Abstract
Exposure to stress during early development causes long-lasting alterations in behavior and hypothalamic pituitary adrenal (HPA) axis activity, including increased expression of corticotropin releasing hormone (CRH). To determine whether early life stress causes epigenetic changes in the CRH promoter leading to increased CRH transcription, 8-week old female and male rats, subjected to maternal deprivation (MD) between days 2 and 13 post-birth, were studied for HPA axis responses to stress and CRH promoter methylation in the hypothalamic paraventricular nucleus (PVN) and central nucleus of the amygdala (CeA). Plasma corticosterone and PVN CRH hnRNA responses to acute restraint stress were higher in MD rats of both sexes. DNA methylation analysis of the CRH promoter revealed a significantly lower percent of methylation in 2 CpGs preceding (CpG1) and inside (CpG2) the cyclic AMP-responsive element (CRE) at −230 bp in the CRH promoter in the PVN but not the CeA of MD rats. Gel-shift assays, using nuclear proteins from forskolin treated hypothalamic 4B cells and CRH promoter CRE oligonucleotides, unmethylated or methylated at CpG1, revealed a strong band which was supershifted by phospho-CREB antibody. This band was 50% weaker using oligonucleotides methylated at CpG2 (intra-CRE), or methylated at both CpG1 and CpG2. These findings demonstrate that HPA axis hypersensitivity caused by neonatal stress causes long-lasting enhanced CRH transcriptional activity in the PVN of both sexes. Hypomethylation of the CRH promoter CRE, a region critical for CRH transcriptional activation, could serve as a mechanism for the increased transcriptional responses to stress observed in MD rats.
Keywords: maternal deprivation, corticotropin releasing hormone, DNA methylation, HPA axis, CRH
INTRODUCTION
Studies in experimental animals and humans have shown that events occurring prenatally and early in life have long lasting effects on behavior and neuroendocrine responses and can contribute to the pathogenesis of diseases during adulthood. Epidemiologic studies have indicated that negative early childhood experiences, such as parental loss, extreme poverty, recurrent physical and/or emotional abuse, chronic neglect, severe maternal depression, parental substance abuse, and family violence, can affect adaptiveresponses to subsequent stressors during life. This can predispose an individual to developmentof neuropsychiatric diseases, as exemplified by the higher incidence of mood and eating disorders, anxiety, depression, schizophrenia and autism in subjects with a history of early life stress (1–8). In humans and experimental animals these alterations are commonly associated with hyperactivity of the hypothalamic-pituitary-adrenocortical (HPA) axis, the major neuroendocrine response to stress. Activation of the HPA axis involves the release of corticotropin releasing hormone (CRH) from the hypothalamic paraventricular nucleus to the pituitary portal circulation with stimulation of ACTH secretion and finally the release of glucocorticoids from the adrenal cortex, which are essential for homeostasis and stress adaptation (9–10). In addition to its effects through pituitary-adrenal stimulation, CRH is an important integrator of neuroendocrine stress responses in the brain, modulating behavior and the autonomic nervous system (11–12). A number of studies, using maternal deprivation or models of deficient maternal care in rodents, have shown that HPA axis hyperactivity following early life stress is associated with increased CRH mRNA levels in the PVN (13–15). Thus it is likely that a major part of the alterations associated with early life stress are related to CRH hyperproduction.
The mechanisms by which early life experiences lead to long-term alterations involves complex interactions between environment and genetics (16–17). Thus, depending on the genetic background, changes in neurotransmitter levels, hormones, or environment at critical stages in development can lead to epigenetic modifications, such as DNA methylation (18–19). Such epigenetic changes can be sex dependent, as suggested by the higher incidence of some stress related disorders in women compared to men (20). It has been shown that early life stress in rodents can change methylation patterns at specific CpG dinucleotides in the genomic DNA, causing permanent alterations in gene expression in the brain (21–23). DNA methylation could inhibit gene transcription by interfering with the recruitment of transcription factors or by recruiting methylated-DNA binding proteins that decrease transcription efficiency (24). CRH expression undergoes dynamic regulatory changes during stress, and rapid but transient transcriptional activation plays an important role in maintaining appropriate levels of CRH mRNA and protein (25–27). Regulation of CRH transcription involves /protein kinase A dependent signaling and requires the integrity of a response element (CRE) located between −228 and −221 bp of the CRH gene promoter (28–29). Since this essential CRE contains a CpG dinucleotide within the sequence, changes in methylation of this dinucleotide could lead to long term alterations in transcriptional activity.
The aim of this study was to test the hypothesis that early life stress increases transcriptional responses of the CRH gene by affecting the methylation state of the critical CRE in the CRH promoter. For this purpose rat litters left undisturbed or subjected to maternal deprivation for 4h/day from day 2 to 13 were studied during adulthood for their HPA axis responsiveness to stress and CRH promoter methylation status. The results showed that maternal deprivation caused HPA axis hypersensitivity associated with long-lasting enhanced CRH transcriptional activity in the PVN of rats of both sexes, and that hypomethylation of a critical CRE in the CRH promoter is likely to be a mechanism for the increased transcriptional responses to stress observed in MD rats.
MATERIALS AND METHODS
Animals and in vivo procedures
Female and male Sprague–Dawley rats were purchased from Harlan Laboratories, Frederick, MD. In order to synchronize the reproductive cycle and induce ovulation, 12 female rats received an intraperitoneal (i.p.) injection 80 μg/per rat of D-Ala6-LH-RH (Sigma, L1898) 5 days before mating. At this point males were introduced and left in the cages with the females for 5 days. During the last week of gestation, 10 female rats which became pregnant were single-housed in standard rat cages (42 cm × 27 cm × 18 cm) and maintained under standard laboratory conditions (12:12 light/dark cycle, lights on at 0600 h, 22 °C, 60% humidity, food and water ad lib.).
One day after parturition, litters were culled to no more than 11 pups. Starting on postnatal day 2, pups were separated daily from the mother for 4 h/day (09.30–13.30 h) up to postnatal day 13 (MD group). Control litters were left undisturbed, except for change of bedding once a week. Pups were weaned at postnatal day 22, separated by sex and placed in groups of four to five per cage. At day 45, rats were divided in groups with two to three per cage so that pups from each one of the 10 litters were represented in each experimental subgroup (one rat per litter for stress responsiveness experiments, and one or two rats per litter for CRH promoter methylation).
Rats were left undisturbed under the animal facility conditions, 14–10 h light/dark cycle with food and water available ad lib. At 60 days of life, groups of 11 females and 11 males control rats and 10 female and 9 male MD rats were killed by decapitation. Female rats were not tested for their stage in the estrous cycle. Thymus and adrenals were removed, the adrenals cleaned from surrounding fat, and weighed. Trunk blood was collected in plastic tubes containing 100 μl of ethylenediaminetetraacetic acid (500 mM; pH 7.4) and 25,000 KIU of Aprotinin; plasma was separated by centrifugation and stored at −80 °C for ACTH and corticosterone determination. Brains were rapidly removed, frozen in isopentane at −40°C and stored at −80°C until microdissection.
On the following day additional groups of 5 females and 5 males from controls and MD were killed by decapitation either under basal conditions, or following restraint stress for 30 or 60 min by placing them into plastic restrainers (64 × 152 mm). Trunk blood was collected as described above for ACTH and corticosterone measurements. The hypothalamic region containing the PVN was microdissected from a coronal section in between the optic chiasma and 1 mm rostral from the mammillary bodies. Sections were placed flat on a chilled rubber cork and the ventral portion containing hypothalamus and amygdala cut at the top of the 3rd ventricle at the level of the rhinal fissure. A hypothalamic block weighing about 25 mg containing the PVN was obtained from this ventral section by blade cuts placed 1 mm lateral at each side of the third ventricle and 1 mm from the ventral edge, to exclude the suprachiasmatic, supraoptic and arcuate nuclei, and quickly frozen in 1.5-ml microtubes on dry ice. The region containing the CeA was obtained bilaterally by blade cuts placed about 1/3 mm lateral from the optic tract, 1 mm from the bottom and 2mm from the lateral border, and frozen in tubes on dry ice. The whole procedure from decapitation to freezing of the tissue was performed in about 3 min. Experiments were performed in the morning with rats killed between 08.30 and 12.00 h. All procedures and experimental protocols were performed according to NIH guidelines and approved by the NICHD Animal Care and Use Committee.
ACTH and corticosterone assays
Plasma levels of ACTH following stress were measured using kit reagents from the ACTH IRMA Immunoradiometric Assay, DiaSorin (Stillwater, MN) according to the manufacturer’s instructions. The intra- and inter-assay coefficients of variation of the ACTH assay were 2.2% and 7.8%, respectively. Corticosterone levels were measured using the Rat Corticosterone Coat-A-Count kit (Siemens, Los Angeles, CA) according to the manufacturer’s instructions.
Brain micropunches for methylation analysis
Coronal sections (300μm) of fresh-frozen brains were cut on a cryostat, mounted on slides, and used for punch microdissection as follows (positions are relative to bregma): three sections between −1.3 and −2.2mm for the paraventricular nucleus (PVN) and the central nucleus of the amygdala (30). The positions of the PVN and CeA were identified in 10 μm-thick sections stained with toluidine blue, previous to collection of the 300 μm sections. Punch microdissection was performed under stereomicroscope control using Harris Uni-Core microdissection needles of 0.5mm diameter for the PVN and 1 mm for the CeA. The punched tissues were stored at −80°C until genomic DNA extraction.
DNA extraction, bisulfite conversion and pyrosequencing
Genomic DNA from mini-punch tissues from PVN or CeA were extracted using the QIAamp DNA Micro kit (Qiagen, Valencia, CA), then treated with bisulfite to convert unmethylated cytosine into uracil using the EZ DNA Methylation-Gold Kit (Zymo Research, Orange, CA) as described in the user’s manual. Briefly, aliquots of 300 ng of genomic DNAwere incubated with bisulfite reagent at 98°C for 10 min followed by 64°C for 2.5 h. The converted DNA was then desulphonated, purified, and recovered with a spin column. Different CRH promoter regions containing CpG sites were then PCR amplified and subjected to pyrosequencing (EpigenDx, Worcest, MA). Forty-five cycles of PCR were performed using HotStar Taq DNA polymerase (Qiagen) with denaturation at 95°C, annealing at 48°C (for ADS582) or 63°C (for ADS 583), and elongation at 72°C, with a MgCl2 concentration of 3.0 mM. Primers for PCR and pyrosequencing were as follows: ADS582, forward PCR primer: 5′ AAGAATTTTTGTTAATGGATAAGT 3′, reverse PCR primer: 5′ AAATCTCACATCCAATTATATCAA 3′, sequencing: 5′ GAAGTTTTTTTATTTTAGGGT 3′; ads583, forward PCR primer: 5′ TTGGATGTGAGATTTAGTGTTGAA 3′, reverse PCR primer: 5′ ACCTTTCCCCTTTCTCTTCAAT 3′, sequencing primer I: 5′ GAGATTTAGTGTTGAAATAG 3′; sequencing primer II: 5′ GTTAGTTTATTAGGTAAATG 3′.
RNA isolation
Hypothalamic and amygdala tissue from individual rats (frozen in 1.5 ml microtubes) were homogenized in 1 ml of TRIzol reagent (Invitrogen, Grand Island, NY), using a motorized pellet pestle rod (Kontes, Vernon Hills, IL) and total RNA was extracted and purified using the RNeasy minikit (QIAGEN, Valencia, CA) according to the manufacturer’s protocol with on-column deoxyribonuclease (Qiagen) digestion to eliminate genomic DNA contamination (31). Total RNA concentration was measured by Nanodrop spectrophotometry.
Real-time quantitative PCR for CRH heteronuclear (hn) RNA and mRNA
CRH primary transcript levels (CRH hnRNA) were measured by quantitative RT-PCR (qRT-PCR), using the LUX gene expression system (Invitrogen). LUX fluorogenic and unlabeled primer pairs were designed using the D-LUX designer software (Invitrogen) by targeting the intron of the CRH gene. The sequence of FAM-labeled forward primer is 5′-CACAGGCGGCGAATAGCTTAA-ACCTG (FAM)G-3′, and the unlabeled reverse primer is 5′-CAGGTGACCCTTCCTTGGAGA-3′. Platinum Quantitative PCR SuperMix-UDG (Invitrogen) was used for the amplification mixture with each LUX primer at a final concentration of 200 nm and 2 μl of cDNA for a total reaction volume of 25 μl. PCRs were performed on a spectrofluorometric thermal cycler (ABI PRISM 7300 real time PCR system; Applied Biosystems, Foster City, CA). Samples were amplified by an initial denaturation at 50 °C for 2 min and 95 °C for 2 min and then cycled (45 times) using 95 °C for 15 sec, 57 °C for 30 sec, and 60 °C for 15 sec. CRH mRNA was measured by qRT-PCR, using Power SYBR Green PCR (Applied Biosystems, Foster City, CA). The sequences of the primers to measure CRH mRNA were as follow: 5′-CTCTCTGGATCTCACCTTCCAC-3′ ( f o r w a r d ) a n d 5′-CTAAATGCAGAATCGTTTTGGC-3′ (reverse). Samples were amplified by an initial denaturation at 50°C for 2 min, 95°C for 10 min, and then cycled (40×) using 95°C for 15 secand 60°C for 1 min. Calibration curves to assess the efficiency of the PCR reaction and for quantification of absolute amounts of CRH hnRNA and CRH mRNA in hypothalamic RNA from individual rats were performed using a 5.5-kb BglII rat genomic fragment containing the full rat CRH gene (498 to 4969) cloned into pUC18, kindly provided by Dr. Audrey Seasholtz. For the experimental samples, 1000 ng of total RNA per sample were used for reverse transcription into cDNA. RNA was primed with random primers, and cDNA was synthesized using the SuperScript III first-strand synthesis system for RT-PCR (Invitrogen) as previously described (32). PCRs with CRH intronic or exonic primers were performed in parallel with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) primers, used as control gene. Predesigned Joe-labeled forward and unlabeled reverse GAPDH primers were obtained from Invitrogen. The absence of RNA detection when the reverse transcription step was omitted indicated the lack of genomic DNA contamination in the RNA samples. Data were expressed as absolute amounts of CRH hnRNA in femtograms per hypothalamus or central amygdala.
Electrophoretic mobility shift assay
Hypothalamic 4B cells were cultured in 10 cm dishes in DMEM (Invitrogen) supplemented with 10% fetal bovine serum, 10% horse serum, 100 U mL−1 penicillin and 100 μg ml of streptomycin. Prior to treatment, cells were changed into serum-free medium containing 0.1% BSA and 2 hours later cells were incubated for 1 hour with 10 μM forskolin. Nuclear proteins were extracted using the NE-PER Nuclear and Cytoplasmic Extraction kit (Pierce, Rockford, IL), as per the manufacturer’s instructions. Protein concentration was quantified by spectrophotometry using the BCA protein assay (Pierce) prior to use in EMSA. Single stranded oligonucleotide probes containing the CRH CRE and end-labeled with IR700 dye (LI-COR Biosciences, Lincoln, Nebraska) were synthesized without methylation (5′-TCGTTGACGTCACCAA-3′, CRE underlined) or methylated at Met-C1 (5′-T[Met-C]GTTGACGTCACCAA-3′), Met-C2 (5′-TCGTTGA[Met-C]GTCACCAA-3′) or both Met C-1 and 2 (5′-T[Met-C]GTTGA[Met-C]GTCACCAA-3′). Probes were annealed to unmethylated, IR700 end-labeled antisense oligonucleotides by incubation at 100°C for 3 minutes, followed by slow cooling to room temperature. For EMSA, 5 μg of nuclear protein was incubated in binding buffer (10 mM Tris-HCl, pH 7.5, containing 4% glycerol, 5 mM MgCl2, 0.5 mM EDTA, 0.5 mM DTT and 5 mM NaCl) with 0.05 μg mL−1 poly (deoxyinosinic–deoxycytidylic) acid (Sigma P-4929) and 10 pmol labeled oligonucleotide in a total volume of 20 μL for 20 min at room temperature. For super shift, 1 μL of phospho-CREB antibody (Millipore 06–519) was added before probe. Samples were electrophoresed on a 6% DNA retardation gel (Invitrogen) for 30–40 min at 4°C at 10–15 mA. Bands were visualized and quantified using a LI-COR Odyssey Imager.
Data analysis
Statistical significance of the differences between groups was calculated by one or two-way analysis of variance (ANOVA), or by Student’s t test for paired data, as indicated in the figure legends. P values < 0.05 were considered significant. Data are presented as means ± standard error of the mean (SEM) of the number of observations indicated in results or figure legends.
RESULTS
Effect of maternal deprivation on body, adrenal and thymus weight
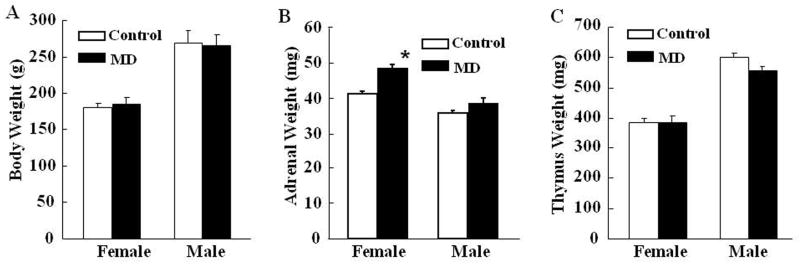
Body weight in rats subjected to early life maternal deprivation (MD) was not significantly different from that in control rats which received normal maternal care (Fig 1A). However, compared with controls, MD during postnatal day 2–13 caused a significant increase of adrenal weight in female rats (P<0.05), but not in males. Adrenal weight was higher in females than in males (P< 0.05) (Fig 1B). Similar differences in adrenal weight were observed when data were compared as adrenal weight: body weight ratios (P<0.05 only in female rats) (not shown). No significant change was found in thymus weight between MD and control rats (Fig 1C).
Fig. 1.
Effect of maternal deprivation from post-natal day 2 to 13 on body weight (A) adrenal weight (B) and thymus weight (C) measured in 60 day old male and female rats. Bars represent the mean ± S.E.M of values obtained from 9 to 11 rats per group. * P< 0.05, compared to the respective control group, following analysis by one-way-ANOVA.
Effect of maternal deprivation on plasma ACTH and corticosterone
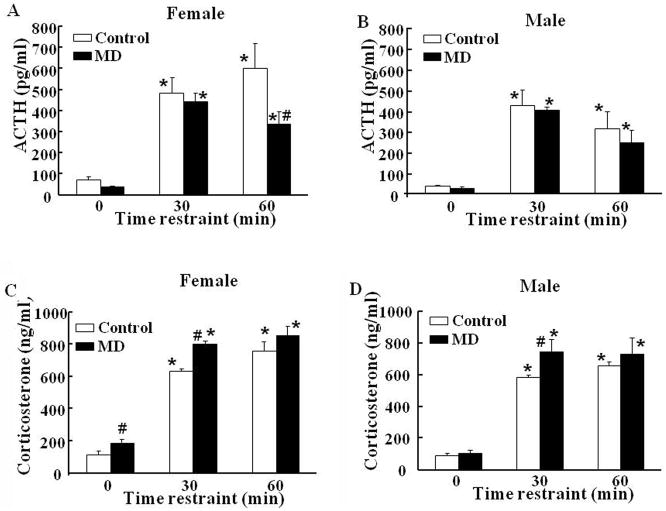
In female rats subjected to MD, basal plasma ACTH levels tended to be lower than those in control female rats (P=0.08) (Fig 2A). Responses to restraint stress at 30 min were similar in both groups of female rats, and showed a tendency to decrease at 60 min (P=0.07) in MD. The overall analysis by two-way ANOVA showed significant effects of restraint stress (P<0.001, F=29.2) and MD (P<0.04, F=5.0) (Fig 2-A). In male rats, there was a significant effect of restraint stress (P<0.01), but not of MD (P<0.5, F=0.5), with plasma ACTH levels being similar in controls and maternal deprivation groups in basal conditions and in response to restraint stress at 30 and 60 min (Fig 2-B).
Fig. 2.
Effect of maternal deprivation from post-natal days 2 to 13, and restraint stress on plasma levels of ACTH (A and B) and corticosterone (C and D) in 60-day old female (A and C) and male B and D) rats. Rats were killed by decapitation in basal conditions or following 30 and 60 min restraint stress. Bars represent the average ± S.E.M of values obtained in 5 rats per group. * P< 0.001, compared to basal; # P<0.05 compared with the respective control group (two-way ANOVA).
In spite of the slightly lower plasma ACTH, plasma corticosterone levels in female rats subjected to maternal deprivation were significantly higher in basal conditions and following 30 min stress compared to those in control female rats (P<0.05). Plasma corticosterone responses to stress at 60 min were similar in controls and MD females (Fig 2C). Two-way ANOVA showed an effect of restraint stress (P<0.001, F=151.9) and MD (P<0.002, F=11.7). In males, MD had no effect on basal plasma corticosterone levels, but as in females, the increases following 30 min restraint were significantly higher in MD males than in controls (Fig 2-D). Two-way ANOVA showed significant effects of restraint stress and MD (P<0.001, F=102.4, and P<0.05, F=4.3, for restraint and MD, respectively).
Effect of early-life maternal deprivation on CRH hnRNA and mRNA levels
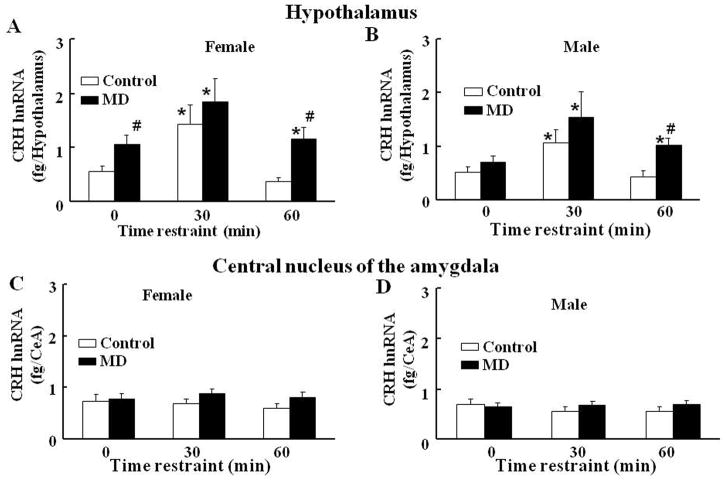
In female rats subjected to MD, basal levels of CRH hnRNA in the hypothalamus were significant higher compared to those in normal maternal care controls (Fig 3A). While in control female rats CRH hnRNA levels increased by 2-fold at 30 min and had returned to basal levels by 1h, in rats subjected to early life maternal deprivation, CRH hnRNA increased to levels similar as in controls at 30 min, though increases were lower when expressed as fold change, due to the elevated basal values. By 60 min restraint CRH hnRNA levels in female MD rats had declined to values similar to the initially elevated basal values, but were significantly elevated compared with the 60 min value in the control group (Fig 3-A). Two-way ANOVA revealed a significant effects of stress (P<0.001, F=45.9) and MD (P<0.001, F=19.3). In male rats, basal CRH hnRNA and stimulated levels at 30 min restraint stress showed similar levels in the MD group compared with controls. However, CRH hnRNA levels were still significantly higher than basal at 60 min restraint stress (Fig 3-B). Similar to the responses in females, two-way ANOVA revealed significant effects of restraint stress and of MD (P<0.001, F=18.4, and P<0.01, F=19.0, respectively).
Fig. 3.
Effect of maternal deprivation from post-natal days 2 to 13, and restraint stress on CRH hnRNA levels in the hypothalamus (A and B) and central nucleus of the amygdala (CeA) (C and D) of 61-day old female (A and C) and male (C and D) rats in basal conditions or following 30 or 60 min restraint stress. Bars represent the average ± S.E.M. of values obtained in 5 rats per group. Data are expressed as absolute levels of CRH hnRNA measured by intronic qRT-PCR using a standard curve as described in Methods. * P< 0.05, compared to respective basal; #, P<0.05 compared with the respective control group (two-way ANOVA).
In contrast to the hypothalamus, in the central amygdala (CeA) there were no significant differences in basal CRH hnRNA levels between control and MD rats, and levels were unaffected by stress irrespective of the early life experiences in either males or females (Fig 3-C and D).
In both the hypothalamus and the amygdala, there were no differences in basal CRH mRNA levels between MD and control for either male or female rats. As expected, CRH mRNA levels were unaffected by restraint stress at 30 or 60 min (not shown).
Maternal deprivation and CRH promoter methylation
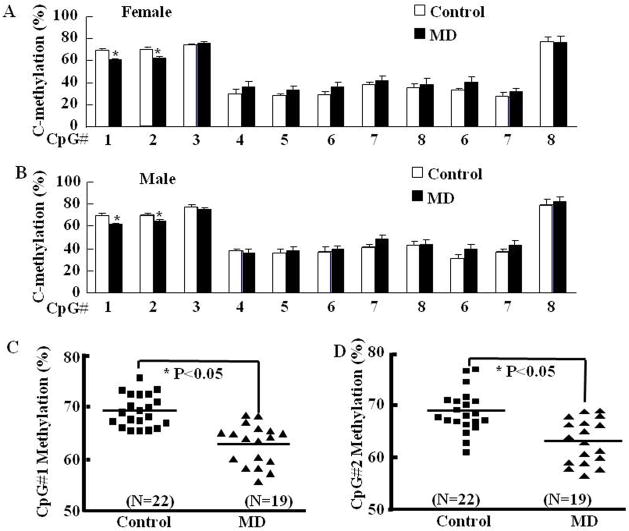
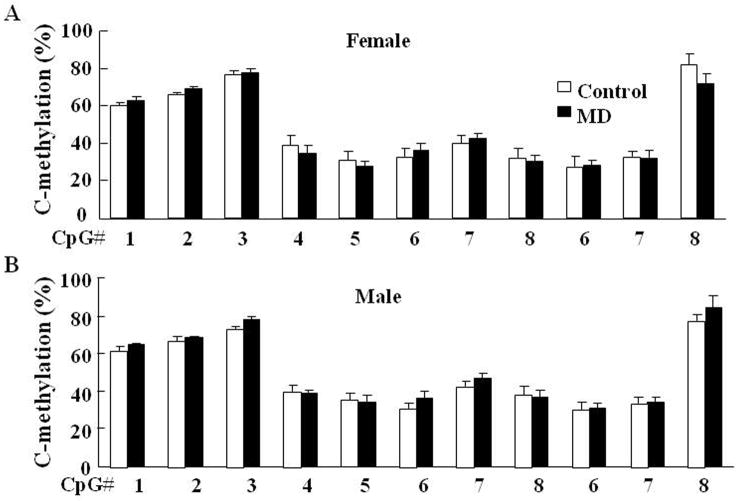
To determine whether maternal deprivation-induced CRH hnRNA expression is related to epigenetic alterations, we performed methylation analysis of CpG dinucleotides of the CRH promoter region in micro-punches of the paraventricular nucleus (PVN) and central amygdala (CeA) (Fig 4). Maternal deprivation resulted in a lower percentage of DNA methylation, specifically in two CpG sites located immediately preceding (#1) and inside (#2) the cyclic AMP-responsive element (CRE) at −230bp of the CRH promoter in the PVN of MD rats compared with control (normal maternal care) rats. MD had similar effects on CRH promoter methylation in female (Fig 5-A) and male (Fig 5-B) rats. Although total methylation of the CRH promoter was not statistically significant by one-way ANOVA, t-test comparison of CpG1 and CpG2 showed significantly lower methylation in the MD groups. The combined analyses of CpG 1 and 2 in all rats including males and females revealed 2.7% and 3.1% lower methylation in MD rats (Fig 5-C and D). In contrast to the PVN, the percentage methylation of CRH promoter CpG dinucleotides in the central amygdala was not significantly different between controls and MD rats (Fig 6-A and B for females and males, respectively).
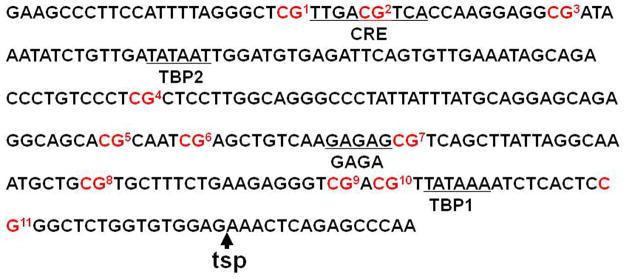
Fig. 4.
Sequence of the proximal CRH promoter (−254 bp to −55 bp) showing the CpG dinucleotides (in red) present in this region. The numbers following each CpG identify the dinucleotides subjected to methylation analysis. TSP: Transcription start point; TBP: TATA binding protein site; GAGA: GAGA factor site; CRE: cyclic AMP response element. CRE is located at −230 bp to −222 bp. CpG1 is located just beside of CRE at −232bp and CpG2 is located inside of CRE at −226 bp.
Fig. 5.
Methylation analysis of the CpG dinucleotide island within the rat CRH promoter region in the hypothalamic paraventricular nucleus of female (A) and male (B) rats. In PVN of MD rats, significantly lower methylation of CpG#1 (C) and CpG#2 (D) at −230 bp the cyclic AMP-responsive element of the CRH promoter was observed. Bars represent the average ± S.E.M of values obtained in 9 to 11 rats per group. Differences were not significant in the overall analysis by two-way-ANOVA. The pooled data for male and females for CpG1 and CpG2 are shown in C and D, respectively. * P< 0.05, MD vs controls by Student’s t test analysis.
Fig. 6.
Methylation analysis of the CpG dinucleotides within the CRH promoter region in the central amygdala of female (A) and male (B) rats. Bars represent the average ± S.E.M. No significant differences were found with two-way-ANOVA or Student’s t test analysis of the data.
Electrophoretic mobility shift assay
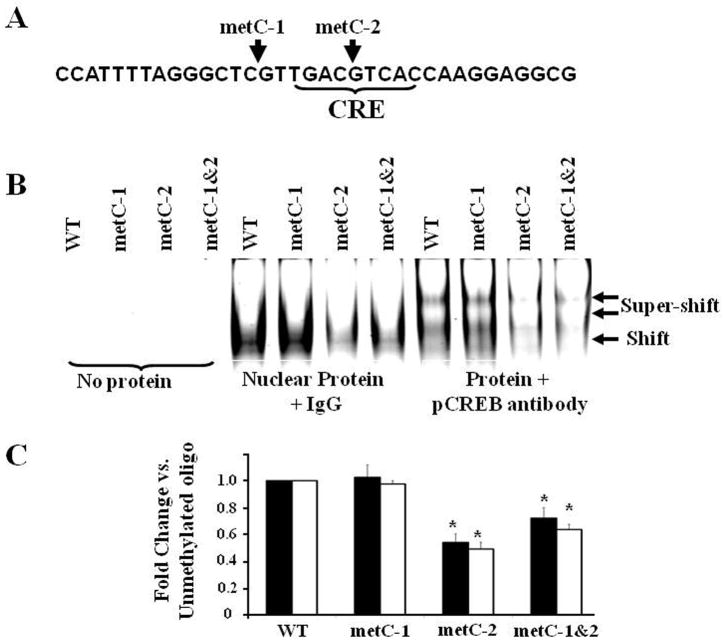
The molecular consequences of CpC methylation proximal to the CRH CRE on phospho-CREB binding were examined by EMSA using nuclear extracts from forskolin-treated 4B cells and oligonucleotide probes that contained the CRH CRE and methylated or unmethylated CpGs. EMSA revealed a major shifted complex of high density that is supershifted with phospho-CREB antibody (Fig. 7). Methylation of the CpG preceding the CRE (Met-C1) did not affect phospho-CREB binding, whereas methylation of the intra-CRE CpG (Met-C2), or both Met-C1 and Met-C2, significantly inhibited the ability of phospho-CREB to bind to the CRH CRE.
Fig. 7.
Effect of methylation at CpGs immediately preceding (metC-1) or within (metC-2) the CRE, or methylation of both CpGs (metC1&2), on phospho-CREB (pCREB) binding to CRH promoter oligonucleotides. The sequence of the oligonucleotide is shown in A, and a representative image of a gel is shown in B. Bars represent the mean ± S.E.M of values obtained from shifted (filled bars) and super-shifted (open bars) bands in three experiments (C). *P< 0.05 compared to wild type oligonucletide (WT) (one-way ANOVA analysis).
DISCUSSION
Stress exposure during early development causes long-lasting alterations in behavior and hypothalamic pituitary adrenal (HPA) axis activity, including increases in corticotropin releasing hormone (CRH) expression. This study was designed to determine whether the mechanism of the long-lasting increases in HPA axis activity and hypothalamic CRH mRNA levels reported in experimental animals subjected to early life stress (13–15) involves epigenetic modifications of the CRH promoter leading to enhanced transcriptional activity of the CRH gene. Using maternal deprivation (MD) in rats as a model of early life stress, we show that while basal HPA axis activity is only increased in adult MD females, both males and females subjected to MD have increased CRH transcriptional responsiveness to stress and reduced methylation of the CRH promoter in the PVN specifically at a CpG site located inside a critical CRE at position −230 bp.
Our data demonstrate that in both male and female rats, plasma corticosterone responses to acute restraint stress were significantly higher in the MD group compared with controls. This indicates that the experimental conditions used were effective in inducing hyper-responsiveness of the HPA as shown by previous studies (14, 33–34). It should be noted that in all experimental groups basal levels of plasma corticosterone levels were relatively high. This was probably due to the fact that rats were not routinely handled prior to sacrifice. Thus, it is likely that the significantly elevated basal plasma corticosterone and CRH hnRNA levels observed in female rats reflects enhanced responses to the mild stress of removal from the home cages, rather than chronic elevations in plasma corticosterone. Although the increases in adrenal size in MD females suggest chronic increases in HPA axis activity, the lack of elevated basal CRH mRNA levels and similar thymus weight in controls vs. MD females are against this possibility. In addition, the consistency of the values within the female groups suggests little influence of estrous cycle differences on the HPA axis measurements. It is noteworthy that the enhanced corticosterone responses to stress were not associated with increased ACTH responses, but in fact, plasma ACTH tended to be lower in MD rats of both sexes. This is in agreement with the recognized dissociation between plasma ACTH and corticosterone responses observed in a number of conditions (35) as well as the view that adrenocortical responses does not solely depend on the prevailing plasma ACTH levels. This data suggest that early life stress causes an increase in adrenal sensitivity to ACTH, especially in females which show significantly lower ACTH levels. In addition, the unchanged or slightly lower plasma ACTH responses to stress in MD rats in spite of the enhanced CRH hnRNA (and probably secretion) responses to stress, suggest that there is also a dissociation between hypothalamic and pituitary responses. The mechanism for the lack of enhanced ACTH responses remains to be elucidated but may involve CRH receptor desensitization due to excessive CRH production (9, 36).
Consistent with the plasma corticosterone data, basal CRH hnRNA levels were high only in female MD rats. However, basal CRH mRNA levels in this group were not different from controls, suggesting that as for corticosterone, elevated CRH hnRNA levels were due to higher sensitivity of female rats to handling at the time of sacrifice. Because of the higher basal values, the fold-increases in CRH hnRNA at 30 min restraint in MD female rats were lower than in control females. In male MD rats, CRH hnRNA responses were clearly more prolonged than in control males, and this may also be the case in MD females depending on whether or not the true basal levels are masked by handling stress. Irrespective of the cause of the higher basal CRH hnRNA and plasma corticosterone levels in females, our data support sex-specific differences in the effects of early life stress on HPA axis activity. Sex-specific differences in HPA axis responsiveness are consistent with observations in humans showing higher prevalence of major depression with hypercortisolism in women than in men, and that women who had experienced early life stress are more likely to develop depression (20).
In the current study we observed that the increases in HPA axis responsiveness to stress in male and female MD rats is associated with reduced methylation of specific CpG dinucleotides immediately preceding and inside the critical CRE at −230 of the CRH promoter. Consistent with our results, in adult male mice subjected to chronic social stress there was increased CRH expression associated with long-term demethylation of the CRH gene at a similar location (22). In contrast to the lack of sex differences in the changes in methylation of the CRE in the CRH promoter observed in our experiments with early life stress, chronic variable mild stress (CVMS) in adult rats induces altered CRH gene methylation in a site-specific and sex-specific manner (37). While in the PVN CVMS caused an increase in total methylation of the CRH promoter due to hypermethylation at CpGs outside the CRE, in the amygdala, CVMS significantly decreases total methylation of the CRH gene in females but not males (37). As in the present experiments and the social defeat model, one CpG displaying decreased methylation was the one inside the CRE. Using a different model of maternal stress during early pregnancy in mice, Mueller and Bale have also shown increased CRH gene expression in the amygdala in association with reduced methylation of a CpG associated with a GRE at position −236 (21). This variability in the site and sex specificity of the different studies is likely to depend on the type of stress, animal species and strain, and/or age of stress exposure. The data suggest that reduction in methylation of the CRE in the CRH promoter is part of the mechanism mediating the increased CRH transcriptional responses to stress. In addition to reductions in CRH promoter methylation, it is likely other factors such as decreased sensitivity to glucocorticoid feedback contribute to the prolonged CRH transcriptional responses to stress. In this regard, deficient maternal care in rats has been shown to increase glucocorticoid receptor promoter methylation leading to decreased expression in the hippocampus, a recognized target for glucocorticoid feedback (23).
As shown by the present study in rats and other studies in mice, the CRH promoter is highly methylated compared with other genes such as the glucocorticoid receptor and BDNF (21). Since methylation of promoter cyclic AMP response elements has been shown to decrease CREB binding and gene transcription (38–39), it is likely that changes in methylation at this site can interfere with the binding of transcription factors and consequently impact the transcriptional activity of the CRH gene. Supporting this possibility, the present gel shift and super-shift experiments demonstrated a marked reduction in the ability of CRH promoter oligonucleotides to recruit CREB specifically when the internal CpG in the CRE (but not the preceding CpG) was methylated. Further evidence for the importance of CRH promoter methylation has been provided by studies in a methyl-CpG-binding protein 2 (MeCP2) knock out mouse used as a model for Rett syndrome showing that these mice have undetectable MeCP2 binding to the CRH promoter and increased CRH expression (40). MeCP2 knockout mice display behavioral and physiologic phenotypes consistent with heightened levels of CRH. This is in keeping with the anxiety behavior and increased cortisol levels observed in patients with Rett syndrome. In the present study and the previous reports (21, 22, 37) methylation of the CRH promoter CRE was reduced by only about 10%. While the decrease may be underestimated due to the presence of non-CRH expressing cells in the micro-punches, it represents a relatively small percent of total methylation at this site. Although it is clear that further studies are clearly needed to determine the impact of such small decreases in CRE methylation on phospho-CREB binding and CRH transcription, this could occur via changes in MeCP2 and acetylated histones binding to the CRH promoter.
In summary, this study confirms the long-term stimulatory effects of maternal deprivation, used as a model of early life stress, on HPA axis activity and demonstrates that hyper-responsiveness to acute stress during adulthood is associated with increased CRH hnRNA responses to stress in the PVN of MD rats of both sexes, indicating enhanced transcriptional activity. The parallel occurrence of hypomethylation of a critical regulatory site of the CRH gene in the PVN suggests that altered methylation of the CRH promoter is one of the mechanisms underlying the enhanced CRH transcriptional responses to stress observed in the hypothalamus of these rats. It is likely that such epigenetic modification of the CRH gene may contribute to the etiology of psychiatric and metabolic alterations observed in adult subjects subjected to adverse experiences during early life.
Acknowledgments
This work was supported by the Intramural Research Program of National Institute of Child Health and Human Development, NIH. The authors would like to thank Dr Jorge Sztein, NIAID, NIH, for his helpful advice with the cycle synchronization and breeding protocols.
ABBREVIATIONS
- MD
maternal deprivation
- CRH
corticotropin releasing hormone
- HPA
hypothalamic pituitary adrenal
References
- 1.Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49(12):1023–39. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- 2.Agid O, Shapira B, Zislin J, Ritsner M, Hanin B, Murad H, Troudart T, Bloch M, Heresco-Levy U, Lerer B. Environment and vulnerability to major psychiatric illness: a case control study of early parental loss in major depression, bipolar disorder and schizophrenia. Mol Psychiatry. 1999;4(2):163–72. doi: 10.1038/sj.mp.4000473. [DOI] [PubMed] [Google Scholar]
- 3.Kendler KS, McGuire M, Gruenberg AM, O’Hare A, Spellman M, Walsh D. The Roscommon Family Study. III. Schizophrenia-related personality disorders in relatives. Arch Gen Psychiatry. 1993;50(10):781–8. doi: 10.1001/archpsyc.1993.01820220033004. [DOI] [PubMed] [Google Scholar]
- 4.Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. Major depression and generalized anxiety disorder. Same genes, (partly) different environments? Arch Gen Psychiatry. 1992;49(9):716–22. doi: 10.1001/archpsyc.1992.01820090044008. [DOI] [PubMed] [Google Scholar]
- 5.Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. Childhood parental loss and adult psychopathology in women. A twin study perspective. Arch Gen Psychiatry. 1992;49(2):109–16. doi: 10.1001/archpsyc.1992.01820020029004. [DOI] [PubMed] [Google Scholar]
- 6.Koenig AL, Ialongo N, Wagner BM, Poduska J, Kellam S. Negative caregiver strategies and psychopathology in urban, African-American young adults. Child Abuse Negl. 2002;26(12):1211–33. doi: 10.1016/s0145-2134(02)00422-2. [DOI] [PubMed] [Google Scholar]
- 7.Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34(1):13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 8.Walker MA. Treatment of autism spectrum disorders: neurotransmitter signaling pathways involved in motivation and reward as therapeutic targets. Expert Opin Ther Targets. 2008;12(8):949–67. doi: 10.1517/14728222.12.8.949. [DOI] [PubMed] [Google Scholar]
- 9.Aguilera G. Regulation of pituitary ACTH secretion during chronic stress. Front Neuroendocrinol. 1994;15(4):321–50. doi: 10.1006/frne.1994.1013. [DOI] [PubMed] [Google Scholar]
- 10.Jacobson L. Hypothalamic-pituitary-adrenocortical axis regulation. Endocrinol Metab Clin North Am. 2005;34(2):271–92. vii. doi: 10.1016/j.ecl.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Vale W, Rivier C, Brown MR, Spiess J, Koob G, Swanson L, Bilezikjian L, Bloom F, Rivier J. Chemical and biological characterization of corticotropin releasing factor. Recent Prog Horm Res. 1983:39245–70. doi: 10.1016/b978-0-12-571139-5.50010-0. [DOI] [PubMed] [Google Scholar]
- 12.Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol. 1999;160(1):1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- 13.Anisman H, Zaharia MD, Meaney MJ, Merali Z. Do early-life events permanently alter behavioral and hormonal responses to stressors? Int J Dev Neurosci. 1998;16(3–4):149–64. doi: 10.1016/s0736-5748(98)00025-2. [DOI] [PubMed] [Google Scholar]
- 14.Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res Mol Brain Res. 1993;18(3):195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- 15.Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277(5332):1659–62. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 16.McEwen BS. Understanding the potency of stressful early life experiences on brain and body function. Metabolism. 2008;57(Suppl):2S11–5. doi: 10.1016/j.metabol.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lesch KP. Gene-environment interaction and the genetics of depression. J Psychiatry Neurosci. 2004;29(3):174–84. [PMC free article] [PubMed] [Google Scholar]
- 18.Murgatroyd C, Patchev AV, Wu Y, Micale V, Bockmuhl Y, Fischer D, Holsboer F, Wotjak CT, Almeida OF, Spengler D. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat Neurosci. 2009;12(12):1559–66. doi: 10.1038/nn.2436. [DOI] [PubMed] [Google Scholar]
- 19.Li S, Hursting SD, Davis BJ, McLachlan JA, Barrett JC. Environmental exposure, DNA methylation, and gene regulation: lessons from diethylstilbesterol-induced cancers. Ann N Y Acad Sci. 2003:983161–9. doi: 10.1111/j.1749-6632.2003.tb05971.x. [DOI] [PubMed] [Google Scholar]
- 20.Oomen CA, Girardi CE, Cahyadi R, Verbeek EC, Krugers H, Joels M, Lucassen PJ. Opposite effects of early maternal deprivation on neurogenesis in male versus female rats. PLoS One. 2009;4(1):e3675. doi: 10.1371/journal.pone.0003675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008;28(36):9055–65. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elliott E, Ezra-Nevo G, Regev L, Neufeld-Cohen A, Chen A. Resilience to social stress coincides with functional DNA methylation of the Crf gene in adult mice. Nat Neurosci. 2010;13(11):1351–3. doi: 10.1038/nn.2642. [DOI] [PubMed] [Google Scholar]
- 23.Meaney MJ, Szyf M, Seckl JR. Epigenetic mechanisms of perinatal programming of hypothalamic-pituitary-adrenal function and health. Trends Mol Med. 2007;13(7):269–77. doi: 10.1016/j.molmed.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Tate PH, Bird AP. Effects of DNA methylation on DNA-binding proteins and gene expression. Curr Opin Genet Dev. 1993;3(2):226–31. doi: 10.1016/0959-437x(93)90027-m. [DOI] [PubMed] [Google Scholar]
- 25.Shepard JD, Liu Y, Sassone-Corsi P, Aguilera G. Role of glucocorticoids and cAMP-mediated repression in limiting corticotropin-releasing hormone transcription during stress. J Neurosci. 2005;25(16):4073–81. doi: 10.1523/JNEUROSCI.0122-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma XM, Lightman SL, Aguilera G. Vasopressin and corticotropin-releasing hormone gene responses to novel stress in rats adapted to repeated restraint. Endocrinology. 1999;140(8):3623–32. doi: 10.1210/endo.140.8.6943. [DOI] [PubMed] [Google Scholar]
- 27.Brunson KL, Avishai-Eliner S, Hatalski CG, Baram TZ. Neurobiology of the stress response early in life: evolution of a concept and the role of corticotropin releasing hormone. Mol Psychiatry. 2001;6(6):647–56. doi: 10.1038/sj.mp.4000942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seasholtz AF, Thompson RC, Douglass JO. Identification of a cyclic adenosine monophosphate-responsive element in the rat corticotropin-releasing hormone gene. Mol Endocrinol. 1988;2(12):1311–9. doi: 10.1210/mend-2-12-1311. [DOI] [PubMed] [Google Scholar]
- 29.Aguilera G, Liu Y. The molecular physiology of CRH neurons. Front Neuroendocrinol. 2011 doi: 10.1016/j.yfrne.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1998. [Google Scholar]
- 31.Liu Y, Kamitakahara A, Kim AJ, Aguilera G. Cyclic adenosine 3′,5′-monophosphate responsive element binding protein phosphorylation is required but not sufficient for activation of corticotropin-releasing hormone transcription. Endocrinology. 2008;149(7):3512–20. doi: 10.1210/en.2008-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y, Aguilera G. inducible early repressor mediates the termination of corticotropin releasing hormone transcription in hypothalamic neurons. Cell Mol Neurobiol. 2009;29(8):1275–81. doi: 10.1007/s10571-009-9423-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Veenema AH, Neumann ID. Maternal separation enhances offensive play-fighting, basal corticosterone and hypothalamic vasopressin mRNA expression in juvenile male rats. Psychoneuroendocrinology. 2009;34(3):463–7. doi: 10.1016/j.psyneuen.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 34.Vazquez DM, Bailey C, Dent GW, Okimoto DK, Steffek A, Lopez JF, Levine S. Brain corticotropin-releasing hormone (CRH) circuits in the developing rat: effect of maternal deprivation. Brain Res. 2006;1121(1):83–94. doi: 10.1016/j.brainres.2006.08.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bornstein SR, Engeland WC, Ehrhart-Bornstein M, Herman JP. Dissociation of ACTH and glucocorticoids. Trends Endocrinol Metab. 2008;19(5):175–80. doi: 10.1016/j.tem.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 36.Wynn PC, Harwood JP, Catt KJ, Aguilera G. Regulation of corticotropin-releasing factor (CRF) receptors in the rat pituitary gland: effects of adrenalectomy on CRF receptors and corticotroph responses. Endocrinology. 1985;116(4):1653–9. doi: 10.1210/endo-116-4-1653. [DOI] [PubMed] [Google Scholar]
- 37.Sterrenburg L, Gaszner B, Boerrigter J, Santbergen L, Bramini M, Elliott E, Chen A, Peeters BW, Roubos EW, Kozicz T. Chronic stress induces sex-specific alterations in methylation and expression of corticotropin-releasing factor gene in the rat. PLoS One. 2011;6(11):e28128. doi: 10.1371/journal.pone.0028128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang X, Odom DT, Koo SH, Conkright MD, Canettieri G, Best J, Chen H, Jenner R, Herbolsheimer E, Jacobsen E, Kadam S, Ecker JR, Emerson B, Hogenesch JB, Unterman T, Young RA, Montminy M. Genome-wide analysis of cAMP-response element binding protein occupancy, phosphorylation, and target gene activation in human tissues. Proc Natl Acad Sci U S A. 2005;102(12):4459–64. doi: 10.1073/pnas.0501076102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rishi V, Bhattacharya P, Chatterjee R, Rozenberg J, Zhao J, Glass K, Fitzgerald P, Vinson C. CpG methylation of half-CRE sequences creates C/EBPalpha binding sites that activate some tissue-specific genes. Proc Natl Acad Sci U S A. 2010;107(47):20311–6. doi: 10.1073/pnas.1008688107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGill BE, Bundle SF, Yaylaoglu MB, Carson JP, Thaller C, Zoghbi HY. Enhanced anxiety and stress-induced corticosterone release are associated with increased Crh expression in a mouse model of Rett syndrome. Proc Natl Acad Sci U S A. 2006;103(48):18267–72. doi: 10.1073/pnas.0608702103. [DOI] [PMC free article] [PubMed] [Google Scholar]