Abstract
CD8 T-lymphocytes are effector cells of cholangiocyte injury in human and in rhesus rotavirus (RRV) induced experimental biliary atresia (BA). Here, we hypothesize that neonatal deficiency in CD25+CD4+ regulatory T cells (Tregs) leads to aberrant activation of hepatic T-lymphocytes in BA. We found that adoptive transfer of total CD4 cells, but not of CD25-depleted CD4 cells, prior to RRV inoculation reduced expansion of CD8 cells, plasma bilirubin levels, ductal inflammation and bile duct epithelial injury at 7 days postinfection (dpi) compared with age-matched infected controls without adoptive transfer. Searching for mechanisms, we found that in vitro production of IFNγ by naïve CD8 cells upon polyclonal stimulation was enhanced in co-culture with hepatic dendritic cells (DC)s from RRV infected, but not with DCs from non-infected mice which was correlated with an increased proportion of CD11b+ myeloid (m)DCs and up-regulation of the costimulatory molecule CD86 on RRV-primed DCs. Furthermore, DC-dependent T-lymphocyte activation was blocked by anti-CD86 antibody in dose dependent fashion. Importantly, expression of CD86 on mDCs was down-regulated by Tregs in vitro, and adoptive transfer of Treg-containing CD4 cells decreased expression of CD86 on hepatic mDCs at 7dpi. On the contrary, in mice resistant to experimental BA, CD25+ cell depletion aggravated bile duct injury at 12dpi after RRV inoculation, as plasma bilirubin levels were elevated by >20fold compared with non-depleted infected controls. Increased susceptibility to hepatobiliary injury in Treg-depleted mice was linked to hepatic CD8 expansion and enhanced stimulatory capacity of hepatic DCs.
Conclusion
Activation of hepatic T-lymphocytes driving biliary obstruction in BA is regulated by mDCs via CD86-dependent costimulation and is susceptible to inhibition by Tregs.
Introduction
Biliary atresia (BA) is a progressive fibroinflammatory obstruction of the extrahepatic biliary tree that leads to chronic cholestasis and biliary cirrhosis. Because of the severity of the disease, it is the most common indication for pediatric liver transplantation worldwide(1). Development of non-transplant therapies is hampered by the gap of knowledge on the pathogenesis of the disease. Immune mediated destruction of the biliary epithelium is supported by up-regulation of genes associated with Th1 polarization(2) and NK cell activation(3), and by oligoclonal expansion of hepatic CD8 T-cells(4) in livers of infants with BA at the time of diagnosis. Whether immaturity of the immune system in neonates with BA is linked to initiation and progression of biliary injury is currently unknown.
Although BA is probably a multifactorial disease with lymphocyte mediated cholangiocyte injury representing the final pathway of aberrant inflammatory responses to different triggers(1), perinatal viral infection, for instance with rotavirus(5) or cytomegalovirus (CMV)(6), is likely the cause in a subgroup of patients. A well-established murine model of experimental BA(7), in which injection of neonatal BALB/c mice with rhesus rotavirus (RRV) leads to rapid onset of cholestasis associated with inflammatory obstruction of the extrahepatic bile duct (EHBD)(8), has facilitated better understanding of the mechanisms of neonatal cholangiocyte injury. Inflammatory cytokines, specifically interferon (IFN)γ(8), and hepatic lymphocytes, including NK cells(3) and CD8 lymphocytes(9), were shown to be critically important for initiation and progression of the disease process in the murine model. Regulation of activation of the effector lymphocytes is largely undefined.
We have previously shown that regulatory T cells (Tregs) are absent in liver and secondary lymphoid tissue during the first three days of life when BALB/c mice are susceptible to RRV-induced BA, but emerge promptly in the liver upon virus challenge in older mice resistant to experimental BA(10). Tregs represent a small proportion of CD4 cells, which constitutively express IL2Rα (CD25) and the transcription factor forkhead box P3 (FoxP3)(11). They are responsible for maintenance of peripheral tolerance and prevention of autoimmune disease(12). Tregs are implicated in the pathogenesis of immune mediated hepatobiliary disease, including primary biliary cirrhosis(13) and autoimmune sclerosing cholangitis(14). Furthermore, an association between hepatic CMV T-cell responses and decreased frequency of circulating Tregs in infants with BA has recently been reported(15). We have shown before that Tregs modulate the innate immune response by suppressing NK activation during initiation of biliary injury(10). Here we demonstrate that AT of Treg-containing CD4 cells, but not of Treg-depleted CD4 cells, dampens the CD8 adaptive immune response in the liver and attenuates the BA phenotype at the time of ductal obstruction. Furthermore, Treg-depletion in older mice leads to enhanced activation of hepatic T-lymphocytes and aggravates RRV-induced hepatobiliary injury. Importantly, we provide evidence that CD86-dependent costimulation of CD8 cells by hepatic myeloid dendritic cells is a critical pathway for effector T-lymphocyte activation during neonatal bile duct obstruction which can be targeted by Tregs in control of immune activation.
MATERIAL AND METHODS
Mice
BALB/cJ (WT) mice and FoxP3/GFP reporter mice(16), in which GFP is co-expressed under control of the endogenous FoxP3 promoter/enhancer elements, were purchased from Charles River and Jackson Laboratory, respectively. Thy1.1 congenic BALB/cJ mice were a generous gift from Dr. Suzanne Morris (Division of Immunobiology at Cincinnati Children’s Hospital Medical Center). All mice were bred in house and kept in conventional conditions. All protocols were approved by the Animal Care and Use Committee of the Cincinnati Children’s Research Foundation.
Adoptive transfer of CD4 lymphocyte populations
Splenic CD4 cells were purified from 8-week-old FoxP3/GFP (Thy1.2+) mice using Pan-T-cell columns (R&D systems, Minneapolis, MN) and subsequent negative CD4 separation with a Treg cell isolation kit (Miltenyi Biotec, Auburn, CA), according to the manufacturers’ instructions. For preparation of CD25−CD4 lymphocyte populations, CD25+ cells were depleted from CD4 cells by incubation with αCD25-microbeads and subsequent separation over a LD column (both Miltenyi Biotec). 10×106 of total CD4 or of CD25−CD4 cells were injected intraperitoneally (i.p.) into congenic Thy1.1-BALB/c mice on day 2 of life followed by i.p. injection of 1.5×106 focus forming units (ffu) of RRV twelve hours later. For age-matched controls, litter mates were subjected to the same protocol, except pups were administered saline on day 2 prior to RRV (or saline) inoculation.
Antibody mediated depletion of Tregs
100µg of purified αCD25 antibody (clone PC-61.5.3, rat IgG1; BioXcell, West Lebanon, NH), or 100µg of isotype control rat IgG1 (clone HPRN, BioXcell) were injected i.p. into BALB/c (WT) mice on the days 5, 10, and 15 of life. A dose of 1×106ffu/gram body weight of RRV was administered i.p. on day 8 of life.
Dendritic cell (DC) assays
For hepatic DC isolation, BALB/c (WT) mice were anesthetized with isoflurane, the portal vein was cannulated and the livers were perfused with cold RPMI with 0.1% (w/v) Collagenase-D (Roche Diagnostics, Indianapolis, IN) before harvest. Livers were gently minced and digested with RPMI with 0.1% Collagenase-D for 30 min at 37°C prior to passage through a 70 µm cell strainer, Percoll gradient centrifugation and separation with αCD11c+/αPDCA-1+ microbeads (Pan-DC isolation kit, Miltenyi Biotec). For DC/CD8 co-cultures, splenic CD8 cells were purified from non-infected neonatal mice using CD8 FlowComp Dynabeads (Invitrogen, Carlsbad, CA), and 100,000 CD8 cells/ well were cultured alone or with DCs in 2:1 ratio in 96-well-U-bottom plates in RPMI (Invitrogen) with 10% (v/v) heat-inactivated fetal calf serum (FCS, Life Technologies) at 37°C with 5% CO2 in presence of immobilized αCD3 (clone: 145-2C11, 1µg/ml, eBioscience) and recombinant hIL-2 (25 IU/ml, Roche Applied Sciences, Indianapolis, IN). Activation and proliferation of CD8 cells were determined by flow cytometric analysis of cellular expression of CD69 and IFNγ and CFSE dilution, respectively. For costimulatory blockade, culture media containing 1 µg/ml of αCD3 and 25 IU/ml of rhIL-2 were conditioned with purified αCD86 (clone PO3, Rat IgG2b), or αCD80 (clone 16-10A1, Armenian Hamster IgG2, both BD Biosciences), or with the respective isotype-IgG control in various concentrations. For Treg/DC in vitro assays, DCs were cultured with CD25+ or with CD25− CD4 cells from non-infected mice in 1:2 ratio in presence of rhIL-2 (25IU/ml) prior to flow cytometric analysis of expression of CD86 on DC subsets.
Mononuclear cell (MNC) isolation, flow cytometric analysis, colorimetric assays
quantitative RT-PCR, were performed as described before(8, 10). Details are provided in Supplemental Material.
Statistical analysis
Values are expressed as mean±standard error (SEM) and statistical significance was determined by unpaired t test, with a significance set at p<0.05. One way analysis of variance (ANOVA) with post-hoc Turkey‘s multiple comparison test was used to assess statistical significance between more than two groups.
Results
Tregs dampen the adaptive immune response and attenuate the BA phenotype
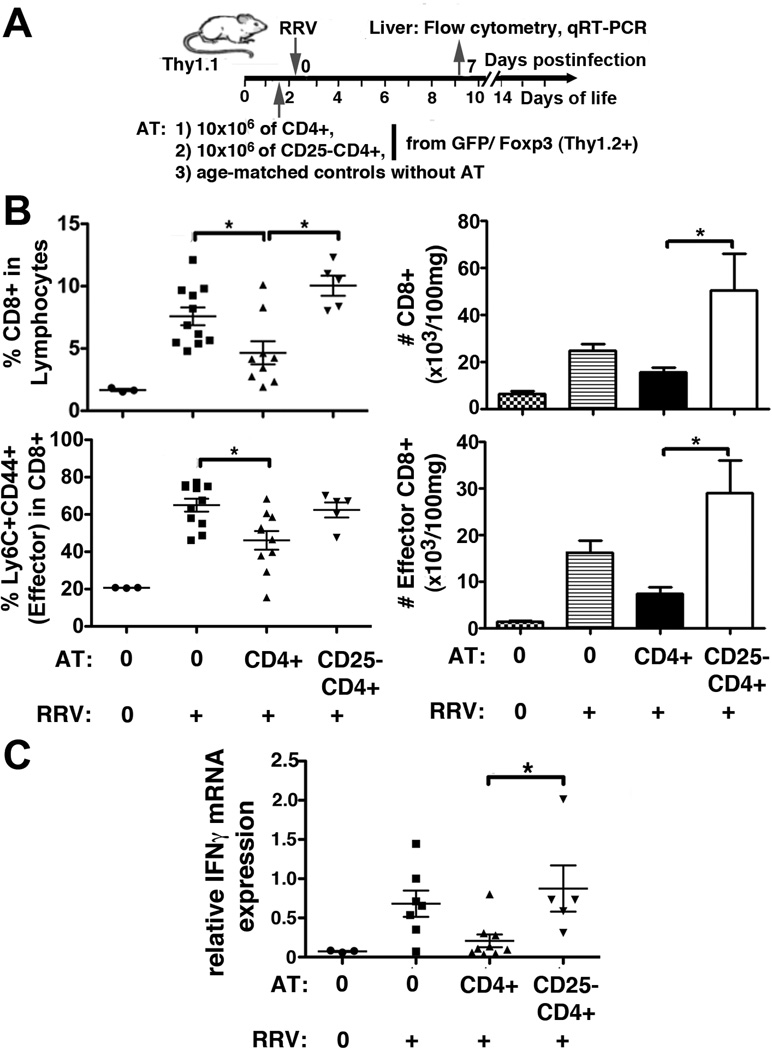
We have previously shown that AT of total CD4 cells prior to RRV infection early after birth improves weight gain and survival in experimental BA(10). Here, we elucidate the role of Tregs in this AT system by comparing the effects of total CD4 with that of Treg-depleted CD4 cells on T-lymphocyte activation and BA phenotype. Depletion of CD25+ cells reduced the frequencies of CD25+FoxP3+ and of total FoxP3+Tregs within the donor CD4 cells by more than 100- and 12-fold, respectively (Supplemental Fig 1). Following AT of total CD4 cells, but not after AT of CD25−CD4 cells, the frequencies of total CD8 and of effector (Ly6C+CD44+) CD8 lymphocytes were both significantly reduced at 7 dpi compared with RRV infected control mice without AT (Fig 1B and Supplemental Fig 2). Ly6C+CD44+ effector CD8 cells represent a subset of T-lymphocytes in BALB/c mice with enhanced cytotoxic killing and IFNγ production(17). AT of CD25−CD4 cells resulted in increased numbers of total and effector CD8 cells in the liver, compared with AT of Treg-containing CD4 cells (Fig 1B), which was associated with up-regulation of hepatic mRNA expression for IFNγ in these mice (Fig 1C). Decreased inflammatory responses following AT of CD4 cells were associated with a 2.5-fold increase of CD4 lymphocytes in the liver at 7dpi compared with controls without AT (Supplemental Fig 3A). The number of donor CD4 cells and donor Tregs detected in the liver at 7dpi is depicted in Supplemental Fig 3B and C, respectively. Although the numbers of donor CD4 cells emerging in the liver were similar between mice subjected to AT of total CD4 or of CD25−CD4 cells, a significantly lower proportion of donor Tregs populated the liver following AT of CD25−CD4 cells (Supplemental Fig 3D). Collectively, these data demonstrate that Tregs are critical for control of CD4 help to hepatic effector T-lymphocyte expansion and IFNγ production following post-natal RRV infection. Next, we explored whether Treg-inhibition of the adaptive immune response at 7dpi impacted the BA phenotype of ductal obstruction.
Figure 1. Treg-containing CD4 cells reduce adaptive immune responses in BA.
Neonatal mice were adoptively transferred before RRV inoculation with either total CD4 or with CD25−CD4 lymphocytes from Foxp3/GFP reporter mice (A; experimental design). Hepatic MNC were isolated at 7dpi and frequencies of total CD8 (gated on lymphocytes, see Supplemental Fig 2) and of effector (Ly6C+CD44+) CD8 cells were enumerated by flow cytometry. Values in graphed frequencies represent individual mice, the horizontal bars display mean±SEM. The total cell counts for these mice are calculated based on the lymphocyte frequencies and numbers of total MNC per 100 mg of liver weight and are displayed as mean+SEM. (C) Hepatic IFNγ expression was determined by SYBR green based qRT-PCR using HPRT for normalization in ΔΔCT analysis. Symbols denote expression values per mouse, the horizontal bars indicate mean±SEM of relative IFNγ expression per group. In (B) and (C), differences between the groups “No AT and RRV”, “CD4 AT and RRV”, and “CD25−CD4 AT and RRV” were tested for statistical significance using one-way ANOVA, with * indicating p<0.05. Differences not denoted with * are non-significant.
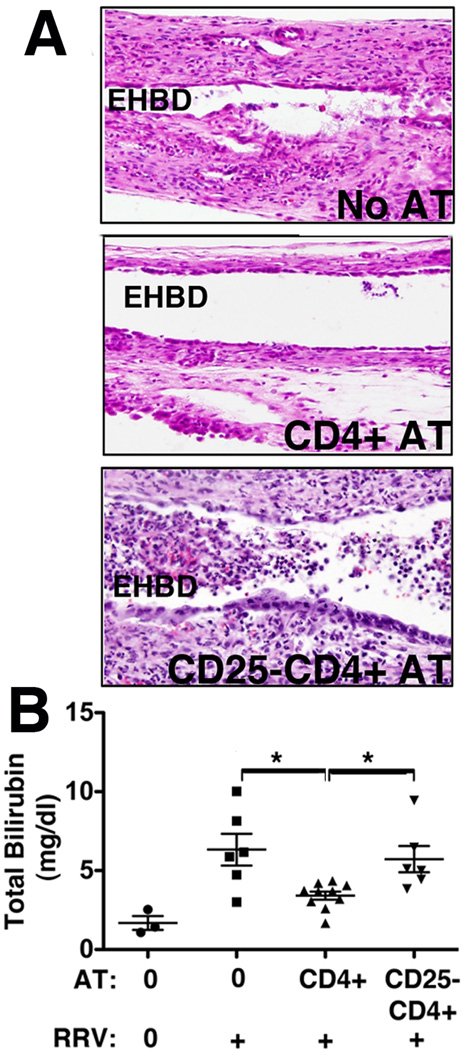
Without AT, RRV infection caused invasion of the EHBD with inflammatory cells denuding the epithelial lining at 7dpi. Following AT of total CD4 cells, periductal inflammation was reduced and the epithelial lining of the bile duct remained intact, as opposed to AT of CD25−CD4 cells which did not prevent inflammatory obstruction of the EHBD and cholangiocyte injury (Fig 2A). Consistent with a decrease in bile duct injury, plasma total bilirubin levels were reduced after AT of CD4, but not after AT of CD25−CD4 lymphocytes, compared with RRV infected controls without AT (Fig 2B). Thus, AT of total CD4 cells attenuates the BA phenotype at 7dpi, at the time of inflammatory ductal obstruction, in Treg-dependent fashion.
Figure 2. Treg-containing CD4 cells attenuate bile duct obstruction in BA.
(A) Extrahepatic bile ducts (EHBDs) were micro-dissected at 7dpi following RRV challenge preceded by AT of total CD4 or CD25−CD4 cells (or no AT in controls). Representative photomicrographs of H&E stained longitudinal sections of EHBDs near the hilum show invasion of the bile ducts with inflammatory cells denuding the epithelial lining in the ‘No AT’ group, reduction of inflammation and of epithelial injury in the ‘CD4 AT’, and inflammatory obstruction of the lumen in the ‘CD25−CD4 AT’ group. (B) Plasma total bilirubin concentrations at 7dpi were determined in a colorimetric assay. Symbols denote plasma levels per mouse, horizontal bars indicate mean±SEM with * indicating p<0.05 for comparisons of the groups “No AT and RRV”, “CD4 AT and RRV”, and “CD25−CD4 AT and RRV”, using one-way ANOVA. Differences not denoted with * are non-significant.
Hepatic mDCs control costimulation of T-lymphocytes during bile duct obstruction
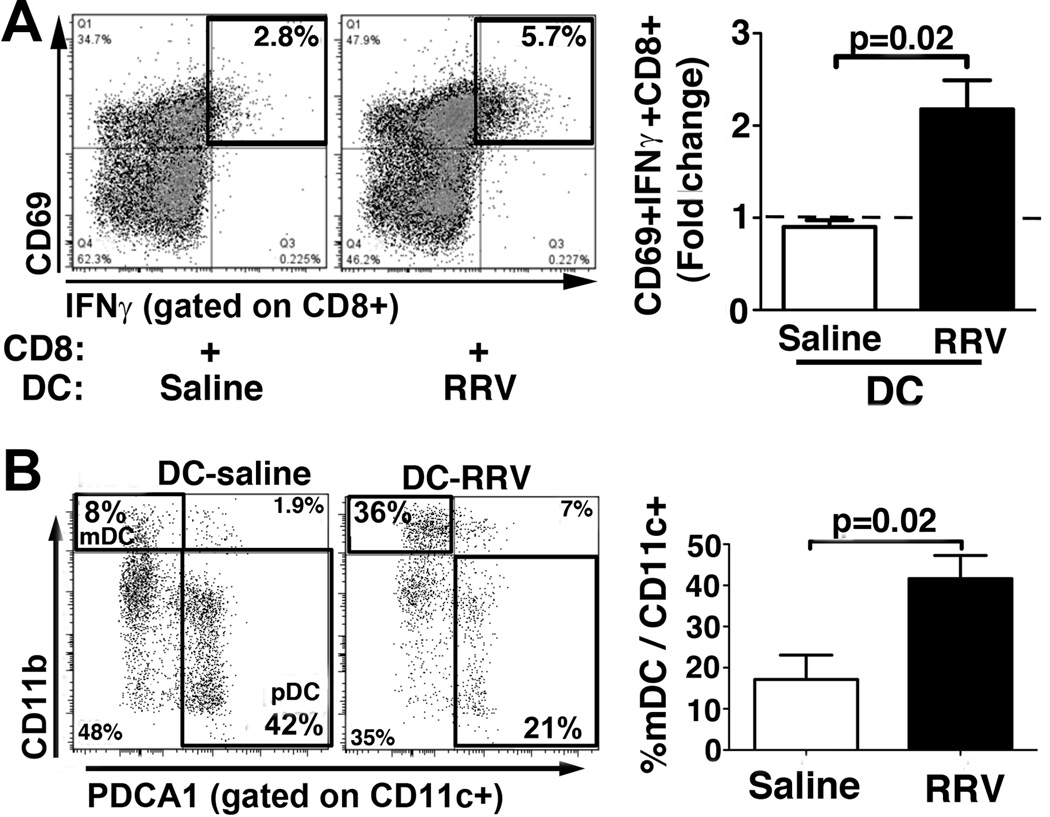
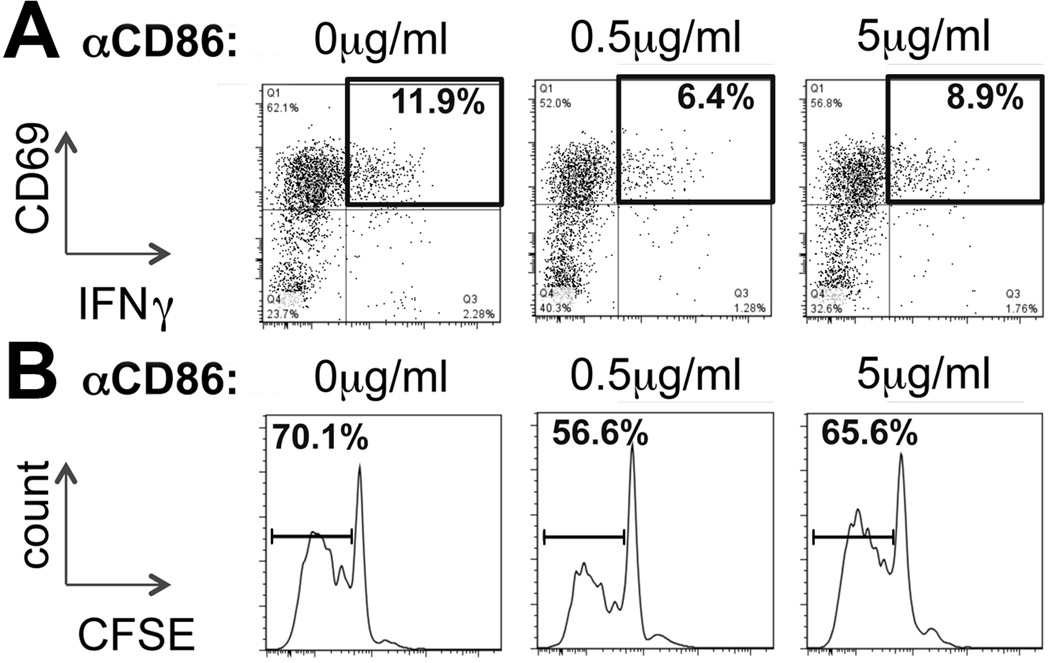
Based on a report showing that Treg-control of T-lymphocyte activation may be mediated by DCs(18), we hypothesized that hepatic DCs activate naïve CD8 cells in experimental BA, and that this DC/CD8 crosstalk is inhibited by Tregs. We found that DCs purified from RRV infected mice at 7dpi enhanced in vitro CD8 activation by about 2-fold compared with DCs from saline treated mice (Fig 3A). The increased stimulatory capacity of purified DCs from RRV infected mice was associated with a 2-fold increase in the percentage of CD11b+ mDCs (Fig 3B) and a trend toward decreased frequency of PDCA1+ plasmacytoid (p)DCs compared with age matched, non-infected controls (mean±SEM for % pDCs/CD11c+: 17.2±3.0 vs 27.7±5.7 in RRV vs saline; p=0.14 in t test). Changes in composition of DC subsets in RRV infected mice were accompanied by up-regulation of the B7 costimulatory molecules CD80 (B7.1) and CD86 (B7.2) on CD11c+ DCs (Supplemental Fig 4). In order to derive direct evidence for the role of B7 molecules in DC-dependent activation of T-lymphocytes, hepatic DCs from RRV infected mice were cultured with CFSE labeled naïve CD8 cells in presence of various concentrations of purified αB7 antibodies. Conditioning of the culture media with αCD86 at a concentration of 0.5µg/ml decreased both the frequency of CD8 cells expressing CD69 and IFNγ (Fig 4A) and the percentage of proliferating CD8 cells (Fig 4B) by about 40%. Lack of inhibition of DC-induced activation and proliferation of CD8 cells in cultures conditioned with a higher concentration of αCD86 (5µg/ml) may be related to the Fc portion of the αCD86 immunoglobulin, which was found to sensitize immature DCs for priming of CD8 lymphocytes(19), thus counteracting the effects of CD86 blockade. Conditioning of the media with equivalent concentrations of αCD80 (clone 16-10A1), shown before to block T-cell stimulation in other experimental systems(20), did not reduce costimulation of CD8 cells by RRV primed hepatic DCs (Supplemental Fig 5). Addition of identical concentrations of rat IgG2b (as control for αCD86) to the culture media did not change CD8 proliferation or activation (data not shown). Based on these results we conclude that in experimental BA intrahepatic T-lymphocyte activation at the time of inflammatory ductal obstruction is regulated by mDCs and mediated by CD86-dependent costimulation.
Figure 3. Hepatic myeloid dendritic cells modulate activation of CD8 lymphocytes in experimental BA.
(A) Hepatic pan-DCs were purified from RRV infected mice and from age-matched, saline injected mice at 7dpi using magnetic beads and co-cultured with naïve CD8 cells from non-infected, neonatal mice. CD8-expression of CD69 and IFNγ was determined by flow cytometry after 48 hours of culture. The numbers in dot plots denote frequencies of CD69+IFNγ+ cells gated on CD3+CD8+ cells. The bar graph displays the fold change in frequencies of CD69+IFNγ+CD8+ cells in co-cultures of CD8 lymphocytes with DCs from saline or RRV treated mice, compared with CD8 cells cultured without DCs, denoted by the dashed line. Values are expressed as mean+SEM from 3 independent experiments with 10–12 pooled livers for DC isolation per condition. (B) Hepatic pan-DCs, purified from RRV infected mice and from saline treated controls at 7dpi, were subjected to flow cytometry to determine the frequencies of CD11b+ myeloid (m) DCs and of PDCA1+ plasmacytoid (p) DCs within CD11c+ cells. Numbers in dot plots indicate the frequencies of positive cells within the gate, values in bar graphs denote mean+SEM of frequencies of DC subsets from 4 independent experiments.
Figure 4. Hepatic costimulation of T-lymphocytes in BA is mediated by CD86.
(A) Hepatic pan-DCs were purified from RRV infected mice at 7dpi and cultured with CFSE-labeled naïve CD8 cells from non-infected neonatal mice in presence of various concentrations of antibodies against CD86. CD8 activation, correlating with expression of CD69 and IFNγ, was determined by flow cytometry after 72 hours of culture. The numbers in representative dot plots denote frequencies of CD69+IFNγ+ cells gated on CD8 cells. (B) Proliferation of CD8 cells was determined in a CFSE dilution assay. The numbers above the intervals in the histograms denote percentages of CD8 cells with decreased cellular CFSE content, correlating with the frequency of proliferating CD8 cells in the co-culture. Plots and histograms are representative for 2 independent experiments.
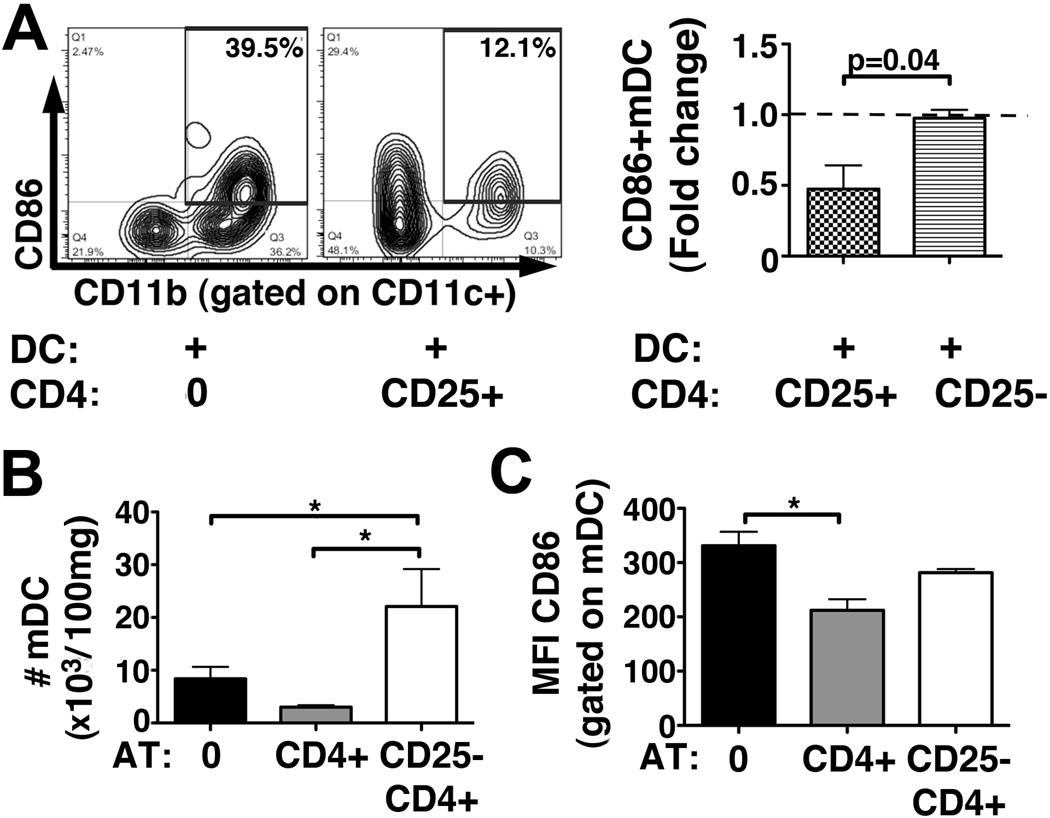
Hepatic mDCs are cellular targets of Tregs during neonatal bile duct obstruction
In order to test the hypothesis that intrahepatic DC-dependent T-lymphocyte activation in BA can be modulated by Tregs, we co-cultured hepatic pan-DCs from RRV infected mice with Tregs (CD25+) or non-Treg (CD25−) CD4 cells, and found down-regulation of CD86 on mDCs when cultured with Tregs (Fig 5A). In vivo, AT of CD25−CD4 cells increased the number of hepatic mDCs by >4-fold at 7dpi compared with mice subjected to AT of Treg-containing CD4 cells (Fig 5B and Supplemental Fig 6). Consistent with our in vitro data, expression of CD86 on mDCs was reduced after AT of CD4 cells, but not after AT of CD25−CD4 cells, when compared with RRV infected controls without AT (Fig 5C). Reduction of CD80 on mDCs after AT of CD4 cells in these mice was not significant (mean±SEM for MFI of CD80 on mDC: 523±32 vs 419±33 vs 487±41 in No AT [n=7] vs CD4 AT [n=4] vs CD25−CD4 AT [n=5]; p=0.18 in One-way-ANOVA). Numbers of hepatic pDCs did not significantly differ between the three groups (mean±SEM for #pDC in 103/100mg liver: 1.5±0.2 vs 0.7±0.2 vs 2.0±0.4 in No AT vs CD4 AT vs CD25−CD4 AT, p=0.11 in One way ANOVA). The MFIs for CD80 and CD86 on pDCs were similar between the groups (data not shown). In summary, using a co-culture system recapitulating the cellular network of CD8 activation in the neonatal liver, we found that CD86 expressing mDCs are critical for costimulation of CD8 cells in experimental BA and represent cellular and molecular targets for Tregs in control of T-lymphocyte activation in BA.
Figure 5. Tregs modulate the molecular phenotype of hepatic DCs in neonatal mice.
(A) Hepatic pan-DCs purified from RRV infected mice at 7dpi were co-cultured with Tregs (CD25+) or non-Treg (CD25−) CD4 lymphocytes from non-infected mice and subjected to flow cytometry after 72 hours. The numbers in the dot plots denote frequencies of CD11b+ mDCs expressing CD86. The bar graph displays the fold change of frequency of CD86+ mCDs in presence of Tregs or non-Treg CD4 cells compared with DCs cultured without CD4 cells, denoted by the dashed line, from 2 independent experiments. (B) Hepatic MNCs were isolated from neonatal mice on day 7 after RRV challenge preceded by AT of CD4 cells (n=4), AT of CD25−CD4 cells (n=5) or no AT (n=7), and subjected to flow cytometry to enumerate total numbers of hepatic mDCs. Representative dot plots are shown in Supplemental Fig 6. (C) Expression of CD86 on hepatic mDCs was determined by flow cytometry and is displayed as mean fluorescent intensity (MFI) for CD86. Values in (B) and (C) are expressed as mean+SEM with * p<0.05, comparing the three groups using one-way ANOVA. Differences not denoted with * are non-significant.
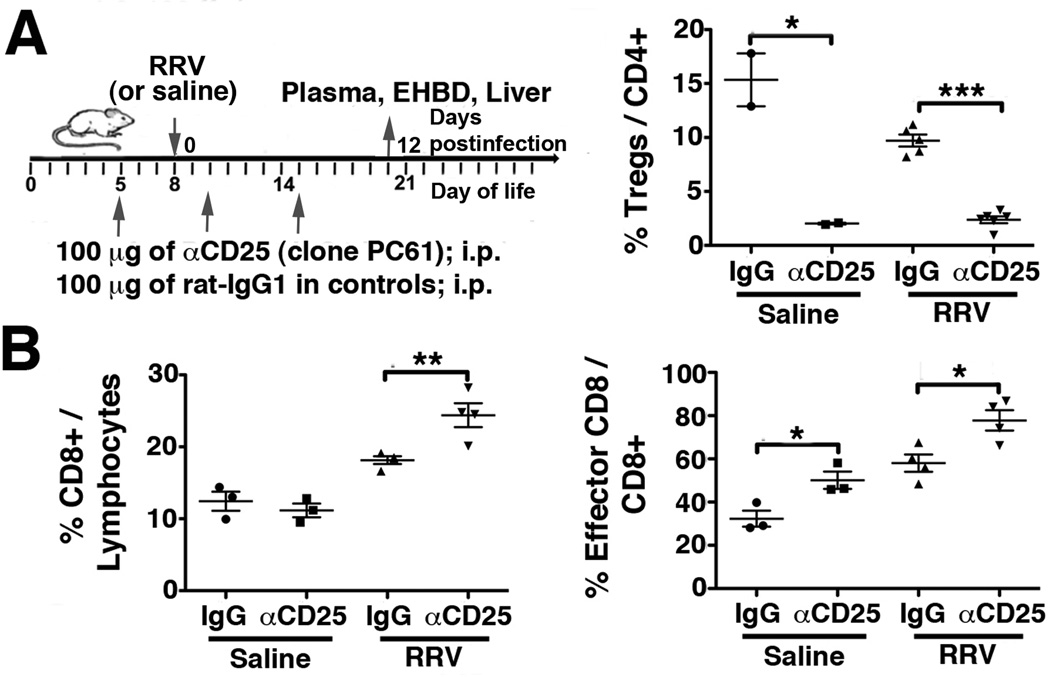
Treg-depletion in older mice enhances DC-mediated CD8 activation and increases susceptibility to RRV induced bile duct injury
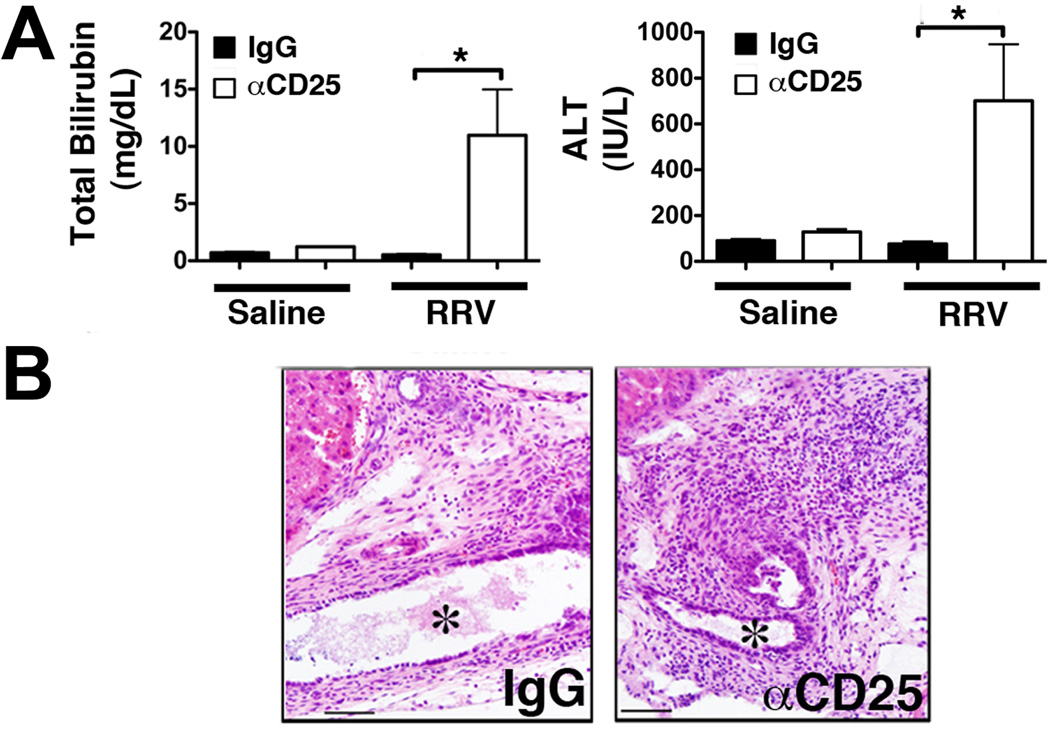
Based on our previous findings of a prompt hepatic Treg-response upon RRV challenge in older mice resistant to BA(10), we reasoned that depletion of Tregs in these mice should increase their susceptibility to virus induced biliary injury. We treated neonatal mice with αCD25 (clone PC61; injection schedule in Fig 6A), an antibody known to deplete Tregs in newborn mice(21). Treatment with αCD25 reduced the frequency of CD25+Foxp3+Tregs in the liver by >7-fold in non-infected and by >4-fold in RRV infected mice (Fig 6A). Following RRV infection on day of life 8, the frequencies of total and of effector (Ly6C+CD44+) CD8 cells in the liver were increased in Treg-depleted mice compared with isotype-IgG treated control mice (Fig 6B). Furthermore, mean plasma bilirubin and ALT levels were increased by >20- and >9-fold, respectively, in αCD25 treated compared with control mice, consistent with aggravated hepatobiliary injury in Treg-depleted mice following RRV challenge (Fig 7A). H&E stained sections of microdissected EHBDs revealed increased periductal inflammation and luminal narrowing in Treg-depleted compared with non-depleted infected controls (Fig 7B). Complete bile duct obstruction or increased mortality were not observed in CD25-depleted mice within 3 weeks after RRV inoculation on day 8.
Figure 6. Antibody mediated depletion of Tregs in older mice increases population of the liver with CD8 lymphocytes following RRV challenge.
8-day-old mice were challenged with RRV following Treg-depletion with αCD25-antibody or treatment with isotype-matched IgG. Hepatic MNC were isolated from αCD25- and IgG-treated mice on day 12 after RRV (or saline) injection and subjected to flow cytometry to enumerate frequencies of CD25+FoxP3+ Tregs (A), of total CD8 cells and of effector Ly6C+CD44+ CD8 lymphocytes (B). Values are expressed as mean±SEM with * p<0.05, ** p≤0.01 and *** p<0.005 in unpaired t test.
Figure 7. Depletion of Tregs in older mice increases susceptibility to RRV induced hepatobiliary injury.
8-day-old mice were challenged with RRV following Treg-depletion with αCD25-antibody or treatment with isotype-matched IgG. (A) Plasma total bilirubin and ALT concentrations at 12dpi were determined in colorimetric assays. Values are expressed as mean+SEM with * p<0.05, in unpaired t test. (B) EHBDs were microdissected from RRV-infected αCD25- or IgG-treated mice at 12 dpi and stained with H&E. Representative photomicrographs of longitudinal sections of the EHBDs near the liver hilum demonstrate increased peri-ductal inflammation and narrowing of the lumen (*) in CD25-depleted mice compared with controls.
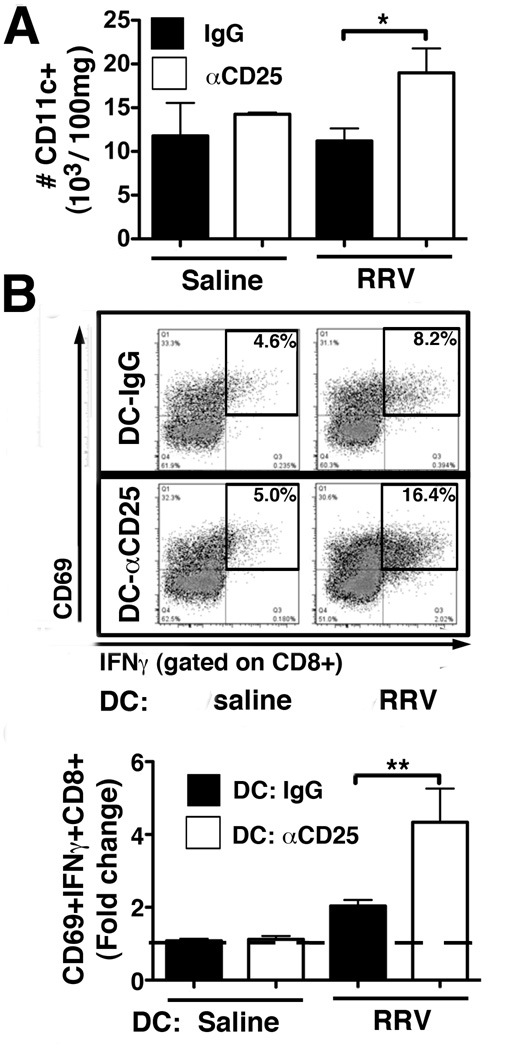
Investigating whether the effects of Treg-depletion on T-lymphocyte activation were mediated by DCs, we studied number and function of hepatic DCs in these mice. At 12dpi following RRV infection on day 8, the number of hepatic CD11c+ DCs was increased by almost 2-fold in Treg-depleted mice compared with IgG-treated controls (Fig 7A). To directly determine the impact of Treg-depletion on DC function, we co-cultured hepatic DCs from the four experimental groups with naïve CD8 cells from age-matched, non-infected mice. Frequency of activated CD8 cells, co-expressing IFNγ and CD69, was increased by >2-fold in co-cultures with DCs from Treg-depleted, RRV-infected mice compared with DCs from IgG-treated, infected controls (Fig 7B). These results show that depletion of neonatal Tregs in the liver results in enhanced DC-mediated CD8 lymphocyte activation and renders older neonatal mice susceptible to RRV-induced bile duct injury.
Discussion
This study demonstrates that effector T-lymphocyte response and biliary injury in experimental BA are susceptible to inhibition by Tregs. AT of total CD4 lymphocytes soon after birth and before RRV infection reduced intrahepatic frequency of effector T-lymphocytes, cholestasis and inflammatory bile duct obstruction in Treg dependent fashion. Using complementary in vitro and in vivo experiments, we found that these effects of Tregs on the hepatic immune response were mediated by modulation of the stimulatory capacity of hepatic DCs, specifically by down-regulation of CD86 expression on mDCs. These mechanisms of Treg-control of immune activation were further validated in an experimental system in which Treg-depletion in older mice resulted in enhanced DC-mediated CD8 lymphocyte activation and aggravated hepatobiliary injury.
We have previously reported that AT of total CD4 cells before post-natal RRV infection reduced the surge of hepatic NK cells at 5dpi(10). Here we show that Treg-containing CD4 cells also constrain the adaptive T-cell response at 7dpi, at the time of maximum inflammatory ductal obstruction(8, 9). Of note, reduction of hepatic CD8 T-lymphocyte expansion by AT of Treg-containing CD4 cells persists throughout the later phase of BA pathogenesis, as shown by us before for day 13 after viral challenge, and is associated with decreased mortality and improved weight gain at this time point(10). Based on the lack of reduction of inflammatory cytokine production and effector T-cell response following AT of Treg-depleted CD4 cells, we conclude that the suppression of immune activation in experimental BA by AT of CD4 cells requires Tregs. Significant increases in numbers of total and effector CD8 cells in the liver were associated with raised plasma bilirubin levels following AT of CD25−CD4 cells compared with AT of total Treg-containing CD4 cells. Whether these differences are only the result of lack of (donor) Treg-inhibition of CD8 activation in mice transplanted with CD25−CD4 cells or whether donor CD25−CD4 cells provide T-cell help to differentiation and expansion of hepatic CD8 lymphocytes, potentially aggravating these effector T cell responses, cannot be definitively answered based on our data. Given the absence of significant differences in hepatic CD8 counts and plasma bilirubin levels between the groups “No AT and RRV” and “CD25−CD4 AT and RRV”, we conclude that deficiency in donor Treg suppressor function is the primary mechanism determining hepatic immune responses and bile duct injury in recipients of CD25−CD4 cells. Furthermore, the degree of ductal obstruction at 7dpi is likely a cumulative result of the effector function of hepatic NK cells initiating bile duct epithelial injury early after viral challenge(3) and of cytotoxic CD8 lymphocytes driving progression of ductal obstruction at a later phase of BA pathogenesis(9). Since both of these processed may be modulated by Tregs, interpretation of correlations between CD8 responses and plasma bilirubin levels following AT of CD4 populations is limited.
The results of the studies in the AT model strongly suggest that Treg deficiency confers susceptibility to neonatal bile duct injury, which is further supported by our Treg-depletion experiments in older mice. Such depletion increased RRV-induced expansion of total and effector CD8 cells in the liver and dramatically aggravated hepatobiliary injury. Interestingly, the frequency of hepatic effector CD8 cells was also increased in CD25-depleted non-infected mice compared with IgG-treated controls, consistent with the established role of Tregs in prevention of immune activation under physiologic conditions(12). It should be noted that although Treg-depletion in older mice enhanced T cell activation, RRV challenge did not result in development of the full BA phenotype, comprising of complete bile duct obstruction and early death. Whether this is related to additional susceptibility factors specific to the immediate post-natal time period, i.e. molecular properties of neonatal cholangiocytes modulating RRV replication(22), is currently unknown. Our findings also support the hypothesis that host genetic factors controlling Treg function contribute to the unique strain specificity of RRV-induced, murine BA (23). Although the delay in Treg ontogeny in neonatal BALB/c mice, as reported by us(10) and others(24), has also been observed in C57BL/6 mice(25), which are resistant to BA, the suppressor function of Tregs during infection significantly differs between these strains. For instance, Tregs only restrict IFNγ production in BALB/c, but not in C57BL/6 mice, following infection with mycobacterium tuberculosis(26). Therefore, we speculate that BALB/c mice, which mount higher hepatic T cell responses after perinatal RRV challenge than C57BL/6 ones (23), depend on Tregs in control of virus induced T-cell activation, which renders them particularly vulnerable during the period of post-natal paucity of Tregs.
Seeking the cellular and molecular targets for Tregs in control of T-lymphocyte activation in the neonatal liver we identified CD11b+ mDCs expressing the costimulatory molecule CD86 as mediators of intrahepatic CD8 lymphocyte activation. Although hepatic DCs are often tolerogenic and inhibit T cell responses under physiologic conditions, DCs are critical for effector cell activation during hepatobiliary inflammation. Here, we show that during peak inflammatory ductal obstruction at 7dpi, mDCs constitute the predominant DC subset in regulation of T-lymphocyte activation, which is in keeping with reports by other investigators showing cholestasis induced expansion of hepatic mDCs in a bile duct ligation model(27). Further, we found that antibody mediated blockade of CD86, more than of CD80, decreased DC-mediated proliferation of naïve, neonatal CD8 cells and diminished their production of IFNγ in an in vitro co-culture assay, recapitulating the cellular network in the neonatal liver. We propose that in experimental BA hepatic mDCs are critical for intrahepatic T-lymphocyte activation via the B7/CD28 pathway. Importantly, in infants with BA increased B7 expression in the liver is correlated with poor prognosis(28). Our data in an experimental model suggest that this increase is directly involved in pathogenesis and not just a reflection of immune activation.
Finally, we examined how Tregs control this pathogenic DC/T-lymphocyte crosstalk in the neonatal liver. Important findings include: a) Tregs down-regulated expression of CD86 on neonatal hepatic mDCs in vitro, b) AT of Treg-containing CD4+ cells reduced expression of CD86 on mDCs in vivo, and c) on the contrary, Treg-depletion in older mice enhanced stimulatory capacity of hepatic DCs. Based on the association between decreased CD8 responses and down-regulated CD86 on mDCs in livers of mice subjected to AT of Treg-containing CD4 cells compared with infected controls without AT, we conclude that modulation of maturation of hepatic DCs is critical for Treg-inhibition of T cell activation in BA. Similar crosstalks between Tregs and tissue specific DC populations have been described in other experimental systems(12, 18). Increased number of hepatic mDCs following AT of CD25−CD4 likely drives aberrant CD8 expansion in these mice, although the mechanisms for this interaction and its effects on bile duct injury require further investigations. Importantly, this study focuses on immune regulation during ductal obstruction at 7dpi. The cellular targets for Tregs during other stages of bile duct injury and time points may be different. For instance, our previously reported effect of Tregs on hepatic NK cell expansion at 5dpi (10) may be mediated by other DC subsets, for instance pDCs, as supported by a recent report demonstrating that pDCs are key cellular triggers of the innate immune response in the neonatal bile duct (29). In this context, Tregs are likely to have synergistic regulatory effects on different arms of the immune system based on the stages of bile duct injury.
Collectively our in vitro and in vivo studies in a well-established murine model of BA provide compelling evidence that Treg deficiency leads to unopposed DC-dependent costimulation and aberrant activation of effector T-lymphocytes in the neonatal liver conferring susceptibility to BA. Furthermore, our study revealed cellular and molecular candidates for future investigations to elucidate whether costimulatory blockade of T cell activation by targeting Tregs or Treg-dependent pathways may prevent progression of bile duct obstruction in BA.
Supplementary Material
Figure 8. Treg-depletion enhances stimulatory capacity of hepatic DCs.
(A) Hepatic MNCs were purified from αCD25- and IgG-treated mice at 12 dpi after RRV (or saline) injection on day 8 of life. Total numbers of hepatic CD11c+ DCs were determined by flow cytometry. Values are displayed as mean+SEM with *p<0.05 in unpaired t test (n=2–4 mice per group). (B) Purified hepatic pan-DCs from the 4 experimental groups were co-cultured with CD8 cells from non-infected neonatal mice for 48 hours. CD8-activation was determined by flow cytometry and numbers in representative dot plots denote frequencies of CD69+IFNγ+ gated CD8 cells. The fold change of %CD69+IFNγ+CD8+ in DC/CD8 co-cultures compared to CD8 cells cultured without DCs (denoted by the dashed line) is displayed in the bar graph. Values are expressed as mean+SEM of fold changes with 6–9 pooled livers per DC condition in 3 independent experiments with ** p<0.01 in unpaired t test.
Acknowledgments
Financial Support
Dr. Miethke was supported by the American Liver Foundation Liver Scholar Award and is recipient of a Trustee Grant from the Cincinnati Children’s Research Foundation. The work was also supported by the NIH grant R01 AI068524 (to C. A. C.)
List of Abbreviations
- BA
biliary atresia
- RRV
Rhesus rotavirus
- CMV
Cytomegalovirus
- Tregs
regulatory T cells
- dpi
days postinfection
- AT
adoptive transfer
- MNC
mononuclear cells
- DC
dendritic cell
- mDC
myeloid dendritic cell
- pDC
plasmacytoid dendritic cell
- APC
antigen presenting cells
- EHBD
Extrahepatic bile duct
Footnotes
Financial disclosure: none
References
- 1.Balistreri WF, Grand R, Hoofnagle JH, Suchy FJ, Ryckman FC, Perlmutter DH, Sokol RJ. Biliary atresia: current concepts and research directions. Summary of a symposium. Hepatology. 1996;23:1682–1692. doi: 10.1002/hep.510230652. [DOI] [PubMed] [Google Scholar]
- 2.Bezerra JATG, Ryckman FC, Alonso M, Sabla GE, Shneider B, Sokol RJ, Aronow BJ. Genetic induction of proinflammatory immunity in children with biliary atresia. Lancet. 2002;23:1653–1659. doi: 10.1016/S0140-6736(02)11603-5. [DOI] [PubMed] [Google Scholar]
- 3.Shivakumar P, Sabla GE, Whitington P, Chougnet CA, Bezerra JA. Neonatal NK cells target the mouse duct epithelium via Nkg2d and drive tissue-specific injury in experimental biliary atresia. J Clin Invest. 2009;119:2281–2290. doi: 10.1172/JCI38879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mack CL, Falta MT, Sullivan AK, Karrer F, Sokol RJ, Freed BM, Fontenot AP. Oligoclonal expansions of CD4+ and CD8+ T-cells in the target organ of patients with biliary atresia. Gastroenterology. 2007;133:278–287. doi: 10.1053/j.gastro.2007.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hertel PM, Estes MK. Rotavirus and biliary atresia: can causation be proven? Curr Opin Gastroenterol. 2012;28:10–17. doi: 10.1097/MOG.0b013e32834c7ae4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu Y, Yu J, Zhang R, Yin Y, Ye J, Tan L, Xia H. The Perinatal Infection of Cytomegalovirus Is an Important Etiology for Biliary Atresia in China. Clin Pediatr (Phila) 2011 doi: 10.1177/0009922811406264. [DOI] [PubMed] [Google Scholar]
- 7.Riepenhoff-Talty M, Schaekel K, Clark HF, Mueller W, Uhnoo I, Rossi T, Fisher J, et al. Group A rotaviruses produce extrahepatic biliary obstruction in orally inoculated newborn mice. Pediatr Res. 1993;33:394–399. doi: 10.1203/00006450-199304000-00016. [DOI] [PubMed] [Google Scholar]
- 8.Shivakumar PCK, Sabla GE, Miethke A, Tiao G, McNeal MM, Ward RL, Bezerra JA. Obstruction of extrahepatic bile ducts by lymphocytes is regulated by IFN-gamma in experimental biliary atresia. J Clin Invest. 2004;114:322–329. doi: 10.1172/JCI21153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shivakumar P, Sabla G, Mohanty S, McNeal M, Ward R, Stringer K, Caldwell C, et al. Effector role of neonatal hepatic CD8+ lymphocytes in epithelial injury and autoimmunity in experimental biliary atresia. Gastroenterology. 2007;133:268–277. doi: 10.1053/j.gastro.2007.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miethke AG, Saxena V, Shivakumar P, Sabla GE, Simmons J, Chougnet CA. Post-natal paucity of regulatory T cells and control of NK cell activation in experimental biliary atresia. J Hepatol. 2010;52:718–726. doi: 10.1016/j.jhep.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 12.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 13.Lan RY, Cheng C, Lian ZX, Tsuneyama K, Yang GX, Moritoki Y, Chuang YH, et al. Liver-targeted and peripheral blood alterations of regulatory T cells in primary biliary cirrhosis. Hepatology. 2006;43:729–737. doi: 10.1002/hep.21123. [DOI] [PubMed] [Google Scholar]
- 14.Zhang W, Sharma R, Ju ST, He XS, Tao Y, Tsuneyama K, Tian Z, et al. Deficiency in regulatory T cells results in development of antimitochondrial antibodies and autoimmune cholangitis. Hepatology. 2009;49:545–552. doi: 10.1002/hep.22651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brindley SM, Lanham AM, Karrer FM, Tucker RM, Fontenot AP, Mack CL. Cytomegalovirus-specific T cell reactivity in biliary atresia at the time of diagnosis is associated with deficits in regulatory T cells. Hepatology. 2011 doi: 10.1002/hep.24807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haribhai D, Lin W, Relland LM, Truong N, Williams CB, Chatila TA. Regulatory T cells dynamically control the primary immune response to foreign antigen. J Immunol. 2007;178:2961–2972. doi: 10.4049/jimmunol.178.5.2961. [DOI] [PubMed] [Google Scholar]
- 17.Cornejo MG, Kharas MG, Werneck MB, Le Bras S, Moore SA, Ball B, Beylot-Barry M, et al. Constitutive JAK3 activation induces lymphoproliferative syndromes in murine bone marrow transplantation models. Blood. 2009;113:2746–2754. doi: 10.1182/blood-2008-06-164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Onishi Y, Fehervari Z, Yamaguchi T, Sakaguchi S. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc Natl Acad Sci U S A. 2008;105:10113–10118. doi: 10.1073/pnas.0711106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Regnault A, Lankar D, Lacabanne V, Rodriguez A, Thery C, Rescigno M, Saito T, et al. Fcgamma receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J Exp Med. 1999;189:371–380. doi: 10.1084/jem.189.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Razi-Wolf Z, Freeman GJ, Galvin F, Benacerraf B, Nadler L, Reiser H. Expression and function of the murine B7 antigen, the major costimulatory molecule expressed by peritoneal exudate cells. Proc Natl Acad Sci U S A. 1992;89:4210–4214. doi: 10.1073/pnas.89.9.4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim B, Feng N, Narvaez CF, He XS, Eo SK, Lim CW, Greenberg HB. The influence of CD4+ CD25+ Foxp3+ regulatory T cells on the immune response to rotavirus infection. Vaccine. 2008;26:5601–5611. doi: 10.1016/j.vaccine.2008.07.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jafri M, Donnelly B, Allen S, Bondoc A, McNeal M, Rennert PD, Weinreb PH, et al. Cholangiocyte expression of alpha2beta1-integrin confers susceptibility to rotavirus-induced experimental biliary atresia. Am J Physiol Gastrointest Liver Physiol. 2008;295:G16–G26. doi: 10.1152/ajpgi.00442.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leonhardt J, Kuebler JF, Turowski C, Tschernig T, Geffers R, Petersen C. Susceptibility to experimental biliary atresia linked to different hepatic gene expression profiles in two mouse strains. Hepatol Res. 2010;40:196–203. doi: 10.1111/j.1872-034X.2009.00577.x. [DOI] [PubMed] [Google Scholar]
- 24.Asano MTM, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med. 1996;184:387–396. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fontenot JD, Dooley JL, Farr AG, Rudensky AY. Developmental regulation of Foxp3 expression during ontogeny. J Exp Med. 2005;202:901–906. doi: 10.1084/jem.20050784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paula MO, Fonseca DM, Wowk PF, Gembre AF, Fedatto PF, Sergio CA, Silva CL, et al. Host genetic background affects regulatory T-cell activity that influences the magnitude of cellular immune response against Mycobacterium tuberculosis. Immunol Cell Biol. 2010 doi: 10.1038/icb.2010.116. [DOI] [PubMed] [Google Scholar]
- 27.Bleier JI, Katz SC, Chaudhry UI, Pillarisetty VG, Kingham TP, 3rd, Shah AB, Raab JR, et al. Biliary obstruction selectively expands and activates liver myeloid dendritic cells. J Immunol. 2006;176:7189–7195. doi: 10.4049/jimmunol.176.12.7189. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi H, Li Z, Yamataka A, Lane GJ, Miyano T. Role of immunologic costimulatory factors in the pathogenesis of biliary atresia. J Pediatr Surg. 2003;38:892–896. doi: 10.1016/s0022-3468(03)00117-9. [DOI] [PubMed] [Google Scholar]
- 29.Saxena V, Shivakumar P, Sabla G, Mourya R, Chougnet C, Bezerra JA. Dendritic cells regulate natural killer cell activation and epithelial injury in experimental biliary atresia. Sci Transl Med. 2011;3:102ra194. doi: 10.1126/scitranslmed.3002069. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.