Abstract
We have used mouse hepatoma cells in culture to study acute, short-term high-dose effects of hexavalent chromium on gene regulation directed by the polycyclic aromatic hydrocarbon benzo[a]pyrene (BaP). We find that the mixture engages three major signaling pathways: (i) activation of detoxification genes; (ii) induction of signal transduction effectors; and, (iii) epigenetic modification of chromatin marks. Preliminary results in mice exposed to mixtures of low doses of Cr (VI) plus BaP indicate that all three pathways are likely to be engaged also in long-term effects resulting from exposure to environmentally relevant doses of the mixture that inhibit the expression of tumor suppressor genes. Given the toxicity and carcinogenicity of these mixtures, we expect that a two-way analytical approach, from cells in culture to biological effects in vivo and vice versa, will provide a better understanding of the molecular mechanisms responsible for the biological effects of mixtures. By focusing both the in vivo and the in vitro work into long-term, low-dose, environmentally relevant exposures, we expect to develop much needed information pertinent to the type of diseases found in human populations exposed to mixtures of environmental toxicants.
Keywords: Epigenetics, Complex Mixtures, Benzo[a]pyrene, Hexavalent Chromium, Gene Expression
Introduction
One of the major quandaries in current toxicology and its application to risk assessment is the lack of a sound methodology to deal with the health effects of chemical mixtures [1]. Data is scarce, and this scarcity points out at the need to evaluate critically the assumptions used to determine risk from exposure to complex toxicant mixtures, ultimately leading to an understanding of the health effects of exposure. Assessing the effects of combined exposures to non-metabolizable halogenated aromatic hydrocarbons has been proposed based on the relative potency of the individual compounds [2] and to PAHs based on their relative carcinogenic potency [3]. These approaches, while valuable, are limited to mixtures of similar compounds [4] and are of little use when dealing with complex mixtures of multiple components, because combined exposures generate substance-specific changes in gene expression that cannot be attributed to a single mechanism.
Epidemiologically, toxicity from chromium exposure is associated with chromium (VI) compounds [5], and, although chromium (III) is the most prevalent form in the environment and in biological tissues, the vast majority of the evidence indicates that trivalent chromium is not toxic in animals. Chromium (VI) enters cells via the sulfate anion transporter system [6] and is reduced to intermediate oxidation states, such as Cr(V) and Cr(IV) in the process of forming stable Cr(III) forms. Chromium is genotoxic, and has been shown to induce DNA-protein cross-links by binding to several reactive amino acids, including cysteine, tyrosine and histidine, and linking these to the DNA phosphate backbone [7–11]. Chromium also causes double strand breaks in DNA [12;13], which might be involved in transcriptional inhibition [14]. Chromium also induces the formation of nuclear foci containing phosphorylated histone H2A.X, a biomarker of DNA damage, similar to the H2A.X foci induced by ionizing radiation [15].
As we and others have shown, the effect of chromium on gene expression has significant practical implications for assessing the risk of environmental exposures [16–18]. Our results indicate that combined exposures to chromium and BaP generate substance specific changes in gene expression that cannot be attributed to a single mechanism [18;19]. Therefore, the analysis of patterns of gene expression and biological effect resulting from exposure are critical to dissect the mechanisms of complex mixture toxicity and to identify sensitive biomarkers of effect and exposure. We have developed a novel approach to study the biological effects of mixtures that departs from the classical concept that the effect of combined exposures may be predicted from the additive effects of each of their components. The paradigm that we have developed is that the characterization of the health effects of mixtures would be simplified if, instead of attempting to study the interactions of the components of every possible mixture in the environment, analyses were focused on the interaction between the signaling pathways affected by the components in a mixture and on the ultimate biological consequences of the interactions between those pathways.
Our work has focused on aspects of chromium toxicity that affect specific gene regulatory changes resulting from exposure to mixtures of chromium and BaP. In this context, the available experimental evidence indicates that chromium exposure alters inducible gene expression due to the formation of chromium-DNA adducts and chromium-DNA cross-links [20]. Chromium was found to block the expression of several inducible genes, such as metal-inducible metallothionein in chick embryo liver, or hormone-inducible phosphoenolpyruvate carboxykinase (PEPCK) without affecting the expression of housekeeping genes, such as β-actin or albumin [6;21–23]. These observations led to the hypothesis that the chromatin structure of inducible promoters, perhaps by virtue of being more open, may offer a better target for chromium binding than the more closed chromatin of constitutive promoters [24]. Chromium (VI) was also shown to persistently activate the MAP kinases ERK1 and ERK2 by a redox-sensitive mechanism that can be enhanced by glutathione depletion in cells treated with L-buthionine-[S,R,]-sulfoximine and attenuated in cells treated with the glutathione replenisher N-acetylcysteine [25]. Electron spin resonance and spin trapping measurements indicated that generation of free radicals from the reduction of Cr(VI) to Cr(V) and Cr(IV) induce the activation of NF-κB in cultured Jurkat cells [26;27]. It has been suggested that the intermediate oxidation states of chromium may be critical factors in the effect of this metal on gene expression, pointing at a mechanism of chromium-induced effects that involve the reduction process in the persistent stimulation of regulatory pathways of gene expression [9–11].
The Ah receptor (AHR) plays a central role in the toxicity of BaP and of numerous other aromatic compounds by controlling the expression of a battery of Phase I and Phase II detoxification enzymes responsible for metabolism of these compounds [28]. Recent reports have shown that modification of Ah receptor-dependent gene expression can result from oxidative stress, suggesting that co-exposure to receptor ligands and pro-oxidant environmental pollutants could disrupt the coordinate regulation of detoxification genes. Experimental studies on the effect of combined chromium plus BaP exposure has been largely limited to the analysis of genotoxic effects. Chromium treatment was shown to inhibit the formation of BaP-induced mutations in the HPRT locus when cells were treated simultaneously, but not when chromium was added before or after BaP [29]. Vitamin E and catalase blocked this effect, suggesting that chromium-mediated oxidative stress was responsible for the inhibition. More recent work has shown that chromium enhances binding of BPDE to mutational hotspots in exons 7 and 8 of the human p53 gene in human lung cells [30]. Few of these studies have addressed the question of changes in the relative abundance of BaP metabolites due to co-exposure or the toxicity and carcinogenicity of the mixture.
Results
Long-term low-concentration Cr(VI) treatment of mouse hepatoma Hepa-1 cells
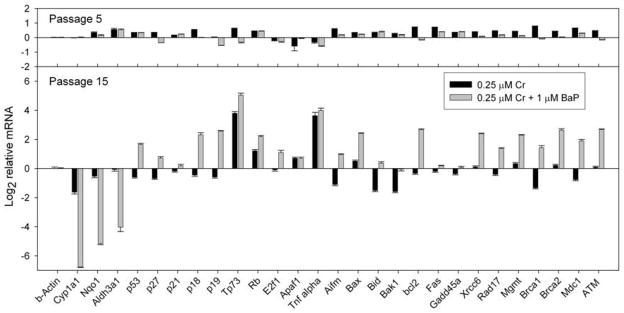
Much of the mechanistic results and conclusions of work with hexavalent chromium in cultured cells have been determined after acute short-term exposures to high chromium concentrations, ranging from 10 – 50 μM. This includes our own work describing the modifications of chromatin and histone marks presented earlier [18;19]. Yet, we have shown that results at low (below 1 μM) and high (above 10 μM) concentrations are very different in regards to clonogenic responses and gene regulation [19]. To develop an environmentally relevant gene expression baseline we grew mouse hepatoma Hepa-1 cells for 15 passages in the presence of very low sodium dichromate dehydrate concentrations, namely, 0, 0.05 μM and 0.25 μM Cr(VI). At passage 5 and 15, we treated half the culture with 1 μM BaP and the other half with vehicle and 8 h later we extracted total RNA from all cultures and measured the expression of a number of genes (drug metabolism, cell cycle, apoptosis, DNA damage) by real-time RT-PCR. After 5 passages, Cr-treated and untreated cells show little difference and the relative levels of expression in Cr+BaP-treated cells compared with just BaP treatment are practically identical. In contrast, after 15 passages in the presence of Cr, several apoptosis-related genes become up-regulated by both Cr concentrations and there is a robust response to BaP in Cr-treated cells as compared to only BaP treatment (Fig. 1). Interestingly, only the drug metabolism genes (Cyp1a1, Nqo1 and Aldh3.1) show a large inhibition of their response to BaP, greater than 26=64-fold for Cyp1a1, in cells grown in Cr. All other genes tested, including cell cycle/tumor suppressor-, apoptosis-, and DNA damage-related genes were significantly up-regulated. These data, at Cr concentrations well below the 100 ppb limit in drinking water, suggest that Cr causes persistent changes in the response of cells to a BaP challenge, and prompted us to explore a similar experimental paradigm in vivo, using mice exposed to low Cr doses in drinking water.
Figure 1. mRNA expression levels in Mouse hepatoma Hepa-1 cells grown in the presence of 0.25 μM K2CrO4 for 5 or 15 passages and then treated with 1 μM BaP or vehicle for 8 h.
The ordinate denotes the ratio of mRNA levels in cells treated with Cr or Cr+BaP relative to the levels in cells propagated for the same number of generations in the absence of Cr.
Long-term low-dose Cr(VI) exposure of mice
To determine whether persistent low-dose Cr(VI) exposure to mice would have a comparable effect on gene expression as determined in cultured cells, we exposed mice for 2 months to 0, 50 ppb, 500 and 5000 ppb in drinking water, the middle two doses corresponding to one-half and two and one-half the USEPA Cr(VI) limit of 100 ppb in drinking water. After the 2-month exposure, mice were inoculated with 50 mg/kg BaP or vehicle for 24 h. RNA prepared from these mice was examined by real-time RT-PCR for expression of an additionally set of drug metabolism genes that included Cyp1b1, Cyp1a2 and Gsta1 and some of the same genes measured in cultured cells, including Cyp1a1, Nqo1, Aldh3.1, the pro-apoptotic Tap73 variant, Apaf1 and Tnfa. To address the potential of uncovering specific repressive effects of Cr(VI) on expression of tumor suppressor genes, we also included p15, p16, p18, p19, p21, and p27 in this screen. Fig. 2 shows the ratio of mRNA levels in livers of exposed mice relative to levels of the same genes in livers of unexposed mice (means of 3 mice per group). With the exception of Cyp1b1, expression of all drug metabolism genes was unaffected by Cr(VI) exposure alone in the absence of BaP and was significantly repressed when mice were also exposed to BaP relative to their expression in mice exposed to BaP in the absence of Cr. Expression of Cyp1b1 was the exception to these observations, being induced both by Cr and more so by Cr+BaP, whereas expression of the proapototic genes, as in cultured cells, was unaffected by Cr and induced by Cr+BaP (Fig. 2A). Note that Cyp1b1 expression is not inducible in Hepa-1 cells, hence this finding in vivo did not have a counterpart in vitro. Unlike in cells, expression of all tumor suppressor genes was clearly inhibited by Cr (Fig. 2B) and by Cr+BaP (Fig. 2C) in a dose-dependent manner, as compared to mice not exposed to Cr.
Figure 2. mRNA expression levels in livers of mice exposed to the indicated doses of Cr(VI) (as Sodium chromate dihydrate) in drinking water for 8 weeks followed by corn oil control or 50 mg/kg BaP for 24 h.
(A) mRNA levels for the genes shown in the abscissa. (B) mRNA of tumor suppressor genes after 8 weeks of the indicated Cr(VI) doses. (C) Same Cr(VI) as in (A) but treated with 50 mg/kg BaP for 24 h.
Discussion
Few and often contradictory data are found in the existing literature regarding the combined effects of metals and PAHs such as BaP, highlighting the need for additional studies to understand the parameters driving these responses. We and others have shown that epigenetic modification of gene expression is a key element of the developmental and carcinogenic outcomes of exposure to chromium, alone or in combination with PAHs [16–18]. In this context, regulation of the expression of tumor suppressor genes is a major target of chromium toxicity, with several studies reporting the association between aberrant p16ink4a methylation with chromium exposure and smoking [31;32], in itself a major source of co-exposure to chromium and BaP. Our findings in mice showing the repression of tumor suppressor genes (vide supra) are consistent with the hypermethylation-dependent silencing of the p16ink4a promoter found in one third of chromium-exposed workers who developed lung cancer, whether smokers or non-smokers [33]. Because p16ink4a promoter methylation accounts for only one third of cancer in this population, it is obvious that there may be additional chromium targets and mechanisms underlying chromium-induced toxicity, including perhaps promoter hypermethylation of other tumor suppressor genes, microRNA silencing or activation of histone modifying enzymes or DNA methyltransferases.
Based on the considerations discussed above, we can point at a number of innovative characteristics of the research approach used here. First, by studying interactions between pathways, rather than between toxicants, we create a new paradigm to study the health effects of complex mixtures that considerably simplifies the magnitude of the studies. Second, by addressing exclusively long-term, low-dose effects of exposure, we are in the forefront of environmentally relevant studies away from the less significant work using high acute doses or concentrations, questionably relevant to human exposures. Third, by integrating molecular, epigenetic and signal transduction mechanistic analyses of the toxicity of complex mixtures, we will be able to develop a thorough state-of-art experimental approach. This approach will lead to an understanding of operative mechanisms that will allow us to expand the range of possible biomarkers of disease incidence, prevention and diagnosis, from DNA methylation and histone code patterns to signal transduction kinase inhibitors and the regulation of detoxification genes.
Acknowledgments
This research was supported by NIEHS grants R01 ES06273 and R01 ES10807. J.L.O. was supported by NIEHS Training Grant 5T32 ES016646 on Gene-Environment Interactions.
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sexton K, Beck BD, Bingham E, Brain JD, DeMarini DM, Hertzberg RC, O'Flaherty EJ, Pounds JG. Chemical mixtures from a public health perspective: the importance of research for informed decision making. Toxicology. 1995;105:429–441. doi: 10.1016/0300-483x(95)03240-g. [DOI] [PubMed] [Google Scholar]
- 2.Birnbaum LS, DeVito MJ. Use of toxic equivalency factors for risk assessment for dioxins and related compounds. Toxicology. 1995;105:391–401. doi: 10.1016/0300-483x(95)03237-a. [DOI] [PubMed] [Google Scholar]
- 3.Collins JF, Brown JP, Alexeeff GV, Salmon AG. Potency equivalency factors for some polycyclic aromatic hydrocarbons and polycyclic aromatic hydrocarbon derivatives. Regul Toxicol Pharmacol. 1998;28:45–54. doi: 10.1006/rtph.1998.1235. [DOI] [PubMed] [Google Scholar]
- 4.Cizmas L, McDonald TJ, Phillips TD, Gillespie AM, Lingenfelter RA, Kubena LF, Phillips TD, Donnelly KC. Toxicity characterization of complex mixtures using biological and chemical analysis in preparation for assessment of mixture similarity. Environ Sci Technol. 2004;38:5127–5133. doi: 10.1021/es035287p. [DOI] [PubMed] [Google Scholar]
- 5.IARC. Chromium, nickel and welding. International Agency for Research on Cancer; Lyon, France: 1990. Evaluation of the carcinogenic risk to humans. [Google Scholar]
- 6.Hamilton JW, Wetterhahn KE. Differential effects of chromium(VI) on constitutive and inducible gene expression in chick embryo liver in vivo and correlation with chromium(VI)-induced DNA damage. Mol Carcinog. 1989;2:274–286. doi: 10.1002/mc.2940020508. [DOI] [PubMed] [Google Scholar]
- 7.Salnikow K, Zhitkovich A, Costa M. Analysis of the binding sites of chromium to DNA and protein in vitro and in intact cells. Carcinogenesis. 1992;13:2341–2346. doi: 10.1093/carcin/13.12.2341. [DOI] [PubMed] [Google Scholar]
- 8.Xu J, Manning FC, Patierno SR. Preferential formation and repair of chromium-induced DNA adducts and DNA--protein crosslinks in nuclear matrix DNA. Carcinogenesis. 1994;15:1443–1450. doi: 10.1093/carcin/15.7.1443. [DOI] [PubMed] [Google Scholar]
- 9.Quievryn G, Messer J, Zhitkovich A. Lower mutagenicity but higher stability of Cr-DNA adducts formed during gradual chromate activation with ascorbate. Carcinogenesis. 2006;27:2316–2321. doi: 10.1093/carcin/bgl076. [DOI] [PubMed] [Google Scholar]
- 10.Reynolds M, Stoddard L, Bespalov I, Zhitkovich A. Ascorbate acts as a highly potent inducer of chromate mutagenesis and clastogenesis: linkage to DNA breaks in G2 phase by mismatch repair. Nucleic Acids Res. 2007;35:465–476. doi: 10.1093/nar/gkl1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salnikow K, Zhitkovich A. Genetic and epigenetic mechanisms in metal carcinogenesis and cocarcinogenesis: nickel, arsenic, and chromium. Chem Res Toxicol. 2008;21:28–44. doi: 10.1021/tx700198a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ha L, Ceryak S, Patierno SR. Chromium (VI) activates ataxia telangiectasia mutated (ATM) protein. Requirement of ATM for both apoptosis and recovery from terminal growth arrest. J Biol Chem. 2003;278:17885–17894. doi: 10.1074/jbc.M210560200. [DOI] [PubMed] [Google Scholar]
- 13.Ha L, Ceryak S, Patierno SR. Generation of S phase-dependent DNA double-strand breaks by Cr(VI) exposure: involvement of ATM in Cr(VI) induction of gamma-H2AX. Carcinogenesis. 2004;25:2265–2274. doi: 10.1093/carcin/bgh242. [DOI] [PubMed] [Google Scholar]
- 14.Xu J, Manning FC, O'Brien TJ, Ceryak S, Patierno SR. Mechanisms of chromium-induced suppression of RNA synthesis in cellular and cell-free systems: relationship to RNA polymerase arrest. Mol Cell Biochem. 2004;255:151–160. doi: 10.1023/b:mcbi.0000007271.53241.ae. [DOI] [PubMed] [Google Scholar]
- 15.Wakeman TP, Kim WJ, Callens S, Chiu A, Brown KD, Xu B. The ATM-SMC1 pathway is essential for activation of the chromium[VI]-induced S-phase checkpoint. Mutat Res. 2004;554:241–251. doi: 10.1016/j.mrfmmm.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Shumilla JA, Broderick RJ, Wang Y, Barchowsky A. Chromium(VI) inhibits the transcriptional activity of nuclear factor- kappaB by decreasing the interaction of p65 with cAMP-responsive element-binding protein-binding protein. J Biol Chem. 1999;274:36207–36212. doi: 10.1074/jbc.274.51.36207. [DOI] [PubMed] [Google Scholar]
- 17.O'Hara KA, Nemec AA, Alam J, Klei LR, Mossman BT, Barchowsky A. Chromium (VI) inhibits heme oxygenase-1 expression in vivo and in arsenic-exposed human airway epithelial cells. J Cell Physiol. 2006;209:113–121. doi: 10.1002/jcp.20710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schnekenburger M, Talaska G, Puga A. Chromium cross-links histone deacetylase 1-DNA methyltransferase 1 complexes to chromatin, inhibiting histone-remodeling marks critical for transcriptional activation. Mol Cell Biol. 2007;27:7089–7101. doi: 10.1128/MCB.00838-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen L, Ovesen JL, Puga A, Xia Y. Distinct contributions of JNK and p38 to chromium cytotoxicity and inhibition of murine embryonic stem cell differentiation. Environ Health Perspect. 2009;117:1124–1130. doi: 10.1289/ehp.0800157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klein CB. Carcinogenicity and Genotoxicity of Chromium. In: Chang LW, editor. Toxicology of Metals. CRC Press Inc; Boca Raton, FL: 1996. pp. 205–219. [Google Scholar]
- 21.Alcedo JA, Misra M, Hamilton JW, Wetterhahn KE. The genotoxic carcinogen chromium(VI) alters the metal-inducible expression but not the basal expression of the metallothionein gene in vivo. Carcinogenesis. 1994;15:1089–1092. doi: 10.1093/carcin/15.5.1089. [DOI] [PubMed] [Google Scholar]
- 22.McCaffrey J, Wolf CM, Hamilton JW. Effects of the genotoxic carcinogen chromium(VI) on basal and hormone- inducible phosphoenolpyruvate carboxykinase gene expression in vivo: correlation with glucocorticoid- and developmentally regulated expression. Mol Carcinog. 1994;10:189–198. doi: 10.1002/mc.2940100403. [DOI] [PubMed] [Google Scholar]
- 23.Maier A, Schumann BL, Chang X, Talaska G, Puga A. Arsenic co-exposure potentiates benzo[a]pyrene genotoxicity. Mutat Res. 2002;517:101–111. doi: 10.1016/s1383-5718(02)00057-8. [DOI] [PubMed] [Google Scholar]
- 24.Manning FC, Xu J, Patierno SR. Transcriptional inhibition by carcinogenic chromate: relationship to DNA damage. Mol Carcinog. 1992;6:270–279. doi: 10.1002/mc.2940060409. [DOI] [PubMed] [Google Scholar]
- 25.Kim G, Yurkow EJ. Chromium induces a persistent activation of mitogen-activated protein kinases by a redox-sensitive mechanism in H4 rat hepatoma cells. Cancer Res. 1996;56:2045–2051. [PubMed] [Google Scholar]
- 26.Chen F, Ye J, Zhang X, Rojanasakul Y, Shi X. One-electron reduction of chromium(VI) by alpha-lipoic acid and related hydroxyl radical generation, dG hydroxylation and nuclear transcription factor-kappaB activation. Arch Biochem Biophys. 1997;338:165–172. doi: 10.1006/abbi.1996.9849. [DOI] [PubMed] [Google Scholar]
- 27.Ye J, Zhang X, Young HA, Mao Y, Shi X. Chromium(VI)-induced nuclear factor-kappa B activation in intact cells via free radical reactions. Carcinogenesis. 1995;16:2401–2405. doi: 10.1093/carcin/16.10.2401. [DOI] [PubMed] [Google Scholar]
- 28.Nebert DW, Puga A, Vasiliou V. Role of the Ah receptor and the dioxin-inducible [Ah] gene battery in toxicity, cancer, and signal transduction. Ann N Y Acad Sci. 1993;685:624–640. doi: 10.1111/j.1749-6632.1993.tb35928.x. [DOI] [PubMed] [Google Scholar]
- 29.Tesfai Y, Davis D, Reinhold D. Chromium can reduce the mutagenic effects of benzo[a]pyrene diolepoxide in normal human fibroblasts via an oxidative stress mechanism. Mutat Res. 1998;416:159–168. doi: 10.1016/s1383-5718(98)00072-2. [DOI] [PubMed] [Google Scholar]
- 30.Feng Z, Hu W, Rom WN, Costa M, Tang MS. Chromium(VI) exposure enhances polycyclic aromatic hydrocarbon-DNA binding at the p53 gene in human lung cells. Carcinogenesis. 2003;24:771–778. doi: 10.1093/carcin/bgg012. [DOI] [PubMed] [Google Scholar]
- 31.Jarmalaite S, Kannio A, Anttila S, Lazutka JR, Husgafvel-Pursiainen K. Aberrant p16 promoter methylation in smokers and former smokers with nonsmall cell lung cancer. Int J Cancer. 2003;106:913–918. doi: 10.1002/ijc.11322. [DOI] [PubMed] [Google Scholar]
- 32.Kim DH, Nelson HH, Wiencke JK, Zheng S, Christiani DC, Wain JC, Mark EJ, Kelsey KT. p16(INK4a) and histology-specific methylation of CpG islands by exposure to tobacco smoke in non-small cell lung cancer. Cancer Res. 2001;61:3419–3424. [PubMed] [Google Scholar]
- 33.Kondo K, Takahashi Y, Hirose Y, Nagao T, Tsuyuguchi M, Hashimoto M, Ochiai A, Monden Y, Tangoku A. The reduced expression and aberrant methylation of p16(INK4a) in chromate workers with lung cancer. Lung Cancer. 2006;53:295–302. doi: 10.1016/j.lungcan.2006.05.022. [DOI] [PubMed] [Google Scholar]