional conflict task
The cued emotional conflict task was programmed using E-prime (Psychology Software Tools Inc.). Each trial started with 1 of 3 word cues presented for 500 ms (Fig. 1): “actual,” which instructed participants to press a key corresponding to the emotional expression of the upcoming target face (e.g., press “happy” when a happy face is presented); “opposite,” which indicated that participants should make the response corresponding to the opposite emotional expression of the target face (e.g., press “happy” when a sad face is presented); or “press,” which prompted participants to press a separate key when a face appeared. After the cue word, a black screen was presented for 2500 ms. After this fixed interstimulus interval, either a happy or sad face was presented for 500 ms. Fourteen faces (7 female, 7 male) from the Karolinska Directed Emotional Faces data set23 were used. Each of these faces was shown in a happy or sad expression (matched for arousal).24 Faces were selected if normative ratings indicated that more than 75% of the raters categorized the facial expression correctly with an average intensity rating greater than 6 on a 9-point scale (intense). Faces were followed by a blank screen that remained until a response was made. Participants were instructed to respond as quickly and accurately as possible after the face presentation; the assignment of labels to the 3 buttons was counterbalanced across participants. The intertrial interval was jittered between 2000 and 3000 ms in 250-ms steps.
Participants completed 30 practice trials using 5 faces not shown in the experimental blocks, followed by 8 blocks of 36 trials. Each block contained 6 trials of each cue/face combination (3 cues × 2 faces). Response times from the practice block were used to determine a threshold for late responses in the experimental blocks, which was equal to the 85th percentile of each participant’s distribution of practice response times. When the response time was outside this individually titrated response window, feedback was presented for 250 ms (“too slow”). To account for possible performance drifts over time, the response window threshold was recalculated at the end of each block. After the cued emotional conflict task, participants rated the faces for valence and arousal using 9-point scales (valence: 1 = unhappy, 5 = neutral, 9 = happy; arousal: 1 = calm, 5 = intermediate, 9 = excited).
Self-report measures of mood and affect
Before the experiment, anxiety and mood were assessed using state versions of the Spielberger Trait–State Anxiety Inventory (STAI-X1)25 and the Positive and Negative Affect Schedule (PANAS),26 respectively. After the experiment, trait anxiety, dispositional affect and depressive symptoms were probed using the STAI-X2, the trait version of the PANAS and the BDI-II.
Apparatus
A Geodesic Sensor Net System (Electrical Geodesic, Inc. [EGI]) was used to record 128-channel electroencephalography (EEG) within an electrically and acoustically shielded room (sampling rate 250 Hz; analog filter 0.01–100 Hz; recording reference vertex; impedances < 45 kΩ). Responses were recorded using E-Prime Biological Add-ons for Net Station (Psychology Software Tools, Inc.).
Data reduction
Behavioural data
In total, the cued emotional conflict task consisted of 288 trials: 96 trials/cue type (3 cues) and 48 trials/target type (6 targets). Only target trials in which participants made a correct response within the response window were considered for statistical analyses. We excluded outliers, which were defined as mean response times (standard deviation [SD] 3.5) of the individual’s mean response time per condition. Throughout the remainder of this report, effects are described by the cue and then facial emotion (e.g., “opposite/happy” refers to the opposite cue followed by a happy face, which would require pressing the button labelled “sad”).
Scalp ERP data
Electroencephalography data were analyzed using Brain Vision software (Brain Products, Gmbh) and filtered offline with a 30-Hz low-pass filter (12 dB/octave). After removal of large artifacts and identification of corrupted channels for interpolation, we used an independent component analysis27 to correct for eye blinks, electrocardiogram, horizontal eye movements and 60-Hz noise. Two researchers (M.-A.V. and S.J.D.) separately identified factors for removal from the independent component analysis and reached a consensus to ensure reliability. Subsequently, channels with corrupted signals were replaced using spatially weighted linear interpolations (Hjorth nearest-neighbours algorithm). Next, we extracted cue-locked (−500 ms to 3000 ms) and target-locked (−250 ms to 1500 ms) segments for correct responses.
We then performed semiautomatic artifact detection to identify remaining artifacts (maximal amplitude: ± 100 μV; within-segment absolute amplitude difference: 150 μV; gradients: 50 μV). Before average reference recomputation, data were baseline-corrected (cue: −500 ms to 0 ms; target: −250 ms to 0 ms), and we applied a bandpass filter of 1–30 Hz. These preprocessing steps were performed to be consistent with prior ERP studies from our laboratory probing cognitive control in individuals with depression.20 Finally, we calculated grand mean ERP waveforms for each group and cell in the design.
Start and end points for the N2 and N450 components were empirically defined using a space-oriented field analysis, which identified periods of stable electric field configurations (“microstates”).28 These microstates captured N2 and N450 waveform deflections, as determined by visual inspection of the current data, but were defined using a fully data-driven procedure. Next, for each microstate, we identified the global field power (GFP) peak, assumed to index periods of maximal signal-to-noise ratio.29 For cue-locked N2, a stable field configuration was identified within 224–640 ms (GFP peak 280 ms). For target-locked N2 and N450 components, microstates were identified within 192–332 ms (GFP peak 252 ms) and 336–632 ms (GFP peak 364 ms) time ranges, respectively. Based on microstate results, cue-locked N2 was calculated as the average amplitude 260–300 ms (GFP peak ± 20 ms) postcue. Similarly, we calculated target-locked N2 as the average voltage amplitude 232–272 ms (GFP peak ± 20 ms) posttarget. For the target-locked N450, the GFP peak (364 ms) was not aligned with the N450 peaks (about 440 ms); accordingly, the N450 was calculated after visual waveform inspection as the average voltage amplitude between 420 ms and 460 ms (visual peak ± 20 ms) posttarget. Based on prior studies15,20 and visual inspection of maximal deflection locations, N2 (cue and target) and N450 (target) mean amplitudes (in μV) were extracted from frontocentral (FCz) and central (Cz) sensors, respectively. To increase reliability,30 mean N2 amplitude was computed for FCz (EGI sensor 6) and its immediately adjacent sensors (sensors 5, 12, 13, 11), whereas N450 amplitude was computed for Cz (sensor 129) and its adjacent sensors (sensors 7, 32, 55, 81, 107).
Statistics
Behavioural data
Separate cue (opposite, actual) × emotion (sad, happy) × group (control, rMDD) mixed-model analyses of variance (ANOVAs) were performed for response time and accuracy scores. To isolate possible group differences in the ability to disengage from emotional stimuli, “opposite” minus “actual” response time difference scores were computed separately for each valence (i.e., opposite/sad minus actual/sad; opposite/happy minus actual/happy). Larger response time difference scores were assumed to indicate weaker cognitive control.
ERP data
Moreover, the control and rMDD groups did not differ in the mean number of segments available for ERP analyses time-locked to cues (mean 90.54 [SD 8.31] v. mean 91.23 [SD 6.93], t31 = 1.05, p = 0.16) or targets (mean 43.84 [SD 5.65] v. mean 42.65 [SD 5.38], t31 = 0.90, p = 0.56). Mirroring the behavioural analyses, mixed-model cue (opposite, actual) × emotion (sad, happy) × group (control, rMDD) ANOVAs were performed on cue-locked N2, target-locked N2 and target-locked N450 amplitudes. In light of group differences in target-locked N450 (see Results), additional analyses were performed on N450 difference scores (i.e., opposite/sad minus actual/sad; opposite/happy minus actual/happy). Less negative difference scores were interpreted as reflecting weaker cognitive control in response to the emotional stimulus.
Across analyses, significant ANOVA effects were followed up using Student t tests, and Cohen d values are reported for t test effect sizes: estimates of 0.1 were considered small, 0.3 medium and 0.5 large.31 Effect sizes for ANOVAs are reported in the form of partial η squared (ηp2), where 0.05, 0.1 and 0.2 correspond to small, medium and large effects, respectively.31 We considered results to be significant at α = 0.05.
Results
Demographics and self-report data
We recruited 20 individuals with rMDD and 18 controls. We excluded 5 from the rMDD group because they did not fulfill the criteria for remission (they reported 1 past depressive episode), leaving 15 participants with rMDD. Table 1 summarizes the demographic, clinical and self-report data of participants. Groups did not differ on any demographic variables (i.e., age, education, marital status, race and sex). On average, remitted individuals experienced 4.13 (SD 4.1) prior major depressive episodes and had been in remission for an average of 21 (SD 12.5) months. No group differences in self-report measures emerged (t < 1.98, all p > 0.05).
Table 1.
Demographic, clinical and mood characteristics of participants
| Group; mean (SD)* | |||
|---|---|---|---|
|
|
|||
| Characteristic | Control, n = 18 | rMDD, n = 15 | Statistic, t/χ2/F |
| Age, yr | 27.17 (10.88) | 27.87 (7.91) | 0.39 |
| Education, yr | 15.38 (6.99) | 15.62 (7.20) | −1.00 |
| Married, no. (%) | 10 (57) | 8 (53) | 0.02 |
| White, no. (%) | 13 (72) | 11 (73) | 1.24 |
| Female, no. (%) | 9 (50) | 8 (53) | 0.04 |
| No. MDE | NA | 4.13 (4.14) | NA |
| Age of onset | NA | 17.33 (4.84) | NA |
| Remission time, yr | NA | 2.01 (1.11) | NA |
| BDI-II score | 2.00 (3.58) | 4.75 (4.36) | 1.98 |
| STAI | |||
| State score | 30.50(6.40) | 34.33 (6.79) | 1.67 |
| Trait score | 34.33 (4.91) | 37.47 (5.21) | 1.78 |
| Positive affect | |||
| State score | 31.11 (6.40) | 28.46 (6.83) | 1.15 |
| Trait score | 36.06 (5.80) | 32.43 (6.09) | 1.72 |
| Negative affect | |||
| State score | 10.22 (1.70) | 10.13 (1.60) | 0.15 |
| Trait score | 11.61 (2.79) | 13.21 (3.56) | 1.43 |
Behavioural data
Groups did not differ in the number of outliers that were excluded from response time analyses (control group: mean 0.19 [SD 0.31%] v. rMDD group: mean 0.23 [SD 0.41%]; t31 = 1.07, p = 0.24).
Response times
Response time data are presented in Figure 2A. In a first set of analyses, behavioural responses to the “press” cue were compared between groups to evaluate possible global differences in response to emotional stimuli. Independent t tests indicated that response time on “press” trials (press/sad and press/happy) were not significantly different between groups (t < 1.19, all p > 0.24). Therefore, and because the press condition required no cognitive control, responses to the “press” condition were not included in further analyses.
Fig. 2.
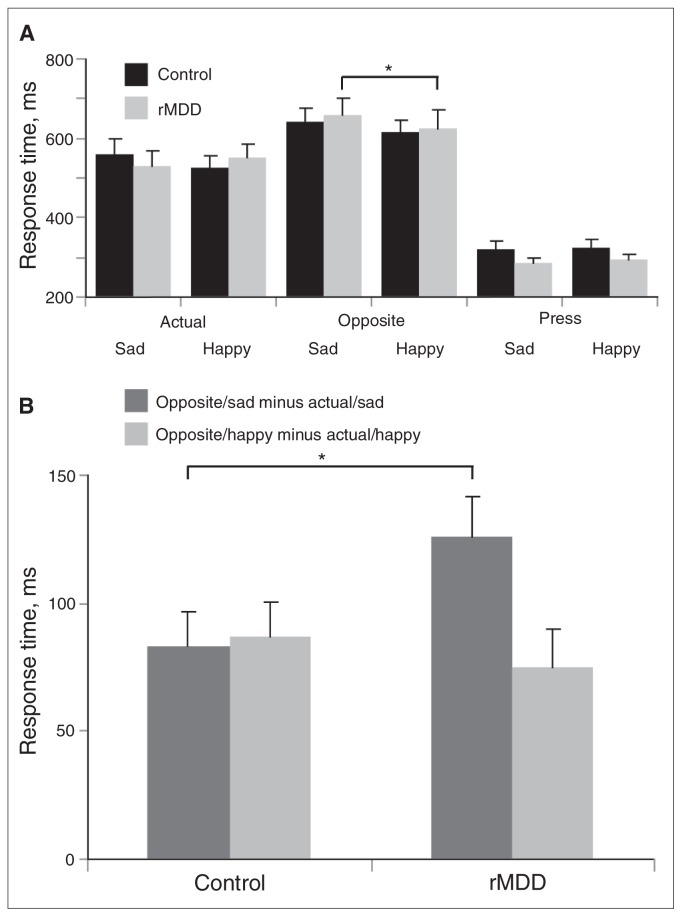
(A) Mean (and standard error) response times in controls with no history of depression (n = 18) and participants with remitted major depressive disorder (rMDD; n = 15) as a function of cue and emotion. For the rMDD, but not the control group, a significant cue (opposite, actual) × emotion (sad, happy) interaction emerged, driven by significantly longer response times for opposite/sad than opposite/happy. (B) Mean (and standard error) difference scores for the control and rMDD groups: response times in trials requiring the emotion identification were subtracted from trials requiring cognitive control (i.e., opposite/happy minus actual/happy; opposite/sad minus actual/sad).
The mixed cue (opposite, actual) × emotion (sad, happy) × group (control, rMDD) ANOVA revealed a main effect of cue (F1,31 = 134.96, p < 0.001, ηp2 = 0.81), a main effect of emotion (F1,31 = 4.61, p = 0.040, ηp2 = 0.13) and a significant 3-way interaction (F1,31 = 4.97, p = 0.033, ηp2 = 0.14). No other main or interaction effects yielded significant effects (all F < 3.67, all p > 0.07). The triple interaction was followed up by separate cue × emotion ANOVAs in each group. The cue × emotion interaction was significant for the rMDD group (F1,14 = 13.19, p = 0.003, ηp2 = 0.49), but not the control group (F1,17 = 0.04, p = 0.84). Paired t tests revealed that remitted individuals responded more slowly on opposite/sad than opposite/happy trials (t14 = 2.34, p = 0.035), whereas there was no difference between actual/sad and actual/happy trials (t14 = 1.14, p = 0.28; Fig. 2A).
In a second step, to isolate possible group differences in the ability to disengage from emotional stimuli, “opposite” minus “actual” response time difference scores were computed separately for each valence (i.e., opposite/sad minus actual/sad; opposite/happy minus actual/happy). Larger response time difference scores indicate weaker cognitive control. Response time difference scores (opposite/sad minus actual/sad; opposite/happy minus actual/happy) were compared across groups. As hypothesized, the rMDD group showed larger response time difference scores for the sad (t31 = 2.12, p = 0.044, Cohen d = 0.74) but not happy (t31 = 0.61, p = 0.55) trials compared to the control group (Fig. 2B). Collectively, the response time results indicate that, relative to healthy controls, individuals with rMDD had greater difficulty disengaging from sad but not happy faces.
Accuracy scores
Overall, accuracy rates for all 6 cued emotional conflict task trial types were high (92.64%–95.82%), preventing analysis of ERPs time-locked to errors. A cue × emotion × group ANOVA on accuracy scores revealed no significant main effect or interactions with group (all F < 1.49, all p > 0.24).
ERP data
Mirroring the behavioural analyses, mixed cue (opposite, actual) × emotion (sad, happy) × group (control, rMDD) ANOVAs were performed on cue-locked N2, target-locked N2 and target-locked N450 amplitudes.
Cue-locked ERPs (N2)
The t tests indicated that N2 amplitudes in response to the “press” cue were not significantly different between groups (t31 = 0.98, p = 0.34), thus these were excluded from subsequent analyses.
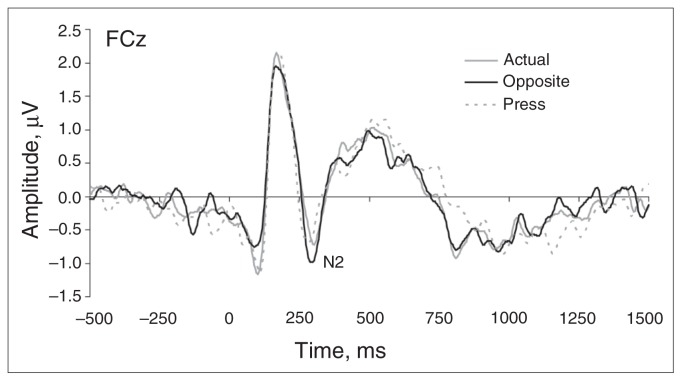
The cue (opposite, actual) × group (control, rMDD) ANOVA yielded only a main effect of cue (F1,31 = 8.16, p = 0.008, ηp2 = 0.21). As shown in Figure 3, N2 amplitudes were more negative in response to the “opposite” cue (−1.24 [SD 1.37] μV) compared with the “actual” cue (−0.78 [SD 0.98] μV; t32 = 2.90, p < 0.01, Cohen d = 0.51). No main effect of group or cue × group interaction emerged (all F < 0.96, all p > 0.35). These findings suggest that the groups prepared similarly for conflict on opposite trials, and argue against an anticipatory deficit in the rMDD group.
Fig. 3.
Cue-locked grand mean waveforms at electrode FCz for the cues “actual” and “opposite,” averaged across groups. No group differences emerged for the cue-related N2 component, highlighting normative preparatory activity in participants with remitted major depressive disorder. Tick marks on the x axis denote 250 ms increments.
Target-locked ERPs (N2, N450)
The t tests conducted on N2 and N450 amplitudes to happy and sad faces presented on “press” trials also revealed no group differences (all t < 1.62, all p > 0.12), thus these values were excluded from subsequent analyses.
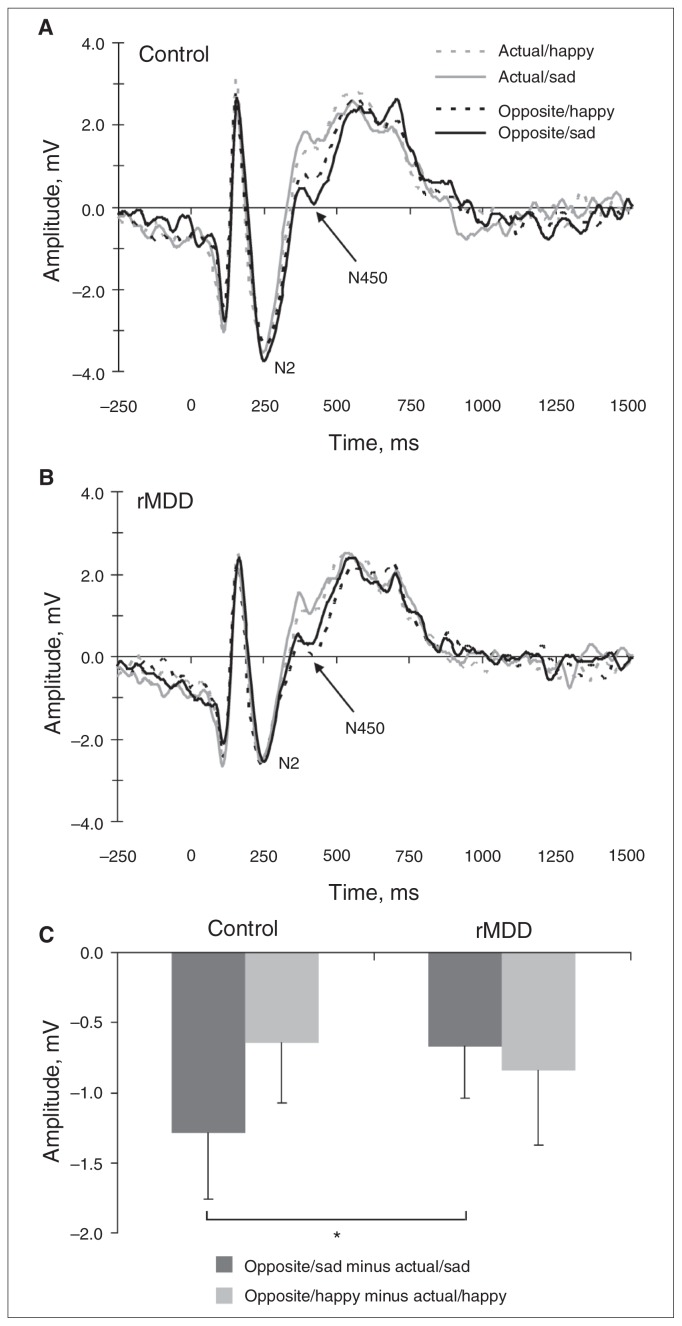
Next, separate cue (opposite, actual) × emotion (sad, happy) × group (control, rMDD) ANOVAs were run on N2 and N450 amplitudes. For the target-locked N2, no significant effects emerged (all F < 2.87, all p > 0.10). For the N450, there was a significant main effect of cue (F1,31 = 52.98, p < 0.001, ηp2 = 0.63) and an emotion × group interaction (F1,31 = 7.63, p = 0.010, ηp2 = 0.20). There was a trend toward significance for a cue × emotion × group interaction (F1,31 = 4.02, p = 0.05, ηp2 = 0.12). No other main or interaction effects yielded a significant effect (all F < 1.35, all p > 0.25). As with the behavioural data, follow-up ANOVAs were performed for the control and rMDD groups separately. The cue × emotion interaction was significant for the control group (F1,17 = 5.67, p = 0.029, ηp2 = 0.25; Fig. 4A), but not the rMDD group (F1,14 = 0.31, p = 0.58; Fig. 4B). Paired t tests revealed that the control group showed greater (i.e., more negative) N450 amplitudes on opposite/sad relative to opposite/happy trials (t17 = 3.03, p = 0.008), but there was no difference in N450 amplitude between actual/sad and actual/happy trials (t17 = 0.45, p = 0.65; Fig. 4B). More negative N450 amplitudes highlight increased recruitment of cognitive control to overcome interference.
Fig. 4.
Target-locked grand mean waveforms at electrode Cz for actual/sad, actual/happy, opposite/sad and opposite/happy trials for the (A) control and (B) remitted major depressive disorder (rMDD) groups. Tick marks on the x axis denote 250 ms increments. (C) Mean (and standard error) difference scores: N450 amplitudes in trials requiring the emotion identification were subtracted from trials requiring cognitive control in response to the emotional stimulus (i.e., opposite/happy minus actual/happy; opposite/sad minus actual/sad) for the control and rMDD groups. Larger (i.e., less negative) difference scores were interpreted as reflecting weaker cognitive control in response to the emotional stimulus. Note that the baseline-corrected data departed from the zero line. This might reflect the use of a fixed interstimulus interval, and might have affected the relative peak amplitudes. However, the 4 conditions showed virtually identical pre-stimulus baseline values; accordingly, the drifting baselines should not have affected between-condition differences.
In light of group differences in the target-locked ERPs, additional analyses were performed on N450 difference scores (i.e., opposite/sad minus actual/sad; opposite/happy minus actual/happy). Less negative difference scores are interpreted as reflecting weaker cognitive control in response to the emotional stimulus. As shown in Figure 4C, a group difference emerged when considering sad faces (t31 = 2.06, p = 0.042), but not happy faces (t31 = 0.62, p = 0.54). Overall, these scalp ERP findings suggest weaker cognitive control in the rMDD group than in the control group in response to emotionally negative information and converge with the response time findings. We observed no correlation between response time and N450 amplitudes to opposite/sad trials for either group (p = 0.25).
Ratings
An ANOVA on valence ratings with emotion (happy, sad) and group (control, rMDD) as factors revealed a main effect of emotion (F1,31 = 31.60, p < 0.001, ηp2 = 0.89), owing to more negative ratings for sad than happy faces (3.54 [SD 1.24] v. 5.09 [SD 1.61]; t32 = 5.76, p < 0.001). An analogous ANOVA on arousal ratings revealed a significant group × emotion interaction (F1,31 = 11.88, p = 0.002, ηp2 = 0.28). Paired t tests revealed that, within the control group, participants rated the happy faces (3.81 [SD 0.87]) as more arousing than the sad faces (2.65 [SD 0.84]; t17 = 3.66, p = 0.005). The rMDD group rated sad and happy faces similarly on arousal (3.46 [SD 0.79] v. 2.80 [SD 0.66]; t14 = 1.84, p = 0.063). The rMDD group rated sad faces as more arousing (t31 = −2.86, p = 0.007) and happy faces as less arousing (t31 = 3.67, p = 0.001) than the control group.
Regression analyses
The groups differed in arousal ratings of sad and happy faces. Because it is not possible to statistically control for the group differences in arousal ratings,32 we examined the possibility that the response time and target-locked N450 results would be influenced by this group difference. Two hierarchical regression analyses were run with arousal ratings for sad faces (entered in the first step) and group (dummy coded, entered in the second step) as predictors and opposite/sad minus actual/sad response time difference score (first regression) and opposite/sad minus actual/sad N450 difference wave (second regression) as criterion variables.
The first regression revealed that group differences in response time did not remain significant after considering arousal (step 2: ΔR2 = 0.04, ΔF1,30 = 1.45, p = 0.20; β = 0.22, t32 = 1.20, p = 0.20). Conversely, the second regression showed that group differences in N450 amplitudes remained significant after considering arousal (step 2: ΔR2 = 0.13, ΔF1,30 = 4.42, p = 0.044; β = 0.40, t32 = 2.10, p = 0.044).
Discussion
The goal of this study was to probe putative deficits in cognitive control over emotionally negative information in euthymic individuals with a history of depression. Specifically, we used 128-channel ERPs to investigate the temporal course of cognitive control processes to disentangle preparatory processes (cued anticipation) and cognitive control in response to the target.
Findings suggest that individuals in remission from depression have difficulty disengaging from sad information to generate the oppositely valenced response. Specifically, the rMDD group generated slower response times on trials that required a “happy” response to a sad face (opposite/sad trials) compared with trials that required a “sad” response to a happy face (opposite/happy trials). This effect was not observed in the control group. Moreover, relative to the control group, the rMDD group responded significantly more slowly on opposite/sad trials (after correction for individual differences in response time to actual/sad), and rated sad faces as more arousing. Collectively, these data support the hypothesis that individuals with rMDD have relatively poor cognitive control in response to sad information, which may place them at increased risk for future depressive episodes.
Behavioural analyses were complemented by ERP recordings, which enabled us to disentangle cued preparation from cognitive control in response to targets. This approach was motivated by concerns that poor cognitive control in participants with rMDD could simply reflect a deficit in effortful anticipatory processing rather than weak cognitive control per se. Interestingly, this hypothesis was not supported, as the control and rMDD groups demonstrated similar increases in N2 amplitude following the “opposite” relative to the “actual” cue. Although this result suggests that cognitive preparation is spared in individuals with a history of depression, future studies with larger sample sizes will be needed to replicate this null finding.
Group differences were apparent when participants had to exert cognitive control to overcome interference from sad, but not happy, faces. Relative to the control group, the rMDD group demonstrated reduced N450 difference scores (“opposite” minus “actual”) to emotionally negative, but not positive, trials. These results point toward a relative deficit in cognitive control in response to emotionally negative information.18,20 These findings are in line with the literature showing that a decreased inhibition for or disengagement from negative material plays an important role in depression.3–6 The current findings add to the literature by showing that these cognitive control deficits only appear in response to emotionally negative material, but suggest preserved anticipatory control processes (see previous paragraph). The fact that group differences emerged for the N450, but not the N2, component is consistent with evidence from prior ERP studies using nonemotional material33 and with the current understanding of cognitive deficits in depression, which emphasizes effects on late stages of information processing.5,6 Of note, only the control group showed a valence-specific difference in cognitive control: they demonstrated more negative N450 amplitudes in response to faces on opposite/sad relative to opposite/happy trials. This finding suggests that the control group exerted enhanced cognitive control in response to emotional conflict elicited by sad relative to happy faces.
Taken together, the response time and ERP data suggest that individuals with rMDD are not characterized by general impairments in cognitive control (at least as assessed by the cued emotional conflict task paradigm), but instead show a selective deficit on trials that required cognitive control over responses elicited by emotionally negative material. The observed deficit in cognitive control in participants with rMDD echo prior findings in patients with MDD, namely a reduced ability to disengage from negative material.9 Because MDD is characterized by high rates of relapse,2 it is important to understand the underlying mechanisms that render individuals vulnerable to depression. The present findings indicate that cognitive control deficits in response to emotionally negative material remain in patients who have been depressed in the past but who are currently remitted. Moreover, these cognitive deficits emerged in the absence of mood priming or cognitive load manipulation. Although additional work is necessary, the current data provide initial support for the hypothesis that weak cognitive control in response to sad stimuli may be a stable factor that increases risk for future depressive episodes.5,9 Interestingly, these cognitive control dysfunctions may be key mechanisms underlying the habitual use of maladaptive emotion regulation strategies34 (for reviews, see De Raedt and Koster5 and Gotlib and Joormann6). In particular, difficulties inhibiting negative information might be related to the inability to reappraise negative thoughts and feelings, which might lead to the inability to stop maladaptive rumination.35
It is important to note a key difference between the response time and ERP results. In the response time data, group differences were driven by a selective deficit (i.e., slower response time) on opposite/sad versus opposite/happy trials in the rMDD group (Fig. 2A). In the target-locked N450 analyses, group differences were primarily driven by a selective benefit (i.e., more negative N450 amplitude) on sad versus happy trials in the control group. In other words, whereas the response time and ERP data sets both point to a selective group difference on cognitive control to emotionally negative stimuli, the precise nature of this difference within each group was different. The reasons for this discrepancy are not immediately clear, and warrant further investigation. Possibly, a successful disengagement from emotionally negative material is an adaptive process that requires increased cognitive control (increased N450 amplitudes to opposite/sad trials in the control group). The rMDD group may have been unable to recruit additional cognitive control efforts and therefore demonstrated longer response times for a successful response (longer response time to opposite/sad trials in the rMDD group).
Findings of reduced N2 and N450 during cognitive interference (e.g., Stroop) tasks in individuals with MDD and rMDD20,33 contrast with robust evidence of potentiated error-related negativity (ERN) in individuals with MDD.36,37 This reduced conflict monitoring and potentiated error processing in individuals with MDD have been associated with dorsal ACC hypoactivation and rostral ACC hyperactivation, respectively.20,36,38,39 Accordingly, these findings point to region-specific hypo- and hyperactivation within frontocingulate pathways in people with depression and are consistent with literature emphasizing functional differentiation between different ACC subdivisions.38,40 In addition to these region-specific abnormalities in people with depression, a growing literature points to anticorrelated functional connectivity between the rostral and the dorsal ACC during cognitive control for emotional salient material.38 Moreover, depressed individuals demonstrate increased connectivity between the rostral ACC and the amygdala during the processing of negative material.41 These activation patterns demonstrate the crucial role of frontocingulate circuitry in emotion–cognition interplay, which includes mechanisms underlying the regulation of sustained (negative) emotions.
Limitations
Two limitations of the present study should be emphasized. First, the current sample was relatively modest. Although most of the effects sizes observed were in the medium to large range, the null finding in cue-locked N2 analyses may have been due to low power. Second, although the current findings echo prior data highlighting cognitive control dysfunctions in people with depression, it is important to emphasize that the precise source of this dysfunction remains to be elucidated. Specifically, it is not clear whether the group differences evident on the opposite/sad trials reflect deficits in response conflict, emotional conflict monitoring, ability to disengage attention from negative stimuli, or a combination of these processes. Moreover, the fact that the rMDD group rated the sad faces as more arousing than the control group suggests that “bottom-up” signals may have been stronger on sad trials in the rMDD group. If so, this could yield group differences in response time and ERPs sensitive to cognitive control even if both groups recruit “top–down” processes to a similar degree. However, the restriction of group differences to opposite/sad trials, combined with the lack of a group difference in cue-locked N2 amplitude, indicates that group differences were valence-specific and did not reflect poor target anticipation or weak preparation in the rMDD group. The ability to exclude these alternative explanations represents an important advantage of the cued emotional conflict task relative to other paradigms used to probe emotional interference in psychopathology.
Conclusion
The present study highlights a selective deficit in cognitive control over sad stimuli in individuals with rMDD. This deficit was evident in slow response time and decreased N450 amplitudes, and was not evident when individuals exerted cognitive control in response to happy faces. This is of clinical importance because reduced cognitive control over negative responses might represent a vulnerability factor in people with depression, possibly via reduced emotion regulation in response to an emotional stimulus. Prospective studies in larger samples are warranted to test how individual differences in cognitive control impairments can hinder sustained recovery from a depressive episode. Similarly, studies probing activation within and connectivity between frontocingulate and frontolimbic pathways during cued emotional conflict task trials in both remitted and depressed samples will be important to unveil putative mechanisms underlying trait-related markers of depression.
Acknowledgements
M.-A. Vanderhasselt is a postdoctoral fellow of the Research Foundation Flanders (FWO08/PDO/168). This study was partially supported by National Institute of Mental Health grants R01MH68376 and R21MH07897 awarded to D.A. Pizzagalli. We gratefully acknowledge Dr. Avram Holmes and Dr. Pia Pechtel for scientific discussions and technical assistance.
Footnotes
Competing interests: None declared for R. De Raedt, D.G. Dillon, S.J. Dutra and N. Brooks. M.-A. Vanderhasselt declares having received travel support from the Research Foundation Flanders. Over the past 3 years, D.A. Pizzagalli has received research support from Advanced Neuro Technology (ANT) North America, Inc., consulting fees from ANT North America, AstraZeneca, Ono Pharmaceuticals and Shire, and honoraria from AstraZeneca for studies unrelated to this project.
Contributors: M.-A. Vanderhasselt, R. De Raedt, D.G. Dillon and D.A. Pizzagalli designed the study. M.-A. Vanderhasselt, S.J. Dutra and N. Brooks acquired the data. M.-A. Vanderhasselt, D.G. Dillon and D.A. Pizzagalli analyzed the data. M.-A. Vanderhasselt, D.G. Dillon and D.A. Pizzagalli wrote the article. All authors reviewed the article and approved its publication.
References
- 1.Goodwin RD, Jacobi F, Bittner A, et al. Epidemiology of depression. In: Stein DJ, Kupfer DJ, Schatzberg AF, editors. Text book of mood disorders. Washington: American Psychiatric Publishing; 2006. pp. 33–54. [Google Scholar]
- 2.Boland RJ, Keller MB. Course and outcome of depression. In: Gotlib I, Hammen C, editors. Handbook of depression. 2nd ed. New York: Guilford Press; 2009. pp. 23–43. [Google Scholar]
- 3.Goeleven E, De Raedt R, Baert S, et al. Deficient inhibition of emotional information in depression. J Affect Disord. 2006;93:149–57. doi: 10.1016/j.jad.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Leyman L, De Raedt R, Schacht R, et al. Attentional biases for angry faces in unipolar depression. Psychol Med. 2007;37:393–402. doi: 10.1017/S003329170600910X. [DOI] [PubMed] [Google Scholar]
- 5.De Raedt R, Koster EHW. Understanding vulnerability for depression from a cognitive neuroscience perspective: a reappraisal of attentional factors and a new conceptual framework. Cogn Affect Behav Neurosci. 2010;10:50–70. doi: 10.3758/CABN.10.1.50. [DOI] [PubMed] [Google Scholar]
- 6.Gotlib IH, Joormann J. Cognition and depression: current status and future directions. Annu Rev Clin Psychol. 2010;6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joormann J, Gotlib IH. Selective attention to emotional faces following recovery from depression. J Abnorm Psychol. 2007;116:80–5. doi: 10.1037/0021-843X.116.1.80. [DOI] [PubMed] [Google Scholar]
- 8.Bhagwagar Z, Cowen PJ. ‘It’s not over when it’s over’: persistent neuro-biological abnormalities in recovered depressed patients. Psychol Med. 2008;38:307–13. doi: 10.1017/s0033291707001250. [DOI] [PubMed] [Google Scholar]
- 9.Joormann J, Yoon KL, Zetsche U. Cognitive inhibition in depression. Appl Prev Psychol. 2007;12:128–39. [Google Scholar]
- 10.Gotlib IH, Yue DN, Joormann J. Selective attention in dysphoric individuals: the role of affective interference and inhibition. Cognit Ther Res. 2005;29:417–32. [Google Scholar]
- 11.Etkin A, Egner T, Peraza DM, et al. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–82. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 12.Siegle GJ, Ingram RE, Matt GE. Affective interference: An explanation for negative attention biases in dysphoria? Cognit Ther Res. 2002;26:73–87. [Google Scholar]
- 13.Fales CL, Barch DM, Rundle MM, et al. Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biol Psychiatry. 2008;63:377–84. doi: 10.1016/j.biopsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hartlage S, Alloy LB, Vázquez C, et al. Automatic and effortful processing in depression. Psychol Bull. 1993;113:247–78. doi: 10.1037/0033-2909.113.2.247. [DOI] [PubMed] [Google Scholar]
- 15.van Veen V, Carter CS. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiol Behav. 2002;77:477–82. doi: 10.1016/s0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- 16.Nieuwenhuis S, Yeung N, Cohen JD. Stimulus modality, perceptual overlap, and the go/no-go N2. Psychophysiology. 2004;41:157–60. doi: 10.1046/j.1469-8986.2003.00128.x. [DOI] [PubMed] [Google Scholar]
- 17.Folstein JR, Van Petten C. Influence of cognitive control and mismatch on the N2 component of the ERP: a review. Psychophysiology. 2008;45:152–70. doi: 10.1111/j.1469-8986.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.West R, Alain C. Effects of task context and fluctuations of attention on neural activity supporting performance of the Stroop task. Brain Res. 2000;873:102–11. doi: 10.1016/s0006-8993(00)02530-0. [DOI] [PubMed] [Google Scholar]
- 19.Hanslmayr S, Pastotter B, Bauml KH, et al. The electrophysiological dynamics of interference during the stroop task. J Cogn Neurosci. 2008;20:215–25. doi: 10.1162/jocn.2008.20020. [DOI] [PubMed] [Google Scholar]
- 20.Holmes AJ, Pizzagalli DA. Response conflict and frontocingulate dysfunction in unmedicated participants with major depression. Neuropsychologia. 2008;46:2904–13. doi: 10.1016/j.neuropsychologia.2008.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 22.Beck AT, Steer RA, Brown GK. Beck Depression Inventory Manual. 2nd ed. San Antonio (TX): The Psychological Corporation; 1996. [Google Scholar]
- 23.Lundqvist D, Flykt A, Öhman A. The Karolinska Directed Emotional Faces (KDEF) Stockholm: Department of Neurosciences, Karolinska Hospital; 1998. [Google Scholar]
- 24.Goeleven E, De Raedt R, Leyman L, et al. The Karolinska Directed Emotional Faces: a validation study. Cogn Emot. 2008;22:1094–118. [Google Scholar]
- 25.Spielberger CD, Gorsuch RL, Lushene R, et al. Manual for the State-Trait Anxiety Inventory, Form Y. Palo Alto (CA): Consulting Psychologists Press, Inc; 1983. [Google Scholar]
- 26.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 27.Jung TP, Makeig S, Westerfield M, et al. Removal of eye activity artifacts from visual event-related potentials in normal and clinical participants. Clin Neurophysiol. 2000;111:1745–58. doi: 10.1016/s1388-2457(00)00386-2. [DOI] [PubMed] [Google Scholar]
- 28.Koenig T, Lehmann D. Microstates in language-related brain potential maps show noun-verb differences. Brain Lang. 1996;53:169–82. doi: 10.1006/brln.1996.0043. [DOI] [PubMed] [Google Scholar]
- 29.Lehmann D, Skrandies W. Spatial-analysis of evoked-potentials in man — a review. Prog Neurobiol. 1984;23:227–50. doi: 10.1016/0301-0082(84)90003-0. [DOI] [PubMed] [Google Scholar]
- 30.Luck SJ. An introduction to the event-related potential technique. Cam-bridge (MA): The MIT Press; 2005. [Google Scholar]
- 31.Cohen J. Statistical power analysis for the behavioral sciences. Rev ed. Hillsdale (NJ): Erlbaum; 1988. [Google Scholar]
- 32.Miller GA, Chapman JP. Misunderstanding analysis of covariance. J Abnorm Psychol. 2001;110:40–8. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- 33.Vanderhasselt MA, De Raedt R. Impairments in cognitive control persist during remission from depression and are related to the number of past episodes: an event related potentials study. Biol Psychol. 2009;81:169–76. doi: 10.1016/j.biopsycho.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 34.Joormann J, Gotlib IH. Emotion regulation in depression: relation to cognitive inhibition. Cogn Emot. 2010;24:281–98. doi: 10.1080/02699930903407948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joormann J, Siemer M. Affective processing and emotion regulation in dysphoria and depression: cognitive biases and deficits in cognitive control. Soc Personal Psychol Compass. 2011;5:13–28. [Google Scholar]
- 36.Holmes AJ, Pizzagalli DA. Spatio-temporal dynamics of error processing dysfunctions in major depressive disorder. Arch Gen Psychiatry. 2008;65:179–88. doi: 10.1001/archgenpsychiatry.2007.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiu PH, Deldin PJ. Neural evidence for enhanced error detection in major depressive disorder. Am J Psychiatry. 2007;164:608–16. doi: 10.1176/ajp.2007.164.4.608. [DOI] [PubMed] [Google Scholar]
- 38.Pizzagalli DA. Frontocingulate dysfunction in depression: towards biomarkers of treatment response. Neuropsychopharmacology. 2011;36:183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holmes AJ, Bogdan R, Pizzagalli DA. Serotonin transporter genotype and action monitoring dysfunction: a possible substrate underlying increased vulnerability to depression. Neuropsychopharmacology. 2010;35:1186–97. doi: 10.1038/npp.2009.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–22. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 41.Yoshimura S, Okamoto Y, Onoda K, et al. Rostral anterior cingulate cortex activity mediates the relationship between the depressive symptoms and the medial prefrontal cortex activity. J Affect Disord. 2010;122:76–85. doi: 10.1016/j.jad.2009.06.017. [DOI] [PubMed] [Google Scholar]