Abstract
Objective
We investigated the effect of various doses of limb segment load on soleus H-reflex amplitude and post activation depression in healthy individuals. We also explored the influence of limb segment load on spinal circuitry in one individual with chronic SCI.
Methods
28 healthy adults and one SCI subject received compressive loads applied to the top of their knee at varied doses of load (10%, 25%, and 50% of the body weight). Soleus H-reflexes were measured before (baseline) and during the loading phase.
Results
There were no significant differences in H-reflex amplitudes during the 50 % BW load-on phase as compared to either baseline session or the load-off phase. However, the post activation depression was decreased over 9% (p < 0.05) during the load-on phase compared to the load-off phase and scaled according to load (50%>25%>10%). The post activation depression ratio also appears less responsive to varying loads after chronic SCI.
Conclusions
Limb segment load decreases post-activation depression in humans. These findings suggest that the mechanism associated with post activation depression is modulated by limb segment load, and may be influenced by spinal reorganization after SCI.
Significance
Future studies will determine if various levels of spasticity modulate the response of limb segment load on post activation depression in those with acute and chronic SCI.
Keywords: Compression, homosynaptic depression, spinal neural modulation
INTRODUCTION
It is well known that spinal circuitry reorganizes over the ensuing months after a spinal cord injury (SCI), causing a velocity dependent increase in muscle stiffness (spasticity) that is not present early after the injury (Ashby et al., 1974; Calancie et al., 1993; Skinner et al., 1996; Hiersemenzel et al., 2000; Schindler-Ivens and Shields, 2000; Shields, 2002). Early mechanical loading interventions have successfully prevented the loss of bone, loss of muscle mass, and changes in spinal excitability in individuals with SCI (Shields and Dudley-Javoroski, 2006; Shields et al., 2006; Dudley-Javoroski and Shields, 2008a, b; Adams et al., 2011; Shields et al., 2011). Functional load through weight bearing activities plays a significant role in modulating spinal motor neuron excitability during human posture and movement. However, few studies have examined the effect of direct limb segment load on spinal motor neuron excitability.
Low-threshold cutaneous afferent receptors and joint mechanoreceptors are activated by mechanical stimuli during weight bearing activities. Limb load is an important mechanical stimulus that induces a sensory feedback volley to control body dynamics during both static standing and walking (Andersen and Sinkjaer, 1999; Petersen et al., 1999; Trimble et al., 2001; Faist et al., 2006; Phadke et al., 2006; Huang et al., 2009; Knikou et al., 2009a, b). Although several studies suggest that skin and knee joint receptors modulate the alpha motor neuron pool during upright weight bearing tasks, the results remain wide-ranging (Ali and Sabbahi, 2000; Field-Fote et al., 2000; Kawashima et al., 2003; Nakazawa et al., 2004; Phadke et al., 2006; Hwang et al., 2011). Specifically, some authors report a systematic decrease in motor neuron excitability in response to increments of body-weight loads (Kawashima et al., 2003; Nakazawa et al., 2004; Hwang et al., 2011); whereas others report no effect of body-weight load (Ali and Sabbahi, 2000; Field-Fote et al., 2000; Phadke et al., 2006). During weight bearing tasks, supra spinal systems are active at various levels (Iles and Pisini, 1992a, b; Lowrey and Bent, 2009). A short latency pre-synaptic inhibition (400 ms) to the motor neuronal pool is one mechanism thought to contribute to the modulation of the H-reflex pathway during whole body weight bearing studies (Iles, 1996; Knikou, 2007).
We developed a method to deliver compressive loads to the lower extremity while minimizing supra spinal drive and controlling for central set to assess if limb load directly modulates spinal reflex excitability. Our long term goal is to understand spinal reflex modulation in response to physiologically applied mechanical loads in people with CNS impairment. However, in this study we examine the effect of limb segment mechanical load in people without CNS impairment, and contrast these findings with a single subject with SCI. Post activation depression, a phenomenon responsible for long-lasting H-reflex depression via a pre-synaptic inhibitory pathway (Crone and Nielsen, 1989; Hultborn et al., 1996; Kohn et al., 1997), is present in able-bodied humans, but lost in humans with long term spinal cord injury (Schindler-Ivens and Shields, 2000; Grey et al., 2008). The impact of isolated repetitive mechanical loads on reflex modulation has important implications as new strategies are developed to improve the health of paralyzed extremities (Shields and Dudley-Javoroski, 2006).
The purpose of this study is to 1) determine the reproducibility of limb segment load on alpha motor neuron excitability, 2) establish the relationship between post activation depression and limb segment load in healthy adults, and 3) explore the influence of limb segment load on spinal excitability in an individual with a chronic, reorganized spinal cord, from a longstanding injury. We hypothesize that limb segment load will decrease alpha motor neuron excitability and decrease post activation depression and the response will be sensitive to the dose of the compressive load. We also hypothesize that the modulating capacity of load will be lost as a result of chronic spinal cord reorganization after SCI.
METHODS
Subjects
A total of 28 healthy adults participated in the study. In the first experiment, 13 subjects aged 22 – 45 years (mean and SD, 29.8 ± 6.9 years; five females and eight males) were tested using a single level of compressive load to determine the reproducibility of the unloaded condition. In the second experiment, 15 subjects aged 22 – 46 years (26.1 ± 6.1 years; eight females and seven males) and one individual with chronic SCI (aged 26 years; T8/complete lesion; ASIA A; 15 years post injury) were recruited to examine the modulation of soleus H-reflex by various levels of load. All able bodied subjects enrolled in the study had no known acute or ongoing orthopedic, neuromuscular, or neurological deficits or disorders. Each individual gave informed consent before participation and the University of Iowa Human Subjects Institutional Review Board approved the study.
Paradigm
Experiment 1
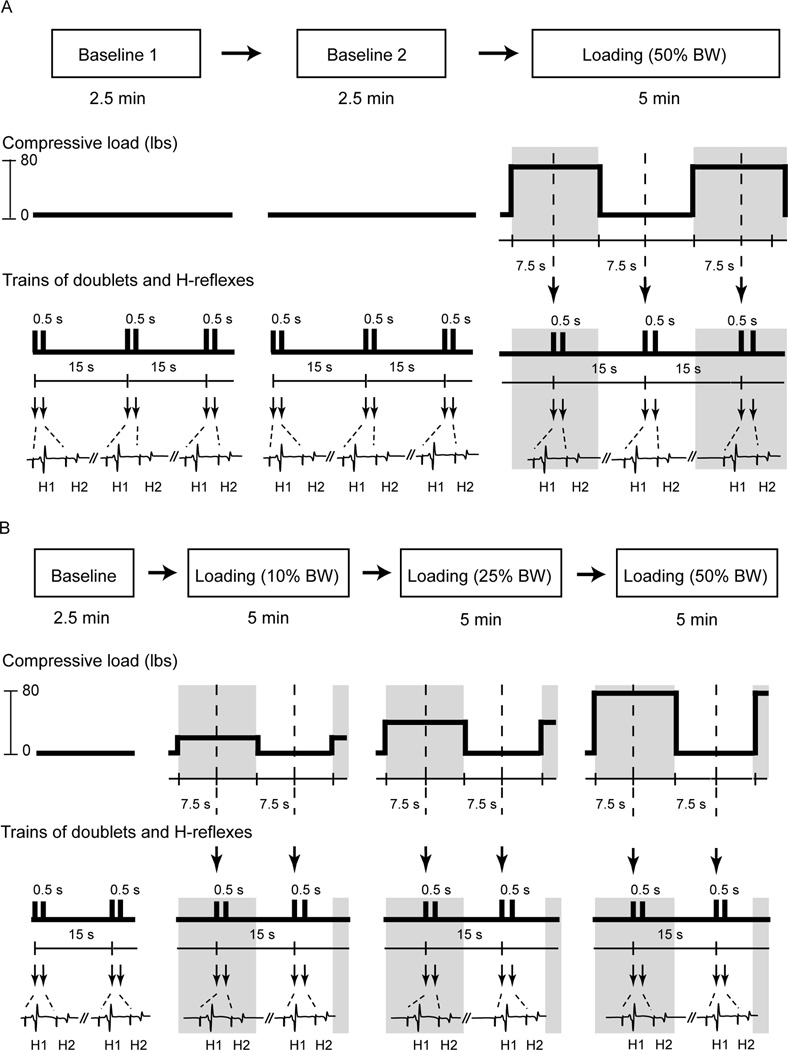
The study consisted of three testing sessions in order: two baseline sessions followed by a single loading session (Top panel, Figure 1A). Each baseline recording was separated by 5 minutes. A one-minute rest period was given before proceeding to the loading session. The purpose of the repeated baseline testing was to examine the stability of the soleus H-reflex responses within each individual prior to introducing the loading stimuli. Paired-pulse electrical stimulations (doublets) with inter-pulse intervals of 500 ms were delivered every 15 seconds to elicit H-reflexes across all three sessions (Bottom panel, Figure 1A). In each baseline session, there was no compressive load applied on the lower leg segment and 10 doublets were delivered to establish baseline H-reflexes (Middle panel, Figure 1A). After completion of two baseline H-reflex measures, a compressive force, equivalent to 50% of the individual’s body weight, was applied over top of the right knee for 15 seconds (load-on phase; gray areas, Figure 1A) followed by a 15-second rest period (load-off phase; White areas, Figure 1A). The doublet was always delivered exactly in the middle of each phase in which the compressive force was continuously initiated or terminated for 7.5 seconds (dash lines, Figure 1A). Thus, the loading session involved ten 30-second cycles constituted by ten 15-second loading phases and ten 15-second recovery phases. As such, subjects experienced a constant compressive force intermittently applied on their right lower leg segments throughout the entire loading session. Hence, each subject underwent three testing sessions where 40 doublet activations were delivered in total: 20 doublets before loading stimuli (10 doublets in each, two baseline sessions) and 20 doublets during loading stimuli (10 doublets in each, two phases in the loading session).
Figure 1. Illustrations of study paradigm, cyclically compressive loads, and electrical stimulation protocols.
The first experiment consisted of two baseline and one loading session (A). The second experiment consisted of one baseline session and three loading sessions (B). The middle panels illustrate the amplitudes and timing of the compressive load applied to the subject’s leg across all testing sessions. The bottom traces depict soleus muscle activity and H-reflexes elicited by doublets over time (in A and B). Arrows indicate the timing when electrical stimuli were delivered to the tibial nerve. H1: test reflex; H2: conditioned reflex, BW: body weight.
Experiment 2
Because baseline H-reflexes were stable, we were confident we could deliver various doses of load to establish a dose (load)-response (H-reflex) relationship. We studied the relationship between post activation depression and varied levels of limb segment loads. Therefore, the second study occurred on a separate day with a different cohort of subjects. The protocol was comprised of a baseline session followed by three loading sessions (top panel, Figure 1B). Each session was separated by one minute. Each loading session consisted of one of three pre-determined compressive forces: 10%, 25%, and 50% of the individual’s body weight (middle panel, Figure 1B). The order of the three loading sessions was random. The protocol always tested the soleus H-reflexes in a 2.5-minute baseline session (i.e. no compressive force) followed by three loading sessions in a random order (bottom panel, Figure 1B). Each subject underwent four testing sessions where 70 doublets were delivered in total: 10 doublets in a baseline session and 60 doublets during three loading sessions (10 doublets in each phase, two phases in each loading session, and three loading sessions).
Instrumentation
Bipolar Ag-AgCl surface electrodes (8 mm in diameter, with a fixed inter-electrode distance of 20 mm) were used to record H-reflexes and M waves from the soleus muscle of the right leg. Electromyographic (EMG) signals were on-site preamplified with a gain of 35. The EMG signals were then further amplified by a GCS 67 differential amplifier (Therapeutics Unlimited, Iowa City, IA) with a gain range from 1000 to 10,000. The differential amplifier had an input impedance of 15 MΩ at 100Hz, a frequency response of 15 to 1000Hz, a common mode rejection ratio of 87 dB at 60 Hz, and a bandwidth of 20 to 4000Hz.
A constant-current electrical stimulator (DS7A, Digitimer Ltd, Welwyn Garden City, Herts, UK) with a constant current range from 50µA to 1A and a total output capacity of 400V was used to deliver square waves with a pulse width of 1000µs. The stimulator was triggered by a digital pulse from a data acquisition board (USB-6259, National Instruments Corp., Austin, TX) controlled by custom LabVIEW software (National Instruments, Austin, TX). Through custom LabVIEW programming, the stimulator delivered paired-pulse electrical stimulation (doublets) with inter-pulse intervals of 500 ms every 15 seconds. Stimulation was administered via a single surface probe (cathode, 1 cm in diameter) secured over the tibial nerve in the right popliteal fossa. The anode was positioned (10 × 10 cm) over top of the femoral condyles as the dispersive electrode.
Servo-Controlled Limb Loading
The custom designed servo-controlled system utilized an air compressor (Super Silent 20A, Silentaire Tech, Houston, TX), an electronic pressure regulator (550X Miniature E/P Transduder, ControlAir Inc, Amherst, NH), a pneumatic cylinder (Clippard USA Corp, Cincinnati, OH), a load cell (1210, Interface, Scottsdale, AZ), and custom-built integrated circuit to control the system under software control. The design allows precise control of the duration and amplitude of a compressive load over the lower leg for a pre-determined number of cycles. The compression control system generated the compressive loads in less than 500 milliseconds. In addition, the compression control system employed a unique safety mechanism in which the pressure was continuously monitored by a pressure switch (Solon Manufacturing Co, Chardon, OH) and was allowed to shut off instantaneously when necessary. The pneumatic cylinder was positioned directly over the knee in a seated position and programmed to deliver the compressive load.
Data Collection
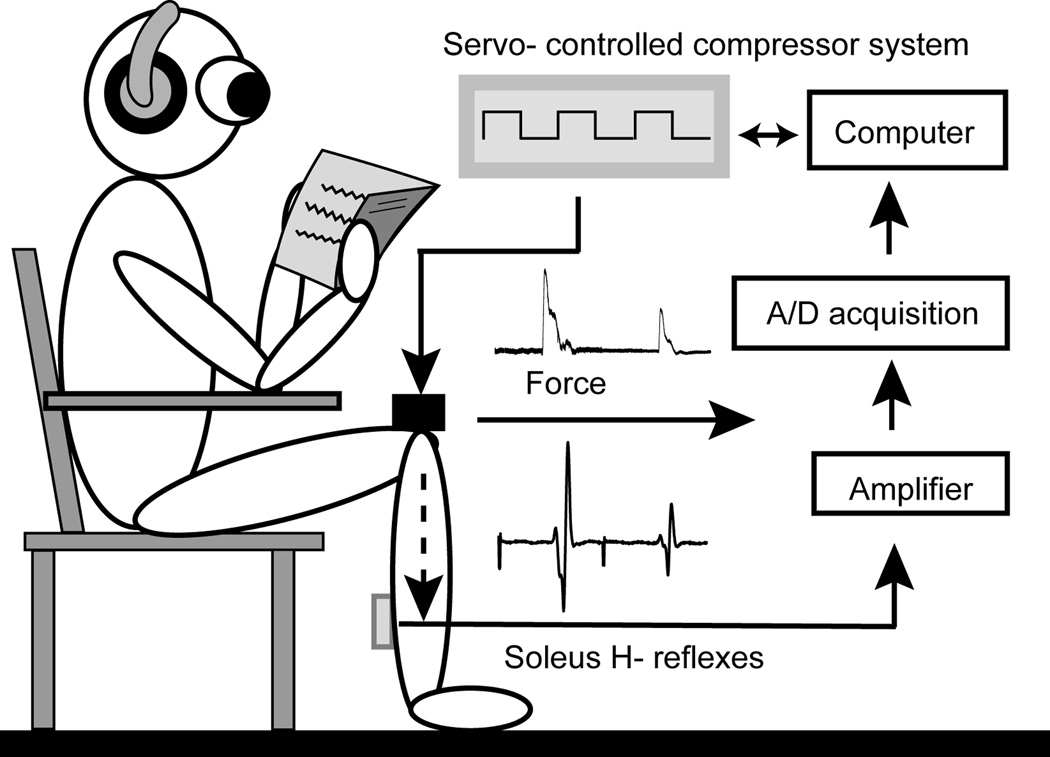
Subjects sat on a custom designed chair with the hip and knee flexed at 120° and the ankle in the neutral position (Figure 2). The testing leg was secured by a Velcro strap around the foot to maintain a fixed position on the platform attached to the compressor system. It is known that the excitability of motor neurons is influenced by central set, characterized as the “preparatory set” within the central nervous system before execution of a motor act (Frank, 1986; Meunier and Pierrot-Deseilligny, 1989; Leis et al., 1995). The central set is affected by many factors such as environmental noise (Delwaide and Schepens, 1995; Kolev and Milanov, 1995), the level of arousal and anxiety(Ribot-Ciscar et al., 2000; Rossi-Durand, 2002; Sibley et al., 2007), body position (Hayashi et al., 1992; Mynark and Koceja, 1997; Phadke et al., 2006), and sensory inputs (Iles and Pisini, 1992b; Hoffman and Koceja, 1995; Iles, 1996; Knikou, 2007; Conway and Knikou, 2008). To minimize the influence from supra spinal and vestibular systems on motor neuron excitability, subjects were instructed to relax and sit quietly throughout the entire experiment. To eliminate the noise from the compressor system and maintain a similar level of auditory feedback across all testing sessions, subjects wore ear plugs, a headset, and engaged in light reading. The skin over the soleus muscle was abraded with sandpaper and cleaned with alcohol swabs. The recording EMG electrode was placed 2 cm lateral to midline of the posterior calf and 2 cm distal to the lowest end of the palpable border of the gastrocnemius muscle. A reference electrode was placed on the anterior aspect of the tibia of the right leg.
Figure 2. Lateral view of the experimental setup.
An air cylinder connected to a force transducer was placed on the top of the right knee joint and aligned perpendicularly to the longitudinal axis of the lower leg to deliver a cyclically compressive load (gray panel); the force transducer then recorded the net force acting on the lower leg.
For soleus H-reflex measures, the tibial nerve was electrically activated via a stimulating electrode positioned in the popliteal fossa. The location for the stimulating electrode was determined by the conditions whereby H-reflexes were elicited without M waves at very low stimulation intensities and M waves appeared as increasing stimulation intensity. The maximal M wave (M-max) was first defined as the peak-to-peak value at the time when the M wave amplitude failed to increase with increasing stimulation current. To ensure the tibial nerve was activated supra maximally, the stimulation intensity was then adjusted to a value greater than 120% of the last current intensity, and at least three pulses were delivered. To compare the H-reflex depression elicited by doublets across sessions, the stimulation intensity was adjusted to the level that elicited a stable soleus H-response equivalent to ~50% of the maximal H-reflex response. The H-reflexes corresponded to 25 – 35% of the M-max. All data were recorded at 4000 Hz using custom LabVIEW software and time-synchronized with the compressor system.
Data Analysis
All analyses were conducted using Custom Matlab software (MathWorks, Natick, MA). Offline, raw EMG signals were first zero offset prior to any advanced processing. The paired H-reflexes were separated by 500-ms and defined as the test H-reflex (H1) and conditioned H-reflex (H2) (bottom panels, Figure 1A and 1B). The peak-to-peak amplitude of each H-reflex was then computed and expressed as a percentage of M-max across all available trials for each testing session. To quantify post-activation depression of soleus H- reflexes, we calculated the depression ratio by dividing the amplitude difference between the test H1 reflex and the conditioned H2 reflex divided by the test H1 reflex amplitude. A depression ratio of 0 indicated that no post-activation depression occurred; whereas a ratio of 1.0 indicated that the greatest depression occurred such that the decrement of H2 was equal to the H1 amplitude.
In order to evaluate the reproducibility of the H-reflexes, we evaluated two baseline measures (H1 and depression ratios) prior to the loading session. In addition, to monitor changes in neural excitability at the peripheral site across all testing sessions, background soleus muscle activity was quantified by calculating the root-mean square (RMS) over a 50-ms window prior to each stimulus. The RMS values were then expressed as a percentage of the maximal M-wave. To quantify the modulation of the soleus H-reflex corresponding to cyclical compressive loads, H-reflex amplitudes and depression ratios were computed independently during each Load-on phase and Load-off phase within each loading cycle. Thus, we could determine whether the modulation of soleus motor neuron excitability coincides with the presence of load stimuli cyclically.
The test H1 reflex, conditioned H2 reflexes, and the depression ratios obtained during the three loading sessions were normalized to baseline values and expressed as ratios. This allowed us to normalize across the various load levels. A ratio greater than 1.0 indicates that the depression ratio is increased during the load session as compared to the baseline session. A ratio less than 1.0 indicates that the depression ratio is decreased during the load session as compared to the baseline session. A ratio of 1.0 indicates that the H-reflex amplitude or depression ratio during the load-on phase is unchanged from the baseline. All variables were first averaged across all available trials from each subject and then were averaged over all subjects to create group means for each session.
Statistical Analysis
Statistical comparisons were made using SAS/STAT software (SAS, Cary, NC, USA). A one-way mixed model ANOVA with repeated measures on one factor (time) was used. When the ANOVA was significant, post hoc analyses were performed using Tukey’s honest significant difference test. The level for statistical significance was set at P < 0.05. Correlation Analysis was carried out to determine the relationship between load and depression ratio.
RESULTS
Individual traces of H reflexes elicited by doublet stimuli across the baseline session, the load-on phase, and load-off phase during the loading session are depicted in Figure 3 from a typical subject. Stable M-waves were observed throughout the entire test supporting that stimulation conditions remained constant. Also noteworthy is that the test H1 amplitudes were consistent across all testing sessions (the first H-reflex shown in each overlaying trace; Figure 3A– 3C, respectively). It is clear that the conditioned H2 amplitudes were less suppressed (Gray zone, Figure 3B) during the load-on phase (50% BW load) as compared to the baseline and load-off phase, despite stable stimulation conditions (M-waves).
Figure 3. M-waves and H-reflexes from a typical participant across a baseline session, load-on, and load-off session.
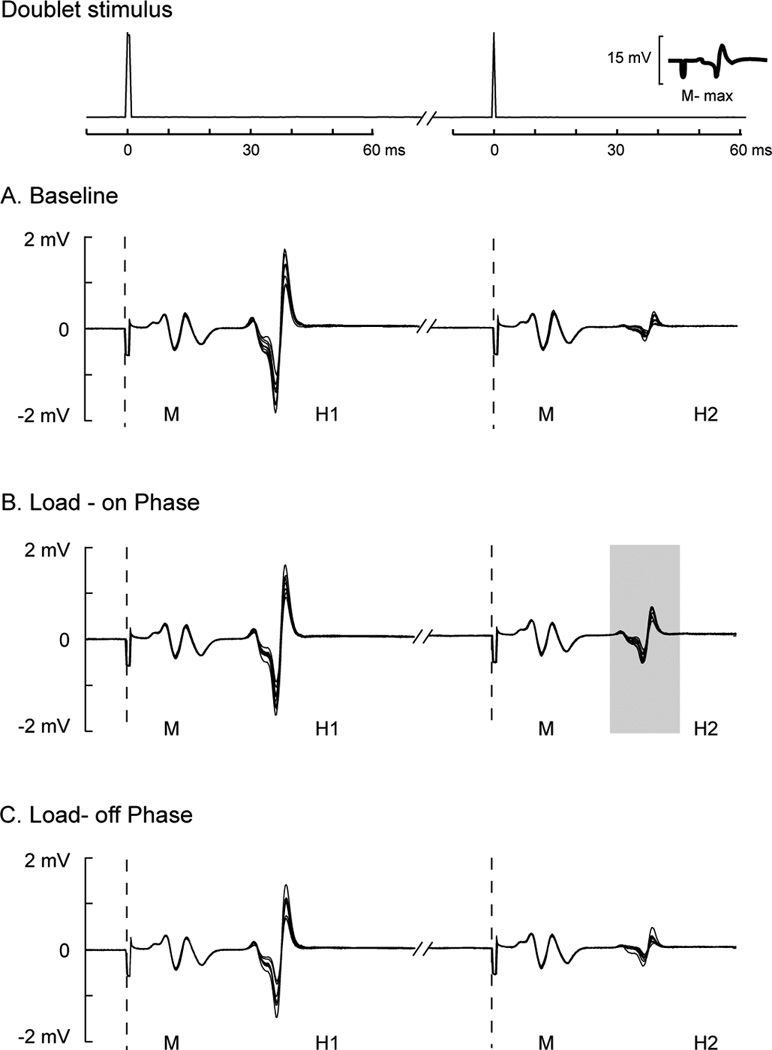
Top panels show time series data of paired-pulse electrical stimulation (doublet) in which voltage signals were plotted 10 ms before and 60 ms after each electrical stimulus was delivered. The amplitude of the maximal M-wave (M-max) was overlaid on the bottom right in the top panel as a reference. Dashed vertical lines indicate the time when each electrical stimulus was delivered for each doublet. Ten traces of M-waves (M) and H-reflexes (H1: test reflex and H2: conditioned reflex) repeatedly elicited by doublets were overlaid across baseline (A), load-on (B), and load-off (C) phases. BW: body weight.
Experiment 1
Similar trends were also observed in group averages of H-reflex amplitudes across different sessions (Figure 4A). The average test H1 amplitude was similar across all sessions, which ranged from 29.7% to 33.5% of M-max. By comparing across sessions, there was no significant effect of time (P = 0.36) on the test H1 amplitude (black bars, Figure 4A). Interestingly, there was a significant effect of time (P = 0.007) on the conditioned H2 amplitude (white bars, Figure 4B). The average conditioned H2 amplitude during the Load-on phase was significantly greater than the two baseline sessions (post-hoc, P = 0.01 and P = 0.03, respectively); whereas the conditioned H2 amplitude during the load-off phase was indifferent from two baseline sessions (post-hoc, P = 0.23 and P = 0.46, respectively).
Figure 4. Experiment 1: Average H-reflexes (A), depression ratios (B), and background soleus muscle activity across different sessions (C).
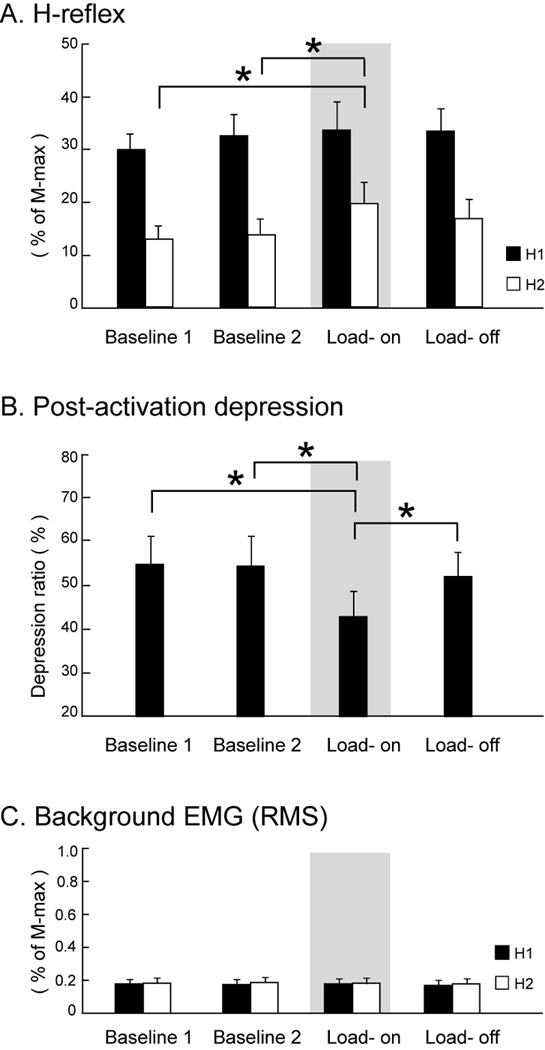
Gray areas indicate the testing condition in which the compressive load equal to 50% of the individual’s body weight was applied (Load-on phase). Error bars, ± 1 SEM. Asterisks indicate significant post-hoc differences between conditions. H1: test reflex; H2: conditioned reflex; RMS: root-mean-square.
The depression ratio also showed a significant main effect of time (P = 0.006) when comparing across different sessions (Figure 4B). The average depression ratio was comparable between two baseline sessions (54.8% and 54.2%, respectively). In contrast, the average depression ratio changed in accordance with the loading cycle: the extent of depression was less during the load-on phase (Gray area; Figure 4B) compared to the load-off phase (42.8% and 51.8%, respectively; post-hoc, P = 0.038). The depression ratio during the load-on phase was also significantly smaller than two baseline sessions (post hoc, P = 0.01 and P = 0.02, respectively) whereas the depression ratio in the load-off phase was indifferent from baseline sessions (post hoc, P = 0.82 and P = 0.9, respectively).
The analysis of average background muscle activity confirmed that altered descending neural drive did not influence the post-activation depression. The average RMS values were similar across baseline, load-on phase, and load-off phase, ranging from 0.17% to 0.19% of the M-max (Figure 4C). Statistically, background muscle activity was not different when compared across different sessions (P = 0.26 and P = 0.53 for test H1 and conditioned H2 measures, respectively). The persistent low-level of background muscle activity supported that post-activation depression was suppressed in the presence of minimal descending CNS drive and, therefore, attributable to the modulation of afferent inputs (i.e. loading).
Experiment 2
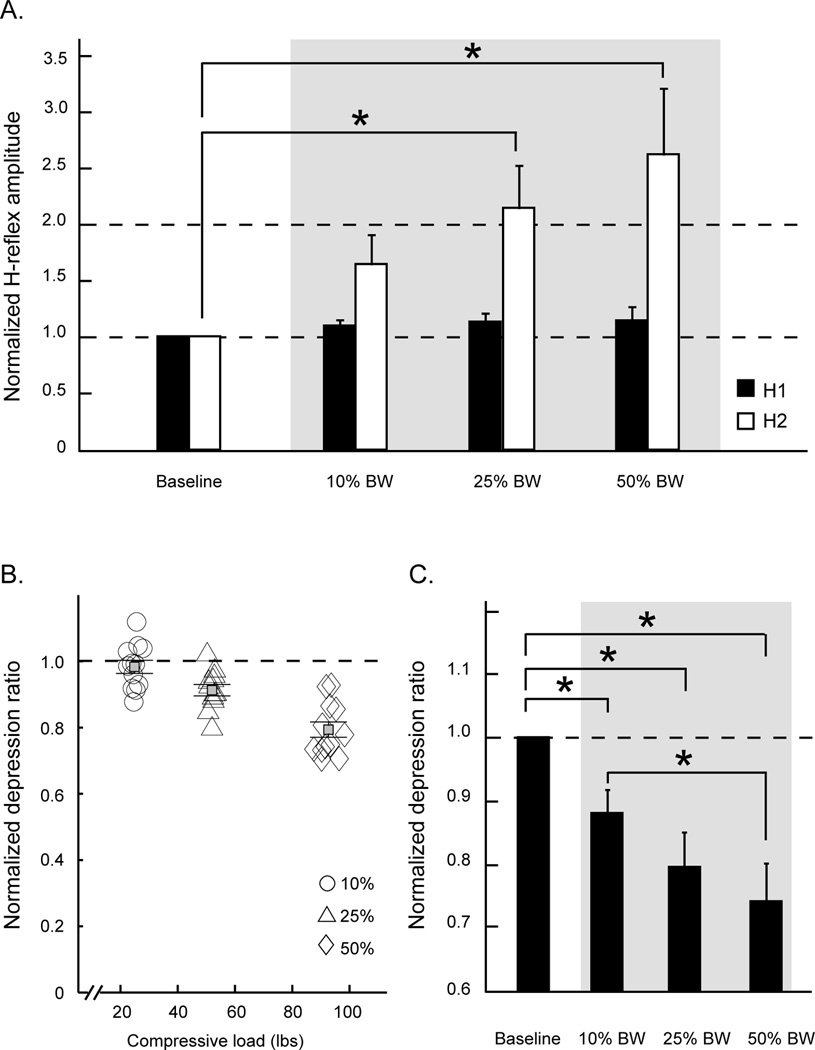
Figure 5A shows the relationship between the soleus H-reflex amplitudes and levels of compressive load. Notice that H1, H2, and the depression ratios obtained during three loading sessions were divided by their baseline mean values and expressed as ratios of the baseline means. There is a clear trend demonstrating that an increment of the compressive load systematically facilitates the conditioned H2 amplitude in healthy adults. Group averages of the conditioning stimulus, H1 amplitude, did not change across different loads. Statistically, there was no significant effect of load (P = 0.25) on the normalized test H1 amplitude (black bars, Figure 5A), but there was a significant effect of load (P = 0.001) on the normalized conditioned H2 amplitude (white bars, Figure 5A). The average conditioned H2 amplitude during the 25% and 50% BW load-on phase was significantly greater than the baseline session (post-hoc, P = 0.027 and P = 0.0009, respectively); whereas the test H1 amplitude during the 10% of the BW load-on phase was indifferent from the baseline session (post-hoc, P = 0.36).
Figure 5. Experiment 2: The relationship between the excitability of soleus motor neurons and compressive loads.
Group averages of normalized H-reflex amplitudes and normalized depression ratios at various levels of compressive forces are shown in A and C, respectively. Normalized depression ratios for all available trials at each level of the compressive force from a typical individual are illustrated in B. H-reflexes and depression ratios obtained during three loading sessions were divided by their baseline mean values and expressed as ratios of the baseline means. Gray areas indicate the loading conditions. H1: test reflex; H2: conditioned reflex; BW: body weight. Error bars, ± 1 SEM. Asterisks indicate significantly post-hoc differences between conditions.
Figure 5B illustrates a scatter plot of all normalized depression ratios across varied loads from a typical subject. A clear pattern of the data points on the scatter plot reveals a negative relationship between depression ratios and compressive loads. That is, the depression ratio systematically decreases with increments of load, and it decreases to a greater extent at the 50% of BW load. Group averages for depression ratios showed main effects for load (P < 0.0001; Figure 5C). Normalized depression ratios during three loading conditions were significantly smaller than the baseline (post-hoc, P = 0.04, P = 0.0001, and P < 0.0001 for 10%, 25%, and 50% of BW load, respectively). Post-hoc analysis also indicated that load influenced the excitability such that the normalized depression ratio was significantly smaller at 50% of BW load as compared to 10% of BW load (P = 0.01). There was a significant inverse correlation (Pearson) between load and depression ratio (r = − 0.46, P = 0.0002).
Individual with SCI
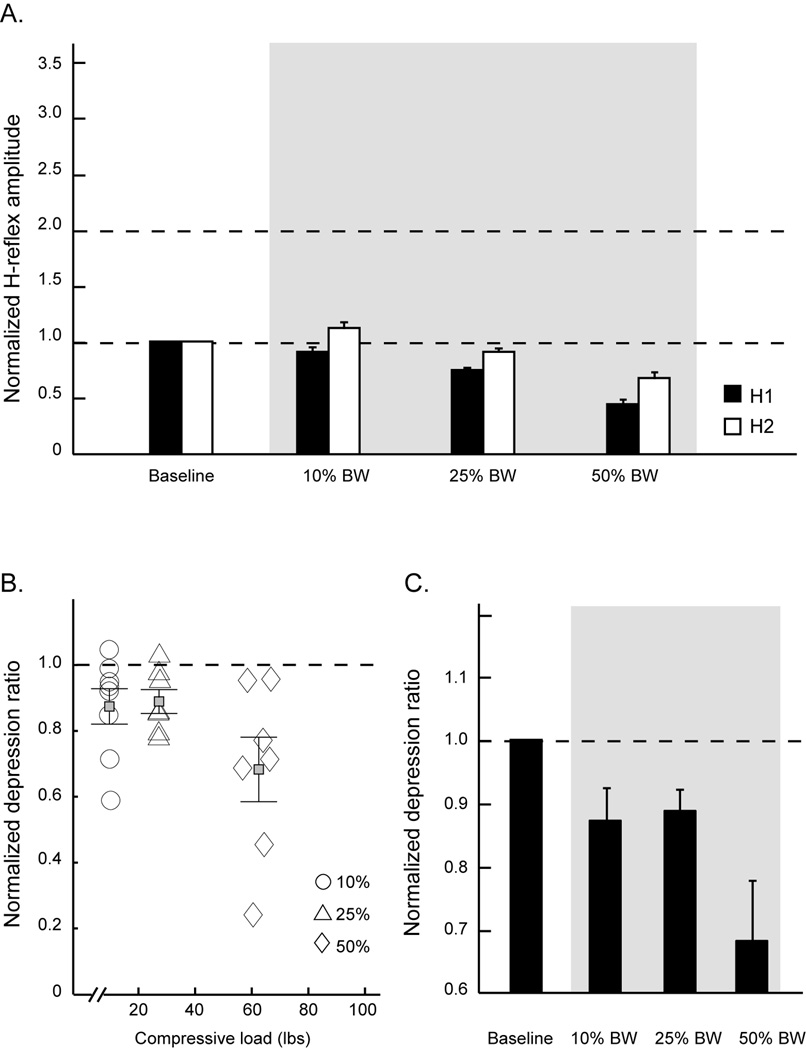
Figure 6 shows the mean H-reflex responses and depression ratios across baselines and three loading sessions from an individual with chronic SCI. There is a consistent trend demonstrating that an increment in the compressive load systematically inhibits both H1 and H2 amplitudes in the SCI subjects (Figure 6A, 6C). Importantly, the depression ratios are less responsive to different doses of loads (Figure 6B). Figure 6D illustrates a scatter plot of all normalized depression ratios across varied loads from this SCI subject. A clear pattern of the scatter plot reveals a negative relationship between depression ratios and compressive loads. That is, the depression ratio systematically decreases with increments of load, and it decreases to a greater extent at the 50% of BW load.
Figure 6. H-reflexes and post activation depression ratios from an individual with SCI.
Average normalized H-reflex amplitudes (A) and normalized depression ratios (C) during load-on phases across three levels of compressive forces for one subject with chronic SCI. B, the scatter plot of normalized depression ratios from all available trials at each level of the compressive force from the same SCI subject. Gray areas indicate the loading conditions. Error bars, ± 1 SEM. H1: test reflex; H2: conditioned reflex; BW: body weight.
Taken together, these findings support that isolated limb segment load modulates post-activation depression (homosynaptic inhibition) in healthy humans, but may be altered by the reorganization associated with chronic spinal cord injury.
DISCUSSION
In this study, we investigated the effects of lower limb segment load on spinal neuronal excitability in healthy individuals. The results from healthy adults showed that: (1) the test H1 amplitude was stable during unloaded and loaded conditions, before, during, or after episodes of load, (2) segmental load decreased post-activation depression to a conditioning stimulation, (3) post-activation depression was load dependent with less depression of the conditioned H2 at higher loads, and (4) the background soleus muscle activity from descending motor drive remained constant and presumably did not influence the modulation to load. The preliminary findings from one subject with SCI showed that: (1) limb segment load inhibits both the test H1 and conditioned H2 responses, and (2) the dose-response for the effect of load on post activation depression may be less in a reorganized spinal cord following chronic SCI. These findings suggest that limb segment load, induced through either cutaneous or joint receptors, decrease post activation depression. These findings have implications to those with SCI, who lose post activation depression as a result of spinal cord reorganization, several months following SCI. Importantly, post activation depression loss after SCI is associated with the level of spasticity (Grey et al., 2008).
In the absence of volitional muscle contraction, we demonstrated that segmental loading had no effect on soleus test H1 reflex amplitude in healthy humans. This finding is consistent with results from studies showing no modulation of soleus H-reflex to the level of body-weight loading during quiet standing (Ali and Sabbahi, 2000; Field-Fote et al., 2000; Phadke et al., 2006), but different from soleus H-reflex amplitude during a sitting or prone-lying position (Hayashi et al., 1992; Koceja et al., 1993; Angulo-Kinzler et al., 1998; Ali and Sabbahi, 2000; Kawashima et al., 2003; Nakazawa et al., 2004; Hwang et al., 2011). A reduction of the H-reflex amplitude during standing may be attributed to inhibitory effects derived from supra-spinal systems due to a change in body orientation, rather than absolute body-weight load (Hayashi et al., 1992; Mynark and Koceja, 1997; Phadke et al., 2006). The vestibular system interacts with multiple spinal inhibitory pathways during postural control, which may influence motor neuron pool excitability according to different postural positions (Aiello et al., 1983; Iles and Pisini, 1992a, b). Postural position is associated with increased background muscle activity and an increment of soleus H-reflex amplitude regardless of whether in the loaded or unloaded conditions (Hayashi et al., 1992; Angulo-Kinzler et al., 1998; Hwang et al., 2011). Background muscle activity (EMG) is typically increased when moving from a sitting position to a standing position (Mynark and Koceja, 1997; Knikou et al., 2009a). Thus, the direct influence of limb load on spinal reflexes during different postural positions is complicated by the modulating descending drive during upright weight bearing tasks, which is known to change H-reflex excitability. It is known that the H-reflex is not only dependent on the characteristics of sensory inputs but also on the state of the spinal inter-neuronal circuits (Knikou, 2007).
Possible neural mechanisms modulate the H-reflex during loading
It is arduous to ascribe specific neuronal mechanisms to the findings in this study. Even though we controlled several factors by 1) applying load directly to the limb segment, and 2) assuring a common descending drive (EMG), we acknowledge that H-reflex excitability still relies on the interplay of multiple peripheral sensory resources. For example, afferent input from the tibial-femoral joint and the talo-crural joint may have contributed to reducing post activation depression with load. Importantly, cutaneous afferent signals from the skin above the knee, in contact with the compression applicator, or the afferent input on the sole of the foot may have contributed to the modulation observed in post activation depression in this study. Indeed, we observed a similar trend from one SCI subject with complete lesion (i.e. without any supra-spinal drive), demonstrating that post activation depression decreases at the higher dose of compressive load, but clearly not to the same extent. Hence, although we eliminated several sources that can influence reflex modulation (multiple joints, supra-spinal drive, vestibular input, and visual input) there remained two joints and two cutaneous afferent sites where applied load may have contributed to the modulation of post activation potentiation observed in this study.
Previous studies support that cutaneous afferent input from the sole of the foot can modulate soleus H-reflex responses in humans (Knikou and Conway, 2001; Hiraoka, 2003; Knikou, 2007; Conway and Knikou, 2008; Sayenko et al., 2009; Bastani et al., 2010). It is also well established that joint receptors facilitate pre synaptic inhibition of Ia fibers to the soleus motor neurons in humans (Nakazawa et al., 2004) and that increasing intra-articular joint pressure augments neural discharges of joint receptors in the knees of cats (Wood and Ferrell, 1984, 1985). Based on these previous studies, we expected that the test H1 amplitude during the loading phase, as compared to the baseline sessions, would be suppressed due to enhanced pre synaptic inhibition. The magnitude of the compressive load (50% BW) was expected to activate sensory afferents on the sole of the foot and the top of the knee, and increase intra-articular pressure of the knee and ankle joints to modulate the H-reflex (H-1). To our surprise, however, we found the test H1 amplitudes were not changed with limb segment load, which may be related to a site specific effect (Iles, 1996; Hiraoka, 2003; Nakajima et al., 2006; Sayenko et al., 2007; 2009). It is well known that applying low-intensity sensory stimulation around the heel results in a facilitation of soleus H-reflexes; whereas stimulating the metatarsal region of the sole of the foot induces inhibition of soleus H-reflexes (Sayenko et al., 2009). In addition, the effects of cutaneous stimulation on H-reflexes have also been found to be time dependent (Iles, 1996; Sayenko et al., 2009). For example, a conditioned cutaneous stimulus delivered 50 ms prior to a test H-reflex facilitated the soleus H-reflex, whereas a cutaneous stimulus delivered more than 50 ms before the H-reflex caused inhibition (Sayenko et al., 2009). In the current study, the applied load was centered over the top of the knee joint and was directly in line with the longitudinal axis of the lower leg segment, including the heel region of the foot. As a result of load over the knee, low-threshold cutaneous afferent receptors, which induce a heteronymous facilitation of the soleus H-reflex via interneuronal circuits of the femoral nerve, were likely triggered (Delwaide and Crenna, 1984). It is plausible that our finding of no change in test H-reflex amplitude (H1) during the loading phase reflects a mixed effect of both presynaptic facilitation and inhibition derived from cutaneous afferent receptors and mechanoreceptors firing concurrently from multiple sites activated by this novel method of applying compressive loads in human tissue.
Inhibition, via group Ib afferents, increased in response to a 300 N load to the foot in the supine position (Faist et al., 2006). Supra spinal descending commands may also modulate the excitatory state of the soleus alpha motor neurons via interneuron pathways (Aymard et al., 2000; Bretzner and Drew, 2005). Although we verified a constant low level of background EMG in this study, we do not know the status of the inter-neuronal networks in response to small changes in background EMG across the entire experimental session. However, randomly introducing the loads precluded any systematic changes and suggests that descending influences had a minimal impact on the findings. Taken together, the sensory inputs from the skin and joints, generated during various doses of limb segment compressive loads, minimally influenced the excitability of the motor neuron pool as supported by the constant test H-reflex amplitude (H1).
Despite no change in soleus test H1 amplitudes across all sessions, we showed that post activation depression was decreased from the limb segment load. Because we used a long inter-stimulus interval (15 seconds) between each doublet stimulation, all test H1 amplitudes elicited by a train of doublets were allowed to fully recover before subsequent activation conditions (i.e. homonymous depression at the presynaptic Ia terminals) (Crone and Nielsen, 1989; Hultborn et al., 1996; Kohn et al., 1997; Aymard et al., 2000; Cortes et al., 2011). Given the understanding that the H-reflex amplitude is depressed at short latencies following a conditioning stimulus, the decrease of the second conditioned H-reflex amplitude (H2), expressed as the depression ratio, provided support that the typical pre-synaptic inhibition causing post activation depression was present.
The novel finding from this study, however, was the decreased depression ratio during the loading phase, which suggested that load diminished the capacity to gate afferent input via pre-synaptic inhibitory pathways. Importantly, the decreased post activation depression was modulated by the magnitude of compressive load (i.e. 50% of BW load caused greater loss of depression as compared to 10% or 25% of BW loads). In unpublished pilot data, we disrupted cutaneous afferent input at the sole of the foot (by numbing with ice) but still observed the same loss of depression during limb segment loading. This finding suggests that joint receptors, rather than cutaneous afferent input may be responsible for decreasing post activation depression. Overall, limb segment load, via cutaneous afferent and/or joint mechanoreceptor input, decreased the capacity to depress a monosynaptic pathway to a conditioning stimulus.
The preliminary findings from one individual with chronic complete SCI indicate that H-reflex amplitudes (both H1 and H2) are less sensitive to the dose of loading. Contrary to findings in the healthy adults, H2 amplitudes decrease as the level of load increases (Figure 6A). In addition, the healthy adults showed no changes in the test H1 amplitudes across all levels of loads whereas the test H1 amplitudes decrease as the compressive load increases in the individual with chronic complete SCI. This finding may be attributed to the spinal cord reorganization that occurs several months following SCI (Schindler-Ivens and Shields, 2000; Shields, 2002; Shields et al., 2011). It is also possible that the reduced supra spinal drive contributes to the impaired dose response of the H-reflex to load (Capaday et al., 1999). Although the findings for this one subject must be considered exploratory, they raise the question as to whether repetitive use of mechanical load may regulate the loss of post activation depression that occurs several months after SCI. Future studies are necessary to understand the extent to which the spinal cord can be reorganized by timely mechanical inputs after loss of supra spinal drive from spinal cord injury.
Clinically, a decreased ability to depress the alpha motor neuron pool via pre synaptic inhibition is one mechanism associated with spasticity in people with SCI (Schindler-Ivens and Shields, 2000; Grey et al., 2008). The current study demonstrated that limb segment load impairs the post activation depression mechanism, a presynaptic inhibition at the Ia afferent-motor neuron synapse, which was induced by repetitive activation. Accordingly, if limb segment load further impairs the capacity to depress an H-reflex via post activation depression in people with SCI, then we would expect enhanced spasticity when limbs are loaded. Therefore, we would expect to observe increased muscle stiffness when individuals with complete SCI load their limbs, an observation confirmed anecdotally by clinicians. Further study is necessary, however, to determine if people with spasticity, who lose the ability to suppress their H-reflexes from a reorganized spinal cord, will have a further loss of suppression during limb segment load. We speculate that this load-induced reduction of post activation depression will be nonexistent in people with chronic SCI because, as a result of spinal cord reorganization, because they have already lost the capacity to depress the H-reflex to a conditioning stimulus.
CONCLUSION
The present study demonstrated that, in the absence of muscle contractions, limb segment load significantly decreases post activation depression of the soleus H-reflex in healthy adults. Furthermore, the magnitude of the loss of post activation depression is greatest with the 50% limb load and least with a 10% limb load. Our findings support that limb segment load induces spinal modulations via multiple neural pathways and influences synaptic efficiency of large diameter afferents. Future studies will determine if individuals with SCI, who have lost post activation depression, also show a limb load response as demonstrated preliminarily in this single subject with SCI. Mechanical stimuli may play an important role in capitalizing on neuronal tissue plasticity after spinal cord injury.
Highlights.
Limb segment load resulted in a predominant inhibition of post activation depression in humans, but minimally modulated the response in a subject with SCI.
The inhibition of post activation depression was directly related to the magnitude of the limb segment load.
These findings highlight whether limb load can influence spinal neuronal reorganization from the acute to the chronic state after SCI.
Acknowledgement
This study was supported in part by awards to RKS from the National Institutes of Health (R01HD062507), The Department of Veterans Affairs, and the Craig H. Neilsen Foundation. We thank engineers Jason Wu, MS and Colleen L. McHenry, MS for technical assistance with the feedback controlled loading system and engineer Zhijun Cai, PhD for controller software implementation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams CM, Suneja M, Dudley-Javoroski S, Shields RK. Altered mRNA expression after long-term soleus electrical stimulation training in humans with paralysis. Muscle Nerve. 2011;43:65–75. doi: 10.1002/mus.21831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello I, Rosati G, Serra G, Tugnoli V, Manca M. Static vestibulospinal influences in relation to different body tilts in man. Exp Neurol. 1983;79:18–26. doi: 10.1016/0014-4886(83)90375-8. [DOI] [PubMed] [Google Scholar]
- Ali AA, Sabbahi MA. H-reflex changes under spinal loading and unloading conditions in normal subjects. Clin Neurophysiol. 2000;111:664–670. doi: 10.1016/s1388-2457(99)00304-1. [DOI] [PubMed] [Google Scholar]
- Andersen JB, Sinkjaer T. The stretch reflex and H-reflex of the human soleus muscle during walking. Motor Control. 1999;3:151–157. doi: 10.1123/mcj.3.2.151. [DOI] [PubMed] [Google Scholar]
- Angulo-Kinzler RM, Mynark RG, Koceja DM. Soleus H-reflex gain in elderly and young adults: modulation due to body position. J Gerontol A Biol Sci Med Sci. 1998;53:M120–M125. doi: 10.1093/gerona/53a.2.m120. [DOI] [PubMed] [Google Scholar]
- Ashby P, Verrier M, Lightfoot E. Segmental reflex pathways in spinal shock and spinal spasticity in man. J Neurol Neurosurg Psychiatry. 1974;37:1352–1360. doi: 10.1136/jnnp.37.12.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aymard C, Katz R, Lafitte C, Lo E, Penicaud A, Pradat-Diehl P, et al. Presynaptic inhibition and homosynaptic depression: a comparison between lower and upper limbs in normal human subjects and patients with hemiplegia. Brain. 2000;123:1688–1702. doi: 10.1093/brain/123.8.1688. [DOI] [PubMed] [Google Scholar]
- Bastani A, Hadian MR, Talebian S, Bagheri H, Olyaie GR. Modulation of the ipsilateral and contralateral H reflexes following ipsilateral mechanical pressure of the foot in normal subjects. Electromyogr Clin Neurophysiol. 2010;50:251–256. [PubMed] [Google Scholar]
- Bretzner F, Drew T. Motor cortical modulation of cutaneous reflex responses in the hindlimb of the intact cat. J Neurophysiol. 2005;94:673–687. doi: 10.1152/jn.01247.2004. [DOI] [PubMed] [Google Scholar]
- Calancie B, Broton JG, Klose KJ, Traad M, Difini J, Ayyar DR. Evidence that alterations in presynaptic inhibition contribute to segmental hypo- and hyperexcitability after spinal cord injury in man. Electroencephalogr Clin Neurophysiol. 1993;89:177–186. doi: 10.1016/0168-5597(93)90131-8. [DOI] [PubMed] [Google Scholar]
- Capaday C, Lavoie BA, Barbeau H, Schneider C, Bonnard M. Studies on the corticospinal control of human walking. I. Responses to focal transcranial magnetic stimulation of the motor cortex. J Neurophysiol. 1999;81:129–139. doi: 10.1152/jn.1999.81.1.129. [DOI] [PubMed] [Google Scholar]
- Conway BA, Knikou M. The action of plantar pressure on flexion reflex pathways in the isolated human spinal cord. Clin Neurophysiol. 2008;119:892–896. doi: 10.1016/j.clinph.2007.12.015. [DOI] [PubMed] [Google Scholar]
- Cortes M, Thickbroom GW, Valls-Sole J, Pascual-Leone A, Edwards DJ. Spinal associative stimulation: A non-invasive stimulation paradigm to modulate spinal excitability. Clin Neurophysiol. 2011;122:2254–2259. doi: 10.1016/j.clinph.2011.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C, Nielsen J. Methodological implications of the post activation depression of the soleus H-reflex in man. Exp Brain Res. 1989;78:28–32. doi: 10.1007/BF00230683. [DOI] [PubMed] [Google Scholar]
- Delwaide PJ, Crenna P. Cutaneous nerve stimulation and motoneuronal excitability. II: Evidence for non-segmental influences. J Neurol Neurosurg Psychiatry. 1984;47:190–196. doi: 10.1136/jnnp.47.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwaide PJ, Schepens B. Auditory startle (audio-spinal) reaction in normal man: EMG responses and H reflex changes in antagonistic lower limb muscles. Electroencephalogr Clin Neurophysiol. 1995;97:416–423. doi: 10.1016/0924-980x(95)00136-9. [DOI] [PubMed] [Google Scholar]
- Dudley-Javoroski S, Shields RK. Dose estimation and surveillance of mechanical loading interventions for bone loss after spinal cord injury. Phys Ther. 2008a;88:387–396. doi: 10.2522/ptj.20070224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley-Javoroski S, Shields RK. Muscle and bone plasticity after spinal cord injury: review of adaptations to disuse and to electrical muscle stimulation. J Rehabil Res Dev. 2008b;45:283–296. doi: 10.1682/jrrd.2007.02.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faist M, Hoefer C, Hodapp M, Dietz V, Berger W, Duysens J. In humans Ib facilitation depends on locomotion while suppression of Ib inhibition requires loading. Brain Res. 2006;1076:87–92. doi: 10.1016/j.brainres.2005.12.069. [DOI] [PubMed] [Google Scholar]
- Field-Fote EC, Hufford T, Kerfoot N, Kizer S, Calancie B. Effect of lower extremity weightbearing load on motoneuron excitability in able-bodied subjects. Electromyogr Clin Neurophysiol. 2000;40:459–464. [PubMed] [Google Scholar]
- Frank JS. Spinal motor preparation in humans. Electroencephalogr Clin Neurophysiol. 1986;63:361–370. doi: 10.1016/0013-4694(86)90021-0. [DOI] [PubMed] [Google Scholar]
- Grey MJ, Klinge K, Crone C, Lorentzen J, Biering-Sorensen F, Ravnborg M, et al. Post-activation depression of soleus stretch reflexes in healthy and spastic humans. Exp Brain Res. 2008;185:189–197. doi: 10.1007/s00221-007-1142-6. [DOI] [PubMed] [Google Scholar]
- Hayashi R, Tako K, Tokuda T, Yanagisawa N. Comparison of amplitude of human soleus H-reflex during sitting and standing. Neurosci Res. 1992;13:227–233. doi: 10.1016/0168-0102(92)90062-h. [DOI] [PubMed] [Google Scholar]
- Hiersemenzel LP, Curt A, Dietz V. From spinal shock to spasticity: neuronal adaptations to a spinal cord injury. Neurology. 2000;54:1574–1582. doi: 10.1212/wnl.54.8.1574. [DOI] [PubMed] [Google Scholar]
- Hiraoka K. Placement of a plate under the forefoot in stance: decreasing the excitability of the soleus motoneuron pool. Am J Phys Med Rehabil. 2003;82:837–841. doi: 10.1097/01.PHM.0000087454.19029.7E. [DOI] [PubMed] [Google Scholar]
- Hoffman MA, Koceja DM. The effects of vision and task complexity on Hoffmann reflex gain. Brain Res. 1995;700:303–307. doi: 10.1016/0006-8993(95)01082-7. [DOI] [PubMed] [Google Scholar]
- Huang CY, Cherng RJ, Yang ZR, Chen YT, Hwang IS. Modulation of soleus H reflex due to stance pattern and haptic stabilization of posture. J Electromyogr Kinesiol. 2009;19:492–499. doi: 10.1016/j.jelekin.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Illert M, Nielsen J, Paul A, Ballegaard M, Wiese H. On the mechanism of the post-activation depression of the H-reflex in human subjects. Exp Brain Res. 1996;108:450–462. doi: 10.1007/BF00227268. [DOI] [PubMed] [Google Scholar]
- Hwang S, Jeon HS, Kwon OY, Yi CH. The effects of body weight on the soleus H-reflex modulation during standing. J Electromyogr Kinesiol. 2011;21:445–449. doi: 10.1016/j.jelekin.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Iles JF. Evidence for cutaneous and corticospinal modulation of presynaptic inhibition of Ia afferents from the human lower limb. J Physiol. 1996;491:197–207. doi: 10.1113/jphysiol.1996.sp021207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iles JF, Pisini JV. Cortical modulation of transmission in spinal reflex pathways of man. J Physiol. 1992a;455:425–446. doi: 10.1113/jphysiol.1992.sp019309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iles JF, Pisini JV. Vestibular-evoked postural reactions in man and modulation of transmission in spinal reflex pathways. J Physiol. 1992b;455:407–424. doi: 10.1113/jphysiol.1992.sp019308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima N, Sekiguchi H, Miyoshi T, Nakazawa K, Akai M. Inhibition of the human soleus Hoffman reflex during standing without descending commands. Neurosci Lett. 2003;345:41–44. doi: 10.1016/s0304-3940(03)00485-3. [DOI] [PubMed] [Google Scholar]
- Knikou M. Plantar cutaneous input modulates differently spinal reflexes in subjects with intact and injured spinal cord. Spinal Cord. 2007;45:69–77. doi: 10.1038/sj.sc.3101917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knikou M, Angeli CA, Ferreira CK, Harkema SJ. Soleus H-reflex gain, threshold, and amplitude as function of body posture and load in spinal cord intact and injured subjects. Int J Neurosci. 2009a;119:2056–2073. doi: 10.1080/00207450903139747. [DOI] [PubMed] [Google Scholar]
- Knikou M, Angeli CA, Ferreira CK, Harkema SJ. Soleus H-reflex modulation during body weight support treadmill walking in spinal cord intact and injured subjects. Exp Brain Res. 2009b;193:397–407. doi: 10.1007/s00221-008-1636-x. [DOI] [PubMed] [Google Scholar]
- Knikou M, Conway BA. Modulation of soleus H-reflex following ipsilateral mechanical loading of the sole of the foot in normal and complete spinal cord injured humans. Neurosci Lett. 2001;303:107–110. doi: 10.1016/s0304-3940(01)01718-9. [DOI] [PubMed] [Google Scholar]
- Koceja DM, Trimble MH, Earles DR. Inhibition of the soleus H-reflex in standing man. Brain Res. 1993;629:155–158. doi: 10.1016/0006-8993(93)90495-9. [DOI] [PubMed] [Google Scholar]
- Kohn AF, Floeter MK, Hallett M. Presynaptic inhibition compared with homosynaptic depression as an explanation for soleus H-reflex depression in humans. Exp Brain Res. 1997;116:375–380. doi: 10.1007/pl00005765. [DOI] [PubMed] [Google Scholar]
- Kolev OI, Milanov I. Vestibular and auditory influences on segmental motoneuron excitability--a comparative study. Neurosci Lett. 1995;184:193–196. doi: 10.1016/0304-3940(94)11204-v. [DOI] [PubMed] [Google Scholar]
- Leis AA, Grubwieser GJ, Schild JH, West MS, Stokic DS. Control of Ia afferent input to triceps surae (soleus) locomotor nucleus precedes agonist muscle activation during gait. J Electromyogr Kinesiol. 1995;5:95–100. doi: 10.1016/1050-6411(94)00008-a. [DOI] [PubMed] [Google Scholar]
- Lowrey CR, Bent LR. Modulation of the soleus H-reflex following galvanic vestibular stimulation and cutaneous stimulation in prone human subjects. Muscle Nerve. 2009;40:213–220. doi: 10.1002/mus.21275. [DOI] [PubMed] [Google Scholar]
- Meunier S, Pierrot-Deseilligny E. Gating of the afferent volley of the monosynaptic stretch reflex during movement in man. J Physiol. 1989;419:753–763. doi: 10.1113/jphysiol.1989.sp017896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mynark RG, Koceja DM. Comparison of soleus H-reflex gain from prone to standing in dancers and controls. Electroencephalogr Clin Neurophysiol. 1997;105:135–140. doi: 10.1016/s0924-980x(96)96096-8. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Sakamoto M, Tazoe T, Endoh T, Komiyama T. Location specificity of plantar cutaneous reflexes involving lower limb muscles in humans. Exp Brain Res. 2006;175:514–525. doi: 10.1007/s00221-006-0568-6. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Miyoshi T, Sekiguchi H, Nozaki D, Akai M, Yano H. Effects of loading and unloading of lower limb joints on the soleus H-reflex in standing humans. Clin Neurophysiol. 2004;115:1296–1304. doi: 10.1016/j.clinph.2004.01.016. [DOI] [PubMed] [Google Scholar]
- Petersen N, Morita H, Nielsen J. Modulation of reciprocal inhibition between ankle extensors and flexors during walking in man. J Physiol. 1999;520:605–619. doi: 10.1111/j.1469-7793.1999.00605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phadke CP, Wu SS, Thompson FJ, Behrman AL. Soleus H-reflex modulation in response to change in percentage of leg loading in standing after incomplete spinal cord injury. Neurosci Lett. 2006;403:6–10. doi: 10.1016/j.neulet.2006.04.058. [DOI] [PubMed] [Google Scholar]
- Ribot-Ciscar E, Rossi-Durand C, Roll JP. Increased muscle spindle sensitivity to movement during reinforcement manoeuvres in relaxed human subjects. J Physiol. 2000;523:271–282. doi: 10.1111/j.1469-7793.2000.t01-1-00271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi-Durand C. The influence of increased muscle spindle sensitivity on Achilles tendon jerk and H-reflex in relaxed human subjects. Somatosens Mot Res. 2002;19:286–295. doi: 10.1080/0899022021000037755. [DOI] [PubMed] [Google Scholar]
- Sayenko DG, Vette AH, Kamibayashi K, Nakajima T, Akai M, Nakazawa K. Facilitation of the soleus stretch reflex induced by electrical excitation of plantar cutaneous afferents located around the heel. Neurosci Lett. 2007;415:294–298. doi: 10.1016/j.neulet.2007.01.037. [DOI] [PubMed] [Google Scholar]
- Sayenko DG, Vette AH, Obata H, Alekhina MI, Akai M, Nakazawa K. Differential effects of plantar cutaneous afferent excitation on soleus stretch and H-reflex. Muscle Nerve. 2009;39:761–769. doi: 10.1002/mus.21254. [DOI] [PubMed] [Google Scholar]
- Schindler-Ivens S, Shields RK. Low frequency depression of H-reflexes in humans with acute and chronic spinal-cord injury. Exp Brain Res. 2000;133:233–241. doi: 10.1007/s002210000377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields RK. Muscular, skeletal, and neural adaptations following spinal cord injury. J Orthop Sports Phys Ther. 2002;32:65–74. doi: 10.2519/jospt.2002.32.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields RK, Dudley-Javoroski S. Musculoskeletal plasticity after acute spinal cord injury: effects of long-term neuromuscular electrical stimulation training. J Neurophysiol. 2006;95:2380–2390. doi: 10.1152/jn.01181.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields RK, Dudley-Javoroski S, Law LA. Electrically induced muscle contractions influence bone density decline after spinal cord injury. Spine. 2006;31:548–553. doi: 10.1097/01.brs.0000201303.49308.a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields RK, Dudley-Javoroski S, Oza PD. Low-frequency H-reflex depression in trained human soleus after spinal cord injury. Neurosci Lett. 2011;499:88–92. doi: 10.1016/j.neulet.2011.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley KM, Carpenter MG, Perry JC, Frank JS. Effects of postural anxiety on the soleus H-reflex. Hum Mov Sci. 2007;26:103–112. doi: 10.1016/j.humov.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Skinner RD, Houle JD, Reese NB, Berry CL, Garcia-Rill E. Effects of exercise and fetal spinal cord implants on the H-reflex in chronically spinalized adult rats. Brain Res. 1996;729:127–131. [PubMed] [Google Scholar]
- Trimble MH, Brunt D, Jeon HS, Kim HD. Modulations of soleus H-reflex excitability during gait initiation: central versus peripheral influences. Muscle Nerve. 2001;24:1371–1379. doi: 10.1002/mus.1158. [DOI] [PubMed] [Google Scholar]
- Wood L, Ferrell WR. Response of slowly adapting articular mechanoreceptors in the cat knee joint to alterations in intra-articular volume. Ann Rheum Dis. 1984;43:327–332. doi: 10.1136/ard.43.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood L, Ferrell WR. Fluid compartmentation and articular mechanoreceptor discharge in the cat knee joint. Q J Exp Physiol. 1985;70:329–335. doi: 10.1113/expphysiol.1985.sp002918. [DOI] [PubMed] [Google Scholar]