Abstract
Objective
To determine feeding practices and nutritional status of infants born to HIV-1–infected women.
Methods
Feeding plans and practices were evaluated by questionnaires and focus group discussions. Infants were weighed at 1 and 6 weeks and tested for HIV-1 at 6 weeks.
Results
Of 128 women seen after delivery, 111 completed the study. Mothers who planned to breast feed were more likely to feed their infants as planned (86% vs. 55%; P < 0.001). Women opted to breast feed due to financial constraints, partner influence, and fear of losing confidentiality. Women who reported that their partners were willing to have HIV-1 testing were less likely to be breast feeding at 6 weeks (odds ratio [OR] = 0.3, 95% confidence interval [CI]: 0.1–0.8; P = 0.01). At 6 weeks, more infants were mixed fed (31% vs. 21%; P = 0.05) than at 1 week. Lower infant weight at 6 weeks was associated with not breast feeding (P = 0.001), HIV-1 infection (P = 0.05), birth weight <3000 g (P = 0.01), maternal employment (P = 0.02), and paying <$12.5 per month in house rent (among infants not breast fed; P = 0.05).
Conclusions
Replacement feeding was difficult, particularly without partner support in HIV-1 testing. Mixed feeding was common and increased by 6 weeks. Mothers of low socioeconomic status who opt not to breast feed require support to avoid nutritional compromise of infants.
Keywords: breast feeding, prevention of perinatal transmission, infant feeding options
Mother-to-child transmission of HIV-1, which accounts for virtually all cases of pediatric HIV-1 infection, is an important problem in sub-Saharan Africa, where 4.5 million children have been infected since the beginning of the HIV-1 pandemic.1 In sub-Saharan Africa, where prolonged breast feeding is the norm, breast milk transmission of HIV-1 accounts for one third to one half of all pediatric HIV-1 infections.2,3 In a randomized trial comparing breast feeding and formula feeding, with a 17-month median duration of breast feeding, the risk of breast milk transmission was found to be 16% at 2 years. In the same study, formula feeding increased the proportion of infants alive and HIV-1 uninfected at 2 years from 58% to 70%.4 An observational study in South Africa found that the cumulative probability of HIV infection by 15 months was lower among infants who were exclusively breast fed for 3 months or more compared with other breast-fed infants (0.247 vs. 0.359).5 Based on the findings of these and other studies, either replacement feeding or exclusive breast feeding has been proposed as a strategy to reduce breast milk HIV-1 transmission.
It is known that for HIV-1–uninfected women, breast feeding is associated with reduced infant infectious disease morbidity.6,7 Less is known regarding the impact of feeding practices on infant morbidity among HIV-1–infected women, however.8,9 In a randomized trial comparing breast feeding and formula feeding among HIV-1–infected women, breast-fed and formula-fed infants had similar mortality rates after 2 years, but formula-fed infants tended to have an increased risk of diarrhea during the first 3 months of life and breast-fed infants had better nutritional status during the first 6 months.10
For HIV-1–infected women, infant feeding decisions are difficult, because most breast milk transmission of HIV-1 occurs in the first few months of life, a time when replacement feeding carries the greatest risk of increasing infectious disease morbidity and the benefits of breast feeding are highest.4,11 In developing countries, decisions regarding the best mode of infant feeding are even more difficult due to social and financial constraints.
We conducted a prospective study among women participating in a randomized clinical trial comparing compliance with 2 antiretroviral regimens for prevention of infant HIV-1 (HIV Network for Prevention Trials [HIVNET] 012 nevirapine regimen and Thai Centers for Disease Control and Prevention [CDC] zidovudine regimen) to determine early infant feeding plans and practices.
METHODS
The study was conducted from November 1999 to January 2001, and the recruitment, enrollment, and follow-up procedures have been described elsewhere.12 Briefly, HIV-positive pregnant women were referred from primary care clinics in Nairobi to a tertiary care hospital (Kenyatta National Hospital). Participants received counseling on HIV/AIDS, routine antenatal care, multivitamins, hematinics, and treatment of any incidental illnesses until 36 weeks of gestation, when they were enrolled in the study.
Care before enrollment included health talks, individual counseling on infant feeding, and demonstration of the preparation of formula for women who planned not to breast feed. Health talks included information on benefits of breast feeding, risk of breast milk HIV-1 transmission, and infant feeding options. Women received individual counseling for 15 to 30 minutes by the study nurse or peer counselor and for 5 to 10 minutes by the study doctor at each visit. Infant feeding options explored in these sessions included replacement feeding, exclusive breast feeding, heat treatment of expressed breast milk, and shortened duration of breast feeding. Women were supported in whatever infant feeding choice they made and were given more information on their infant feeding choices at subsequent visits. Those who planned to breast feed were strongly encouraged to breast feed exclusively for the first 4 to 6 months of life. For women who planned not to breast feed, preparation of infant feeds was demonstrated, and they were counseled regarding safe breast milk substitutes as per United Nations AIDS (UNAIDS) guidelines.13 Formula was not provided by the study. Women were encouraged to share HIV-1 test results with their partners, and HIV-1 testing was available for partners at no cost.
At enrollment (36 weeks of gestation) women were interviewed using questionnaires to assess sociodemographic characteristics, obstetric variables, knowledge regarding mother-to-child transmission of HIV-1, infant feeding plans, and attitudes toward infant feeding. After the interview, women were randomized to receive either the Thai CDC or HIVNET 012 regimen.14,15
After delivery, information on infant feeding practices and on the reasons for choosing different modes of infant feeding was obtained at 1 and 6 weeks. At these visits, women were asked how they were currently feeding their infants (previous 24 hours), and relevant infant medical history and physical examination findings were recorded in a medical chart. At 6 weeks, heel prick blood samples were obtained from infants on filter paper for HIV-1 DNA polymerase chain reaction (PCR) testing at the end of the study.
Four focus group discussions on infant feeding were held with women attending the antenatal clinic who had not yet received their HIV-1 test results, and 3 were conducted with HIV-1–infected breast-feeding and non–breast-feeding women 6 to 10 weeks after delivery. The focus group discussions had 8 to 11 participants.
Sociodemographic and obstetric characteristics of women who were breast feeding (exclusive breast feeding and mixed feeding) and replacement feeding were compared, and correlates of infant feeding practices were determined. Weight for age Z scores were calculated from infant weights using the National Center for Health Statistics (NCHS)/World Health Organization (WHO) reference population and ANTHRO software (WHO/CDC, 1999).16 χ2 and Mann-Whitney U tests were used to compare categories and continuous variables, respectively. Logistic and linear regression were used for multivariate analyses.
RESULTS
Study Population
Of 139 pregnant women recruited in the randomized clinical trial, 128 (92%) who were seen after delivery formed the study population for evaluation of infant feeding plans and practices. The median age was 25 years, 40% had at least a secondary school education, and 26% were employed. Seventy-four percent had a previous pregnancy, and 56% said they paid more than KSh 1000 ($12.50) for house rent (median = KSh 1200, range: KSh 200–7000). For reference, the monthly per capita income in Kenya is KSh 2300 ($29).17 The majority of the women (72%) said they had informed their partners of their HIV-1 test results, and 61% said that their partners would be willing to have an HIV-1 test. Ninety-one percent of the women knew that mother-to-child transmission of HIV-1 could occur through breast feeding (Table 1).
TABLE 1.
Characteristics of Study Population
| Characteristic | Number (%) (N = 128) |
|---|---|
| Client characteristics | |
| Age >25 years | 62 (48%) |
| Secondary level education or more | 51 (40%) |
| Employed | 33 (26%) |
| House rent >KSh 1000 | 71 (56%) |
| Had a previous pregnancy | 95 (74%) |
| Partner characertistics (data obtained from women in clinic) | |
| Married | 104 (81%) |
| Age >25 years | 94 (73%) |
| Secondary level education or more | 83 (65%) |
| Employed | 119 (94%) |
| Informed of HIV-1 test results | 91 (72%) |
| Willing to have HIV-1 test | 78 (61%) |
| Knowledge and attitudes regarding infant feeding at recruitment | |
| MTCT of HIV-1 can occur through breast feeding | 124 (91%) |
| Not breast feeding can cause some problems | 101 (79%) |
MTCT indicates mother-to-child transmission.
Follow-Up
Five (4%) women had stillbirths, 1 infant died 3 days after delivery, and 12 (9%) women were lost to follow-up before 6 weeks. Women who were lost to follow-up were younger (median age: 23 vs. 26 years; P = 0.01) and had fewer children (median: 0 vs. 1; P = 0.01) than those who completed the study.
Infant Feeding Plans and Practices
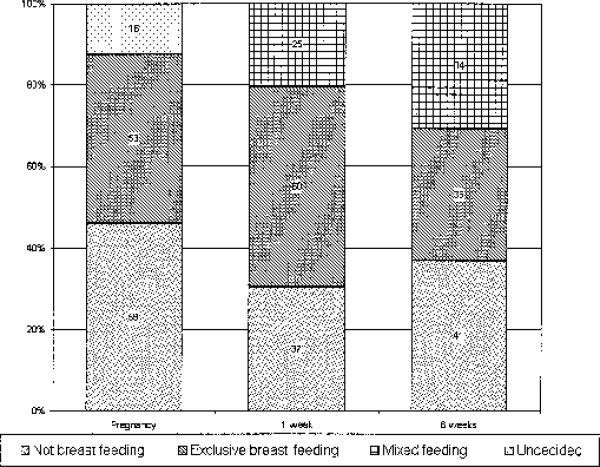
At 36 weeks of gestation, 59 (42%) women planned not to breast feed, 53 (46%) planned to breast feed, and 16 (12%) were undecided. After delivery 76 (64%) women who had made infant feeding plans fed their infants according to their preference during pregnancy. Women who planned to breast feed were more likely to feed their infants according to their preference than those who planned not to breast feed (86% vs. 55%; P < 0.001). More women were mixed feeding at 6 weeks than at 1 week (31% vs. 21%; P = 0.005), whereas the number of women who were not breast feeding increased from 30% at 1 week to 37% at 6 weeks (P = 0.08; Fig. 1).
FIGURE 1.
Infant feeding plans and practices.
Of 41 women who were not breast feeding at 6 weeks, only 1 was not giving infant formula; others were feeding their infants with commercial infant formula (59%), homemade formula from fresh cow's milk (15%), or homemade formula from pasteurized homogenized whole-cream cow's milk (37%) (some gave more than 1 type). To these breast milk substitutes 23 (56%) had added other foods, including water (61%), milk (17%), porridge (13%), fruits (17%), bananas (4%), and potatoes (4%). The reasons given for introducing these other foods were that the baby was crying a lot (78%), to reduce cost (9%), the baby was not gaining weight (9%), and the baby was old enough to start other foods (13%).
Mixed feeding at 6 weeks was more common among women who were exclusively breast feeding at 1 week (odds ratio [OR] = 6.2, 95% confidence interval [CI]: 1.7, 23; P = 0.006) and mixed feeding (OR = 7.5, 95% CI: 1.7, 33; P = 0.007) compared with those who were not breast feeding at 1 week. The 34 women who were mixed feeding at 6 weeks had introduced milk (44%), water (29%), and fruits (18%). The reasons given for mixed feeding were that the baby was crying a lot due to hunger (74%), the baby was old enough to start other foods (14%), maternal travel (9%), lack of privacy for breast feeding (11%), and pressure from relatives and spouses (20%). Only 1 woman said she mixed fed because she had been sick.
Correlates of Infant Feeding Practices
At 1 week, women who were not breast feeding their infants were more likely to be over 25 years old (P = 0.02), to have said that that their partners would be willing to have an HIV-1 test (P = 0.01), and to have said that they planned not to breast feed (P = 0.008; Table 2). In multivariate analysis, a prenatal decision not to breast feed (OR = 5.1, 95% CI: 1.9, 14; P = 0.001) and reporting that the partner was willing to have an HIV-1 test (OR = 3.7, 95% CI: 1.3, 10; P = 0.01) were independently associated with replacement feeding at 1 week.
TABLE 2.
Correlates of Replacement Feeding at 1 and 6 Weeks After Delivery
| 1 Week After Delivery |
6 Weeks After Delivery |
|||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Client characteristics | ||||
| Age >25 years | 2.6 (1.2, 5.9) | 0.02 | 1.4 (0.6, 3.1) | 0.4 |
| Married | 0.8 (0.3, 2.2) | 0.7 | 1.5 (0.6, 4.5) | 0.4 |
| Secondary education or more | 1.5 (0.8, 3.2) | 0.3 | 1.5 (0.7, 3.2) | 0.4 |
| Employed | 1.9 (0.8, 4.5) | 0.2 | 1.7 (0.7, 4.0) | 0.2 |
| House rent >KSh 1000 | 2.0 (0.8, 4.8) | 0.1 | 3.1 (1.2, 7.6) | <0.001 |
| Had a previous pregnancy | 2.0 (0.8, 5.5) | 0.2 | 1.3 (0.5, 3.4) | 0.6 |
| Received Thai CDC versus HIVNET 012 regimen | 0.9 (0.4, 2.0) | 0.8 | 0.6 (0.3, 1.4) | 0.2 |
| Partner characteristics | ||||
| Age >25 years | 2.8 (0.8, 11) | 0.1 | 1.0 (0.3, 3.0) | 1.0 |
| Secondary education or more | 1.6 (0.7, 3.7) | 0.3 | 1.2 (0.5, 2.8) | 0.6 |
| Employed | 0.7 (0.2, 3.0) | 0.6 | 0.5 (0.1, 2.8) | 0.5 |
| Informed of HIV-1 test results | 1.6 (0.7, 4.0) | 0.3 | 1.7 (0.7, 4.1) | 0.2 |
| Willing to have HIV-1 test | 3.1 (1.3, 7.6) | 0.01 | 6.7 (2.5, 18) | <0.001 |
| Knowledge and attitude to infant feeding | ||||
| Planned not to breast feed | 5.1 (2.0, 13) | 0.0008 | 4.1 (1.6, 10) | 0.003 |
Replacement feeding at 6 weeks was associated with paying more than KSh 1000 house rent (P < 0.001), reporting that the partner was willing to have an HIV-1 test (P < 0.001), and planning not to breast feed prenatally (P = 0.003; see Table 2). In multivariate analysis adjusting for factors significantly associated with replacement feeding, planning not to breast feed (OR = 5.2, 95% CI: 1.7, 16; P = 0.004), and reporting that the partner was willing to have an HIV-1 test (OR = 9.3, 95% CI: 2.3, 38; P = 0.002) were independently associated with replacement feeding at 6 weeks (see Table 2).
Among breast-feeding women, those who exclusively breast fed at 6 weeks were younger (mean age: 25 vs. 27 years; P = 0.05) and more likely to have known that antiretrovirals can prevent mother-to-child transmission of HIV-1 at enrollment (75% vs. 44%; P = 0.01). There was no association between exclusive breast feeding and prenatal infant feeding plans, parity, amount of house rent, or partner willingness to have an HIV test.
Marital status, level of education, occupation of the woman or her partner, informing the partner of HIV-1 test results, and the antiretroviral regimen to which a woman was randomized were not associated with infant feeding practices.
Infant Growth and Morbidity
At 1 Week
The mean weight for age Z score of the infants was –0.5 (range: –3.7–2.7), and infants with a birth weight less than 3000 g had lower scores than those whose birth weight was 3000 g or more (–1.9 vs. –0.2; P < 0.001). Infants who tested positive for HIV-1 by PCR at 6 weeks had lower Z scores compared with uninfected infants (–1.0 vs. –0.5; P = 0.05; Table 3).
TABLE 3.
Mean Z Scores by Mode of Infant Feeding and Maternal Characteristics
| Characteristic | Mean Z Scores at 1 week (95% CI) | P | Mean Z Scores at 6 weeks (95% CI) | P | |
|---|---|---|---|---|---|
| Age | >25 years | –0.5 (–0.7, –0.2) | 0.4 | 0.3 (–0.1, 0.7) | 0.6 |
| ≤25 years | –0.7 (–1.0, –0.3) | 0.2 (–0.1, 0.6) | |||
| Marital status | Married | –0.5 (–0.7, –0.2) | 0.5 | 0.3 (0.0, 0.6) | 0.6 |
| Not married | –0.7 (–1.1, –0.3) | 0.2 (–0.4, 0.8) | |||
| Education | Secondary or more | –0.5 (–0.8, –0.2) | 1.0 | 0.4 (0.0, 0.8) | 0.4 |
| Less than secondary | –0.5 (–0.8, –0.3) | 0.2 (–0.4, 0.8) | |||
| Occupation | Employed | –0.7 (–1.1, –0.2) | 0.6 | –0.3 (–0.9, 0.2) | 0.02 |
| Not employed | –0.5 (–0.7, –0.2) | 0.5 (0.2, 0.8) | |||
| 1 week feeding | Breast feeding | –0.6 (–0.9, –0.3) | 0.5 | 0.4 (0.1, 0.7) | 0.1 |
| Not breast feeding | –0.4 (–0.7, 0.0) | 0.0 (–0.6, 0.5) | |||
| 6 week feeding | Breast feeding | –0.5 (–0.8, –0.2) | 0.4 | 0.6 (0.3, 0.9) | <0.001 |
| Not breast feeding | –0.6 (–0.9, –0.3) | 0.4 (–0.9, 0.1) | |||
| Parity | Primigravida | –0.8 (–1.2, –0.3) | 0.1 | 0.0 (–0.3, 0.5) | 0.3 |
| Parous | –0.5 (–0.7, –0.2) | 0.3 (0.0, 0.7) | |||
| Birth weight | ≥3000 g | –0.2 (–0.4, 0.0) | <0.001 | 0.6 (0.3, 0.9) | 0.01 |
| <3000 g | –1.9 (–2.2, –1.6) | –0.4 (–1.2, 0.4) | |||
| Infant sex | Male | –0.5 (–0.8, –0.2) | 0.7 | 0.4 (0.1, 0.7) | 0.5 |
| Female | –0.5 (–0.8, –0.2) | 0.1 (–0.2, 0.5) | |||
| HIV infection | Positive | –1.0 (–1.6 –0.4) | 0.05 | –0.4 (–1.0, 0.4) | 0.05 |
| Negative | –0.5 (–0.7, –0.2) | 0.4 (0.1, 0.6) | |||
| House rent | KSh 1000 or more | –0.4 (–0.7, –0.2) | 0.3 | 0.3 (–0.1, 0.6) | 0.8 |
| Less than KSh 1000 | –0.7 (–1.0, –0.3) | 0.2 (–0.2, –0.8) |
At 6 Weeks
The mean weight for age Z score was 0.2 (range: –2.8–3.0). Infants who were not breast fed had lower scores than breast-fed infants (–0.4 vs. 0.6; P < 0.001) and HIV-1–infected infants had lower scores than uninfected infants (–0.4 vs. 0.4; P = 0.05). Mean weight for age Z scores were lower among infants of employed mothers compared with unemployed mothers (–0.3 vs. 0.5; P = 0.02) and infants with a birth weight <3000 g compared with those with a birth weight ≥3000 g (–0.4 vs. 0.6; P = 0.01; see Table 3). In stratified analysis, paying a house rent of less than KSh 1000 was associated with a lower mean weight for age Z scores among infants not breast fed (–1.3 vs. –0.2; P = 0.04) but not among breast-fed infants (0.6 vs. 0.7; P = 0.9). In multivariate linear regression analysis, lower infant weight for age Z scores were associated with HIV-1 infection among breast-fed infants (P = 0.01) and paying less than KSh 1000 house rent among infants not breast fed (P = 0.05). Maternal characteristics such as age, occupation, parity, and education did not influence infant nutritional status at 1 and 6 weeks (see Table 3).
The number of infants treated in the study clinic did not differ by infant feeding practice by 6 weeks: 3 (8%) exclusively breast-fed infants, 6 (15%) mixed-fed infants, and 6 (15%) infants not breast fed had been seen at the study clinic due to illness (P = 0.6).
Seventeen (15%) of 110 infants tested for HIV-1 at 6 weeks were HIV-1 infected. The study had inadequate power to look at the impact of infant feeding practices on HIV-1 transmission.
Focus Group Discussions
Before Receiving HIV-1 Test Results
Pregnant women said that if they tested positive for HIV-1, advice by medical personnel, ability to afford formula, partner's attitudes, and ability to maintain confidentiality (the woman's desire and ability to keep her HIV status confidential) would influence whether they decided to breast feed or not. Women favored early introduction of other foods, with some saying that this should be done as early as 1 week after delivery. Mothers preferred to introduce other foods early because they believed that breast milk was not adequate and that babies would cry a lot and fail to thrive if exclusively breast fed for long.
Six to 10 Weeks After Delivery
HIV-1–infected women thought that not breast feeding was a better option for their infant's health, but some opted to breast feed due to financial constraints; fear of revealing HIV-1 status; and pressure from parents, partners, and other relatives. Women who had a previous infant die or become infected after breast feeding or formula feeding were reluctant to repeat the previously “unsuccessful” mode of feeding. To maintain confidentiality while not breast feeding, women would breast feed for a short time and stop, say they had inadequate milk, feed the baby only in the privacy of their homes, or say they had been advised against breast feeding due to a medical illness (Table 4).
TABLE 4.
Results of Focus Group Discussions
| Antenatal clinic attendants before receiving HIV-1 test results |
| Weaning |
| Ideal time: 1 week to 6 months |
| Ideal foods: fruits, porridge, potatoes, eggs, and spinach |
| Reasons for early weaning |
| Baby crying a lot due to hunger |
| Baby not gaining weight adequately |
| Breast disease in the mother |
| Mother does not have enough milk |
| What would influence infant feeding decision? |
| Advice by medical personnel |
| Ability to afford formula |
| Ability to maintain confidentiality |
| Attitudes and opinions of partner and relatives |
| HIV-1–positive women 6–10 weeks after delivery |
| Reasons for current infant feeding practices |
| Fear of loss of confidentiality |
| Financial constraints |
| Experiences such as breast-fed or formula-fed infant who died or became infected |
| Most convinced not breast feeding a better option |
| Exclusive breast feeding difficult due to |
| Conflicting advice from health workers |
| Advice from older relatives and friends |
| Children cry a lot due to hunger |
| Children need water for thirst and stomach upsets |
| Strategies for maintaining confidentiality while not breast feeding |
| Breast feeding for a short time and stop |
| Giving excuse like have breast disease or infant refusal of breast |
| Avoiding going in public places with the baby |
| Saying it is medical advice due to illness |
DISCUSSION
In spite of many women planning on not breast feeding, the use of replacement feeding in this group of HIV-1–infected women was relatively low (30%). As reported elsewhere in Africa, financial considerations were probably the most important reason for the low use of replacement feeding.18,19 From focus group discussions, however, it appears that other reasons, such as fear of loss of confidentiality, attitudes of partners, and experiences of formula-fed infants who died or became infected, may be as important. Women who informed their partners of their HIV-1 test results were more likely to take up replacement feeding at 1 and 6 weeks; however, this was not statistically significant. This suggests that although partner knowledge of HIV-1 status is a factor influencing feeding decisions, a supportive partner attitude may be more important. The importance of partner attitudes is supported by our finding that women who reported that their partners would be willing to have an HIV-1 test were 4 times more likely to take up replacement feeding. Recent operations research findings also suggest that nonfinancial considerations are important in determining uptake of replacement feeding. In one study also in Kenya, uptake of replacement feeding was low (ranging from 18% to 50% at separate sites), even when formula was provided at no cost.20 Conversely, some women opted not to breast feed due to their partner's attitudes or because of experiences with breast-fed infants who died or became infected. Because of the significance of these issues in determining infant feeding choices, it is important to assist women in exploring their experiences with HIV-1 and infant feeding, partner's attitudes, perceptions of risk of breast milk transmission, and confidentiality concerns when counseling women on infant feeding options.
We found several predictors of poor infant growth, including HIV-1 infection, not breast feeding, and maternal employment. At 6 weeks, we found that infants who were not breast fed had significantly lower weight for age Z scores. This is consistent with previous reports showing that the growth of formula-fed infants in the first 3 months of life is slower than that of breast-fed infants.10,21–24 This slowed growth among formula-fed infants compared with breast-fed infants was not associated with increased mortality and morbidity rates in a previous study in Nairobi, and in our study, the number of infants seeking treatment in the study clinic did not differ by mode of infant feeding.10 For infants who were not breast fed, in univariate and multivariate analyses, low socioeconomic status (as measured by low house rent) was a strong predictor of poor growth. In contrast, the early growth of breast-fed infants did not appear to be compromised by socioeconomic sta tus. Current UNAIDS guidelines regarding feeding counseling for HIV-1–infected mothers take this issue into consideration. The guidelines advise detailed assessment of the ability of women to provide adequate nutritional replacement for breast milk as part of the counseling process in deciding how to feed the infant. Despite this counseling, however, the reality remains that some HIV-1–infected women opt not to breast feed despite inadequate replacement options, and this may compromise infant growth. This suggests the need for increased support of HIV-1–infected women of low social economic status who decide not to breast feed.
In our study, 79% of HIV-1–infected women were practicing 1 of the 2 recommended modes of infant feeding (exclusive replacement or exclusive breast feeding) at 1 week. This declined to 69% at 6 weeks, however, due to the fact that many women who were exclusively breast feeding at 1 week started mixed feeding. There may have been relative complacency in counseling of women who were exclusively breast feeding to encourage continuing exclusive breast feeding. There is need for aggressive counseling prenatally and soon after delivery to address issues that compromise exclusive breast feeding so as to prevent early initiation of mixed feeding and to sustain exclusive breast feeding. Exclusive breast feeding was associated with younger age and better knowledge about prevention of mother-to-child transmission of HIV-1. Thus, it appears that younger women may be more open to receiving education and advice from the clinical staff. The prevalence of mixed feeding at 6 weeks was lower in our study than in a previous study conducted in Nairobi (31% vs. 52%), suggesting that the intensive counseling was effective in increasing the practice of exclusive breastfeeding.25 The reasons for mixed feeding in our study were similar to those described in studies elsewhere in Africa and need to be addressed not only for HIV-1–infected women but for all women.19,26,27 The concept that for HIV-1–infected women, breast feeding maybe safer than replacement feeding in some circumstances was difficult for the women to grasp, and many still believed that replacement feeding was the better option. Additionally, we found that some of the strategies used by women to maintain confidentiality regarding their HIV-1 status may promote mixed feeding. An interesting finding from this study is that introduction of other foods by formula-feeding women was also common, and the reasons given were similar to those given for mixed feeding.
In conclusion, we found that women find it difficult to opt for replacement feeding, infant feeding decisions were influenced by many factors, mixed feeding was common and increased during the first 6 weeks, and replacement feeding by women of low social economic status was associated with infant growth faltering. Concerns regarding community spill-over and inappropriate use of formula have recently led to decreased availability of free or subsidized formula within programs to prevent mother-to-child transmission of HIV-1. Because of the complexity of infant feeding decisions, however, it is important to support women in the decisions they make regarding infant feeding. Such support for mothers who cannot afford adequate replacement feeds but opt not to breast feed should ideally include provision of formula or training on safe use of animal milks, which may be more readily available.
Acknowledgments
James N. Kiarie was a scholar in the International AIDS Research and Training Program, supported by the Fogarty International Center, National Institutes of Health (D43-TW00007).
REFERENCES
- 1.UNAIDS/ WHO . Report on the Global HIV/AIDS Epidemic: Global HIV/AIDS and STD Surveillance. UNAIDS; Geneva: 2000. [Google Scholar]
- 2.De Cock K, Fowler M, Mercier E, et al. Prevention of mother-to-child HIV transmission in resource-poor countries, translating research into policy and practice. JAMA. 2000;283:1175–1182. doi: 10.1001/jama.283.9.1175. [DOI] [PubMed] [Google Scholar]
- 3.Nicoll A, Newell M, Peckham C, et al. Infant feeding and HIV-1 infection—year 2000 [review]. AIDS. 2000;14(Suppl):S57–S74. [PubMed] [Google Scholar]
- 4.Nduati R, John G, Mbori-Ngacha D, et al. Effect of breastfeeding and formula feeding on transmission of HIV-1: a randomized clinical trial. JAMA. 2000;283:1167–1174. doi: 10.1001/jama.283.9.1167. [DOI] [PubMed] [Google Scholar]
- 5.Coutsoudias A, Pillay K, Kuhn L, et al. Method of feeding and HIV-1 transmission from mothers to children by 15 months age: prospective cohort study from Durban, South Africa. AIDS. 2001;15:379–387. doi: 10.1097/00002030-200102160-00011. [DOI] [PubMed] [Google Scholar]
- 6.Clavano N. Mode of feeding and its effect on infant mortality and morbidity. J Trop Pediatr. 1982;28:287–293. doi: 10.1093/tropej/28.6.287. [DOI] [PubMed] [Google Scholar]
- 7.Brown K, Black R, deRomana G. Infant feeding practices and their relationship with other diseases in Huascar (Lima), Peru. Pediatrics. 1989;83:31–40. [PubMed] [Google Scholar]
- 8.Bobat R, Coovadia H, Moodley D, et al. Growth in early childhood in a cohort of children born to HIV-1-infected women from Durban, South Africa. Ann Trop Paediatr. 2001;21:203–210. doi: 10.1080/02724930120077772. [DOI] [PubMed] [Google Scholar]
- 9.Gray R, Brahmbhatt H. Child mortality associated with failure to breast-feed and voluntary and involuntary weaning; an assessment of the risks and benefits of breastfeeding by HIV infected mothers [abstract WeOrC496].. Presented at the XIII International AIDS Conference; Durban, South Africa. July, 2000. [Google Scholar]
- 10.Mbori-Ngacha D, Nduati R, John G, et al. Morbidity and mortality in breastfed and formula-fed infants of HIV-1 infected women: a randomized clinical trial. JAMA. 2001;286:2413–2420. doi: 10.1001/jama.286.19.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO Collaborative Study Team on the Role of Breastfeeding on the Prevention of Infant Mortality Effect of breastfeeding on infant and child mortality due to infectious diseases in less developed countries: a pooled analysis. Lancet. 2000;355:451–455. [PubMed] [Google Scholar]
- 12.Kiarie JN, Kreiss JK, Richardson BA, et al. Compliance with antiretrovirals to prevent perinatal HIV-1 transmission in Kenya. AIDS. 2003;17:65–71. doi: 10.1097/01.aids.0000042938.55529.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO Technical Consultation . New Data on the Prevention of Mother-to-Child Transmission of HIV and Their Policy Implications. WHO; Geneva: 2000. [Google Scholar]
- 14.Guay LA, Musoke P, Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala Uganda: HIVNET 012 randomised trial. Lancet. 1999;354:795–802. doi: 10.1016/S0140-6736(99)80008-7. [DOI] [PubMed] [Google Scholar]
- 15.Shaffer N, Chuachoowong R, Mock PA, et al. Short-course zidovudine for perinatal HIV-1 transmission in Bangkok, Thailand: a randomized controlled trial. Lancet. 1999;353:773–780. doi: 10.1016/s0140-6736(98)10411-7. [DOI] [PubMed] [Google Scholar]
- 16.WHO Expert Committee . Physical Status: The Use and Interpretation of Anthropometry. WHO; Geneva: 1995. p. 854. [PubMed] [Google Scholar]
- 17.Statistics Division of the United Nations Secretariat and International Labour Office . Indicators of Income and Economic Activity. United Nations; New York: 1999. [Google Scholar]
- 18.Chorba T, Sibailly T, Ekpini E, et al. Implementation and effectiveness of a short course of zidovudine and replacement feeding program to prevent mother-to-child transmission of HIV-1 in Abidjan, Ivory Coast.. Presented at the XIII International AIDS Conference; Durban, South Africa. July, 2000; [abstract ThPpC1446] [Google Scholar]
- 19.Gottlieb D, Shetty A, Basset M, et al. Infant feeding: knowledge, attitudes and practices among women in Zimbabwe [abstract MoPeD2785].. Presented at the XIII International AIDS Conference; Durban, South Africa. July, 2000. [Google Scholar]
- 20.Mbori-Ngacha D, Nduati R, Kalibala S, et al. Utilization of services for the prevention of mother-to-child transmission (MTCT) of HIV-1 in 2 sites in Kenya.. Presented at Global Strategies for the Prevention of HIV Transmission from Mothers to Infants; Kampala, Uganda. September 2001. [Google Scholar]
- 21.Donma MM, Donma O. Infant feeding and growth: a study on Turkish infants from birth to 6 months. Pediatr Int. 1999;41:542–548. doi: 10.1046/j.1442-200x.1999.01099.x. [DOI] [PubMed] [Google Scholar]
- 22.Agostoni C, Grandi F, Gianni ML, et al. Growth patterns of breast fed and formula fed infants in the first 12 months of life: an Italian study. Arch Dis Child. 1999;81:395–399. doi: 10.1136/adc.81.5.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Victora CG, Morris SS, Barros FC, et al. The NCHS reference and the growth of breast- and bottle-fed infants. J Nutr. 1998;128:1134–1138. doi: 10.1093/jn/128.7.1134. [DOI] [PubMed] [Google Scholar]
- 24.Victora CG, Morris SS, Barros FC, et al. Breast-feeding and growth in Brazilian infants. Am J Clin Nutr. 1998;67:452–458. doi: 10.1093/ajcn/67.3.452. [DOI] [PubMed] [Google Scholar]
- 25.Sherry B, Embree JE, Mei Z, et al. Sociodemographic characteristics, care, feeding practices, and growth of cohorts of children born to HIV-1 seropositive and seronegative mothers in Nairobi, Kenya. Trop Med Int Health. 2000;5:678–686. doi: 10.1046/j.1365-3156.2000.00631.x. [DOI] [PubMed] [Google Scholar]
- 26.Desclaux A, Taverne B, Alfieri C, et al. Socio-cultural obstacles in the prevention of HIV transmission through breastmilk in West Africa.. Presented at the XIII International AIDS Conference; Durban, South Africa. July, 2000. [Google Scholar]
- 27.Rantona K, Tlou S, Nyblade L, et al. The role of the larger community in the success of mother to child HIV prevention programs.. Presented at the XIII International AIDS Conference; Durban, South Africa. July, 2000. [Google Scholar]