Abstract
Here, we report on the unprecedentedly high-resolution imaging of ion transport through single nanopores by scanning electrochemical microscopy (SECM). The quantitative SECM image of single nanopores allows for the determination of their structural properties, including their density, shape, and size, which are essential for understanding the permeability of the entire nanoporous membrane. Nanoscale spatial resolution was achieved by scanning a 17 nm-radius pipet tip at a distance down to 1.3 nm from a highly porous nanocrystalline silicon membrane in order to obtain the peak current response controlled by the nanopore-mediated diffusional transport of tetrabutylammonium to the nanopipet-supported liquid/liquid interface. A 280 nm × 500 nm image resolved 13 nanopores, which corresponds to a high density of 93 pores/µm2. A finite element simulation of the SECM image was performed to quantitatively assess the spatial resolution limited by the tip diameter in resolving two adjacent pores, and to determine the actual size of a nanopore, which was approximated as an elliptic cylinder with a depth of 30 nm and major and minor axes of 53 and 41 nm, respectively. These structural parameters are consistent with those determined by TEM, which thereby confirms the reliability of quantitative SECM imaging at the nanoscale level.
The development and application of nanoporous membranes for nanofiltration,1 biomedical devices,2 nanofluidics,3 and biomimetic membrane transport4 require the quantitative understanding of membrane permeability at a single nanopore level. In fact, it has been theoretically and experimentally demonstrated that the permeability of the whole nanoporous membrane depends on the convolution of several structural properties of nanopores, including their density, shape, and size.5 Here, we applied scanning electrochemical microscopy6 (SECM) to quantitatively and separately determine these key structural parameters from the high-resolution image of ion transport through single nanopores. Remarkably, the spatial resolution of SECM achieved in this study is the highest reported to date, with the exception of one study,7 where no quantitative image analysis was shown.
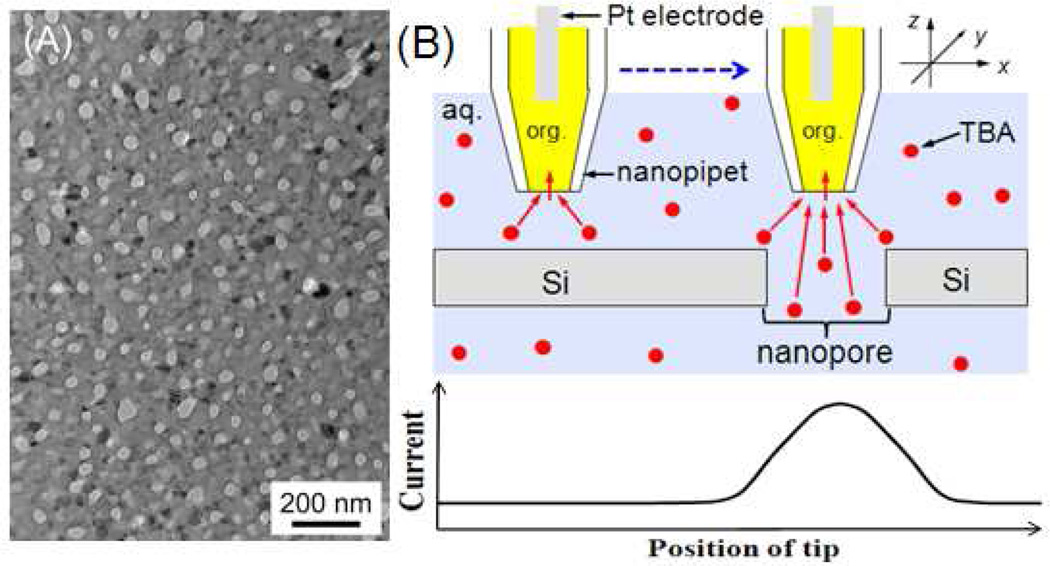
The unprecedentedly high spatial resolution of SECM is required for the imaging of a highly porous nanocrystalline silicon (pnc-Si) membrane8 at a single nanopore level. This emerging class of ultrathin nanoporous membranes, with a thickness of 30 nm, is robust enough to be self-standing in the aqueous solution and found to be useful for unique practical applications that require its high permeability, such as for the efficient separation of macromolecules8a–c and nanoparticles,8d tissue engineering, and cell culture.8e Single-pore imaging, however, has not been reported for a pnc-Si membrane, which not only possesses small nanopores, but also has a high pore density with short pore–pore separations of < 100 nm. Indeed, its density of ~102 pores/µm2 (Figure 1A) is 102–106 times higher than that of the nanopore membranes (mainly track-etched polymer membranes) that were used for the electrochemical imaging of single pores by SECM,9 scanning ion-conductance microscopy (SICM),10 SECM-SICM,11 and SECM-AFM.12 In these previous imaging studies, the shortest separations between two resolvable pores were limited to > 250 nm and ~1.5 µm for SECM(–AFM)12b and SICM,10f respectively. On the other hand, micrometer-sized SECM tips were used to probe the local permeability of a pnc-Si membrane based on several thousands of nanopores.5b,5c
Figure 1.
(A) TEM image of a pnc-Si membrane and (B) scheme of SECM line scan with a nanopipet-supported ITIES tip over the impermeable and nanoporous regions of the membrane.
In this work, nanoscale spatial resolution of a pnc-Si membrane was achieved by scanning a small SECM tip with a radius of 17 nm at an exceptionally short distance down to 1.3 nm. In the nanoscale SECM imaging, the current response at the nanotip is based on diffusion-controlled ion transfer at the interface between two immiscible electrolyte solutions (ITIES), which is supported at the tip of the quartz nanopipet filled with an organic electrolyte solution13 (Figure 1B). The ionic tip current is suppressed when the tip is positioned within a tip diameter from the impermeable region of a pnc-Si membrane, which hinders ion diffusion to the nanoscale ITIES tip (i.e., negative feedback effect).6 In contrast, the tip current increases as the tip is laterally scanned over a nanopore, which mediates ion diffusion to the ITIES tip. Subsequently, a peak-shaped response is obtained during a line scan over a pore (Figure 1B), where a shorter tip–membrane distance enhances the image contrast based on the peak height and improves spatial resolution based on the peak width.6
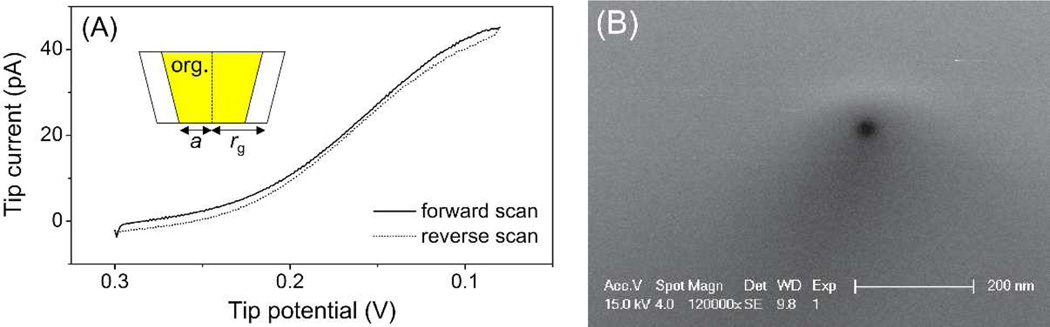
A nanopipet-based SECM tip was prepared as reported elsewhere13b and characterized by ion-transfer voltammetry and SEM. A nanopipet was filled with the 1,2-dichloroethane solution of organic supporting electrolytes and immersed in an aqueous solution of tetrabutylammonium (TBA) in order to voltammetrically drive TBA transfer across the nanopipet-supported ITIES (Figure 2A). The sigmoidal steady-state voltammograms on forward and reverse potential sweeps show small capacitive current and nearly overlap with each other. The pipet-supported ITIES tip was assumed as an inlaid disk, and therefore a limiting current, iT,∞, of 42 pA corresponds to a tip inner radius, a, of 17 nm with a typical tip outer radius, rg, of 1.4a as determined from
| (1) |
where x is a function of RG (= rg/a),16 n is the number of transferred charges (= +1) in the tip reaction, and D (= 5.1 × 10−6 cm2/s) and c* (= 10 mM) are the diffusion coefficient and concentration of the transferred ion in the bulk solution, respectively. The inner radius of the ITIES tip is similar to that of a typical nanopipet (~15 nm) as estimated by SEM (Figure 2B).
Figure 2.
(A) Cyclic voltammetry of 10 mM TBA in 0.3 M KCl at 50 mV/s. The tip potential is defined against an Ag/AgCl reference electrode. (B) SEM image of the tip opening of a typical nanopipet.
The nanopipet-supported ITIES tip was employed for the imaging of a pnc-Si membrane using the constant-height mode of SECM6 (i.e., without the active feedback control of the tip−membrane distance) in a newly developed isothermal chamber, which supresses a distance change due to thermal drift to a subnanometer level.13b In addition, the flat surface of the pnc-Si membrane with a root-mean-square roughness of 0.29 nm as measured by AFM14 was horizontally aligned on the SECM stage using a bubble level15 to be perpendicular to the tip electrode axis. Subsequently, we observed that a sharp nanopipet with small RG of 1.4 approached very closely to the flat substrate and was scanned laterally without contact when a relatively small area of the membrane was imaged (see below; also Figure S-1 for details).
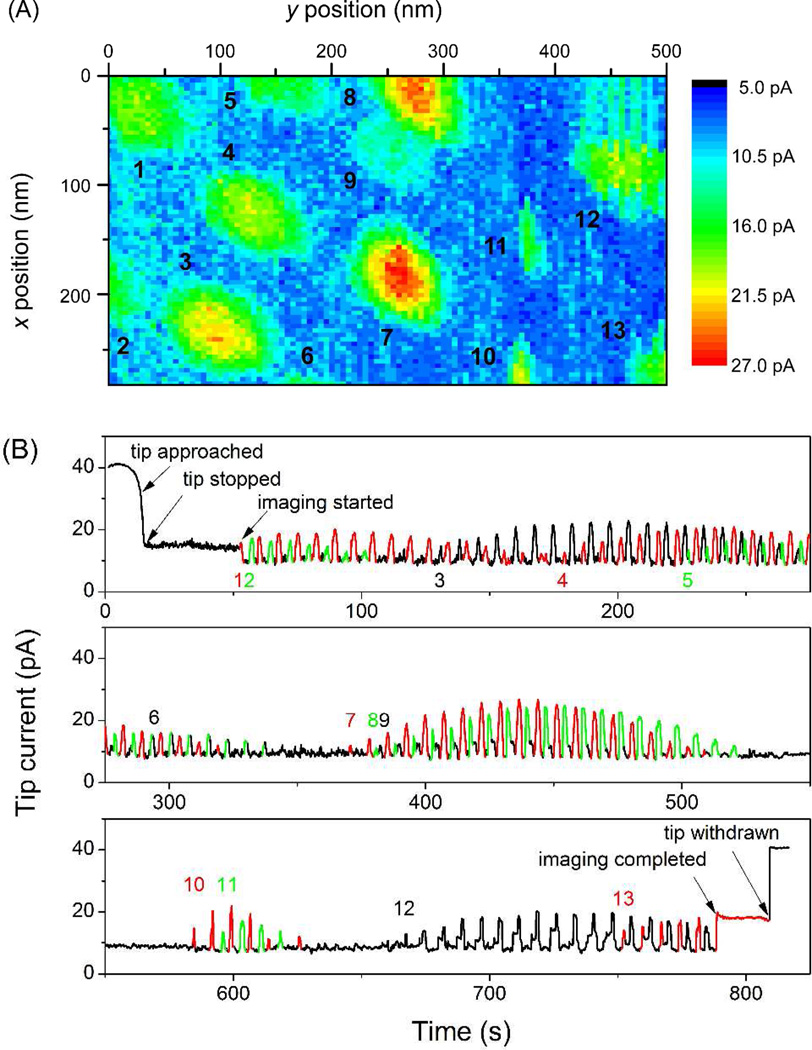
The whole procedure for obtaining an SECM image (Figure 3A) is illustrated in Figure 3B using the corresponding time profile of the current response at the nanopipet-supported ITIES tip. Prior to imaging, the tip was brought in close proximity to a pnc-Si membrane and stopped when the tip current decreased to 40% of iT,∞ (Figure 3B). In this example, the tip approached pore 1 at (x, y) = (0, 0) (Figure 3A). The tip then remained over the pore for 35 s until the tip was scanned from x = 0 nm to x = 280 nm with a step size of 4 nm, which was repeated from y = 0 nm to y = 500 nm with an interval of 5 nm. It took approximately 0.1 s at each tip position to move and settle the x, y-axes piezo positioner and monitor the steady-state tip current.
Figure 3.
(A) SECM image of a pnc-Si membrane and (B) current versus time profile during the whole imaging experiment, where a number is given for each pore at the time when its first peak appears.
During the 280 nm × 500 nm scan (Figure 3A), the height of a peak current response to a pore under the tip varied with a lateral distance between tip and pore, thereby yielding a family of peaks with various heights for each nanopore (Figure 3B). In contrast, the negative feedback current at the foot of a current peak was very stable and reproducible, which confirmed that the tip–membrane distance was nearly constant during imaging. A tip current, iT, of 10 pA in Figure 3B is equivalent to a tip–membrane distance of 1.3 nm in the approximate equation with RG = 1.4.13b A stable distance was also maintained between the tip and pore 13 to give a constant current after the imaging was completed. Finally, the tip current nearly recovered to the initial iT,∞ value when the tip was withdrawn to 1.5 µm away from the substrate. The good stability of the tip current indicates a lack of significant tip damage due to tip–membrane contact during imaging.
Overall, 13 pores were successfully resolved as local regions with higher tip currents in the SECM image of a pnc-Si membrane (Figure 3A). This result corresponds to a high density of 93 pores/µm2, which is consistent with a density of ~90 pores/µm2, as determined from the TEM image (Figure 1A). Qualitatively, a larger pore occupies a larger area in the SECM image, where pores 9, 10, and 11 are significantly smaller than the other pores. The area occupied by a pore in the image, however, is larger than expected from the actual size of the pore, because the spatial resolution is limited by the tip size that is comparable to the pore size. Therefore, the quantitative analysis of the SECM image is needed to reliably evaluate pore size (see below). Noticeably, unidirectional orientation is seen for some pores in the SECM image, which may be due to the imperfect disk shape of the ITIES supported at an elongated tip orifice. We found that the orientation of nanopores varied from tip to tip, but was independent of the tip−membrane distance (1.3–8.5 nm), and was different from the direction of the tip scan (Figure 3A).
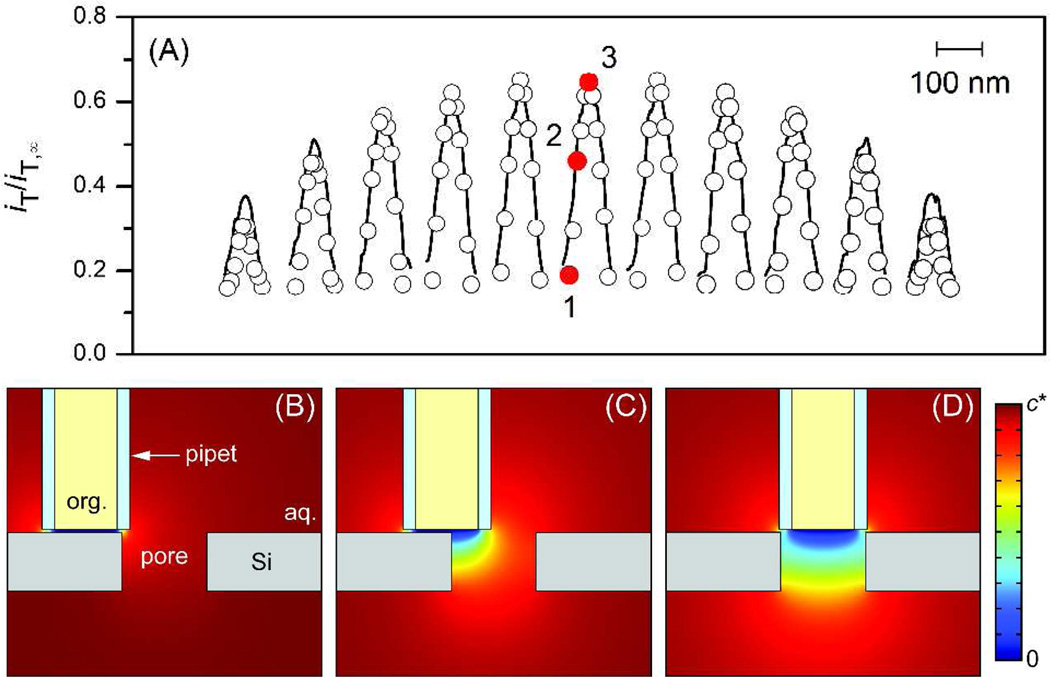
SECM line scans over pore 7 were analyzed by employing the finite element simulation of ion diffusion around the tip and the nanopore (see Supporting Information) to reliably determine the actual pore size without the limitation of spatial resolution set by the tip size (Figure 4). For simplicity, the x, y-cross section of a pore was assumed to be elliptical in shape, thereby yielding the major and minor axes and depth of a pore as structural parameters. TBA is small enough to freely diffuse through a nanopore without electrostatic or steric hindrance from the pore wall.5c Figure 4A shows very good fits of simulation results with experimental results, where each peak current response in a x line scan was plotted against the x position of the center of the tip with respect to that of the center of each nanopore, i.e., Δx, while Δy represents the corresponding relative y position of the tip (see also Figure S-3 for these definitions). The simulation results show that pore 7 has major and minor axes of 53 nm and 41 nm, respectively. An aspect ratio of 1.3 is consistent with the corresponding values of 1.0–2.1, as determined from ~150 pores in the TEM image (Figure 1A). Moreover, the average of the major and minor axes gives an apparent pore diameter5b of 47 nm, which is in agreement with the corresponding values of 14–58 nm in the TEM image. Moreover, the apparent diameter determined by numerical simulation is much smaller than an apparent pore diameter of ~80 nm for pore 7, as determined from its SECM image, where major and minor axes of ~90 and ~70 nm, respectively, were obtained from the area surrounded by a contour line of ~11 pA. Noticeably, a pore depth of 30 nm was also confirmed by the simulation of peak currents in Figure 4A, which are sensitive to the pore depth (see Figure S-4).
Figure 4.
(A) Simulated (circles) and experimental (solid lines) tip current, iT/iT,∞, in the normalized form for x-line scans over pore 7. In the simulation, the y position of the tip center was kept at Δy = −35, −25, −15, −10, −5, 0, +5, +10, +15, +25, and +35 nm from the leftmost peak to the rightmost peak, while its x position for each line scan was Δx = −42.5, −34, −25.5, −17, −8.5, 0, +8.5, +17, +25.5, +34, and +42.5 nm from the leftmost circle to the rightmost circle. Sliced concentration profiles are shown for tip positions at Δx = (B) −42.5, (C) −25.5, and (D) 0 nm and Δy = 0 nm, as indicated by red dots 1–3, respectively, in part (A).
The simulated concentration profiles of TBA around pore 7 during the x-line scan at Δy = 0 nm are shown in parts B–D of Figure 4 to quantitatively demonstrate how the tip size limits spatial resolution in determining acture pore size and separating two adjacent nanopores. In Figure 4B, the center of the tip is positioned by approximately a tip radius away from the edge of the pore, and thus the whole tip surface is positioned above the impermeable region of the membrane. The resultant negative feedback current determines the base of a current peak (red dot 1 in Figure 4A). Therefore, the apparent pore size estimated from the current peak (or the SECM image) is larger than the actual pore size by a tip inner diameter of 34 nm, which is confirmed for pore 7 with apparent diameters of ~80 and 47 nm, as determined from the SECM image and its simulation, respectively (see above). In fact, when the center of the tip is positioned above the edge of the nanopore (Figure 4C), the corresponding tip current (red dot 2) is significantly enhanced by ion diffusion from the inside of the pore and already reaches more than half of the peak current (red dot 3) that is obtained when the tip center is positioned above the center of the nanopore (Figure 4D). These results also indicate that two adjacent nanopores can be completely separated in the SECM image only when their edge-edge separation is larger than the tip inner diameter. For instance, pore 8 partially overlaps with pore 9 in Figure 3A, because their center-center separation of only ~55 nm is comparable to a typical pore diamater.
In summary, SECM was successfully used to generate the unprecedented high-resolution and quantitative imaging of single nanopores at a high density of 93 pores/µm2. The SECM image can be quantitatively analyzed to determine the structural properties of single nanopores, including the smallest pore axis of 41 nm, without the limitation of spatial resolution set by the tip diameter. The numerical simulation also indicates that two adjacent pores with an edge-edge separation of the tip diameter or larger, i.e., ≥ 34 nm in this work are completely resolvable. Advantageously, the highest resolution of SECM under normal experimental conditions was achieved in this study by employing the simple constant-height mode without feedback distance control, not only because the pnc-Si membrane surface was flat, but also because the SECM stage was isolated from the ambient environment using an isothermal chamber to suppress thermal drift.13b Based on these findings, we envision the application of this simple, quantitative, and high-resolution SECM approach to the imaging of biological nanopores.17
Supplementary Material
ACKNOWLEDGMENT
This work was supported by grants from the National Institutes of Health (GM073439). We thank Dr. Christopher C. Striemer and Mr. Charles Chan, SiMPore, for the TEM images of pnc-Si membranes and their analysis.
Footnotes
ASSOCIATED CONTENT
Supporting Information. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.(a) Vandezande P, Gevers LEM, Vankelecom IFJ. Chem. Soc. Rev. 2008;37:365. doi: 10.1039/b610848m. [DOI] [PubMed] [Google Scholar]; (b) Shannon MA, Bohn PW, Elimelech M, Georgiadis JG, Marinas BJ, Mayes AM. Nature. 2008;452:301. doi: 10.1038/nature06599. [DOI] [PubMed] [Google Scholar]; (c) Peng XS, Jin J, Nakamura Y, Ohno T, Ichinose I. Nat. Nanotechnol. 2009;4:353. doi: 10.1038/nnano.2009.90. [DOI] [PubMed] [Google Scholar]; (d) Karan S, Samitsu S, Peng X, Kurashima K, Ichinose I. Science. 2012;335:444. doi: 10.1126/science.1212101. [DOI] [PubMed] [Google Scholar]
- 2.(a) Kooman JP, van der Sande FM, Leunissen KML. Blood Purification. 2007;25:377. doi: 10.1159/000107774. [DOI] [PubMed] [Google Scholar]; (b) Fissell WH, Dubnisheva A, Eldridge AN, Fleischman AJ, Zydney AL, Roy S. J. Membr. Sci. 2009;326:58. doi: 10.1016/j.memsci.2008.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Perin L, Da Sacco S, De Filippo RE. Adv. Drug Deliv. Rev. 2011;63:379. doi: 10.1016/j.addr.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 3.(a) van den Berg A, Wessling M. Nature. 2007;445:726. doi: 10.1038/445726a. [DOI] [PubMed] [Google Scholar]; (b) Schoch RB, Han JY, Renaud P. Rev. Mod. Phys. 2008;80:839. [Google Scholar]; (c) Li YQ, Zheng YB, Zare RN. ACS Nano. 2012;6:993. doi: 10.1021/nn300356d. [DOI] [PubMed] [Google Scholar]
- 4.(a) Jovanovic-Talisman T, Tetenbaum-Novatt J, McKenney AS, Zilman A, Peters R, Rout MP, Chait BT. Nature. 2009;457:1023. doi: 10.1038/nature07600. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kowalczyk SW, Kapinos L, Blosser TR, Magalhaes T, van Nies P, Lim RYH, Dekker C. Nat. Nanotechnol. 2011;6:433. doi: 10.1038/nnano.2011.88. [DOI] [PubMed] [Google Scholar]
- 5.(a) Berg HC. Random Walks in Biology. Princeton, NJ: Princeton University Press; 1993. [Google Scholar]; (b) Kim E, Xiong H, Striemer CC, Fang DZ, Fauchet PM, McGrath JL, Amemiya S. J. Am. Chem. Soc. 2008;130:4230. doi: 10.1021/ja711258w. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Ishimatsu R, Kim J, Jing P, Striemer CC, Fang DZ, Fauchet PM, McGrath JL, Amemiya S. Anal. Chem. 2010;82:7127. doi: 10.1021/ac1005052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Bard AJ, Mirkin MV, editors. Scanning Electrochemical Microscopy. New York: Marcel Dekker; 2001. [Google Scholar]; (b) Amemiya S, Bard AJ, Fan FRF, Mirkin MV, Unwin PR. Ann. Rev. Anal. Chem. 2008;1:95. doi: 10.1146/annurev.anchem.1.031207.112938. [DOI] [PubMed] [Google Scholar]
- 7.Fan FRF, Bard AJ. Proc. Natl. Acad. Soci. U.S.A. 1999;96:14222. doi: 10.1073/pnas.96.25.14222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Striemer CC, Gaborski TR, McGrath JL, Fauchet PM. Nature. 2007;445:749. doi: 10.1038/nature05532. [DOI] [PubMed] [Google Scholar]; (b) Fang DZ, Striemer CC, Gaborski TR, McGrath JL, Fauchet PM. Nano Lett. 2010;10:3904. doi: 10.1021/nl101602z. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Snyder JL, Clark A, Fang DZ, Gaborski TR, Striemer CC, Fauchet PM, McGrath JL. J. Membrane Sci. 2011;369:119. doi: 10.1016/j.memsci.2010.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Gaborski TR, Snyder JL, Striemer CC, Fang DZ, Hoffman M, Fauchet PM, McGrath JL. ACS Nano. 2010;4:6973. doi: 10.1021/nn102064c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Agrawal AA, Nehilla BJ, Reisig KV, Gaborski TR, Fang DZ, Striemer CC, Fauchet PM, McGrath JL. Biomaterials. 2010;31:5408. doi: 10.1016/j.biomaterials.2010.03.041. [DOI] [PubMed] [Google Scholar]
- 9.(a) Bath BD, White HS, Scott ER. In: Scanning Electrochemical Microscopy. Bard AJ, Mirkin MV, editors. New York: Marcel Dekker; 2001. p. 343. [Google Scholar]; (b) Uitto OD, White HS. Anal. Chem. 2001;73:533. doi: 10.1021/ac0009301. [DOI] [PubMed] [Google Scholar]; (c) Uitto OD, White HS, Aoki K. Anal. Chem. 2002;74:4577. doi: 10.1021/ac0256538. [DOI] [PubMed] [Google Scholar]; (d) Lee S, Zhang Y, White HS, Harrell CC, Martin CR. Anal. Chem. 2004;76:6108. doi: 10.1021/ac049147p. [DOI] [PubMed] [Google Scholar]; (e) Ervin EN, White HS, Baker LA. Anal. Chem. 2005;77:5564. doi: 10.1021/ac050453s. [DOI] [PubMed] [Google Scholar]; (f) Ervin EN, White HS, Baker LA, Martin CR. Anal. Chem. 2006;78:6535. doi: 10.1021/ac060577k. [DOI] [PubMed] [Google Scholar]; (g) White RJ, White HS. Anal. Chem. 2007;79:6334. doi: 10.1021/ac070610i. [DOI] [PubMed] [Google Scholar]; (h) McKelvey K, Snowden ME, Peruffo M, Unwin PR. Anal. Chem. 2011;83:6447. doi: 10.1021/ac201489c. [DOI] [PubMed] [Google Scholar]
- 10.(a) Proksch R, Lal R, Hansma PK, Morse D, Stucky G. Biophys. J. 1996;71:2155. doi: 10.1016/S0006-3495(96)79416-X. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Böcker M, Muschter S, Schmitt EK, Steinem C, Schäffer TE. Langmuir. 2009;25:3022. doi: 10.1021/la8034227. [DOI] [PubMed] [Google Scholar]; (c) Chen C-C, Derylo MA, Baker LA. Anal. Chem. 2009;81:4742. doi: 10.1021/ac900065p. [DOI] [PubMed] [Google Scholar]; (d) Chen -CC, Baker LA. Analyst. 2011:136. [Google Scholar]; (e) Chen C-C, Zhou Y, Baker LA. ACS Nano. 2011;5:8404. doi: 10.1021/nn203205s. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Zhou Y, Chen CC, Baker LA. Anal. Chem. 2012;84:3003. doi: 10.1021/ac300257q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(a) Takahashi Y, Shevchuk AI, Novak P, Zhang Y, Ebejer N, Macpherson JV, Unwin PR, Pollard AJ, Roy D, Clifford CA, Shiku H, Matsue T, Klenerman D, Korchev YE. Angew. Chem. Int. Ed. 2011;50:9638. doi: 10.1002/anie.201102796. [DOI] [PubMed] [Google Scholar]; (b) Morris CA, Chen C-C, Baker LA. Analyst. 2012 doi: 10.1039/c2an16178h. Advance Article, http://dx.doi.org/10.1039/C2AN16178H. [DOI] [PubMed] [Google Scholar]
- 12.(a) Macpherson JV, Unwin PR. Anal. Chem. 2000;72:276. doi: 10.1021/ac990921w. [DOI] [PubMed] [Google Scholar]; (b) Macpherson JV, Jones CE, Barker AL, Unwin PR. Anal. Chem. 2002;74:1841. doi: 10.1021/ac0157472. [DOI] [PubMed] [Google Scholar]; (c) Gardner CE, Unwin PR, Macpherson JV. Electrochem. Comm. 2005;7:612. [Google Scholar]
- 13.(a) Elsamadisi P, Wang Y, Velmurugan J, Mirkin MV. Anal. Chem. 2011;83:671. doi: 10.1021/ac102704z. [DOI] [PubMed] [Google Scholar]; (b) Kim J, Shen M, Nioradze N, Amemiya S. Anal. Chem. 2012;84:3489. doi: 10.1021/ac300564g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang DZ. Ph.D. Dissertation. University of Rochester: Rochester, NY; 2010. [Google Scholar]
- 15.Sun P, Mirkin MV. Anal. Chem. 2006;78:6526. doi: 10.1021/ac060924q. [DOI] [PubMed] [Google Scholar]
- 16.Lefrou C. J. Electroanal. Chem. 2006;592:103. [Google Scholar]
- 17.Guo J, Amemiya S. Anal. Chem. 2005;77:2147. doi: 10.1021/ac048370j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.