Background
The Framingham Heart Study, the longest running prospective study of chronic disease in a population, has been in operation for more than 60 years. Designed shortly after the end of World War II, the study was initiated in response to the rapid rise in cardiovascular disease deaths which was twice as frequent as the second leading cause of death, cancer. At the time coronary heart disease accounted for one in three deaths in men below the age of 60 years. One of six coronary attacks was manifested as sudden death which accounted for half of all coronary deaths and was often the initial symptom of the disease. The Framingham Study represented one of the earliest modern application of epidemiology to chronic disease.
Conduct of the Study
The Framingham Study was planned as a 20 year study to identify factors predisposing to the occurrence of cardiovascular disease and hypertension. Fortunately, the study has been continued and expanded. Details of the history of the study, significant contributions, personnel and publications are available on the website: www.framinghamheartstudy.org and will not be dealt with here.
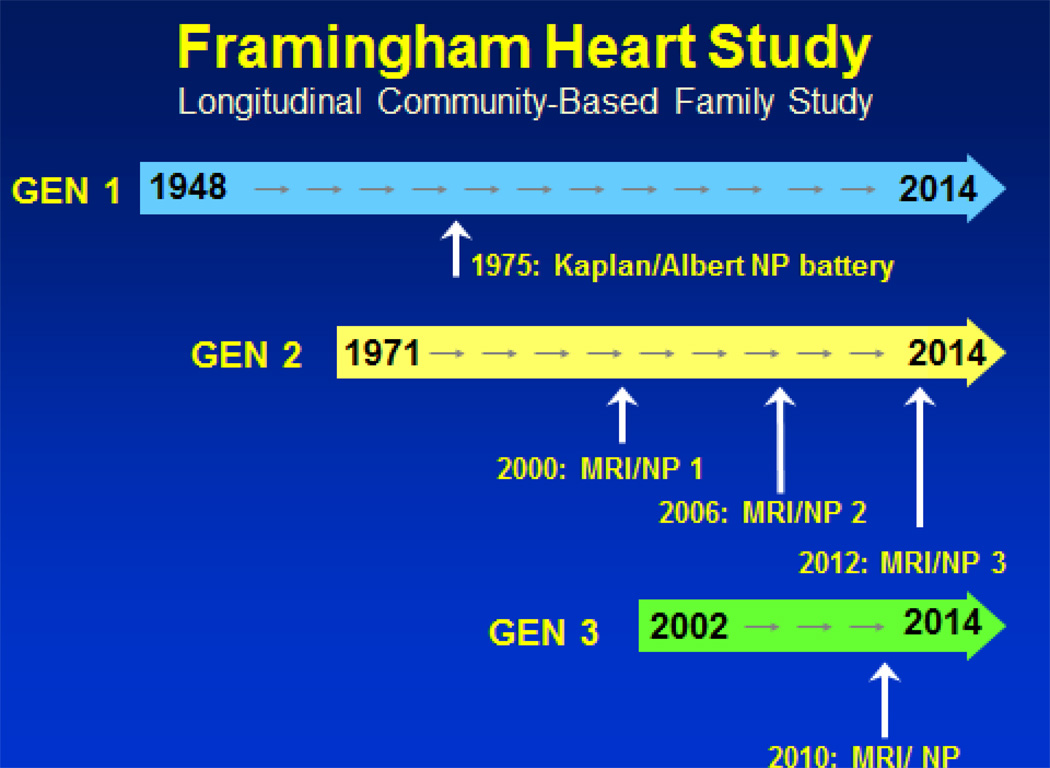
It is key to highlight a number of important features of the study design which have unique pertinence for the study of neurological diseases. Despite the focus on coronary heart disease and its impact on men under 60, women were invited to participate in the Framingham Study in part to encourage their husbands to join. Women accounted for approximately half the 5,209 subjects in the Original Cohort, ages 30 to 60 years at entry in 1948. This resulted in large numbers of spouse pairs and extended families in this uniformly Caucasian population of European descent. [Figure 1] In 1971 to enhance the study of familial factors, particularly blood lipids and blood pressure, and to capitalize on the large number of spouse pairs, an Offspring cohort (n=5,214) was recruited). These children of the Original Cohort, along with their spouses thereby provided two generations of subjects for study of familial and genetic influences on disease and phenotype occurrence. The recruitment of families was extremely fortunate, and resulted from an appreciation of clustering of cardiovascular and hypertensive disease in families. In this way, disease occurrence in parents in the Original Cohort could be related to disease or predisposing factors in their children in the Offspring Cohort. The advent of non-invasive testing including physiological measures, e.g., ankle-brachial index, imaging of the heart and carotid arteries, permitted identification of subclinical vascular disease in different arterial beds. Recruitment of 4,201 of their children, the grandchildren of the Original Framingham Cohort, in 2002 has provided a further opportunity to explore genetic factors and to identify earlier evidence of disease and of evidence of premonitory factors of subclinical disease. This has important implications for our understanding of precursors of neurological diseases and aging which occur over decades, manifested in later life as stroke, dementia and cognitive decline. The subjects in these 3 cohorts have shown extraordinary allegiance to the study and cooperate with frequent telephone and in person surveillance and examinations over decades. The original cohort has been examined every 2 years, with annual telephone health history follow-ups, for 62 years; the offspring approximately every 4 years over 4 decades of follow-up. This detailed and prolonged surveillance, standardized and quality controlled measurements and systematic and criteria-based disease reviews has permitted the study of risk factors, disease incidence, secular trends and genetic studies of disease.
Figure 1.
Time line of studies of cognitive performance and MRI studies to evaluate dementia and cognitive decline in three generations of the Framingham Heart Study
Unique features of FHS for the more common neurological diseases of the elderly
Prolonged follow-up from mid-life to disease and ultimately to death has permitted the computation of a useful statistic - the mortality-adjusted residual or remaining lifetime risk (rLTR) of developing a disease during the remaining years of life in a man or woman, at a specific age. This statistic serves to alert physicians, patients and the public health planners to the likelihood and importance of a specific disease or diseases. For example, at age 65, the residual lifetime risk of developing either stroke or dementia is one in three in men and one in two in women.1 The rLTR is an easily understood portrayal of the high likelihood of developing either (or both) of these neurological diseases and may provide an impetus for utilizing preventive measures.
Stroke
The initial Framingham publication on stroke (1965) clearly identified elevated blood pressure, systolic no less importantly than diastolic, as the premier risk factor for all stroke, infarction as well as hemorrhage. Clinical observation of study subjects hospitalized for stroke provided validation of the diagnoses before imaging became widely available in 1978. With increasing age systolic blood pressure continues to rise while the diastolic component peaks in the 50’s or 60’s then falls leading to isolated systolic hypertension in persons above age 65. Isolated systolic hypertension, i.e., systolic blood pressure ≥160 mm Hg. along with a diastolic pressure of <90 mm Hg., was not innocuous as had been commonly taught, but was an important and frequent precursor of cardiovascular disease, particularly stroke. These observations led to a series of key clinical trials demonstrating the substantial benefit of systolic blood pressure reduction on stroke incidence and severity. These trials continue with ever lower targets and increasingly elderly subjects demonstrating the benefit of blood pressure reduction even above the age of 80 years.2 The 40 years of antihypertensive therapy trials beginning with Fries’ Veterans Administration trial of severely hypertensive patients in 1969 to the Hypertension in The Very Elderly Trial (HYVET) nearly 40 years later represent a remarkable application in clinical trials of benefit derived from observational studies including Framingham.
A particular contribution of FHS to stroke epidemiology is the documentation of the importance of non-rheumatic chronic atrial fibrillation (AF) to stroke incidence in 1978. While stroke clinicians had related chronic AF to increased stroke risk and particularly dire clinical outcomes, many physicians, including cardiologists, were skeptical. The demonstration of the importance of AF as an important stroke risk factor in Framingham and of its particular importance in the elderly was key to the demonstration in a series of clinical trials of the benefit of warfarin anticoagulation. Further, the increasing prevalence of AF with age in part a result of the improved survival of patients with acute myocardial infarction and congestive heart failure, resulted in the growing importance of AF in stroke occurrence in the elderly. A risk profile developed in Framingham permits the estimation of probability of stroke or death in persons with chronic AF.
Other FHS contributions include the application of a number of statistical developments to assist in risk prediction, notably the development of a quantitative scale to estimate the probability of developing a stroke during a specific time period. The Framingham Stroke Risk Profile has been widely utilized as a clinical tool for estimating stroke probability by applying patient data available to an office-based health care professional. Secular trends in the prevalence and impact of the risk factors as well as more recent findings such as familial occurrence of stroke are currently being incorporated into an updated modification of the FSRP.
Dementia, Alzheimer’s Disease and Cognitive Decline
It has been increasingly apparent that atherosclerotic cardiovascular disease and cardiovascular risk factors are contributors to the development of dementia and cognitive decline and play a role in clinical manifestations of vascular cognitive impairment and Alzheimer’s Disease. Systematic assessment of cardiovascular risk factors, subclinical and clinical disease in midlife for more than 60 years in Framingham has provided an extraordinary opportunity to link them to late life cognitive decline and dementia. . Complementing the extensive accumulated cardiovascular disease data was the systematic assessment of cognitive function dating back to 1975. This battery led to establishment of a dementia-free (and stroke free) cohort for study of incidence and risk factors for dementia. The MMSE was administered routinely on biennial exams until 1989 when an NIA grant award supported administration of a more extensive battery and neurological follow-up of Original cohort. Subjects suspected of dementia were evaluated with neurological and neurocognitive testing and application of standard criteria for dementia diagnosis and classification. The neurological, cognitive and functional status of the Original Cohort members are monitored with annual health history updates, home and nursing home visits and biennial clinic visits. The Offspring cohort has been screened with MMSE’s since the 1970’s and with more comprehensive testing since 1989. Dementia has been documented in >600 subjects from both cohorts. I Criteria for amnestic and non-amnestic MCI were fulfilled in >225 Offspring subjects who continue to be followed. A brain donation program is in place; >150 brains from subjects whose cognitive status was known have been studied to date. The availability of brain specimens from non-demented as well as demented subjects has permitted identification of AD pathology changes in visual association area, Brodmann area 19 in all AD cases as well as in 52% of cognitively intact elderly subjects suggesting this area is particularly vulnerable to these changes and is a early site of AD pathology.3 Unique Contributions of FHS: Cognitive evaluation of Original Cohort in mid-1970’s when they were 55 to 85 years of age permitted designation of study subjects demonstrated (not just presumed) to be free of dementia and stroke, a key step in a cohort study of a disease [Figure 1]. There has been longstanding interest in the relationship between blood pressure level and cognition. The availability of the neuropschological battery, designed by the late Edith Kaplan, PhD and Martin Albert, MD, PhD (Kaplan-Albert Battery) administered on biennial exam 14–15 to nearly 3,000 surviving Original Cohort members, could be related to blood pressure levels measured over a prior 10 year period. Since effective antihypertensive therapy had not yet become available this provided an opportunity to relate chronic levels of untreated blood pressure to cognitive performance.4 The odds of having a significantly poorer performance, i.e., the lowest quartile on logical memory-delayed recall score increased with age and with increasing systolic blood pressure levels.5 A 40mmHg increase in SBP, e.g., from 120 mmHg to 160 mmHg,, was associated with performance equivalent to a person 10 years older. Thus, a higher SBP may be associated with cognitive performance on this test of a person whose brain was 10 years older than the chronological age. Subsequent follow-up also disclosed an inverse relationship of performance on this baseline cognitive battery with an increased incidence of AD after 22 years. Poorer performance on the logical memory-delayed testing was associated with approximately 50% greater incidence of AD even after persons developing AD 5 and 10 years following testing were excluded,6 providing support for the chronicity of the AD process. We have examined this association of poorer performance on the Kaplan-Albert battery in 1976 and incidence of AD after 32 years of follow-up and this robust relationship persists.
Initially, in the 1970’s the diagnosis of dementia was made only in subjects classified as moderate or severe; mild or possible dementia were not designated as “cases”. Over time, as interest in mild dementia and then “pre-dementia” or mild cognitive impairment increased, we re-reviewed all potential mild cases and and applied contemporary criteria and nosology yielding a substantially higher incidence, prevalence and residual lifetime risk (adjusting for mortality due to competing causes) of these conditions.7 [Table] Detection of subjects with MCI, and categorization as amnestic and non-amnestic MCI has followed. Since late onset Alzheimer’s Disease likely represents the end stage of a prolonged process, we have used the Third Generation cohort (mean age 45) to try to identify of persons at increased risk while in a preclinical stage.
Table 1.
Framingham Heart Study: Comparing the cumulative incidences (unadjusted for alternative cause mortality) and the 25-year and 40-year residual lifetime risks (adjusted for mortality due to competing causes) of Alzheimer’s dementia in persons who are cognitively intact at age 65 years based on variation in severity required to diagnose clinical dementia
| Period of follow-up | 1975 to 1995 | 1975 to 2008 | |
|---|---|---|---|
| Outcome used | Age at onset | Age at diagnosis | Age at diagnosis |
| Severity required for clinical diagnosis | Moderate + | Moderate + | Mild + |
| Number demented | 141 | 353 | 388 |
| Cumulative incidence | |||
| 25 year | 14.4 | 12.2 | 20.9 |
| 40 year | 29.9 | 62.3 | 66.6 |
| Residual lifetime risk | |||
| 25 year | 8.9 | 8.7 | 11.1 |
| 40 year | 11.8 | 13.5 | 15.3 |
Reprinted from Alzheimer’s and Dementia: Seshadri S, Beiser A, Au R et al. Operationalizing diagnostic criteria for Alzheimer's disease and other age-related cognitive impairment-Part 2. Alzheimers Dement. 2011 Jan;7(1):37 with permission from Elsevier
Risk Factors for AD
It has been accepted for many years that clinical stroke is followed by dementia. We found a doubling of the incidence of dementia, largely vascular in type following clinical stroke. Once CT-scans became available it became apparent approximately 12% of initial strokes in persons clinically presumed to be stroke-free had CT evidence of a prior unsuspected stroke. Stroke risk factors have been related to incidence of dementia (and AD) particularly diabetes, elevated plasma homocysteine level, low levels of physical activity and plasma phosphatidylcholine docosahexaenoic acid.8
Offspring Cohort MRI and Neurocognitive markers
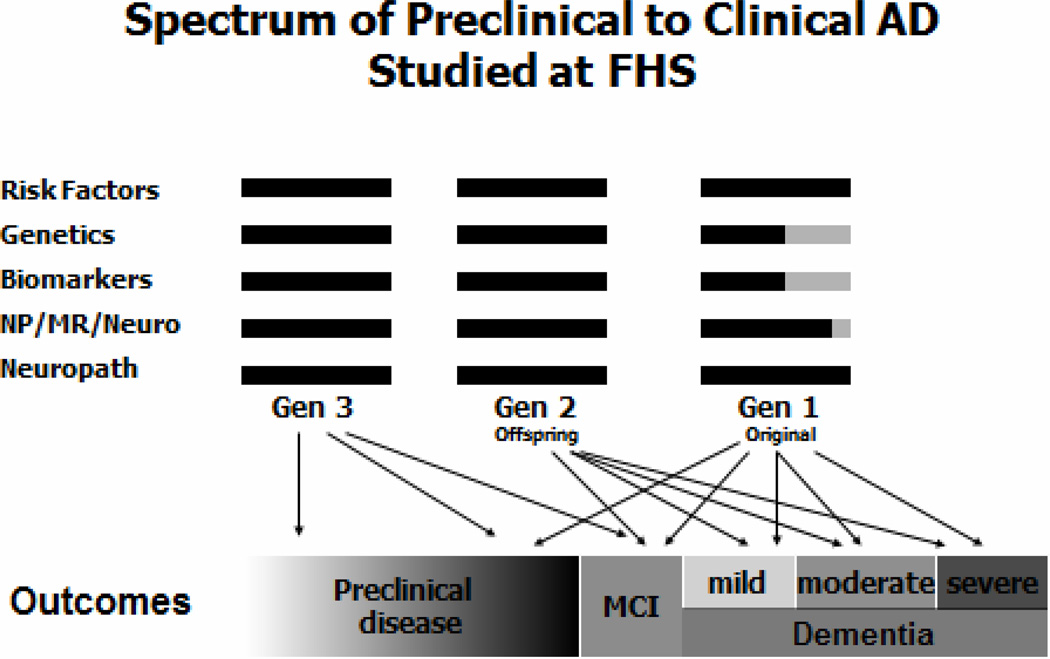
In 1999, quantitative brain MR scans and a comprehensive neuropsychological battery was obtained on nearly 3,000 Offspring cohort members. Figure 2. We related previously measured cardiovascular risk factors and markers to these morphologic and cognitive measures. As a starting point, the Framingham Stroke Risk Profile (FSRP), developed for determining the probability of stroke in Framingham and validated in many other populations, was related to brain volume as a percentage of cranial volume (TCBV) and to neuropsychological test performance in approximately 1,800 subjects. There was a significant inverse relationship between probability of stroke on FSRP and TCBV.9 The apparent brain atrophy was not a result of clinical or covert brain infarcts but rather loss of brain substance. There was an approximately 2% difference between the uppermost and lowest TCBV quartiles which corresponded to the 1.9% decline in TCBV in the decade from age 50 to 60 years. Thus, those with the greatest probability of stroke had TCBV’s equivalent to TCBV levels of persons 10 years older with optimal risk factor levels. The presence of covert brain infarcts and increased white matter hyperintensities was similarly related to risk factor levels and were associated with poorer executive function and with subsequent increased stroke risk.
Figure 2.
Spectrum of measures available for the study of dementia and cognitive decline in three generations of the Framingham Heart Study: risk factors, genome-wide association studies, biomarkers, preclinical indicators and change in cognitive function and brain morphology, and neuropathological examination.
Metabolic dysregulation: obesity, insulin resistance, metabolic syndrome, impaired glucose tolerance and diabetes, was significantly associated with diminished cognitive performance, brain volume, particularly hippocampal volume and white matter hyperintensity volume.10 All were related to significantly reduced TCBV. Diabetes and hemoglobin A1C levels were inversely related to hippocampal volume. Smaller TCBV was present in persons with high fasting insulin levels; this was true even in non-diabetics, amounting to 6 years of structural brain aging. Executive function was also inversely related to these indices of metabolic dysregulation.
Greater degrees of obesity (BMI, waist circumference and waist to hip ratio) were also generally associated with smaller TCBV with a striking interaction between obesity and hypertension Persons in the uppermost quartile of waist-hip ratio, but not body mass index, who were also hypertensive had the poorest performance on tests of executive function and visuomotor skills but not on tests of memory. A number of other biomarkers have also been shown to relate these endophenotypes.
Parental AD and Endophenotypes in their Offspring
Rather than having to rely upon a history of dementia or AD in parents of subjects, FHS utilized more than 30 years of surveillance, neurological and cognitive evaluations and structured case reviews of subjects to determine if criteria for AD and/or dementia were fulfilled. We could thereby relate subjects fulfilling standard criteria for probable AD in the Original cohort to cognitive performance and brain structure in their children. In ApoEε4 carriers only, parental dementia and parental AD was associated with poorer scores on tests of verbal memory and visuospatial memory tasks in their offspring, mean age 59 years. These APOEε4 carriers also showed greater brain atrophy rates over 6 years of follow-up on serial brain MRI’s. There was worsening performance in executive function among offspring of AD parents regardless of ApoEε4 status.
Genetic Studies
In addition to APOEε4, as a result of genome-wide association studies and extensive collaborations among investigative teams including the Framingham Heart Study, a number of variants associated with stroke and AD have been identified.11 It seems likely there will continue to be rapid advances made in understanding of the genetic influences on late-onset AD. The Framingham Study formed working groups, including the Neurology traits Working Group to collaborate with their counterparts in several other prospective epidemiologic studies: forming the Cohorts for Heart and Aging Research in Genomic Epidemiology consortium (CHARGE consortium) sharing our data with the AD community resulting in a recent significant publication.12 Access to all Framingham Heart Study GWAS data is made available simultaneously to all other non-Framingham investigators on the dbGaP website as the SNP Health Association Resource (SHARe).
A comprehensive biomarker initiative, Systems Approach to Biomarker Research (SABRE) NHLBI Biomarker Initiative) is underway and will provide thousands of biomarkers for future investigative efforts.
These efforts over more than 60 years are due to the steadfast support of the Original Framingham Heart Study participants, their children and grandchildren. Their allegiance and dedication have made a landmark study such as Framingham possible. The Framingham Heart Study has received continued support from the National Heart, Lung and Blood Institute, and from grant support from other Institutes of the National Institutes of Health. The studies of aging, dementia, Alzheimer’s Disease have been funded by the National Institute on Aging since 1989; stroke study support is from the National Institute of Neurological Diseases and Stroke since 1981.
Acknowledgements
This work was supported by the National Heart, Lung and Blood Institute's Framingham Heart Study (Contract No. N01-HC-25195) and grants R01NS17950 and R01AG08122.
Key investigators on the FHS neurological team deserving particular thanks: Rhoda Au, PhD, Sanford Auerbach, MD, Alexa Beiser, PhD, Ralph B. D’Agostino, PhD, Charles DeCarli, MD, Merrill F. Elias, PhD, MPH, Carlos Kase, MD, Margaret Kelly-Hayes, EdD, and Sudha Seshadri, MD, whose dedication over decades made these studies possible. William B. Kannel, MD MPH deserves my personal gratitude and recognition for his wisdom and guidance over many years.
Footnotes
Disclosures: There are no conflicts of interest present.
References
- 1.Seshadri S, Wolf PA. Lifetime risk of stroke and dementia: current concepts, and estimates from the Framingham Study. Lancet Neurol. 2007 Dec;6(12):1106–1114. doi: 10.1016/S1474-4422(07)70291-0. [DOI] [PubMed] [Google Scholar]
- 2.Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008 May 1;358(18):1887–1898. doi: 10.1056/NEJMoa0801369. [DOI] [PubMed] [Google Scholar]
- 3.McKee AC, Au R, Cabral HJ, Kowall NW, Seshadri S, Kubilus CA, et al. Visual association pathology in preclinical Alzheimer disease. J Neuropathol Exp Neurol. 2006 Jun;65(6):621–630. doi: 10.1097/00005072-200606000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Elias MF, Wolf PA, D'Agostino RB, Cobb J, White LR. Untreated blood pressure level is inversely related to cognitive functioning: the Framingham Study. Am J Epidemiol. 1993 Sep 15;138(6):353–364. doi: 10.1093/oxfordjournals.aje.a116868. [DOI] [PubMed] [Google Scholar]
- 5.Elias PK, D'Agostino RB, Elias MF, Wolf PA. Blood pressure, hypertension, and age as risk factors for poor cognitive performance. Exp Aging Res. 1995 Oct-Dec;21(4):393–417. doi: 10.1080/03610739508253992. [DOI] [PubMed] [Google Scholar]
- 6.Elias MF, Beiser A, Wolf PA, Au R, White RF, D'Agostino RB. The preclinical phase of alzheimer disease: A 22-year prospective study of the Framingham Cohort. Arch Neurol. 2000 Jun;57(6):808–813. doi: 10.1001/archneur.57.6.808. [DOI] [PubMed] [Google Scholar]
- 7.Seshadri S, Beiser A, Au R, Wolf PA, Evans DA, Wilson RS, et al. Operationalizing diagnostic criteria for Alzheimer's disease and other age-related cognitive impairment-Part 2. Alzheimers Dement. 2011 Jan;7(1):35–52. doi: 10.1016/j.jalz.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schaefer EJ, Bongard V, Beiser AS, Lamon-Fava S, Robins SJ, Au R, et al. Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease: the Framingham Heart Study. Arch Neurol. 2006 Nov;63(11):1545–1550. doi: 10.1001/archneur.63.11.1545. [DOI] [PubMed] [Google Scholar]
- 9.Seshadri S, Wolf PA, Beiser A, Elias MF, Au R, Kase CS, et al. Stroke risk profile, brain volume, and cognitive function: the Framingham Offspring Study. Neurology. 2004 Nov 9;63(9):1591–1599. doi: 10.1212/01.wnl.0000142968.22691.70. [DOI] [PubMed] [Google Scholar]
- 10.Tan ZS, Fox CS, Au R, Himali J, Debette S, DeCarli C, Ramachandran VS, Wolf PA, Seshadri S. Association of Metabolic Dysregulation with Volumetric Brain MRI and Cognitive Markers of Subclinical Brain Aging in Middle-Aged Adults: The Framingham Offspring Study. Diabetes Care. 2011 doi: 10.2337/dc11-0308. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikram MA, Seshadri S, Bis JC, Fornage M, DeStefano AL, Aulchenko YS, et al. Genomewide association studies of stroke. N Engl J Med. 2009 Apr 23;360(17):1718–1728. doi: 10.1056/NEJMoa0900094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hollingworth P, Harold D, Sims R, Gerrish A, Lambert JC, Carrasquillo MM, et al. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer's disease. Nat Genet. 2011 Apr 3; doi: 10.1038/ng.803. [DOI] [PMC free article] [PubMed] [Google Scholar]