Abstract
Background
Recent data from neuroimaging, genetic and clinical trials and animal models suggest a role for altered glutamatergic neurotransmission in the pathogenesis of obsessive–compulsive disorder (OCD). The aim of this study was to investigate whether variants in the GRIN2B gene, the gene encoding the NR2 subunit of the N-methyl-d-aspartate (NMDA) glutamate receptor, may contribute to genetic susceptibility to OCD or to different OCD subphenotypes.
Methods
Between 2003 and 2008, we performed a case–control association study in which we genotyped 10 tag single-nucleotide polymorphisms (SNPs) in the 3′ untranslated region (3′ UTR) of GRIN2B. We performed SNP association and haplotype analysis considering the OCD diagnosis and different OCD subphenotypes: early-onset OCD, comorbid tic disorders and OCD clinical symptom dimensions.
Results
We enrolled 225 patients with OCD and 279 controls recruited from the OCD Clinic at Bellvitge Hospital (Barcelona, Spain). No significant difference in the distribution of alleles or genotypes was detected between patients with OCD and controls. Nonetheless, on analyzing OCD subphenotypes, the rs1805476 SNP in male patients (95% confidence interval [CI] 1.37–4.22, p = 0.002) and a 4-SNP haplotype in the whole sample (rs1805476, rs1805501, rs1805502 and rs1805477; odds ratio 1.92, 95% CI 1.22–3.01; permutation p = 0.023) were significantly associated with the presence of contamination obsessions and cleaning compulsions.
Limitations
Study limitations included the risk of population stratification associated with the case–control design, use of psychiatrically unscreened blood donors as the control group, reduced sample size of participants with certain OCD subphenotypes and tested polymorphisms limited to 3′ UTR and exon 13 of GRIN2B.
Conclusion
Our results converge with recent data suggesting a possible contribution of glutamatergic variants to the genetic vulnerability to OCD or at least to certain OCD manifestations. The dissection of OCD into more homogeneous subphenotypes may constitute a useful tool to disentangle the complex genetic basis of the disorder.
Introduction
An emerging body of evidence from neuroimaging, genetic and clinical trials and animal models supports the hypothesis that dysregulation of glutamate neurotransmission may contribute to the pathophysiology of obsessive–compulsive disorder (OCD).1 It has been suggested that a frontocortical hyperglutamatergic dysfunction underlies the cortico–striatal–thalamo–cortical abnormalities observed in imaging studies of OCD.2 Greater glutamatergic concentrations in the left striatum, which normalized after 12 weeks of paroxetine therapy, have been described in psychotropic-naive, nondepressed pediatric patients with OCD.3 Similarly, increased levels of a combined measure of glutamate and glutamine relative to creatine were noted in orbitofrontal white matter in adult patients with OCD.4 In contrast, lower glutamatergic concentrations were observed in the anterior cingulate cortex of children and adolescents with OCD5 and in women with OCD.6 Finally, significantly higher levels of cerebrospinal fluid glutamate have been described in patients with OCD.7 Animal models confirm the role of corticolimbic glutamatergic hyperactivation in patients with OCD.8 In animal models, D1CT-7 transgenic mice, which show chronic hyperactivation of orbitofrontal, somatosensory–sensorimotor and limbic glutamatergic output neurons, engaged in OCD-like behaviours,9 and mice with genetic deletion of SAPAP3, a postsynaptic scaffolding protein at glutamatergic synapses, exhibited a behavioural phenotype similar to OCD with compulsive overgrooming behaviour and increased anxiety, which improved in response to selective serotonin reuptake inhibitors.10 Finally, glutamatergic neurotransmission modulating agents, such as riluzole, topiramate, N-acetylcysteine, memantine or D-cycloserine, are currently being tested in patients with OCD and have shown some evidence of benefit in those with treatment-resistant OCD.11
Several genetic studies have reported positive associations between glutamatergic genes and OCD. The gene that encodes the neuronal glutamate transporter (SLC1A1) has been the most extensively studied. A sex-specific association between SLC1A1 and OCD in male, but not in female, patients has been reported,12–16 although negative results have also been published.17 Preliminary results also suggest a possible association between OCD and genetic variations of certain ionotropic glutamate receptors, including GRIK2 (glutamate receptor, ionotropic, kainate 2)18,19 and GRIN2B (ionotropic glutamate receptor, N-methyl-d-aspartate subunit 2B).20
Arnold and colleagues20 described a positive association between variants in the 3′ untranslated region (3′ UTR) of the GRIN2B gene — the gene encoding the NR2 subunit of the N-methyl-d-aspartate (NMDA) glutamate receptor — and OCD in 178 affected individuals from 130 families. The NMDA receptors (NMDAR) are ligand- and voltage-gated ion channels, composed of heteromeric complexes containing an obligatory NR1 (GRIN1) subunit, plus an additional NR2 (GRIN2A-D) or NR3 (GRIN3A,B) subunit.21 The NMDA receptors are critical for corticogenesis, neuronal migration and synaptogenesis during brain development. The NR2B subunit has been reported to play an essential role in memory and learning by regulating key aspects of synaptic plasticity.22,23 In this sense, overexpression of GRIN2B in the mouse forebrain has been reported to enhance hippocampal long-term potentiation, spatial learning and memory and to improve learning processes involved in fear conditioning and extinction.24 Furthermore, NR2B receptors are highly expressed in medium spiny neurons of the striatum, neurons that have been implicated in response control processes like error processing and response inhibition.25 Since response inhibition has been proposed as an endophenotype for OCD,26 and since obsessive–compulsive symptoms improve in response to cognitive–behaviour therapy, whose main component — exposure — relies on the basic principles of extinction learning, we considered that the preliminary association reported between GRIN2B and OCD deserves further study and replication in independent samples of patients with the disorder.
Recent models emphasize the heterogeneous nature of OCD, a fact that partially explains the extreme difficulty in identifying vulnerability genes for the disorder. So, exploring OCD subphenotypes (e.g., early OCD onset, comorbid tic disorders or clinical symptom dimensions), which are likely to be more etiologically homogeneous and closely linked to the action of genes, may help us to disentangle the complex genetic basis of the disorder.27 We hypothesized that genetic variation within GRIN2B would be associated with OCD and/or its different subphenotypes. To test this hypothesis, we performed a case–control association study by genotyping 12 SNPs in exon 13 or in the 3′-untranslated region (3′ UTR) of GRIN2B in a large sample of adult OCD probands and healthy controls, and analyzed the possible relation between these variants and OCD and OCD subphenotypes including early-onset OCD, OCD with comorbid tic disorders and obsessive–compulsive symptom dimensions.
Methods
Participants
We recruited participants from the OCD Clinic at Bellvitge Hospital (Barcelona, Spain) between 2003 and 2008. To be included in the study, patients had to fulfill DSM-IV criteria for OCD28 for a period of at least 1 year. Diagnoses were made on the basis of structured interviews conducted independently by 2 trained psychiatrists (P.A. and C.S.) using the Structured Clinical Interview for DSM-IV Axis I disorders, clinician version (SCID-CV).29 Exclusion criteria were age younger than 18 or older than 65 years, psychoactive substance abuse or dependence (current or in the past 6 months), psychotic disorders, mental retardation and severe organic or neurologic pathology other than tic disorder. Comorbidity with other DSM-IV Axis I disorders was not an exclusion criterion provided that OCD was the main diagnosis and the primary reason for seeking medical assistance. We recruited the control participants from a group of healthy blood donors. They were not psychiatrically screened.
Written informed consent was obtained from each participant after a full description of the study, which was approved by the hospital’s ethics committee.
Clinical assessment included information on age at onset of OCD, defined as the age when symptoms became a considerable source of distress and interfered with the patient’s social functioning. The threshold for early-onset OCD was established at age 15 years. A clinician-administered version of the Yale–Brown Obsessive–Compulsive Scale (Y-BOCS)30 and the 21-item Hamilton Rating Scale for Depression (HAM-D)31 were used to assess the severity of obsessive–compulsive and depressive symptoms, respectively. We used the clinician-administered version of the Y-BOCS Symptom Checklist30 to ascertain the presence of 5 previously identified symptom dimensions designated as “symmetry/ordering,” “hoarding,” “contamination/cleaning,” “aggression/checking” and “sexual/religious obsessions.”32 A dimension was considered to be present if the patient reported either current or lifetime history of at least 1 symptom included in the dimension. The Dimensional Y-BOCS (DY-BOCS)33 was used to assess the worst-ever severity of obsessive–compulsive symptoms within each of these symptom dimensions; the miscellaneous obsessions and compulsions dimension was excluded from the analysis owing to its unspecificity.
SNP selection and genotyping
The GRIN2B gene is located in reverse orientation on chromosome 12p13.1, where it spans about 470 kb. Given its large genomic size and complex linkage disequilibrium structure, complete tagging of the region exceeded the budgetary constraints of this study, so we decided to focus on genomic regions previously reported to be associated with OCD, schizophrenia or bipolar disorder, located at exon 13 and the 3′ UTR of the GRIN2B gene.20,34 To select them, we downloaded the genotyping data corresponding to the CEU trios (US residents of northern and western European ancestry) of the HapMap project (http://hapmap.ncbi.nlm.nih.gov/; Data Release 24, phaseII Nov08, on NCBI B36 assembly, dbSNP b126). Among all available SNPs (n = 19), we selected the 12 variants with a minor allele frequency higher than 0.01.
We typed SNPs using a custom VeraCode GoldenGate Genotyping Assay (Illumina) according to the manufacturer’s protocols (www.illumina.com). Raw data were processed using BeadStudio software (Illumina) to infer all SNP genotypes via a genotyping cluster. For statistical analysis, we selected those SNPs with a minimum genotyping rate of 95%, and in Hardy–Weinberg equilibrium (p < 0.05); 10 SNPs among the 12 initial ones were finally included in the analysis. The overall description of each polymorphism is shown in Appendix 1, available at cma.ca/jpn.
Population admixture
The sample studied has been previously genotyped for population admixture with anonymous unlinked SNPs and was found to show no population stratification (for a detailed description, see Alonso and colleagues35).
Single SNP analysis
We assessed the significance of association between 10 SNPs and affected status (cases versus controls) and between cases according to each subphenotype (age at onset, comorbid tic disorder, contamination/cleaning, symmetry/ordering, hoarding, sexual/religious and aggressive/checking) through multivariate logistic regression assuming a codominant, dominant, recessive and log-additive model of inheritance using the SNPassoc R package.36 The models were adjusted using sex and age as covariates. Effect of genotype variants on symptom severity for each DY-BOCS symptom dimension was assessed using analysis of variance (ANOVA), with genotype as the factor variable and scores on each symptom dimension as the dependent variables. To control for the possible confounding effect of depressive comorbidity, we repeated all analyses excluding patients with a comorbid affective disorder (n = 52). Bonferroni correction for 10 independent SNPs and the 7 different phenotypes tested was used to estimate the significance of the results. Thus, the statistical significance level was set at 0.003.
Haplotype analysis
The linkage disequilibrium pattern of the GRIN2B 3′ genomic region was estimated through r2 and D′ values, and haplotype blocks were estimated from the 4 gamete rules as implemented in Haploview software version 4.1.37 To perform the haplotype-based association analysis with the block containing the significant SNP and the contamination phenotype, we considered haplotypes with frequencies above 5%. Furthermore, to estimate the significance of the best results, we used the permutation procedure (n = 1000). Analyses were performed using Haploview software, and then the SNPassoc R package was used to obtain the odds ratio (OR) and 95% confidence intervals (CIs).
Results
Participants
During the selection period, 262 outpatients were assessed by the examiners and fulfilled DSM-IV criteria for OCD. Of these, 11 were ruled out in accordance with the exclusion criteria and 15 refused to take part in the study. In all, 236 consecutive white Spanish outpatients with OCD (125 men and 111 women) were included in the study. The control group consisted of 296 unrelated participants recruited from a group of healthy blood donors.
We submitted all 236 patients and 296 controls for genotyping. After the quality control assessments, the data available for all further analyses came from 225 patients and 279 controls. Of this sample for whom genotyping data were available for analysis, the controls were older than patients with OCD (mean age 40.02 [standard deviation (SD) 11.8], range 18–65 yr v. 34.0 [SD 10.5], range 18–63 yr; t = 6.2; p < 0.001). There were no significant differences in sex distribution between the 2 groups (male:female ratio 161:118 controls v. 116:109 patients with OCD; χ2 = 1.1, p = 0.24).
Single SNP and haplotype association analysis
The complete list of the 10 tag SNPs used in the study, their chromosomal locations, allele frequencies and genotyping rates are presented in Appendix 1. No significant difference in the distribution of alleles or genotypes was detected between controls and patients with OCD considered as a whole (data not shown). We further performed an analysis based on the clinical presentation of OCD, considering age at onset, comorbid tic disorders and symptom dimensions. For early onset OCD, comorbid tic disorders and symptom dimensions, including symmetry/ordering, hoarding, sexual/religious and aggressive/checking, no SNP achieved experiment-wide significance after adjustment for multiple testing. A significant association was found between rs1805476 and the contamination/cleaning dimension (p = 0.002, 95% CI 1.37–4.22) under a dominant model, and this association remained significant after multiple testing corrections (Table 1). The ANOVA test showed that patients carrying at least 1 T allele at this locus had significantly higher worst-ever scores on the contamination/cleaning dimension of the DY-BOCS than those homozygous for the G allele. No significant association was detected between scores on the other DY-BOCS symptom dimensions and rs1805476 variants (Table 2) or between the other tested SNPs and DY-BOCS symptom dimension scores (data not shown).
Table 1.
Effect of rs1805476 on contamination/cleaning symptoms
| Controls,* n = 279 | All patients, n = 225 | Male patients, n = 116 | Female patients, n = 109 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||||||||
| Symptoms, no. (%) | Symptoms, no. (%) | Symptoms, no. (%) | |||||||||||||
|
|
|
|
|||||||||||||
| Genotype | No. (%) | OR (95% CI) | p value | No | Yes | OR (95% CI) | p value | No | Yes | OR (95% CI) | p value | No | Yes | OR (95% CI) | p value |
| GG | 118 (42.2) | 1.0 | 55 (47.0) | 30 (27.8) | 1.0 | 30 (48.4) | 10 (18.5) | 1.0 | 25 (45.5) | 20 (37.0) | 1.0 | ||||
| GT/TT | 161 (57.7) | 1.19 (0.83–1.70) | 0.33 | 62 (53.0) | 78 (72.2) | 2.40 (1.37–4.22) | 0.002† | 32 (51.6) | 44 (81.5) | 4.13 (1.77–9.65) | < 0.001‡ | 30 (54.5) | 34 (63.0) | 1.55 (0.71–2.41) | 0.27 |
CI = confidence interval; OR = odds ratio.
Odds ratios were calculated for controls compared with patients with obsessive–compulsive disorder considered as a whole. No information on subclinical obsessive symptoms for any symptom dimension was available for controls.
Corrected for sex and age.
Corrected for age.
Table 2.
Effect of rs1805476 variants on DY-BOCS scores for symptom dimensions according to a dominant model
| Symptoms; mean (SD) score* | |||||
|---|---|---|---|---|---|
|
|
|||||
| Patient group | Contamination/cleaning | Aggressive/checking | Order/symmetry | Sexual/religious | Hoarding |
| All | |||||
| GG | 3.58 (5.1) | 7.58 (5.0) | 4.05 (5.5) | 2.81 (5.0) | 1.91 (3.8) |
| GT/TT | 5.76 (5.4) | 7.32 (4.6) | 3.19 (5.1) | 2.38 (4.5) | 1.85 (3.6) |
| F | 8.5 | 0.5 | 1.2 | 0.4 | 0.01 |
| p value | 0.004 | 0.46 | 0.26 | 0.51 | 0.92 |
| Male | |||||
| GG | 2.38 (4.6) | 7.71 (5.1) | 3.48 (5.4) | 3.04 (4.7) | 2.62 (4.1) |
| GT/TT | 5.56 (4.9) | 7.74 (5.3) | 3.95 (5.6) | 2.59 (4.6) | 2.23 (3.9) |
| F | 10.7 | 0.001 | 0.1 | 0.2 | 0.2 |
| p value | 0.001 | 0.97 | 0.67 | 0.63 | 0.63 |
| Female | |||||
| GG | 4.64 (5.4) | 6.89 (4.8) | 4.05 (5.5) | 1.65 (4.1) | 0.98 (2.7) |
| GT/TT | 5.98 (5.9) | 7.95 (4.7) | 3.19 (5.1) | 3.03 (4.7) | 1.60 (3.7) |
| F | 1.4 | 1.1 | 1.4 | 2.4 | 0.9 |
| p value | 0.23 | 0.27 | 0.26 | 0.12 | 0.34 |
DY-BOCS = Dimensional Yale–Brown Obsessive Compulsive Scale;33 SD = standard deviation.
Unless otherwise indicated.
We performed a further analysis stratified by sex and found that the association between this SNP and contamination/cleaning symptoms was significant only in male patients (p < 0.001, 95% CI 1.77–9.65; Table 1). The same was true for the association between rs1805476 variants and DY-BOCS scores for each symptom dimension (Table 2).
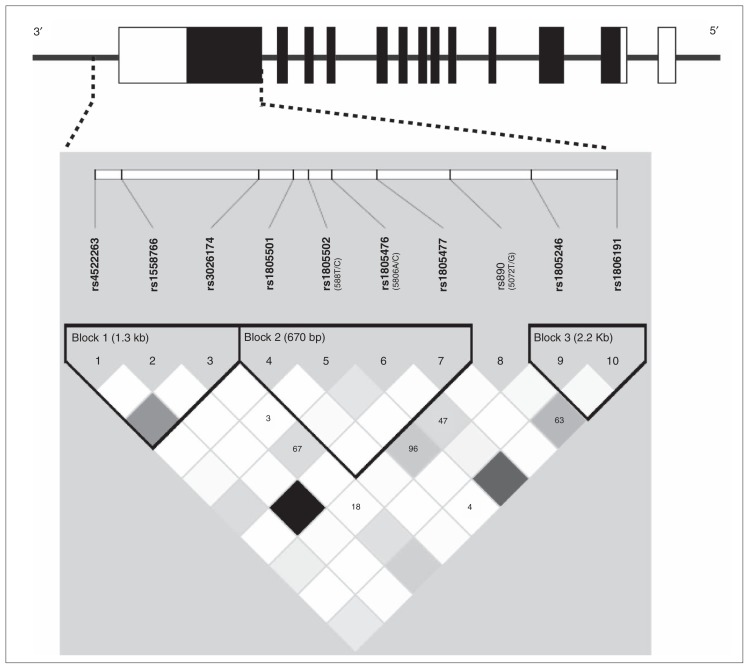
For the haplotype analysis, we estimated the linkage disequilibrium pattern of the region and identified 3 different blocks (Fig. 1). The significant SNP in the single SNP analysis was in block 2 of 670 bp and contained 3 other SNPs (rs1805501, rs1805502 and rs1805477). We performed the haplotype analysis of this block and found a significant association between a composite A-A-T-C and the presence of contamination obsessions and cleaning compulsions (OR 1.92, 95% CI 1.22–3.01; permutation p = 0.023; Table 3). No significant difference between male and female patients was detected for the haplotype analysis when we corrected for sex.
Fig. 1.
Schematic representation of the GRIN2B gene structure. Black boxes indicate coding exons, open boxes indicate first untranslated exons and the 3′ untranslated region. Haplotype blocks, linkage disequilibrium structure and genotyped single nucleotide polymorphisms (SNPs) in the 3′ region of GRIN2B derived by Haploview software. Alternate names for SNPs previously used in the literature are in parentheses. Linkage disequilibrium values are shown inside the squares if they are less than 1. The r2 linkage disequilibrium colour scheme is as follows: r2 = 0: white; 0 < r2 < 1: shades of grey; r2 = 1: black.
Table 3.
Haplotype distribution in patients with and without contamination/cleaning obsessions and compulsions
| rs1805501 | rs1805502 | rs1805476 | rs1805477 | Group; haplotype frequency, % | OR (95% CI) | p value | |||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| All patients | No contamination/cleaning | Contamination/cleaning | Crude | Permuted | |||||
| A | A | G | C | 45.7 | 39.8 | 51.0 | 1.0 | — | — |
| A | A | T | C | 36.4 | 30.8 | 42.6 | 1.92 (1.22–3.01) | 0.0047 | 0.023 |
| A | G | G | C | 16.2 | 16.7 | 15.7 | 1.23 (0.72–2.09 | 0.45 | — |
CI = confidence interval; OR = odds ratio.
To confirm that these genetic findings were not modulated by any other significant clinical difference between patients with and without contamination/cleaning obsessions and compulsions, we compared the sociodemographic and clinical variables of patients with OCD according to the presence of this symptom dimension. No significant differences, including sex distribution, were detected between them except for the DY-BOCS scores on the contamination/cleaning dimension (Table 4).
Table 4.
Sociodemographic and clinical characteristics of the 225 patients with obsessive–compulsive disorder with and without contamination/cleaning obsessions and cleaning compulsions
| Group; mean (SD) [range]* | ||||
|---|---|---|---|---|
|
|
||||
| Characteristic | Contamination/cleaning symptoms, n = 108 | No contamination/cleaning symptoms, n = 117 | t/χ2 | p value |
| Age, yr | 34.7 (10.2) [18–63] | 33.3 (10.2) [18–65] | −0.9 | 0.32 |
| Male:female | 55:53 | 62:55 | 1.07 | 0.73 |
| Years of education | 11.9 (3.6) | 12.0 (3.8) | −0.5 | 0.61 |
| Age at onset of OCD, yr | 18.8 (8.5) | 20.6 (8.0) | 1.6 | 0.10 |
| Onset before 15, no. (%) | 44 (40.7) | 32 (27.3) | 4.1 | 0.06 |
| Basal Y-BOCS score | ||||
| Global | 26.8 (5.9) | 26.3 (5.9) | −0.6 | 0.63 |
| Obsessions | 13.4 (3.0) | 13.4 (2.9) | −0.02 | 0.97 |
| Compulsions | 13.3 (3.0) | 12.9 (3.6) | −1.0 | 0.28 |
| Basal HAM-D score | 13.2 (4.8) | 13.5 (6.2) | 0.4 | 0.56 |
| DY-BOCS score | ||||
| Aggressive/checking | 6.7 (5.0) | 8.2 (4.8) | 2.1 | 0.034 |
| Contamination/cleaning | 10.3 (2.4) | 0.1 (1.2) | −39.0 | 0.001 |
| Sexual/religious | 2.3 (4.2) | 2.7 (4.8) | 0.6 | 0.52 |
| Hoarding | 1.9 (3.6) | 1.7 (3.7) | −0.3 | 0.73 |
| Symmetry/ordering | 3.5 (5.1) | 3.4 (5.5) | −0.07 | 0.91 |
| Comorbid diagnosis, no. (%) | ||||
| Any comorbid diagnosis | 49 (45.3) | 55 (47.0) | 0.05 | 0.32 |
| Affective disorders | 24 (22.2) | 28 (23.9) | 0.05 | 0.32 |
| Major depressive disorder | 16 | 18 | ||
| Dysthymia | 4 | 5 | ||
| Depressive disorder NEC | 2 | 3 | ||
| Bipolar disorder | 2 | 2 | ||
| Anxiety disorders other than OCD | 19 (17.5.) | 21 (17.9) | 0.6 | 0.41 |
| Tics disorder or GT | 15 (13.8) | 16 (13.6) | 0.9 | 0.55 |
| Eating disorders | 7 (6.4) | 8 (6.8) | 0.01 | 0.36 |
| Symptom dimensions present, no. (%) | ||||
| Aggressive/checking | 69 (63.8) | 87 (74.3) | 3.4 | 0.17 |
| Sexual/religious | 22 (20.3) | 31 (26.4) | 2.0 | 0.32 |
| Hoarding | 27 (25.0) | 32 (27.3) | 1.0 | 0.52 |
| Symmetry/ordering | 46 (42.5) | 35 (29.9) | 5.0 | 0.07 |
| Psychiatric family history, no. (%) | ||||
| Any psychiatric diagnosis | 54 (51.4) | 63 (53.8) | 0.07 | 0.31 |
| OCD | 21 (20.0) | 20 (17.0) | 0.09 | 0.27 |
| Anxiety disorders other than OCD | 15 (14.2) | 17 (14.5) | 0.6 | 0.44 |
| Affective disorders | 24 (22.8) | 27 (23.0) | 0.1 | 0.35 |
| Tic disorders or GT | 10 (9.5) | 17 (14.5) | 0.06 | 0.23 |
Results from our analysis considering only the patients without comorbid affective disorders were not significantly different than those in the whole sample (data not shown).
Discussion
Our study provides further evidence of a role for GRIN2B as a candidate gene for at least certain OCD subphenotypes. Although we failed to replicate a global relation between GRIN2B and OCD, on analyzing OCD subphenotypes and after correcting for multiple comparisons, rs1805476 and a 4-SNP haplotype (rs1805476, rs1805477, rs1805501 and rs1805502) were found to be associated with the presence of contamination obsessions and cleaning compulsions. Our haplotype contained the 2-marker haplotype (rs1805476, rs1805502) described by Arnold and colleagues20 in their initial analysis as associated with OCD using a family-based association test.
Although none of the positive variants identified in this study had a direct functional effect on the amino acid sequence of the protein, they might affect other regulatory aspects of GRIN2B expression, such as transcription, mRNA processing, nuclear export or alteration of secondary structure. On the other hand, the variants detected in our study may be in linkage disequilibrium with a functional variant located in non-explored regions of GRIN2B. It has also been hypothesized that variants in the 3′ UTR of some genes, like the ones explored in this study, may influence translational control through alteration of binding sites for micro-RNAs, although direct evidence of such a role for the 3′ UTR in GRIN2B is not currently available. Further research, including resequencing of GRIN2B, is warranted to locate functional variants contributing to the OCD phenotype as well as to identify mutant variants, microdeletions or duplications that might be involved in susceptibility to OCD.
Our results on symptom dimensions are especially relevant given the current conceptualization of OCD as a multidimensional disorder. The dissection of the OCD phenotype into more homogeneous components has been proposed as a way to improve our knowledge of the genetic factors implicated in OCD.27 Early onset OCD, OCD with comorbid tic disorders and OCD with associated sensory phenomena are some of the subtypes more closely related to familial risk. No significant association between these subphenotypes and GRIN2B variants was detected in our sample. On the other hand, the consideration of obsessive–compulsive symptom dimensions, which have proven to be consistent, temporally stable, valid and reliable constructs, has also provided promising leads in recent genetic studies.38–42 Specific genetic influences related to the contamination/cleaning dimension in OCD have been postulated using twin data.43 Recent neuroimaging studies suggest that this symptom dimension may be mediated by a specific neural substrate, mainly comprising the insula;44 prefrontal cortical areas,45 such as the ventromedial prefrontal regions including the anterior cingulate, orbitofrontal gyri and ventrolateral prefrontal cortex;46 and the caudate nucleus.46,47 Arnold and colleagues48 recently reported the association between the C-C haplotype of rs1805476 and decreased left orbitofrontal cortex volume and right anterior cingulate volume compared with carriers of the A allele in a sample of pediatric patients with OCD. The same authors also described a significant association between anterior cingulate glutamatergic concentration and the GRIN2B rs1019385 polymorphism.49 The particular implication of ventromedial regions in the development of contamination/cleaning symptoms may explain the specific contribution of GRIN2B to the genetic susceptibility to this symptom dimension.
Our group has recently reported on the positive association between the contamination/cleaning symptom dimension and another candidate gene, the estrogen receptor α gene (ESR1).35 The NMDA receptors have been hypothesized as possible mediators of estrogen actions in the brain. Fluctuations in hippocampal dendritic spine density related to estradiol action during the estrous cycle have been shown to occur via a mechanism requiring NMDA receptor activation.50 Moreover, animal studies have shown that expression of the NMDA receptor R2B gene is regulated by estrogen and that the NMDA receptor containing the R2B subunit may be involved in the initiation of puberty.51 We explored whether a gene × gene interaction between GRIN2B and ESR1 might contribute to genetic susceptibility to contamination/cleaning symptoms in OCD but failed to detect this interaction in our sample. The 3′ UTR of the human NR2D subunit of the NMDAR gene has been reported to contain at least 4 half palindromic estrogen responsive elements.52 The possibility that susceptibility to the contamination/cleaning symptom dimension might be modulated by the interaction between ESR1 and NMDA receptor genes other than GRIN2B should be further explored.
Genetic variations of GRIN2B have been implicated in the genetic susceptibility to neuropsychiatric disorders other than OCD, including attention-deficit/hyperactivity disorder,53 dyslexia,54 schizophrenia and bipolar disorder (for a review, see Cherlyn and colleagues34). Moreover, Parkinson disease and Huntington disease, 2 neurologic conditions in which obsessive–compulsive symptoms are not rare, have also been associated with changes in the function of the NR2B-containing NMDA receptors (for a review, see Loftis and Janowsky22).
As in the case of SLC1A1,12–14 the association between rs1805476 and OCD was detected in male but not female patients. Several studies have reported sex-related differences in the clinical manifestations of OCD, including an earlier age at onset for male patients,55–58 more cleaning obsessions and contamination compulsions in female patients and symmetry/ordering symptoms in male patients,58–60 and sex differences in response to OCD treatment.61 Moreover a sex-specific contribution to OCD susceptibility has also been reported for other candidate genes, such as MAO and COMT.58
Limitations
The major limitation of our study is the potential susceptibility of the case–control design to population stratification owing to admixture of different ethnicities among patients versus controls. We addressed this issue by genotyping multiple ancestry-informative markers and identified only 1 population stratum among patients and controls. Nevertheless, the Caucasian origin of all the participants cannot be conclusively assigned, and subtle or weak admixtures may not have been identified. Furthermore, the use of psychiatrically unscreened blood donors as the control group was not optimal, but could be considered potentially cost-effective and had a negligible effect on statistical power in the study of a disorder such as OCD with a population lifetime risk of 1%–2%.62 The small sample size of some OCD subphenotypes (particularly patients with sexual/religious and hoarding symptoms) may have limited our ability to detect subtle genetic contributions in these subgroups. Finally, we tested polymorphisms located in the 3′ UTR and exon 13 of GRIN2B, so it is possible that we missed other areas of the gene that could be associated with OCD. Future studies should employ a more comprehensive approach to explore the implication of other GRIN2B variants in the susceptibility to OCD.
Conclusion
We identified a positive association between GRIN2B variants and certain subphenotypes of OCD. This evidence converges with recent bioinformatic approaches suggesting the involvement of GRIN2B and its interaction with other glutamatergic and dopaminergic genes in a gene pathway underlying OCD pathogenesis.63 If confirmed, these findings argue in favour of testing new glutamate modulating agents as potential therapeutic alternatives in patients with refractory OCD, especially in certain subphenotypes.
Acknowledgements
We thank the study participants and the staff from Department of Psychiatry at Hospital de Bellvitge who helped us with the recruitment of participants. We thank Michael Maudsley from the Linguistic Services of University of Barcelona for revising the manuscript. Financial support was received from the Psychiatric Genetics Network (G03/184), the Spanish Ministry of Health, Instituto de Salud Carlos III, Fondo de Investigaciones Sanitarias de la Seguridad Social (PI10/01753, PI10/01003, CIBER-CB06/03/0034, CIBERESP), Barcelona node of the Spanish National Genotyping Center (CEGEN) and Genome Spain.
Footnotes
Presented as a poster at the XVIII World Congress on Psychiatric Genetics in Athens, Greece, Oct. 3–7, 2010.
Competing interests: As above for P. Alonso, C. Segalà, E. Real and J.M. Menchón. None declared for all other authors.
Contributors: P. Alonso, M. Gratacós and J.M. Menchón designed the study. P. Alonso, M. Gratacós, C. Segalàs, E. Real, J. Labad and C. López-Solà acquired the data, which P. Alonso, M. Gratacós, G. Escaramís, M. Bayés, X. Estivill and J.M. Menchón analyzed. P. Alonso and M. Gratacós wrote the article. All authors reviewed the article and approved its publication.
References
- 1.Ting JT, Feng G. Glutamatergic synaptic dysfunction and obsessive-compulsive disorder. Curr Chem Genomics. 2008;2:62–75. doi: 10.2174/1875397300802010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saxena S, Rauch SL. Functional neuroimaging and the neuro-anatomy of obsessive-compulsive disorder. Psychiatr Clin North Am. 2000;23:563–86. doi: 10.1016/s0193-953x(05)70181-7. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg DR, MacMaster FP, Keshavan MS, et al. Decrease in caudate glutamatergic concentrations in pediatric obsessive-compulsive disorder patients taking paroxetine. J Am Acad Child Adolesc Psychiatry. 2000;39:1096–103. doi: 10.1097/00004583-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Whiteside SP, Port JD, Deacon BJ, et al. A magnetic resonance spectroscopy investigation of obsessive-compulsive disorder and anxiety. Psychiatry Res. 2006;146:137–47. doi: 10.1016/j.pscychresns.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg DR, Mirza Y, Russell A, et al. Reduced anterior cingulate glutamatergic concentrations in childhood OCD and major depression versus healthy controls. J Am Acad Child Adolesc Psychiatry. 2004;43:1146–53. doi: 10.1097/01.chi.0000132812.44664.2d. [DOI] [PubMed] [Google Scholar]
- 6.Yücel M, Wood SJ, Wellard RM, et al. Anterior cingulate glutamate-glutamine levels predict symptom severity in women with obsessive-compulsive disorder. Aust N Z J Psychiatry. 2008;42:467–77. doi: 10.1080/00048670802050546. [DOI] [PubMed] [Google Scholar]
- 7.Chakrabarty K, Bhattacharyya S, Christopher R, et al. Glutamatergic dysfunction in OCD. Neuropsychopharmacology. 2005;30:1735–40. doi: 10.1038/sj.npp.1300733. [DOI] [PubMed] [Google Scholar]
- 8.Nordstrom EJ, Burton FH. A transgenic model of comorbid Tourette’s syndrome and obsessive-compulsive disorder circuitry. Mol Psychiatry. 2002;7:617–25. 524. doi: 10.1038/sj.mp.4001144. [DOI] [PubMed] [Google Scholar]
- 9.McGrath MJ, Campbell KM, Parks CR, et al. Glutamatergic drugs exacerbate symptomatic behavior in a transgenic model of comorbid Tourette’s syndrome and obsessive-compulsive disorder. Brain Res. 2000;877:23–30. doi: 10.1016/s0006-8993(00)02646-9. [DOI] [PubMed] [Google Scholar]
- 10.Welch JM, Lu J, Rodriguiz RM, et al. Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature. 2007;448:894–900. doi: 10.1038/nature06104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pittenger C, Krystal JH, Coric V. Glutamate-modulating drugs as novel pharmacotherapeutic agents in the treatment of obsessive-compulsive disorder. NeuroRx. 2006;3:69–81. doi: 10.1016/j.nurx.2005.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arnold PD, Sicard T, Burroughs E, et al. Glutamate transporter gene SLC1A1 associated with obsessive-compulsive disorder. Arch Gen Psychiatry. 2006;63:769–76. doi: 10.1001/archpsyc.63.7.769. [DOI] [PubMed] [Google Scholar]
- 13.Dickel DE, Veenstra-VanderWeele J, Cox NJ, et al. Association testing of the positional and functional candidate gene SLC1A1/EAAC1 in early-onset obsessive-compulsive disorder. Arch Gen Psychiatry. 2006;63:778–85. doi: 10.1001/archpsyc.63.7.778. [DOI] [PubMed] [Google Scholar]
- 14.Stewart SE, Fagerness JA, Platko J, et al. Association of the SLC1A1 glutamate transporter gene and obsessive-compulsive disorder. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:1027–33. doi: 10.1002/ajmg.b.30533. [DOI] [PubMed] [Google Scholar]
- 15.Wendland JR, Moya PR, Timpano KR, et al. A haplotype containing quantitative trait loci for SLC1A1 gene expression and its association with obsessive-compulsive disorder. Arch Gen Psychiatry. 2009;66:408–16. doi: 10.1001/archgenpsychiatry.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samuels J, Wang Y, Riddle MA, et al. Comprehensive family-based association study of the glutamate transporter gene SLC1A1 in obsessive-compulsive disorder. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:472–7. doi: 10.1002/ajmg.b.31184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang YAA, Shugart YY, Samuels JF, et al. A screen for SLC1A1 for OCD-related alleles. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:675–9. doi: 10.1002/ajmg.b.31001. [DOI] [PubMed] [Google Scholar]
- 18.Delorme R, Krebs MO, Chabane N, et al. Frequency and transmission of glutamate receptors GRIK2 and GRIK3 polymorphisms in patients with obsessive compulsive disorder. Neuroreport. 2004;15:699–702. doi: 10.1097/00001756-200403220-00025. [DOI] [PubMed] [Google Scholar]
- 19.Sampaio AS, Fagerness J, Crane J, et al. Association between polymorphisms in GRIK2 gene and obsessive-compulsive disorder: a family-based study. CNS Neurosci Ther. 2011;17:1412–7. doi: 10.1111/j.1755-5949.2009.00130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arnold PD, Rosenberg DR, Mundo E, et al. Association of a glutamate (NMDA) subunit receptor gene (GRIN2B) with obsessive-compulsive disorder: a preliminary study. Psychopharmacology (Berl) 2004;174:530–8. doi: 10.1007/s00213-004-1847-1. [DOI] [PubMed] [Google Scholar]
- 21.Villmann C, Becker CM. On the hypes and falls in neuroprotection: targeting the NMDA receptor. Neuroscientist. 2007;13:594–615. [Google Scholar]
- 22.Loftis JM, Janowsky A. The N-methyl-D-aspartate receptor subunit NR2B: localization, functional properties, regulation, and clinical implications. Pharmacol Ther. 2003;97:55–85. doi: 10.1016/s0163-7258(02)00302-9. [DOI] [PubMed] [Google Scholar]
- 23.Riedel G, Platt B, Micheau J. Glutamate receptor function in learning and memory. Behav Brain Res. 2003;140:1–47. doi: 10.1016/s0166-4328(02)00272-3. [DOI] [PubMed] [Google Scholar]
- 24.Tang YP, Shimizu E, Dube GR, et al. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–9. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- 25.Beste C, Baune BT, Domschke K, et al. Dissociable influences of NR2B-receptor related neural transmission on functions of distinct associative basal ganglia circuits. Neuroimage. 2010;52:309–15. doi: 10.1016/j.neuroimage.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 26.Chamberlain SR, Menzies L, Hampshire A, et al. Orbitofrontal dysfunction in patients with obsessive-compulsive disorder and their unaffected relatives. Science. 2008;321:421–2. doi: 10.1126/science.1154433. [DOI] [PubMed] [Google Scholar]
- 27.Miguel EC, Leckman JF, Rauch S, et al. Obsessive-compulsive disorder phenotypes: implications for genetic studies. Mol Psychiatry. 2005;10:258–75. doi: 10.1038/sj.mp.4001617. [DOI] [PubMed] [Google Scholar]
- 28.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington: The Association; 1994. [Google Scholar]
- 29.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV) Washington: American Psychiatric Press, Inc; 1996. [Google Scholar]
- 30.Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46:1006–11. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- 31.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mataix-Cols D, Rauch SL, Manzo PA, et al. Use of factor-analyzed symptom dimensions to predict outcome with serotonin reuptake inhibitors and placebo in the treatment of obsessive-compulsive disorder. Am J Psychiatry. 1999;156:1409–16. doi: 10.1176/ajp.156.9.1409. [DOI] [PubMed] [Google Scholar]
- 33.Rosario-Campos MC, Miguel EC, Quatrano S, et al. The Dimensional Yale-Brown Obsessive-Compulsive Scale (DY-BOCS): an instrument for assessing obsessive-compulsive symptom dimensions. Mol Psychiatry. 2006;11:495–504. doi: 10.1038/sj.mp.4001798. [DOI] [PubMed] [Google Scholar]
- 34.Cherlyn SY, Woon PS, Liu JJ, et al. Genetic association studies of glutamate, GABA and related genes in schizophrenia and bipolar disorder: a decade of advance. Neurosci Biobehav Rev. 2010;34:958–77. doi: 10.1016/j.neubiorev.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Alonso P, Gratacos M, Segalas C, et al. Variants in estrogen receptor alpha gene are associated with phenotypical expression of obsessive-compulsive disorder. Psychoneuroendocrinology. 2011;36:473–83. doi: 10.1016/j.psyneuen.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 36.SNPassoc: an R package to perform whole genome association studies [program] Vienna: R Foundation for Statistical Computing; 2006. [Google Scholar]
- 37.Barrett JC, Fry B, Maller J, et al. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–5. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 38.Katerberg H, Lochner C, Cath DC, et al. The role of the brain-derived neurotrophic factor (BDNF) val66met variant in the phenotypic expression of obsessive-compulsive disorder (OCD) Am J Med Genet B Neuropsychiatr Genet. 2009;150B:1050–62. doi: 10.1002/ajmg.b.30930. [DOI] [PubMed] [Google Scholar]
- 39.Alsobrook JP, Leckman JF, Goodman WK, et al. Segregation analysis of obsessive-compulsive disorder using symptom-based factor scores. Am J Med Genet. 1999;88:669–75. doi: 10.1002/(sici)1096-8628(19991215)88:6<669::aid-ajmg17>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 40.Leckman JF, Pauls DL, Zhang H, et al. Obsessive-compulsive symptom dimensions in affected sibling pairs diagnosed with Gilles de la Tourette syndrome. Am J Med Genet B Neuropsychiatr Genet. 2003;116B:60–8. doi: 10.1002/ajmg.b.10001. [DOI] [PubMed] [Google Scholar]
- 41.Cullen B, Brown CH, Riddle MA, et al. Factor analysis of the Yale-Brown Obsessive Compulsive Scale in a family study of obsessive-compulsive disorder. Depress Anxiety. 2007;24:130–8. doi: 10.1002/da.20204. [DOI] [PubMed] [Google Scholar]
- 42.Pinto A, Greenberg BD, Grados MA, et al. Further development of YBOCS dimensions in the OCD Collaborative Genetics study: symptoms vs. categories. Psychiatry Res. 2008;160:83–93. doi: 10.1016/j.psychres.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Grootheest DS, Boomsma DI, Hettema JM, et al. Heritability of obsessive-compulsive symptom dimensions. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:473–8. doi: 10.1002/ajmg.b.30622. [DOI] [PubMed] [Google Scholar]
- 44.Husted DS, Shapira NA, Goodman WK. The neurocircuitry of obsessive-compulsive disorder and disgust. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:389–99. doi: 10.1016/j.pnpbp.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 45.Gilbert AR, Mataix-Cols D, Almeida JR, et al. Brain structure and symptom dimension relationships in obsessive-compulsive disorder: a voxel-based morphometry study. J Affect Disord. 2008;109:117–26. doi: 10.1016/j.jad.2007.12.223. [DOI] [PubMed] [Google Scholar]
- 46.Mataix-Cols D, Wooderson S, Lawrence N, et al. Distinct neural correlates of washing, checking, and hoarding symptom dimensions in obsessive-compulsive disorder. Arch Gen Psychiatry. 2004;61:564–76. doi: 10.1001/archpsyc.61.6.564. [DOI] [PubMed] [Google Scholar]
- 47.van den Heuvel OA, Remijnse PL, Mataix-Cols D, et al. The major symptom dimensions of obsessive-compulsive disorder are mediated by partially distinct neural systems. Brain. 2009;132:853–68. doi: 10.1093/brain/awn267. [DOI] [PubMed] [Google Scholar]
- 48.Arnold PD, Macmaster FP, Hanna GL, et al. Glutamate system genes associated with ventral prefrontal and thalamic volume in pediatric obsessive-compulsive disorder. Brain Imaging Behav. 2009;3:64–76. doi: 10.1007/s11682-008-9050-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arnold PD, Macmaster FP, Richter MA, et al. Glutamate receptor gene (GRIN2B) associated with reduced anterior cingulate glutamatergic concentration in pediatric obsessive-compulsive disorder. Psychiatry Res. 2009;172:136–9. doi: 10.1016/j.pscychresns.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Woolley CS, McEwen BS. Estradiol regulates hippocampal dendritic spine density via an N-methyl-D-aspartate receptor-dependent mechanism. J Neurosci. 1994;14:7680–7. doi: 10.1523/JNEUROSCI.14-12-07680.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kanamaru H, Kakeyama M, Seki T, et al. Estrogen potentiates N-methyl-D-aspartate receptor subunit R2B mRNA expression during the late prepubertal period in female rats. Neurosci Lett. 2001;300:9–12. doi: 10.1016/s0304-3940(01)01527-0. [DOI] [PubMed] [Google Scholar]
- 52.Watanabe T, Inoue S, Hiroi H, et al. NMDA receptor type 2D gene as target for estrogen receptor in the brain. Brain Res Mol Brain Res. 1999;63:375–9. doi: 10.1016/s0169-328x(98)00304-0. [DOI] [PubMed] [Google Scholar]
- 53.Dorval KM, Wigg KG, Crosbie J, et al. Association of the glutamate receptor subunit gene GRIN2B with attention-deficit/hyperactivity disorder. Genes Brain Behav. 2007;6:444–52. doi: 10.1111/j.1601-183X.2006.00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ludwig KU, Roeske D, Herms S, et al. Variation in GRIN2B contributes to weak performance in verbal short-term memory in children with dyslexia. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:503–11. doi: 10.1002/ajmg.b.31007. [DOI] [PubMed] [Google Scholar]
- 55.Rasmussen SA, Eisen JL. Epidemiology of obsessive compulsive disorder. J Clin Psychiatry. 1990;51(Suppl):10–3. discussion 14. [PubMed] [Google Scholar]
- 56.Bogetto F, Venturello S, Albert U, et al. Gender-related clinical differences in obsessive-compulsive disorder. Eur Psychiatry. 1999;14:434–41. doi: 10.1016/s0924-9338(99)00224-2. [DOI] [PubMed] [Google Scholar]
- 57.Castle DJ, Deale A, Marks IM. Gender differences in obsessive compulsive disorder. Aust N Z J Psychiatry. 1995;29:114–7. doi: 10.3109/00048679509075899. [DOI] [PubMed] [Google Scholar]
- 58.Lensi P, Cassano GB, Correddu G, et al. Obsessive-compulsive disorder. Familial-developmental history, symptomatology, comorbidity and course with special reference to gender-related differences. Br J Psychiatry. 1996;169:101–7. doi: 10.1192/bjp.169.1.101. [DOI] [PubMed] [Google Scholar]
- 59.Tükel R, Polat A, Genc A, et al. Gender-related differences among Turkish patients with obsessive-compulsive disorder. Compr Psychiatry. 2004;45:362–6. doi: 10.1016/j.comppsych.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 60.Katerberg H, Cath DC, Denys DA, et al. The role of the COMT Val(158)Met polymorphism in the phenotypic expression of obsessive-compulsive disorder. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:167–76. doi: 10.1002/ajmg.b.30971. [DOI] [PubMed] [Google Scholar]
- 61.Mundo E, Bareggi SR, Pirola R, et al. Effect of acute intravenous clomipramine and antiobsessional response to proserotonergic drugs: Is gender a predictive variable? Biol Psychiatry. 1999;45:290–4. doi: 10.1016/s0006-3223(98)00027-4. [DOI] [PubMed] [Google Scholar]
- 62.Moskvina V, Holmans P, Schmidt KM, et al. Design of case-controls studies with unscreened controls. Ann Hum Genet. 2005;69:566–76. doi: 10.1111/j.1529-8817.2005.00175.x. [DOI] [PubMed] [Google Scholar]
- 63.Grados MA. The genetics of obsessive-compulsive disorder and Tourette syndrome: an epidemiological and pathway-based approach for gene discovery. J Am Acad Child Adolesc Psychiatry. 2010;49:810–9. 819.e1–2. doi: 10.1016/j.jaac.2010.04.009. [DOI] [PubMed] [Google Scholar]