Abstract
Cytotoxic CD8 T lymphocytes (CTLs) have an astonishing ability to eliminate pathogen-infected cells. However, if uncontrolled, these CTLs could cause devastating pathology to host tissues. CD8+ effector T cells, therefore, interact with antigen-presenting cells and other immune cells, such as regulatory T cells (Tregs), to regulate further on-site expansion and differentiation of the effector cells. This ensures protection of the host with minimal bystander pathological consequences. During prolonged chronic infections CTLs, however, often lose effector function. Induction of multiple inhibitory pathways is emerging as a major regulator converting effector CTLs into exhausted CTLs during chronic viral infections such as HIV, HCV and HBV. The mechanisms involved in induction of exhaustion during chronic viral infections are the focus of this article. Blockade of inhibitory pathways could potentially restore functional capabilities to exhausted CTLs and represents a potential immune-based intervention in chronic viral infections.
Keywords: HIV, chronic infection, immune regulation, HLA-alleles
Introduction
CD8+ T cells interact via their T cell receptors (TCR) with virus-infected cells by recognizing viral peptides presented on the cell surface of human histocompatibility leukocyte antigen (HLA) molecules. This interaction results in killing of the virus-infected cell through two main pathways; the granule-independent pathway involving Fas/FasL interactions (Poonia et al., 2009) or lytic granule loading of perforin and granzyme B (GzmB) (Migueles et al., 2008). CD8+ T cells play a crucial role against diverse chronic viral infections. In HIV-1 infection the frequency of CD8+ T cells inversely correlates with viremia and disease progression to AIDS (Kiepiela et al., 2007). In addition, enhanced CTL function has been demonstrated in rare individuals who can control infection (elite controllers (EC) (Saez-Cirion et al., 2007) and long-term non-progressors (LTNPs) (Betts et al., 2006, Horton et al., 2006).
Virus specific CD8+ T cells differ in many aspects; in particular their multifunctional capacities upon antigen stimulation, are currently seen as the best correlate of T-cell immunity measurable directly ex vivo (Almeida et al., 2009). CTL from LTNP exhibit multiple effector functions such as cytokine and chemokine production (Betts et al., 2006), higher lytic granule content as a critical determinant of cytotoxicity (Migueles et al., 2008), and preserved proliferative capabilities during chronic HIV-1 infection (Horton et al., 2006). Virus-specific CD8+ T cells also differ with respect to static parameters such as HLA restriction and TCR avidity. Many LTNPs possess HIV-specific CD8+ CTLs restricted by HLA-B*27 or HLA-B*57 (protective alleles) that can continue to proliferate throughout chronic infection, whereas the majority of HIV-specific CD8+ CTLs restricted by non-protective HLA alleles lose their proliferative capacity (Horton et al., 2006). Therefore, the effectiveness of the T cell immune response during chronic viral infections depends on several factors such as target antigen load, TCR avidity and structure, T cell differentiation status, and activation/functional profile of CD8+ CTLs (Genesca, 2011). The effectiveness of an effector CTL is also governed by external forces, in particular, by regulation imposed by cells which are specialized for this role, e.g Tregs. Immune regulation during chronic viral infection is the subject of this brief review.
Pathogenesis: Inhibitory pathways to mediate immune exhaustion
Several cell-intrinsic mechanisms have evolved to minimize immune-driven pathology by upregulating inhibitory molecules on activated CTLs, thereby turning them off (Fig. 1A). In addition, immune regulatory cells, by mechanisms extrinsic to the effector cell, can inhibit CTL responses either via ligation with these inhibitory receptors or other mechanisms depicted in Fig. 1b.
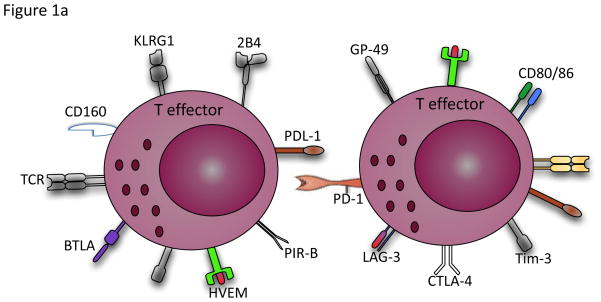
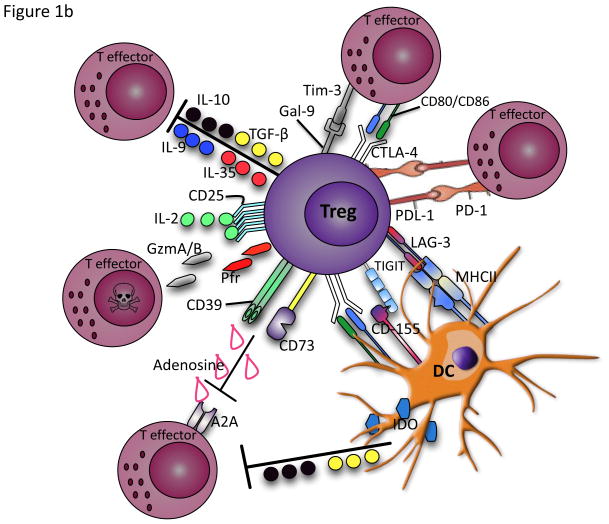
Figure 1.
a) Selective increase and simultaneous expression of a wide range of inhibitory receptors such as PD-1, Tim-3, LAG-3, CTLA-4, KLRG1, 2B4, CD160, BTLA, HVEM, GP-49 and PRI-B on activated and exhausted effector T cells. b) Activated Tregs produce anti-inflammatory cytokines such as IL-10, TGF-β, IL-35 and IL-9 and cytolysis molecules (granzyme A/B) and perforin to inhibit effector T cell responses. They can disrupt metabolic function by IL-2 deprivation of effector T cells, cAMP inhibition or by CD39/CD73-adenosine generated A2A mediated suppression. Tregs can also suppress by direct contact of inhibitory receptors with their ligands, delivering inhibitory signals to the effector T cells and DCs. Excessive control exerted by Tregs via these pathways under some circumstances such as chronic viral infections therefore could result in inhibition of antigen-specific immune response.
a. Inhibitory receptors
Failure to eliminate viral infections such as HIV, HCV and HBV leads to a progressive loss of T cell functionality. This attenuation of viral-specific T-cell responses in chronic viral infections, known as 'exhaustion', is hierarchical, with loss of proliferative capabilities and IL-2 secretion first followed by loss of TNF and eventually loss of IFN-γ production (Shin and Wherry, 2007). While it is known that such exhaustion occurs, the mechanisms that induce such dysfunction are not clear. A number of inhibitory receptors appear to be constitutively upregulated on these cells. Programmed cell death-1 (PD-1) is a well-characterized inhibitory receptor, expressed by a variety of activated immune cells including T cells, and the interaction between PD-1 and its ligands Programmed cell death ligand-1 or -2 (PD-L1 or PD-L2) suppresses T cell functions (Barber et al., 2006). Persistent expression of PD-1 on CD8+ T cells has been shown during HIV, HBV and HCV infections (Rouse and Sehrawat, 2010). Blocking the PD-1–PD-L pathway in vivo enhances CTL function and decreases viral load in the murine LCMV model (Barber et al., 2006). However, functional rejuvenation by PD-1–PD-L blockade is incomplete, and defects in CTL functions remain despite this blockade (Barber et al., 2006), which suggests the involvement of other inhibitory receptors in CD8+ T cell exhaustion. CTL-associated protein 4 (CTLA-4) is another co-inhibitory receptor that acts synergistically with PD-1 to promote T-cell exhaustion in HIV-1, HCV and HBV infections (Kaufmann et al., 2007, Neumann-Haefelin et al., 2006, Schurich et al., 2011). CTLA-4 is upregulated on activated CD4+ T cells, binds to CD80/CD86, and inhibits T cell activation by reducing the production of IL-2 and arresting cell cycle progression (Kaufmann et al., 2007). More recent studies in mice demonstrated constitutive upregulation of several other inhibitory receptors on virus-specific CD8 T cells, such as 2B4, LAG-3 and CD160 (Blackburn et al., 2009). Consistent with these findings, co-expression of inhibitory receptors including PD-1, 2B4, LAG-3 and CD160 was recently reported on HIV-specific CD8+ T cells (Yamamoto et al., 2011). In addition, co-expression of KLRG1 with PD-1, CD160, and 2B4 has been reported on exhausted HCV-specific CD8+ T cells and suggest that T cell exhaustion contributes to the failure of HCV-specific CD8+ T cell response (Bengsch et al., 2010). It appears, the more exhausted the cells, the greater inhibitory molecules they express. These data suggest that blockade of multiple inhibitory molecules might be more effective in restoring CTLs responses than single blockade of the PD-1 pathway. This has been demonstrated in the LCMV model by combination of PD-1 and LAG-3 blockade (Blackburn et al., 2009) and in HIV-1 by simultaneous blockade of the PD-1 and 2B4 pathways in vitro (Yamamoto et al., 2011).
T cell immunoglobulin and mucin domain-containing molecule 3 (Tim-3) is another inhibitory receptor upregulated on exhausted CD8+ T cells in patients with HIV and HCV (Jones et al., 2008, McMahan et al., 2010). Tim-3, through interaction with its ligand, Galectin-9 (Gal-9), is involved in the induction of peripheral tolerance and administration of Tim-3Ig abrogates the development of tolerance in Th1 cells (Sabatos et al., 2003). Gal-9, a member of the β-galactoside-binding animal lectin family, is one of the well known ligands for Tim-3, which is expressed on a wide rage of immune cells (Wiersma et al., 2011). Finally expression of PIR-B and GP49 has been reported on exhausted CD8+ T cells in mice. Both have been linked to negative regulation (Blackburn et al., 2009) however, future studies are required to further delineate their functions. Thus, the diversity of inhibitory receptors suggests multiple non-redundant pathways contribute in qualitatively different ways to induce T cell exhaustion.
b. Regulatory Cells
Tregs are essential to maintain immune homeostasis and play a crucial role in modulating pathophysiological responses by suppressing a wide variety of cell types. Although Tregs employ several strategies to mediate suppression (Fig. 2b), here we exclusively focus on suppression through inhibitory receptors. Tregs express PD-L1 and ligation of PD-1 on effector cells by either PD-L1 or PD-L2 attenuates effector T-cell proliferation and cytokine production (Francisco et al., 2010). CTLA-4 is also constitutively expressed on Tregs and may suppress T effectors by ligating CD80 on the effector T cell, resulting in the inhibition of T-cell proliferation and cytokine production (Kaufmann et al., 2007). In addition, Tregs expressing CTLA-4 can induce the down-regulation of CD80 and CD86 on DCs, thereby educating the DC to become less activated and/or more tolerogenic (Francisco et al. 2010). Gal-9 is also constitutively expressed on Tregs (Wang et al., 2009), and thus Tregs can suppress proliferative capabilities of effector T cells through interaction with TIM-3 on the effector cells (Elahi et al., 2011). Furthermore, expression of LAG-3 has been also reported on Tregs, which is required for maximal suppressive activity of both natural and induced Tregs by binding to MHCII (Huang et al., 2004). Lastly, expression of TIGIT on Tregs and its interaction with CD155, expressed on DCs, increases the secretion of IL-10 and decreases the secretion of proinflammatory cytokines (Yu et al., 2009).
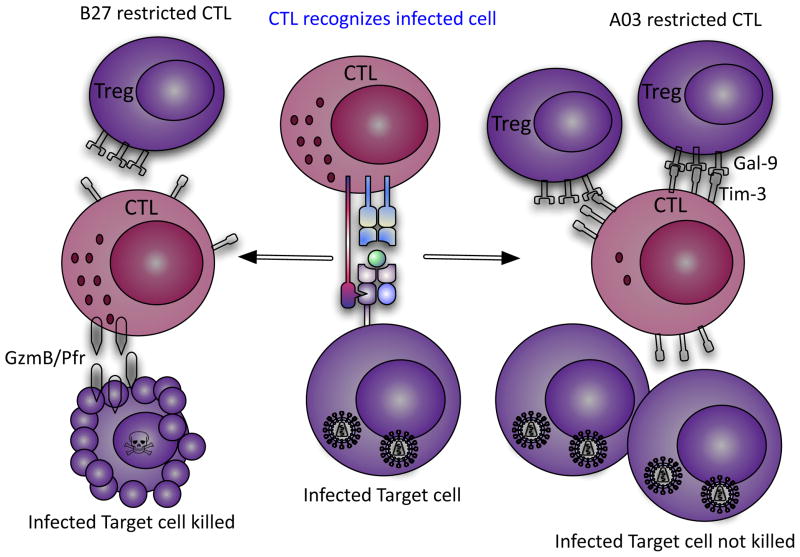
Figure 2.
Model depicting differential suppression of HLA-B27/B57-restricted HIV-specific CD8+ vs. HLA-A03 T cells by Tregs. HIV-specific B27-restricted CD8+ T cell do not upregulate surface expression of Tim-3 upon recognition of their cognate epitopes on HIV-infected CD4+ T cells, whereas HIV-specific A03-restricted CD8+ T cell upregulate high surface expression of Tim-3. Tregs suppress and kill A03-restricted CD8+ T cells due to their high expression of Tim-3 but cannot suppress proliferation of B27-restricted CD8+ T cells. Highly proliferating B27-restricted CD8+ T cells upregulate high levels of GzmB and kill infected CD4+ T cells that they encounter. Thus B27-restricted CD8+ T cells can control HIV replication during chronic infection, whereas A03-restricted CD8+ T cells cannot.
However, it is not yet clear, whether Tregs are detrimental in chronic viral infection because they suppress antigen-specific responses, or if they are beneficial because they inhibit immune activation associated with infection. Some recent in vivo studies suggest that high frequencies of Tregs during HIV-1 infection are detrimental. Specifically, a recent in vivo phase 3 trial indicated that IL-2 therapy preferentially expands Tregs in infected individuals and that individuals with the greatest expansion are more likely to progress to AIDS (Weiss et al. 2010).
Moreover, our recent study provided novel insights into the role of Tregs in chronic viral infection. This study showed that HIV-specific CD8+ T cells restricted by protective HLA-B*27/B*57 alleles are much more resistant to Treg–mediated suppression than CD8+ T cells restricted by non-protective HLA alleles (Elahi et al. 2011). This resistance to Treg suppression was because CD8+ T cells restricted by protective HLA-alleles upregulated low levels of Tim-3 when they encountered their antigen. Thus, Tregs were not able to suppress proliferation of these HIV-specific CD8+ CTLs through Tim-3:Gal-9 interactions. In contrast, HIV-specific CD8+ CTLs restricted by non-protective alleles upregulated high levels of Tim-3 when they encountered their antigen, and were subsequently suppressed by Tregs. The lack of suppression of B*27/B*57-restricted CTL allows them to continue to proliferate and kill infected targets during chronic infection, which may account for delayed disease progression in persons with protective alleles (Elahi et al., 2011). Interestingly, the ability of B*27/B*57 CTLs to evade Treg suppression is not restricted to HIV-1 infection since EBV- and HSV-specific CTLs restricted by these alleles are also not suppressed by Tregs in HIV-1 uninfected individuals (Elahi et al., 2011). Based upon these observations we suggested that there are two divergent outcomes for HIV-specific CD8+ CTLs during chronic infection: the majority of HIV-specific CTLs upregulated Tim-3 when they encountered their cognate epitopes and were subsequently suppressed by Tregs; however, CD8+ CTLs restricted by protective HLA allele groups upregulated less Tim-3 but more GzmB upon recognition of their cognate epitopes (Elahi et al., 2011). Therefore, they are subsequently less susceptible to Treg mediated suppression (Fig. 2), but able to kill Tregs they encounter in a GzmB dependent manner (Elahi et al., 2011). It is interesting to speculate that the inability of CTL restricted by HLA-B27/57 to be suppressed accounts for the observed higher incidence of autoimmune disease that has previously been reported in individuals with these HLA allele groups (Mathieu et al., 2009, Feng et al., 2009). Thus, possession of these allele groups seems to be a double-edged sword. Because T cells restricted by them cannot be tolerized, these allele groups are beneficial in chronic infection but detrimental in autoimmunity. The TCR characteristics and downstream molecular pathways that explain why protective CD8+ CTLs upregulate less Tim-3 upon recognition of their cognate epitopes but instead upregulate more lytic effector molecules such as GzmB require further investigation. Thus, our study uncovers a previously unknown mechanism of immune regulation in chronic viral infections that certain CTLs, notably restricted by HLA-B*27 and HLA-B*57 evade Tregs mediated suppression.
Therapy
Blockade of inhibitory receptors represent potential targets for novel immune therapies in chronic viral infections such as HIV-1 infection. Indeed, antibodies that target PD-1 have demonstrated some promise in enhancing viral control and restoring functions in exhausted CTLs in SIV infection in macaques (Velu et al., 2009). Tim-3 is another attractive candidate for therapeutic intervention. Blockade of the Tim-3:Gal-9 interaction may be one way of manipulating the immune response for functional cure of HIV-1 T cell dysfunction. Although, these inhibitory receptor-mediated pathways represent potential targets for novel immune therapies in chronic viral infections, such a strategy must be approached with caution to prevent untoward autoimmune complications.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almeida JR, Sauce D, Price DA, Papagno L, Shin SY, Moris A, Larsen M, Pancino G, Douek DC, Autran B, Saez-Cirion A, Appay V. Antigen sensitivity is a major determinant of CD8+ T-cell polyfunctionality and HIV-suppressive activity. Blood. 2009;113:6351–6360. doi: 10.1182/blood-2009-02-206557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- Bengsch B, Seigel B, Ruhl M, Timm J, Kuntz M, Blum HE, Pircher H, Thimme R. Coexpression of PD-1, 2B4, CD160 and KLRG1 on exhausted HCV-specific CD8+ T cells is linked to antigen recognition and T cell differentiation. PLoS Pathog. 2010;6:e1000947. doi: 10.1371/journal.ppat.1000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, Roederer M, Koup RA. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elahi S, Dinges WL, Lejarcegui N, Laing KJ, Collier AC, Koelle DM, McElrath MJ, Horton H. Protective HIV-specific CD8+ T cells evade Treg cell suppression. Nat Med. 2011;17:989–995. doi: 10.1038/nm.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng BJ, Sun LD, Soltani-Arabshahi R, Bowcock AM, Nair RP, Stuart P, Elder JT, Schrodi SJ, Begovich AB, Abecasis GR, Zhang XJ, Callis-Duffin KP, Krueger GG, Goldgar DE. Multiple Loci within the major histocompatibility complex confer risk of psoriasis. PLoS Genet. 2009;5:e1000606. doi: 10.1371/journal.pgen.1000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genesca M. Characterization of an effective CTL response against HIV and SIV infections. J Biomed Biotechnol. 2011;2011:103924. doi: 10.1155/2011/103924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton H, Frank I, Baydo R, Jalbert E, Penn J, Wilson S, McNevin JP, McSweyn MD, Lee D, Huang Y, De Rosa SC, McElrath MJ. Preservation of T cell proliferation restricted by protective HLA alleles is critical for immune control of HIV-1 infection. J Immunol. 2006;177:7406–7415. doi: 10.4049/jimmunol.177.10.7406. [DOI] [PubMed] [Google Scholar]
- Huang CT, Workman CJ, Flies D, Pan X, Marson AL, Zhou G, Hipkiss EL, Ravi S, Kowalski J, Levitsky HI, Powell JD, Pardoll DM, Drake CG, Vignali DA. Role of LAG-3 in regulatory T cells. Immunity. 2004;21:503–513. doi: 10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Jones RB, Ndhlovu LC, Barbour JD, Sheth PM, Jha AR, Long BR, Wong JC, Satkunarajah M, Schweneker M, Chapman JM, Gyenes G, Vali B, Hyrcza MD, Yue FY, Kovacs C, Sassi A, Loutfy M, Halpenny R, Persad D, Spotts G, Hecht FM, Chun TW, McCune JM, Kaul R, Rini JM, Nixon DF, Ostrowski MA. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med. 2008;205:2763–2779. doi: 10.1084/jem.20081398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann DE, Kavanagh DG, Pereyra F, Zaunders JJ, Mackey EW, Miura T, Palmer S, Brockman M, Rathod A, Piechocka-Trocha A, Baker B, Zhu B, Le Gall S, Waring MT, Ahern R, Moss K, Kelleher AD, Coffin JM, Freeman GJ, Rosenberg ES, Walker BD. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat Immunol. 2007;8:1246–1254. doi: 10.1038/ni1515. [DOI] [PubMed] [Google Scholar]
- Kiepiela P, Ngumbela K, Thobakgale C, Ramduth D, Honeyborne I, Moodley E, Reddy S, de Pierres C, Mncube Z, Mkhwanazi N, Bishop K, van der Stok M, Nair K, Khan N, Crawford H, Payne R, Leslie A, Prado J, Prendergast A, Frater J, McCarthy N, Brander C, Learn GH, Nickle D, Rousseau C, Coovadia H, Mullins JI, Heckerman D, Walker BD, Goulder P. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat Med. 2007;13:46–53. doi: 10.1038/nm1520. [DOI] [PubMed] [Google Scholar]
- Mathieu A, Paladini F, Vacca A, Cauli A, Fiorillo MT, Sorrentino R. The interplay between the geographic distribution of HLA-B27 alleles and their role in infectious and autoimmune diseases: a unifying hypothesis. Autoimmun Rev. 2009;8:420–425. doi: 10.1016/j.autrev.2009.01.003. [DOI] [PubMed] [Google Scholar]
- McMahan RH, Golden-Mason L, Nishimura MI, McMahon BJ, Kemper M, Allen TM, Gretch DR, Rosen HR. Tim-3 expression on PD-1+ HCV-specific human CTLs is associated with viral persistence, and its blockade restores hepatocyte-directed in vitro cytotoxicity. J Clin Invest. 2010;120:4546–4557. doi: 10.1172/JCI43127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migueles SA, Osborne CM, Royce C, Compton AA, Joshi RP, Weeks KA, Rood JE, Berkley AM, Sacha JB, Cogliano-Shutta NA, Lloyd M, Roby G, Kwan R, McLaughlin M, Stallings S, Rehm C, O'Shea MA, Mican J, Packard BZ, Komoriya A, Palmer S, Wiegand AP, Maldarelli F, Coffin JM, Mellors JW, Hallahan CW, Follman DA, Connors M. Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity. 2008;29:1009–1021. doi: 10.1016/j.immuni.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann-Haefelin C, McKiernan S, Ward S, Viazov S, Spangenberg HC, Killinger T, Baumert TF, Nazarova N, Sheridan I, Pybus O, von Weizsacker F, Roggendorf M, Kelleher D, Klenerman P, Blum HE, Thimme R. Dominant influence of an HLA-B27 restricted CD8+ T cell response in mediating HCV clearance and evolution. Hepatology. 2006;43:563–572. doi: 10.1002/hep.21049. [DOI] [PubMed] [Google Scholar]
- Poonia B, Pauza CD, Salvato MS. Role of the Fas/FasL pathway in HIV or SIV disease. Retrovirology. 2009;6:91. doi: 10.1186/1742-4690-6-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse BT, Sehrawat S. Immunity and immunopathology to viruses: what decides the outcome? Nat Rev Immunol. 2010;10:514–526. doi: 10.1038/nri2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatos CA, Chakravarti S, Cha E, Schubart A, Sanchez-Fueyo A, Zheng XX, Coyle AJ, Strom TB, Freeman GJ, Kuchroo VK. Interaction of Tim-3 and Tim-3 ligand regulates T helper type 1 responses and induction of peripheral tolerance. Nat Immunol. 2003;4:1102–1110. doi: 10.1038/ni988. [DOI] [PubMed] [Google Scholar]
- Saez-Cirion A, Lacabaratz C, Lambotte O, Versmisse P, Urrutia A, Boufassa F, Barre-Sinoussi F, Delfraissy JF, Sinet M, Pancino G, Venet A. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc Natl Acad Sci U S A. 2007;104:6776–6781. doi: 10.1073/pnas.0611244104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurich A, Khanna P, Lopes AR, Han KJ, Peppa D, Micco L, Nebbia G, Kennedy PT, Geretti AM, Dusheiko G, Maini MK. Role of the coinhibitory receptor cytotoxic T lymphocyte antigen-4 on apoptosis-Prone CD8 T cells in persistent hepatitis B virus infection. Hepatology. 2011;53:1494–1503. doi: 10.1002/hep.24249. [DOI] [PubMed] [Google Scholar]
- Shin H, Wherry EJ. CD8 T cell dysfunction during chronic viral infection. Curr Opin Immunol. 2007;19:408–415. doi: 10.1016/j.coi.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Velu V, Titanji K, Zhu B, Husain S, Pladevega A, Lai L, Vanderford TH, Chennareddi L, Silvestri G, Freeman GJ, Ahmed R, Amara RR. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458:206–210. doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Wan L, Zhang C, Zheng X, Li J, Chen ZK. Tim-3-Galectin-9 pathway involves the suppression induced by CD4+CD25+ regulatory T cells. Immunobiology. 2009;214:342–349. doi: 10.1016/j.imbio.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Weiss L, Letimier FA, Carriere M, Maiella S, Donkova-Petrini V, Targat B, Benecke A, Rogge L, Levy Y. In vivo expansion of naive and activated CD4+CD25+FOXP3+ regulatory T cell populations in interleukin-2-treated HIV patients. Proc Natl Acad Sci U S A. 2010;107:10632–10637. doi: 10.1073/pnas.1000027107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiersma VR, de Bruyn M, Helfrich W, Bremer E. Therapeutic potential of Galectin-9 in human disease. Med Res Rev. 2011 doi: 10.1002/med.20249. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Price DA, Casazza JP, Ferrari G, Nason M, Chattopadhyay PK, Roederer M, Gostick E, Katsikis PD, Douek DC, Haubrich R, Petrovas C, Koup RA. Surface expression patterns of negative regulatory molecules identify determinants of virus-specific CD8+ T-cell exhaustion in HIV infection. Blood. 2011;117:4805–4815. doi: 10.1182/blood-2010-11-317297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Harden K, Gonzalez LC, Francesco M, Chiang E, Irving B, Tom I, Ivelja S, Refino CJ, Clark H, Eaton D, Grogan JL. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol. 2009;10:48–57. doi: 10.1038/ni.1674. [DOI] [PubMed] [Google Scholar]