Abstract
Adenoviral technology has been thoroughly evaluated for delivering genetic material to tumor tissue and the surrounding microenvironment. Almost any gene can be cloned into an adenovirus (Ad) vector, which when combined with strong, constitutively active promoters permit up to a million-fold amplification of the transgene in a single adenoviral particle, thus facilitating their use in cancer therapy and imaging. However, widespread infection of the liver and other non-targeted tissues by Ad vectors is a substantial problem that often results in significant liver inflammation and hepatotoxicity at doses required to achieve efficient tumor transduction. miR-122 is a highly expressed liver-specific microRNA that provides a unique opportunity for down-regulating adenoviral transgene expression in liver tissue. The binding of endogenous miRNAs to complementary miRNA targeting elements (miRTs) incorporated into the 3′ untranslated region of adenoviral transgenes interferes with message stability and/or protein translation, and miRT elements against miR-122 (miRT-122) can selectively reduce adenoviral transgene expression in the liver. Previous studies using miR-122-based regulation, with and without other types of transcriptional targeting, have yielded promising preliminary results. However, investigations to date evaluating miRT-122 elements for improving tumor specificity have used either non-tumor bearing animals or direct intratumoral injection as the mode of delivery. In the present study, we confirmed the ability of miRT-122 sequences to selectively down-regulate adenoviral luciferase expression in the liver in vitro and in vivo, and show that this strategy can improve tumor specific transgene expression in a HT1080 human fibrosarcoma model. Rapid growth and the inefficient flow of blood through tumor neovasculature often results in profound hypoxia, which provides additional opportunities for targeting solid tumors and their microenvironment using vectors incorporating hypoxia-responsive promoters to drive transgene expression. We therefore employed a combinatorial approach using miRT-122 elements with hypoxia-responsive transcriptional targeting to further improve the tumor specific expression of an adenoviral reporter gene. Results from this investigation reveal that miRT122 elements alone decrease off-target liver expression and improve tumor specificity of adenoviral vectors. Furthermore, increased tumor specificity can be achieved by combining miRT-122 elements with hypoxia-responsive promoters.
Introduction
Adenovirus serotype 5 (Ad5) remains the most widely-used viral gene delivery system in clinical studies,. Many investigators have evaluated a wide variety of modified adenoviral vectors for efficacy as cancer imaging and therapy agents.2–8 Despite its potential for efficiently delivering genetic payloads to tumor cells, the ability of adenovirus to promiscuously infect a wide variety of cell and tissue types presents a substantial problem for gene therapy. The broad biodistribution of these vectors following intravenous injection requires relatively large doses to ensure sufficient infection of tumor tissue because the majority of systemically administered wild-type Ad5 is taken up by the liver. The ensuing inflammatory responses may result in severe hepatic dysfunction.9–11 Thus, improved methods for reducing liver infection while increasing tumor specificity of adenoviral agents are needed.
Since their discovery in 1993,12 microRNAs (miRNAs) have become recognized as important regulatory molecules for controlling gene expression, and numerous studies link the aberrant regulation of miRNAs to the pathology of most common malignancies.13 miRNAs are a class of short, non-coding RNAs that are endogenously expressed in many organisms. Their primary function in mammals is to regulate the expression of genes by binding to the 3′ untranslated region (3′ UTR) of an expressed mRNA and interfering with protein translation.14 The production of particular miRNAs is often tissue-specific, providing a unique opportunity for the fine tuning of gene therapy applications by introducing miRNA binding sites into the 3′ UTR of transgenes to down-regulate their expression in particular tissues. One of the most tissue-specific miRs is miR-122, which accounts for 72% of all miRs cloned from mouse liver,15, 16 and there is conservation of miR-122 sequences across a variety of mammalian species, including humans.17 A preliminary study in vitro has shown that miR-122-based regulation can reduce adenoviral gene expression in hepatic cells,18 and this approach has been assessed in several studies in vivo. Four miR-122 binding sites incorporated into the 3′ UTR of adenovirus luciferase expression cassettes can reduce transgene gene expression 50–1500-fold in the livers of mice compared to conventional Ad vectors.19, 20 Histopathological analysis and/or biochemical determination of ALT/AST levels also show substantially reduced hepatotoxicity for adenoviral agents incorporating miRNA target elements (miRTs) against miR-122.
This endogenous regulatory mechanism holds great promise for improving tissue specificity, but most studies have been performed in either non tumor-bearing animals or have used direct intratumoral injection as the mode of delivery. In addition, few studies to date have combined this strategy with other methods for improving the tumor specificity of adenoviral vectors. miRT-based regulation of viral vectors might work well in combination with transcriptional targeting, which uses cell-specific promoters to up-regulate transgene expression in particular tissues. Previous work has shown that combining binding sites for multiple miRs along with transcriptional targeting in a baculoviral system improve the selectivity of suicide gene expression in tumors while reducing toxic effects in normal cells.21 Another study showed that combining miRTs specific for miR-122 with a cell specific promoter reduces reporter gene expression in an oncolytic adenoviral vector to almost undetectable levels in hepatic cells.22
Solid tumors must recruit new vasculature to provide oxygen and nutrients for continued growth, but the disorganized and leaky vasculature observed in solid tumors often results in a profound hypoxic state.23 Recent work in our lab has shown that the hypoxia-responsive promoters can achieve a high degree of tumor-specific adenoviral gene expression in vivo.5 A combinatorial approach using hypoxia responsive promoters with miR-122 target sequences might be a highly effective strategy for reducing adenoviral transgene expression in the liver and further improving tumor-specificity. We therefore combined miR-122 based gene silencing approaches with transcriptional targeting using the carbonic anhydrase IX (CAIX) and chimeric hypoxia response element-thymidine kinase (Hre3TK) promoters to reduce liver transgene expression and enhance the tumor specificity of systemically administered adenoviral vectors in mice bearing HT1080 human fibrosarcoma xenografts.
Materials and Methods
Construction of adenoviral vectors
Four tandem miRTs recognized by miR-122 (ACAAACACCATTGTCACACTCCA) were inserted into the 3′UTR of a CMV promoter-driven luciferase reporter cassette in an adenovirus shuttle plasmid using synthetic oligonucleotides and convenient restriction endonuclease sites. A full length recombinant viral genome (AdCMVLux122) was then made using cre/loxP homologous recombination in vitro and transfected into 911 cells. Viral constructs without miR binding sites (AdCMVLux) or with 4 tandem copies of the reversed target binding site sequence for miR-122 (AdCMVLuxcon) were also made for use as negative controls. To place the miRT-122 regulated luciferase reporter under the control of the cell-specific promoters in the shuttle plasmid, a synthetic Hre3TK promoter containing three 50np tripartite hypoxia responsive elements (Hre) upstream of a minimal thymidine kinase (TK) promoter24, 25 and the human CAIX promoter fragment amplified from human genomic DNA 26 was incorporated into the AdCMVLux122 vector in place of the CMV promoter using basic cloning techniques. Transgenes were constructed into viral vectors using the cre-loxP recombination method in vitro to yield AdHre3TKLux122 and AdCAIXLux122 viruses.27 AdHre3TKLuc and AdCAIXLuc lacking miRT-122 elements have already been published.5
Single plaque isolates of all viruses were amplified and titered by plaque assay for experiments on cultured cells. The three virus preparations ranged in titer from 2×109 pfu/mL to 4.0×109 pfu/mL. Viruses were amplified further and purified for in vivo use in subsequent experiments by CsCl gradient centrifugation and Sepharose CL-4B chromatography as previously described.5 These preparations ranged in titer from 7.1×1012 particles/mL to 1.1×1013 particles/mL.
Cell culture
Both a single cell clone of HT1080 human fibrosarcoma cells, primary mouse embryo fibroblasts (MEF), human Hep3B and the adenoviral host cells 911 were propagated in DMEM containing 10% FBS. Primary mouse hepatocytes were prepared from mixed strain wild type mice and plated in Williams’ E medium containing 5% FBS, 10nM dexamethasone, 10nM insulin and penicillin-streptomycin as antibiotics. Infections of all cells in vitro were performed with crude stocks of viruses diluted in DMEM containing 2% FBS. A range of titers were used for infection of cell cultures for 1 hr at 37°C, and then incubated overnight to permit luciferase expression before cells were harvested and luciferase activity determined to quantify gene expression.
Animal experiments
All animal experiments were approved by the Institutional Animal Care and Use Committee. Female nude mice (strain code 088 from Charles River) were used for HT1080 tumor cell implantation and CD-1 female mice (strain code 022, from Charles River) were used for the experiment shown in Figure 3. For tumor xenografts, mice were injected subcutaneously with 3–5×106 cells suspended in 0.5ml DMEM on the dorsal flank and tumors allowed to grow until 0.4–0.8 cc in size as measured by calipers. Purified adenoviral constructs were injected via the tail vein at doses of 1011 or 1012 particles per mouse. Three days after injection of adenovirus, mice were sacrificed and major organs and tumors removed for biochemical determination of luciferase activity performed as previously described.5 Viral DNA content in tissues was measured by real time PCR detection of the adenovirus type 5 hexon gene using a TaqMan assay.5
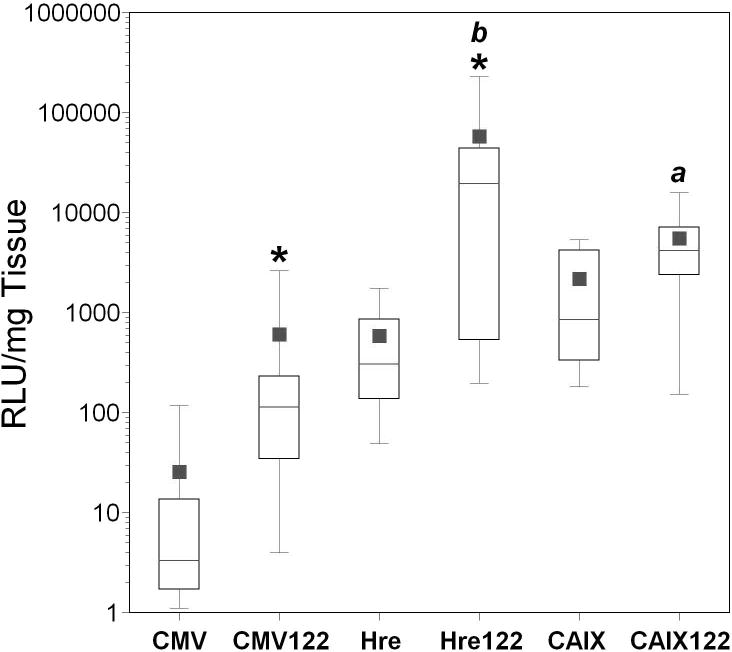
Figure 3. Down-regulation of adenoviral transgene expression in the liver using miRT-122 silencing in non-tumor bearing CD-1 mice.
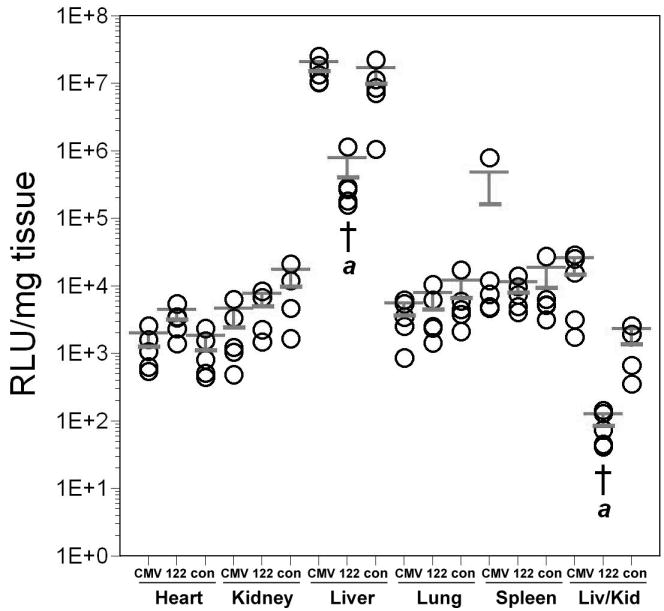
AdCMVLux (CMV), AdCMVLux122 (122), or AdCMVLuxcon (con) was administered intravenously at a dose of 1011 particles per mouse. Luciferase expression was quantified by biochemical means and is expressed as RLU per milligram of tissue. Values for individual mice are shown as circles, with means and standard deviations represented by horizontal and error bars, respectively. Significant differences between AdCMVLux122 and AdCMVLux are indicated by daggers (†, p<0.01), and significant differences between AdCMVLux122 and AdCMVLuxcon are indicated by italicized letter (a, p<0.05).
Determination of AST/ALT levels and histological indicators of liver inflammation
AST/ALT levels as markers of hepatocellular necrosis were measured in serum samples obtained from mice at the time of sacrifice by the Mouse Metabolic Phenotyping Core at UT Southwestern Medical Center at Dallas using the Vitros® 250 chemistry system. Liver pathology was assessed on H&E stained liver sections.
Statistical Analyses
SigmaStat for Windows v3.11 was used to perform Mann-Whitney RankSum Test analyses for pairwise comparisons between individual treatment groups and controls.
Results
In vitro assessment of adenovirus containing miRT-122 elements and control vectors in various cell lines
To confirm the ability of miRTs against miR-122 to regulate adenoviral gene expression, we first infected several cell types with our experimental and control vectors in vitro. HT1080 fibrosarcoma (HT1080), human hepatoma (Hep3B), primary mouse embryonic fibroblast (MEF) and primary murine hepatocyte cultures were infected with varying concentrations of virus expressing luciferase under the control of the CMV promoter alone (AdCMVLux), with miR target elements recognized by miR-122 (AdCMVLux122), or with reverse complementary miRT-122 sequences as an additional control (AdCMVLuxcon, Figure 1). These in vitro results are shown in Figure 2, with luciferase expression measured in relative light units plotted against viral concentration. AdCMVLux122 clearly shows no silencing of transgene expression in HT1080, Hep3B or MEF cells, with similar concentration-dependent luciferase expression following infection with this vector and the two controls. This was expected for MEF and Hep3B cells based on previous work showing undetectable levels of miR-122 in these cell lines,28, 29 and these results also support the use of the HT1080 tumor cell model because no appreciable silencing of transgene expression was observed using the miRT-122-containing vector. In mouse primary hepatocytes, however, transgene expression was significantly down-regulated following infection with AdCMVLux122, requiring approximately 25- or 50-fold higher concentrations of virus to achieve luciferase expression equal to AdCMVLuxcon or AdCMVLux, respectively.
Figure 1.
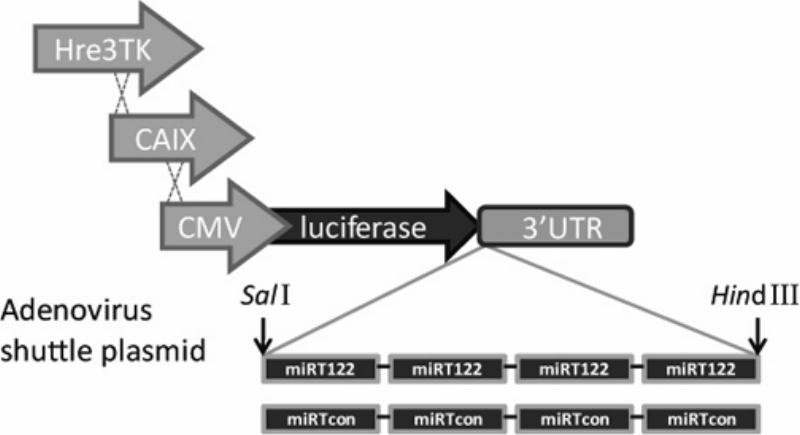
Schematic diagram of the constructs incorporating four tandem copies of sequences complementary to miR-122 (miRT-122) or reverse complementary miRT-122 sequences (miRT-con) into the 3′UTR of the luciferase transgene within the adenoviral shuttle plasmid. Alternative constructs use either the constitutive CMV or hypoxia-responsive promoters (Hre3TK or CAIX).
Figure 2. Effects of miRT-122 sequences on adenoviral luciferase expression in vitro.
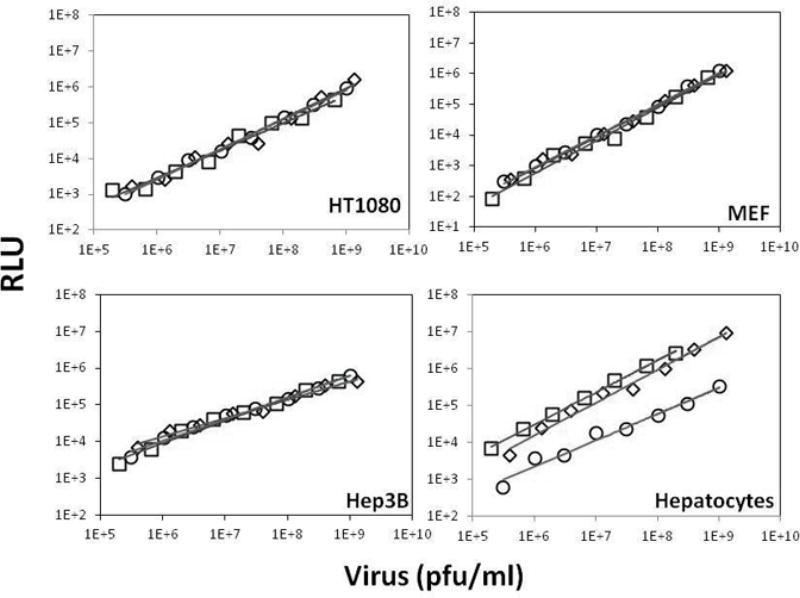
Human fibrosarcoma (HT1080), hepatoma (Hep3B), mouse embryonic fibroblasts (MEF), and mouse primary hepatocytes were infected with various concentrations of crude viruses AdCMVLux (squares), AdCMVLuxcon (diamonds), or AdCMVLux122 (circles). Data are the means of triplicate measurements. Luciferase expression was plotted against virus concentration, and the best fits determined by linear regression analysis are shown by solid lines.
Preliminary assessment of AdCMVLux122 and controls in non-tumor bearing mice
We next evaluated in vivo activity of miRT-122 gene silencing by measuring luciferase expression in various tissues following the intravenous administration of 1011 particles of AdCMVLux, AdCMVLux122, or AdCMVLuxcon into non-tumor bearing CD-1 mice. Five animals were used for each viral construct, and results are shown as scatter plots in Figure 3. In all tissues except liver, luciferase expression was similar for all three viral constructs. In liver, luciferase expression was significantly decreased for AdCMVLux122 compared to either AdCMVLux (median decrease 50-fold, p=0.008) or AdCMVLuxcon (median decrease 32-fold, p=0.016), confirming the silencing effect of the miRT-122 sequences on liver transgene expression in vivo. The specificity of this silencing strategy for liver tissue is apparent from the decrease in the liver to kidney ratio of luciferase expression for AdCMVLux122 compared to either AdCMVLux (median decrease 212-fold, p=0.008) or AdCMVLuxcon (median decrease 18-fold, p=0.016). These results replicate and confirm those previously published20 using similar adenoviral constructs.
Evaluation of AdCMVLux122 in tumor-bearing animals
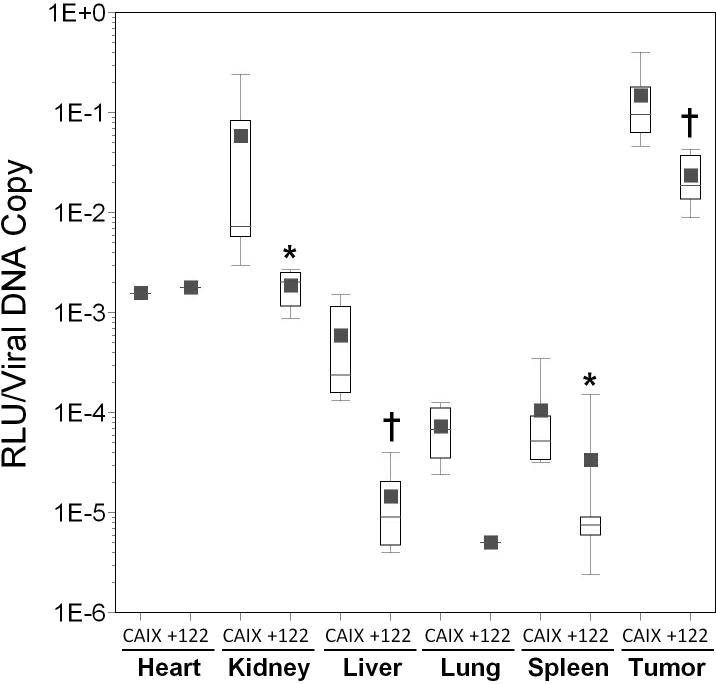
The AdCMVLux122 virus was next injected into tumor-bearing nude mice to confirm the miRT-122-mediated liver silencing effect in our in vivo tumor model and to determine any improvements in tumor specificity compared to control vectors. Following the establishment of HT1080 tumors, each viral construct was intravenously administered at 1011 particles per animal and luciferase activity was measured in tumor and other tissues (Figure 4, panel A). Luciferase expression for all three viruses was similar in most tissues tested, but a significant 22-fold reduction in mean luciferase activity was shown in the liver for AdCMVLux122 compared to AdCMVLux (p=0.001). There was also approximately a four-fold reduction in mean luciferase expression of AdCMVLux122 compared to AdCMVLuxcon in liver, but this difference did not achieve significance, and AdCMVLuxcon showed a 5.5-fold reduction in mean liver transgene expression compared to AdCMVLux (p=0.009). AdCMVLux122 also showed a 4-fold reduction in mean luciferase expression compared to AdCMVLux in the spleen (p=0.015). Most importantly, the mean tumor/liver ratio of luciferase expression was approximately 25-fold higher for AdCMVLux122 compared to either AdCMVLux or AdCMVLuxcon, indicating substantially improved tumor specific transgene expression for the vector containing miRT-122 sequences.
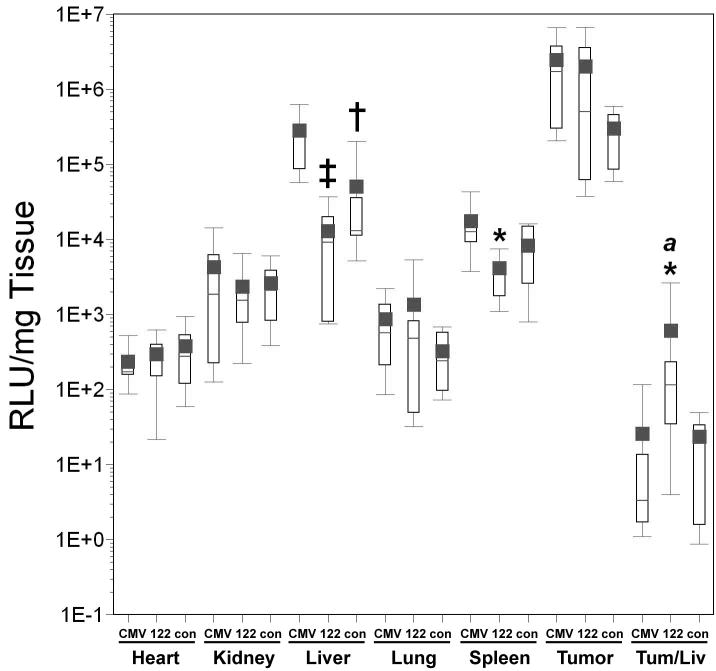
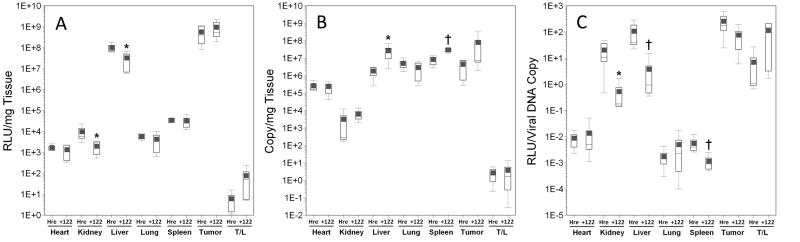
Figure 4. Effect of miR-122 targeting on luciferase expression and biodistribution of adenoviral vectors in HT1080 tumor-bearing mice.
AdCMVLux (CMV), AdCMVLux122 (122), or AdCMVLuxcon (con) was administered intravenously at a dose of 1011 particles per mouse. Luciferase expression was quantified by biochemical means and is expressed as RLU per milligram of tissue. Results for groups are displayed as box plots with mean values shown as solid squares, median values by horizontal lines, 75% confidence intervals by open boxes and 95% confidence intervals by double sided error bars. Significant differences between AdCMVLux122 or AdCMVLuxcon and AdCMVLux vectors are indicated by asterisks (*, p<0.05), daggers (†′ p<0.01) and double daggers (‡, p≤0.001), and significant differences between AdCMVLux122 and AdCMVLuxcon vectors are indicated by italicized letters (a,′ p<0.05, b, p≤0.01 and c,′ p≤0.001). Panel A, Luciferase expression quantified by biochemical means and expressed as RLU per mg of tissue; Panel B, viral transduction shown as viral DNA copy per mg of tissue determined by real-time PCR; Panel C, Specific activity of gene expression, as RLU luciferase per viral DNA copy.
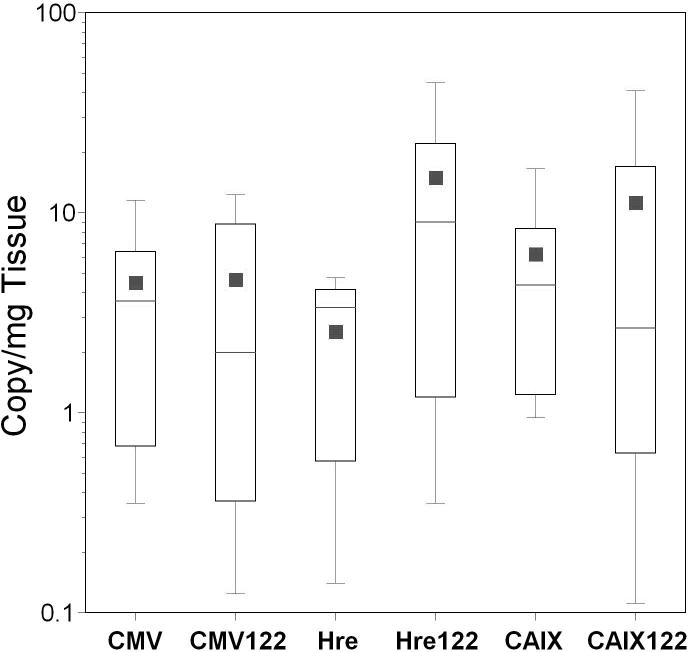
Quantitative real time PCR showed higher levels of cell-associated viral DNA in all tissues for mice treated with AdCMVLux122 compared to AdCMVLux or AdCMVLuxcon, and these increases were significant for most tissues in this particular experiment (Figure 4, panel B). This result was a statistical anomaly that could not be repeated in other experiments. Because a number of different factors can result in variations in copy number that can significantly complicate the analysis of relative gene expression levels when different viral vectors are used, many beyond the experimenters control, the specific activity of gene expression in RLU per viral DNA copy was subsequently used as the most relevant comparison of tumor specificity. As a result, luciferase expression per viral DNA copy was significantly lower for AdCMVLux122 compared to both controls (Figure 4, panel C). This reduced expression was most pronounced in the liver, with an almost 500-fold decrease in mean RLU per viral DNA copy for AdCMVLux122 compared to AdCMVLux. The mean tumor/liver ratio of transgene expression per viral DNA copy was also approximately 27-fold higher for AdCMVLux122 compared to AdCMVLux (p=0.026), and 14-fold higher compared to AdCMVLuxcon (p=0.034). These results confirm the ability of the miRT122 target sequences to down-regulate gene expression specifically in the liver and thereby improve the tumor specificity of an adenoviral vector.
Combinatorial regulatory control using miRT-122 elements and hypoxia-responsive promoters in tumor-bearing animals
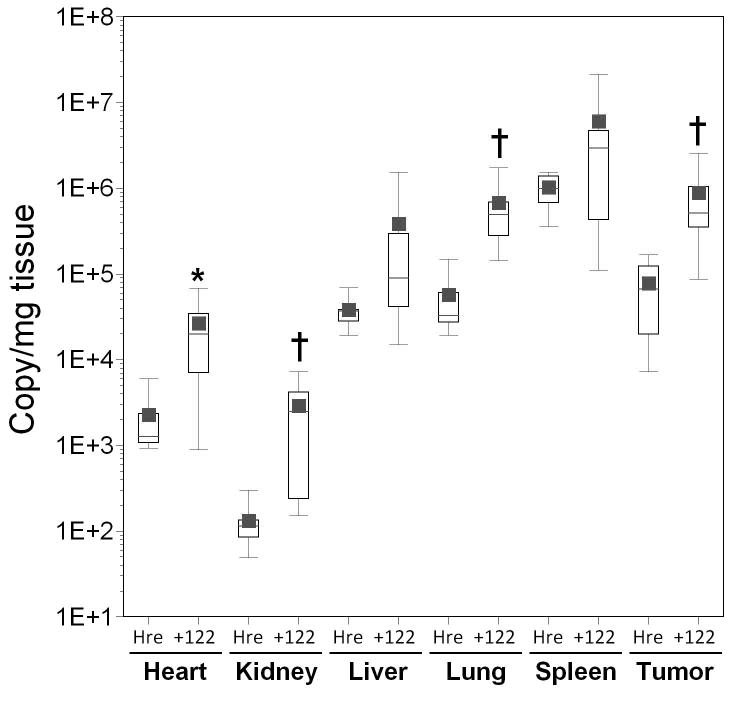
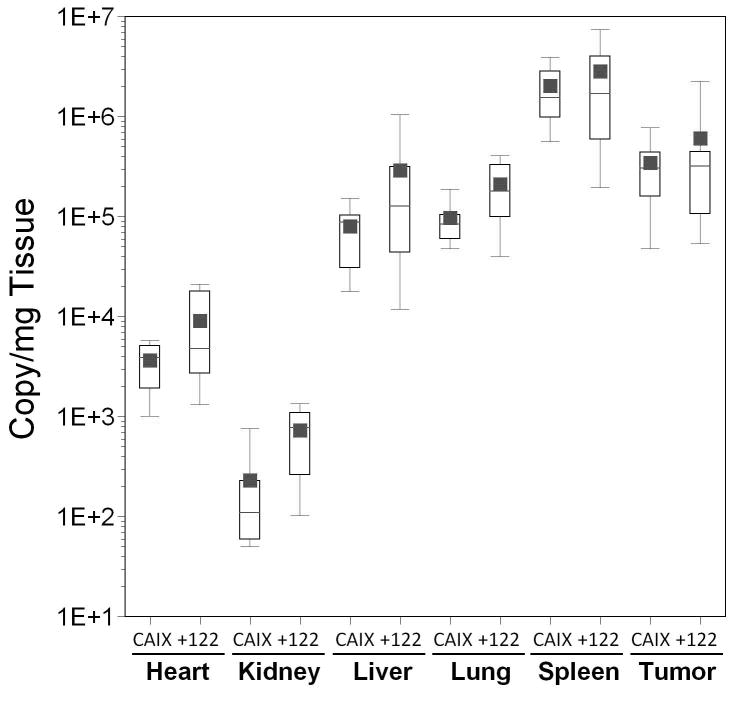
To examine whether a combinatorial approach could provide further additive or synergistic improvements in tumor specificity, we combined the miRT-122 silencing strategy with transcriptional targeting using two different hypoxia-responsive promoters (Hre3TK and CAIX) previously shown in our lab to provide a high degree of tumor specific transgene expression.5 Results for both promoters are shown as box plots in Figure 5, with comparisons shown for the hypoxia responsive vectors with or without miRT-122 elements in various tissues. The addition of miRT-122 elements failed to significantly reduce the liver-specific expression of luciferase compared to virus with the Hre3TK promoter alone, and in fact resulted in significantly higher luciferase expression in most tissues including the heart, kidney, lung and tumor (Figure 5, panel A). However, this result is understandable because viral DNA copy number quantified by real time PCR was again significantly increased in all tissues except liver and spleen for AdHre3TKLux122 (Figure 5, panel B). Despite this unexpected increase in apparent viral DNA content for the miRT-122-containing vector which complicated the interpretation of miRT-122-based gene silencing, mean luciferase expression per viral DNA copy was reduced 4-fold in the liver for the miRT-122 containing vector compared to the control (p=0.028, Figure 5, panel C). A significant reduction in normalized luciferase expression was also observed in the lung for AdHre3TKLux122 compared to the control, although mean values were very similar for the two vectors.
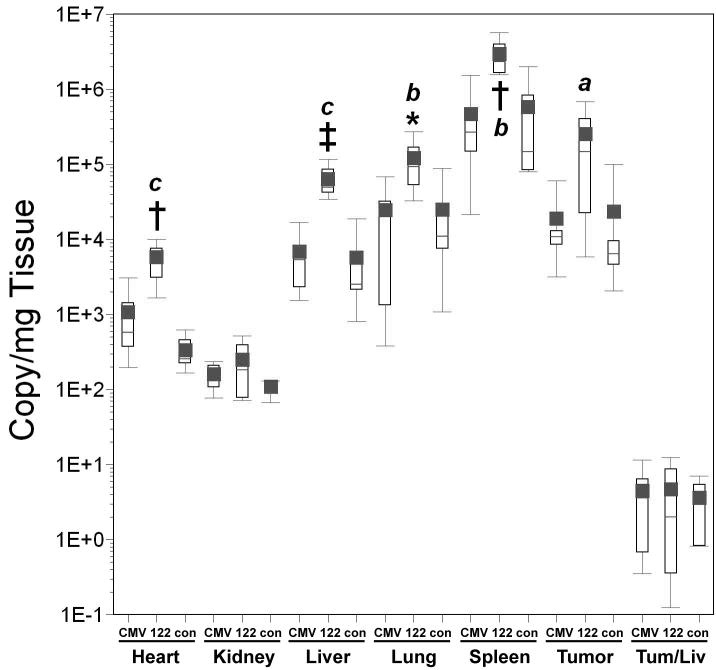
Figure 5. Effect of miRT-122 sequences on luciferase expression and biodistribution of transcriptionally targeted adenoviral vectors in HT1080 tumor-bearing mice.
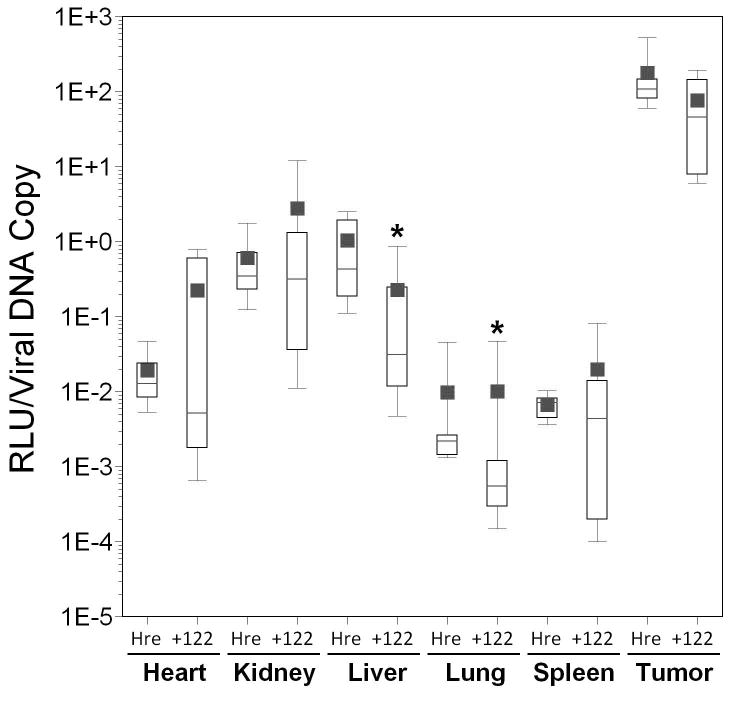
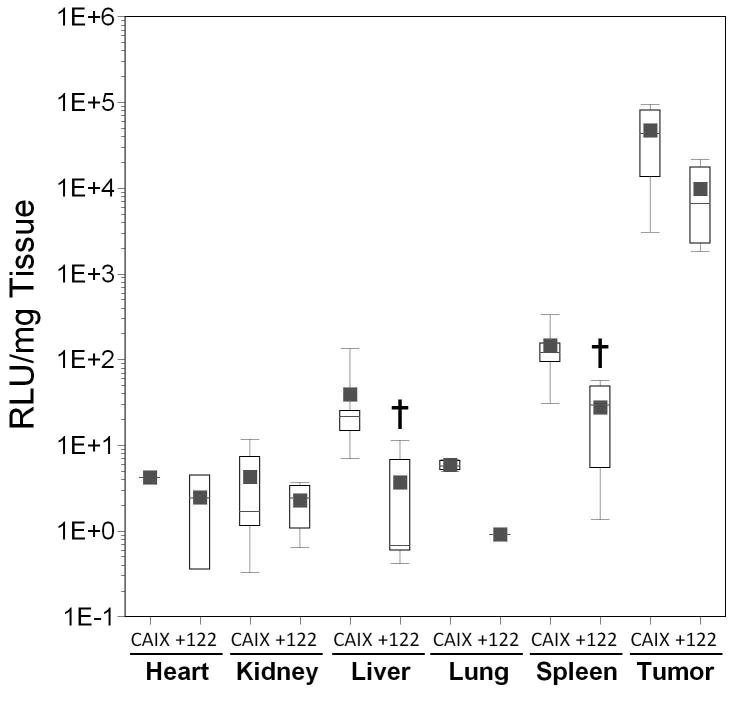
Results are displayed as box plots as in Figure 4. Significant differences between experimental vectors containing miRT-122 sequences and controls (containing hypoxia responsive promoters alone) are indicated by asterisks (*, p<0.05) and daggers (†, p<0.01). Panels A–C; Adenoviral vectors expressing luciferase from the Hre3TK promoter either with (122) and without (Hre) miRT-122 sequences are compared in various tissues. Panel A, Luciferase expression as RLU per mg of tissue; Panel B, viral transduction shown as viral DNA copy per mg of tissue; Panel C, luciferase expression per viral DNA copy. Panels D–F; Vecto rs expressing luciferase under the control of the hypoxia-responsive CAIX promoter either with (122) or without (CAIX) miRT-122 sequences are similarly compared.
Incorporation of miRT-122 sequences into the CAIX promoter vector resulted in significantly lower transgene expression in the liver (mean difference over 10-fold; p=0.005) and spleen (mean difference over 5-fold; p=0.007) compared to AdCAIXLuc (Figure 5, panel D), although overall luciferase activity was low for these vectors and below the threshold of detection in some tissues for several animals. Luciferase values in mice injected with miRT-122 containing vectors should therefore be considered as upper limits of expression. Small increases (1.4- to 3.7-fold) in tissue transduction determined by real time PCR were again observed in all tissues for AdCAIXLux122 compared to its control, but this time these differences were not significant. (Figure 5, panel E). However, a 40-fold reduction in mean RLU per viral DNA copy in the liver (p=0.003), and a 6-fold reduction in mean RLU/viral DNA copy in tumor tissue (p=0.002; Figure 5, panel F) was observed for AdCAIXLux122 compared to AdCAIXLuc. Inclusion of miRT-122 sequences also resulted in significant reductions in RLU/viral DNA copy in the kidney and spleen compared to the control vector (p=0.036 and p=0.035, respectively). These results confirm the ability of miR122 targets to specifically down-regulate expression of the viral transgene in liver tissue even when highly tumor-specific promoter elements are incorporated into the vector.
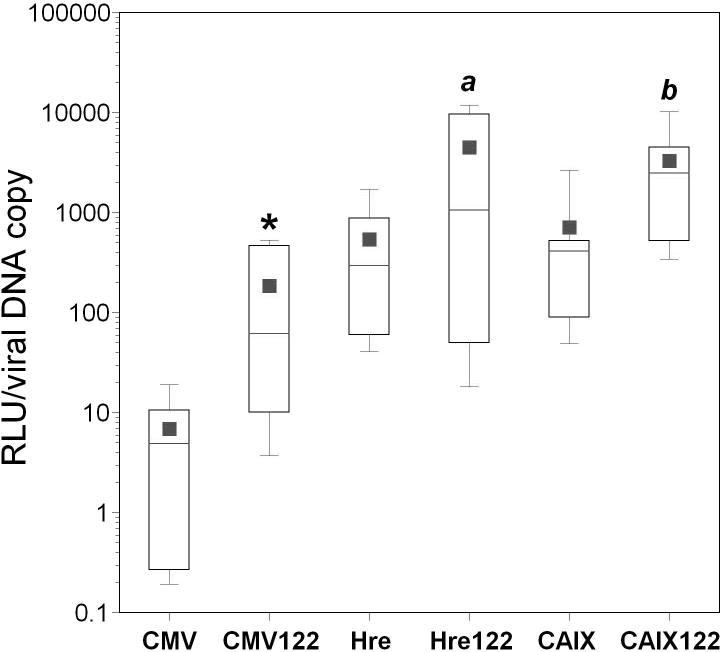
Comparison of the tumor specificity of all vectors with miRT-122-based and/or transcriptional regulatory control
Tumor specificity assessed by the tumor/liver ratios of adenoviral transgene delivery and activity for all promoters with or without the inclusion of miRT-122 sequences is plotted in Figure 6. As noted in Figure 4, a 25-fold increase in the mean tumor-specific luciferase activity was observed for AdCMVLux122 compared to AdCMVLux (p=0.045; Figure 6, panel A). miRT-122 sequences also increased the mean tumor-specific luciferase activity when used in combination with hypoxia responsive promoters, with nearly a 100-fold increase for AdHre3TKLux122 compared to AdHre3TKLux (p=0.045) and an insignificant 2.5-fold increase for AdCAIXLux122 compared to AdCAIXLuc. The combinatorial approach using hypoxia-responsive promoters with the miRT-122 elements also significantly improved tumor specific luciferase activity over the miRT-122-containing CMV-driven vector. Compared to AdCMVLux122, AdHre3TKLux122 increased the mean RLU/mg tissue tumor/liver ratio by 95-fold (p=0.006), and AdCAIXLux122 increased this parameter by nearly 10-fold (p=0.032).
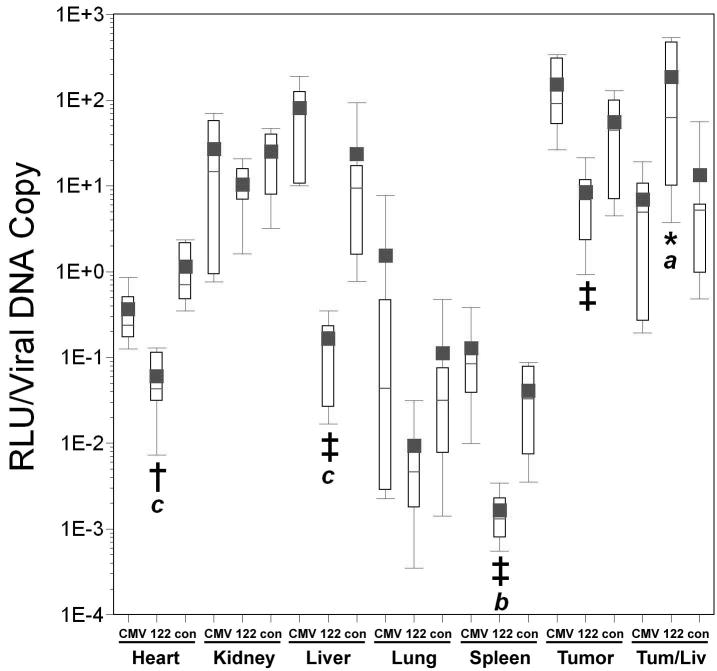
Figure 6. Effect of miRT-122 sequences on the tumor specificity of various adenoviral vectors.
Tumor/liver ratios of luciferase expression (Panel A), cell-associated viral DNA (Panel B), and luciferase expression per viral DNA copy (Panel C) are shown for the various vectors as box plots. Significant differences between viruses harboring the same promoter either with or without miRT-122 sequences are indicated by asterisks (*, p<0.05), and significant differences between the hypoxia-responsive promoter viruses and the CMV vector are shown by italicized letters (a,′ p<0.05 and b, ′ p<0.01).
Inclusion of hypoxia responsive promoters or miRT-122 elements did not result in significant changes in the tumor specificity of cell transduction, as expected (Figure 6, panel B). As previously noted, an approximate 27-fold increase in the mean tumor/liver ratio was observed for AdCMVLux122 compared to AdCMVLux when luciferase expression was normalized to viral DNA copy number (p=0.026). A 5- to 8-fold increase in the mean tumor/liver ratio of normalized luciferase expression was also seen for the inclusion of miRT-122 elements in both of the hypoxia-responsive vectors (Figure 6, panel C), but these differences did not achieve statistical significance. The use of hypoxia responsive promoters instead of CMV in the miRT-122-containing vector, however, again resulted in significant improvements in tumor-specific luciferase expression per viral DNA copy. The Hre3TK promoter increased the mean RLU/viral DNA copy over CMV in a miRT-122 background by approximately 24-fold (p=0.026), and the CAIX increase was nearly 18-fold (p=0.008).
miRT-122-mediated silencing combined with the Hre3TK promoter at the higher dose of 1012 viral particles in tumor-bearing animals
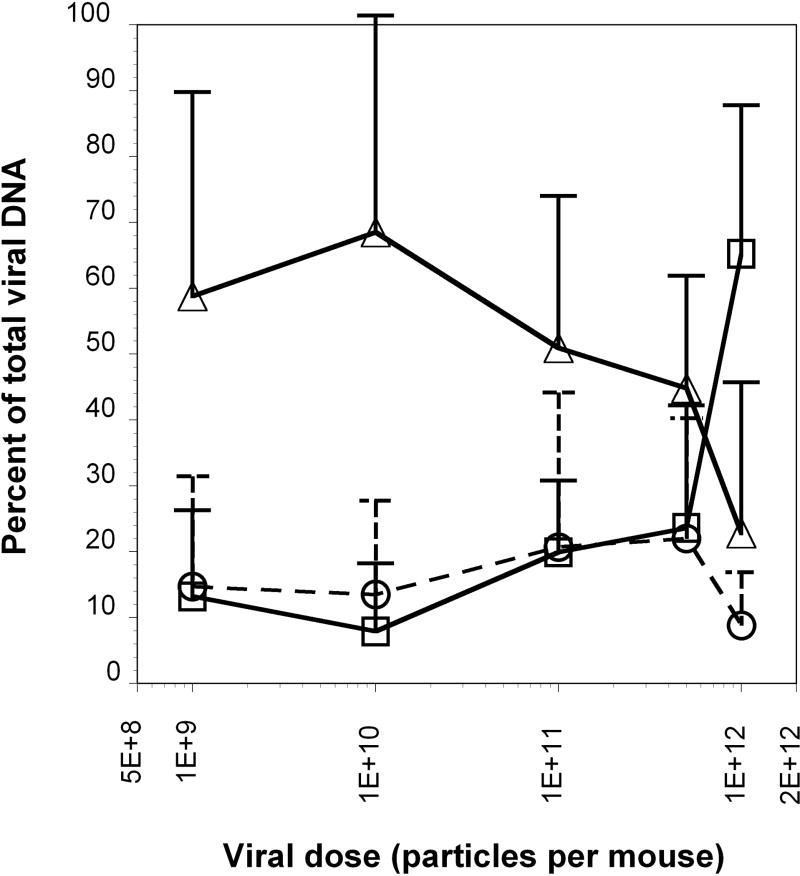
Liver inflammation and damage is one of the primary limitations of adenoviral technology, with immune responses against adenovirus and the resulting cytokine cascade acting to increase hepatotoxicity in a dose-dependent manner.30 Analysis of real time PCR data from a large number of mice, including some previously published,5 that had been injected intravenously with doses from 109 to 1012 particles showed that liver transduction is non-linear with respect to virus dose (Figure 7). Calculation of the viral DNA copy number present in liver, tumor and spleen (the three tissues containing the majority of all injected viral DNA) as a percentage of total detectable virus reveals that HT1080 tumors harbored a fixed percentage of injected viral DNA independent of viral dose (Figure 7). However, a dramatic and statistically significant increase in viral DNA copy occurred in the liver at a dose of 1012 particles per mouse following a relatively constant percentage at lower doses that was associated with a concomitant decrease in the percentage found in the spleen. The increase in liver transduction is the most probable explanation for the inflammation and hepatotoxicity observed at higher adenoviral doses.
Figure 7. Percent of total viral DNA detected in the liver, tumor and spleen following infection with varying doses of adenovirus.
Tumor-bearing mice were infected with varying doses of adenoviral vectors and total viral DNA associated with each organ (heart, kidney, liver, lung, spleen and tumor) was assessed by real time PCR. The mean percentages of viral DNA for the three highest tissues, liver (squares), tumor (circles) and spleen (triangles), relative to the sum total detected in all organs is plotted against viral dose. Standard deviations are shown by error bars.
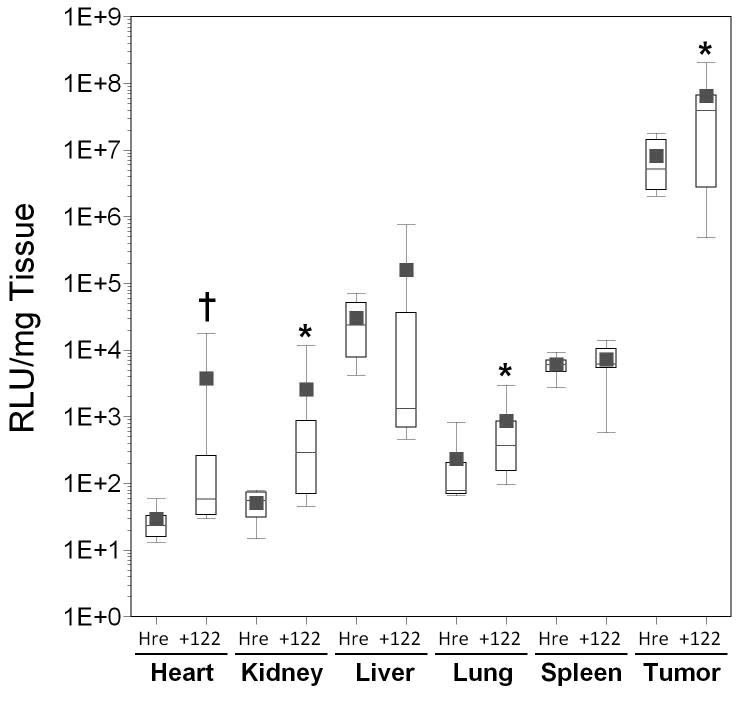
Improvements in the tumor-specific luciferase expression for the AdHre3TKLux122 vector over controls at the 1011 dose prompted us to evaluate this doubly regulated vector at the higher dose of 1012 viral particles to see if miRT-122-based regulation could help improve tumor specificity and reduce hepatotoxicity at higher doses. The results indicate that AdHre3TKLux122 had significantly lower gene expression in the liver compared to AdHre3TKLux at the 1012 dose, but the differences in the mean values was only about 3-fold (Figure 8, Panel A). The inclusion of miRT122 elements improved the RLU/mg tumor/liver ratio by over ten-fold, but this did not achieve significance. Significant increases in apparent cell transduction measured by viral DNA copy per mg tissue was again seen for AdHre3TKLux122 compared to AdHre3TKLux at 1012 viral particles (Figure 8, Panel B), but unlike the 1011 dose, this effect was now restricted to the liver and spleen. Luciferase expression per viral DNA copy (Figure 8, Panel C) was again significantly reduced for AdHre3TKLux122 compared to AdHre3TKLuc in the liver at 1012 particles, but surprisingly, RLU/viral DNA copy at this dose was higher for AdHre3TKLux122 or AdHre3TKLuc compared to either vector at 1011 viral particles.
Figure 8. Effect of miRT122 sequences on luciferase expression and biodistribution of transcriptionally targeted adenoviral vector administered at high dose to HT1080 tumor-bearing mice.
Results for groups of mice injected with 1012 particles virus are displayed as box plots. Adenoviral vectors expressing luciferase under the control of the Hre3TK promoter either with (+122) and without (Hre) miRT-122 sequences are compared in various tissues. T/L, tumor/liver ratio. Significant differences between experimental vector and control are indicated by asterisks (*, p<0.05) and daggers (†, p<0.01). Panel A, Luciferase expression assessed by RLU per mg of tissue; Panel B, viral transduction shown as viral DNA copy per mg of tissue; Panel C, luciferase expression per viral DNA copy.
We also evaluated both aspartate aminotransferase/alanine aminotransferase (AST/ALT) levels and histopathological indicators of liver inflammation and tissue damage for AdHre3TKLuc and AdHre3TKLux122 at the 1012 dose. The results generally indicate marginally high and quantitatively similar levels of AST or ALT serum enzymes for the AdHre3TKLuc and AdHre3TKLux122 vectors at this dose. Baseline values for the nude mice used in these studies are 119±95 for AST and 51±19 for ALT. For mice injected with AdHre3TKLuc, mean AST was 338 U/L (range: 138–578) and mean ALT was 53 U/L (range: 34–88). For AdHre3TKLux122, mean AST was 896 U/L (range: 146–2814) and mean ALT was 246 U/L (range: 44–937). Differences in AST/ALT levels between the experimental and control virus groups were not significant, indicating an overall inability of miRT-122 detargeted vectors to reduce liver inflammation and innate cellular immune responses resulting from high-dose intravenous adenovirus injection. Histopathological analysis also showed similar liver pathology in both sets of virus-injected mice (data not shown), with mononuclear inflammatory cells infiltrated into the hepatic parenchyma, and cellular anomalies (such as vacuoles) seen in the majority of hepatocytes, as we have previously published.5
Discussion
The emergence of microRNAs as important tissue-specific regulatory molecules provides a unique opportunity for improving the tumor specificity of novel cancer targeting agents. In this study, we evaluated the potential of this endogenous regulatory mechanism as a method to reduce off-target toxicities resulting from the systemic administration of adenoviral vectors for cancer gene targeting. In vitro evidence from this and previous investigations demonstrate that the inclusion of four tandem repeats of miR-122 complementary sequences can effectively silence the expression of an adenoviral transgene, and based on the cell lines used in this investigation, this effect appears to be highly specific for non-malignant hepatic cells. In an approach very similar to that employed by Suzuki et al,20 we first evaluated the intravenous administration of these vectors in non tumor bearing mice, confirming miRT-122-mediated down-regulation of transgene expression specifically in the liver with no substantial effects in other tissues. Extension of these experiments in a tumor-bearing mouse model also showed significant increases in tumor specificity.
Few previous studies have assessed the effectiveness of miRT-122-based regulation of systemically administered adenoviral vectors in tumor-bearing animals.31 We therefore evaluated the ability of miRT-122 elements to down-regulate liver transgene expression and improve the tumor specificity of our adenoviral vector when administered intravenously in mice bearing HT1080 human fibrosarcoma xenografts. Because the effect of miRT-122 elements should only occur post-transcriptionally, a priori their presence in the viral genome is expected to have no impact on viral transduction. Nevertheless, we sometimes observed significantly higher levels of cell-associated viral DNA in multiple tissues of mice injected with AdCMVLux122 compared to control vectors, which would complicate the interpretation of the efficacy of miRT-122-based transgene regulation unless DNA copy number were taken into account. Despite the increase in viral DNA content, significantly lower luciferase expression per mg of tissue was still observed in the livers of animals receiving miRT-122-containing vectors. Although lower luciferase expression per viral DNA copy was detected in multiple tissues due to miRT-122-mediated gene silencing, this effect was most obvious in the liver, with up to a 500-fold decrease in RLU per viral DNA copy compared to control vector. This translated into a nearly 30-fold improvement in tumor specificity as indicated by tumor/liver ratio that can be accomplished via miR-122 regulation.
Few studies have combined miRT-122-based regulatory control with other targeting strategies, and none to our knowledge have used such a combinatorial approach with systemically administered adenovirus in tumor-bearing animals. Previous results from our lab show that hypoxia-responsive promoters can provide a high degree of tumor selective transgene expression in vivo,5 so the possibility that hypoxia-responsive transcriptional control combined with miR-122-based regulatory mechanisms might provide additive or synergistic improvements in tumor specificity prompted us to evaluate this combinatorial strategy in HT1080 tumor-bearing animals. Using the Hre3TK promoter, the miRT-122 elements clearly down-regulated transgene expression in a liver-specific manner when adjusted for viral gene delivery. Relative reductions in liver transgene expression using miRT-122 elements were even more obvious under CAIX transcriptional control, because unlike Hre3TK, cell transduction by AdCAIXLux122 was not significantly increased compared to the AdCAIXLuc control. However, overall luciferase expression was much lower in all tissues for this promoter compared to Hre3TK or CMV, with luciferase activity below the threshold of detection in certain tissues (including liver) for several animals. Although improvements resulting from the use of miRT-122 elements in hypoxia-responsive vectors were not as great as that observed for the CMV-driven vector, increases in tumor specificity using both hypoxia-responsive and miRT-122-based regulatory strategies was clearly additive. Improvements in the tumor/liver ratios of both normalized and non-normalized luciferase expression were also significantly greater when the combinatorial strategy was used compared to miRT-122-based regulation alone.
Although the effect of miRT-122 elements for reducing transgene expression was greatest in the liver, miRT-122-dependent reductions in normalized luciferase expression was also observed in other tissues, including tumor. This was somewhat surprising since the expression of miR-122 is known to be expressed in a highly liver-specific manner in mice,16 and we tested for any miRT-122 down-regulation of expression in HT1080 cells. However, we did not directly quantify the expression of miR-122 in HT1080 tumor xenografts which may be substantially different from that in HT1080 cells in vitro due to the contributions of various murine cells to the tumor. Non-specific interactions of the miRT-122 targets with other miRNAs with similar sequences could also account for some down-regulation of transgene expression observed in non-liver tissues.
Inflammatory responses are known to activate NF-kB which drives expression from the CMV promoter,32, 33 and previous work in our lab showed significantly higher CMV-driven luciferase expression per viral DNA copy in the liver with increasing dose.5 Prior studies have also shown a nonlinear dose response in liver transduction that leads to a nonlinear gene expression profile.34 Although this effect was expected for CMV, we did not predict that increased inflammatory responses in the liver due to the higher viral dose would drive expression from hypoxia-responsive promoters. For example, the mean luciferase expression from each viral DNA copy was over 100-fold greater for AdHre3TKLuc at 1012 versus 1011 particles. Our data suggest that inflammatory responses in the liver can drive higher expression from a variety of promoter elements, not just CMV. Marginally high AST and ALT values, as well as histopathological indicators of liver inflammation and cellular necrosis, were similar for miRT-122 and control viruses at the higher 1012 dose despite the observed down-regulation of transgene expression in the liver using the miRT-122 elements at doses of 1011 particles. Evidence from this study and prior investigations indicate that cell transduction, not viral gene expression, is the primary cause of cytokine induction and resulting hepatotoxicity.10 miRNA-based regulatory mechanisms incorporated into adenoviral vectors are therefore unlikely to substantially reduce this clinically problematic effect.
Results from this study confirm the ability of miRT-122 elements to selectively down-regulate adenoviral transgene expression in the liver, and show that this technique can improve the tumor specificity of adenoviral transgene expression both alone and in combination with hypoxia responsive promoter elements. Furthermore, the additive improvements in tumor specificity from the miRT-122-based and transcriptional targeting using hypoxia-responsive promoters suggest that this combinatorial approach is an effective strategy for improving the tumor-specific expression of adenoviral transgenes for cancer imaging or therapy. Whether the use of other miR targets, either alone or in combination with miRT-122, would be more effective than miRT-122 elements alone for improving tumor specificity of transgenes in adenoviral vectors remains to be determined.
Acknowledgments
We thank TingTing Li for the kind gift of primary mouse hepatocytes. Alex Pertsemlidis provided statistical advice, and Joe Garcia and Rolf Brekken were kind enough to critically read the manuscript. This work was supported in part by NIH-NCI grant CA115935 to RDG.
References
- 1. [Accessed October 23, 2010];J Gene Med Gene Therapy Clinical Trials Worldwide. www.wiley.co.uk/genmed/clinical. [Web Page]
- 2.Alemany R. Designing adenoviral vectors for tumor-specific targeting. Methods Mol Biol. 2009;542:57–74. doi: 10.1007/978-1-59745-561-9_2. [DOI] [PubMed] [Google Scholar]
- 3.Gerard RD, Chan L. Adenovirus-mediated gene transfer: strategies and applications in lipoprotein research. Curr Opin Lipidol. 1996;7(2):105–11. doi: 10.1097/00041433-199604000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Gerard RD, Collen D. Adenovirus gene therapy for hypercholesterolemia, thrombosis and restenosis. Cardiovasc Res. 1997;35(3):451–8. doi: 10.1016/s0008-6363(97)00134-x. [DOI] [PubMed] [Google Scholar]
- 5.Hogg RT, Garcia JA, Gerard RD. Adenoviral targeting of gene expression to tumors. Cancer Gene Ther. 2010;17:375–386. doi: 10.1038/cgt.2010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hogg RT, Thorpe P, Gerard RD. Retargeting adenoviral vectors to improve gene transfer into tumors. Cancer Gene Ther. 2011;18:275–87. doi: 10.1038/cgt.2010.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Praus M, Wauterickx K, Collen D, Gerard RD. Reduction of tumor cell migration and metastasis by adenoviral gene transfer of plasminogen activator inhibitors. Gene Ther. 1999;6(2):227–36. doi: 10.1038/sj.gt.3300802. [DOI] [PubMed] [Google Scholar]
- 8.Varenne O, Sinnaeve P, Gillijns H, Iung B, Laurysens V, Meurrens K, et al. Percutaneous gene therapy using recombinant adenoviruses encoding human herpes simplex virus thymidine kinase, human PAI-1, and human NOS3 in balloon-injured porcine coronary arteries. Hum Gene Ther. 2000;11(9):1329–39. doi: 10.1089/10430340050032429. [DOI] [PubMed] [Google Scholar]
- 9.Liu Q, Muruve DA. Molecular basis of the inflammatory response to adenovirus vectors. Gene Ther. 2003;10(11):935–40. doi: 10.1038/sj.gt.3302036. [DOI] [PubMed] [Google Scholar]
- 10.Muruve DA, Barnes MJ, Stillman IE, Libermann TA. Adenoviral gene therapy leads to rapid induction of multiple chemokines and acute neutrophil-dependent hepatic injury in vivo. Hum Gene Ther. 1999;10(6):965–76. doi: 10.1089/10430349950018364. [DOI] [PubMed] [Google Scholar]
- 11.Worgall S, Wolff G, Falck-Pedersen E, Crystal RG. Innate immune mechanisms dominate elimination of adenoviral vectors following in vivo administration. Hum Gene Ther. 1997;8(1):37–44. doi: 10.1089/hum.1997.8.1-37. [DOI] [PubMed] [Google Scholar]
- 12.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–54. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 13.Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet. 2009;10 (10):704–14. doi: 10.1038/nrg2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem. 2010;79:351–79. doi: 10.1146/annurev-biochem-060308-103103. [DOI] [PubMed] [Google Scholar]
- 15.Chang J, Nicolas E, Marks D, Sander C, Lerro A, Buendia MA, et al. miR-122, a mammalian liver-specific microRNA, is processed from hcr mRNA and may downregulate the high affinity cationic amino acid transporter CAT-1. RNA Biol. 2004;1(2):106–13. doi: 10.4161/rna.1.2.1066. [DOI] [PubMed] [Google Scholar]
- 16.Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. Identification of tissue-specific microRNAs from mouse. Curr Biol. 2002;12(9):735–9. doi: 10.1016/s0960-9822(02)00809-6. [DOI] [PubMed] [Google Scholar]
- 17.Girard M, Jacquemin E, Munnich A, Lyonnet S, Henrion-Caude A. miR-122, a paradigm for the role of microRNAs in the liver. J Hepatol. 2008;48(4):648–56. doi: 10.1016/j.jhep.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 18.Ylosmaki E, Hakkarainen T, Hemminki A, Visakorpi T, Andino R, Saksela K. Generation of a conditionally replicating adenovirus based on targeted destruction of E1A mRNA by a cell type-specific MicroRNA. J Virol. 2008;82(22):11009–15. doi: 10.1128/JVI.01608-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cawood R, Chen HH, Carroll F, Bazan-Peregrino M, van Rooijen N, Seymour LW. Use of tissue-specific microRNA to control pathology of wild-type adenovirus without attenuation of its ability to kill cancer cells. PLoS Pathog. 2009;5(5):e1000440. doi: 10.1371/journal.ppat.1000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki T, Sakurai F, Nakamura S, Kouyama E, Kawabata K, Kondoh M, et al. miR-122a-regulated expression of a suicide gene prevents hepatotoxicity without altering antitumor effects in suicide gene therapy. Mol Ther. 2008;16(10):1719–26. doi: 10.1038/mt.2008.159. [DOI] [PubMed] [Google Scholar]
- 21.Wu C, Lin J, Hong M, Choudhury Y, Balani P, Leung D, et al. Combinatorial control of suicide gene expression by tissue-specific promoter and microRNA regulation for cancer therapy. Mol Ther. 2009;17(12):2058–66. doi: 10.1038/mt.2009.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leja J, Nilsson B, Yu D, Gustafson E, Akerstrom G, Oberg K, et al. Double-detargeted oncolytic adenovirus shows replication arrest in liver cells and retains neuroendocrine cell killing ability. PLoS One. 2010;5(1):e8916. doi: 10.1371/journal.pone.0008916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neri D, Bicknell R. Tumour vascular targeting. Nat Rev Cancer. 2005;5(6):436–46. doi: 10.1038/nrc1627. [DOI] [PubMed] [Google Scholar]
- 24.Tian H, McKnight SL, Russell DW. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev. 1997;11(1):72–82. doi: 10.1101/gad.11.1.72. [DOI] [PubMed] [Google Scholar]
- 25.McKnight SL, Gavis ER, Kingsbury R, Axel R. Analysis of transcriptional regulatory signals of the HSV thymidine kinase gene: identification of an upstream control region. Cell. 1981;25(2):385–98. doi: 10.1016/0092-8674(81)90057-x. [DOI] [PubMed] [Google Scholar]
- 26.Dioum EM, Clarke SL, Ding K, Repa JJ, Garcia JA. HIF-2alpha-haploinsufficient mice have blunted retinal neovascularization due to impaired expression of a proangiogenic gene battery. Invest Ophthalmol Vis Sci. 2008;49(6):2714–20. doi: 10.1167/iovs.07-1469. [DOI] [PubMed] [Google Scholar]
- 27.Aoki K, Barker C, Danthinne X, Imperiale MJ, Nabel GJ. Efficient generation of recombinant adenoviral vectors by Cre-lox recombination in vitro. Mol Med. 1999;5(4):224–31. [PMC free article] [PubMed] [Google Scholar]
- 28.Chang J, Guo JT, Jiang D, Guo H, Taylor JM, Block TM. Liver-specific microRNA miR-122 enhances the replication of hepatitis C virus in nonhepatic cells. J Virol. 2008;82(16):8215–23. doi: 10.1128/JVI.02575-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kutay H, Bai S, Datta J, Motiwala T, Pogribny I, Frankel W, et al. Downregulation of miR-122 in the rodent and human hepatocellular carcinomas. J Cell Biochem. 2006;99(3):671–8. doi: 10.1002/jcb.20982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaiss AK, Machado HB, Herschman HR. The influence of innate and pre-existing immunity on adenovirus therapy. J Cell Biochem. 2009;108(4):778–90. doi: 10.1002/jcb.22328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cawood R, Wong SL, Di Y, Baban DF, Seymour LW. MicroRNA controlled adenovirus mediates anti-cancer efficacy without affecting endogenous microRNA activity. PLoS One. 6(1):e16152. doi: 10.1371/journal.pone.0016152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeMeritt IB, Milford LE, Yurochko AD. Activation of the NF-kappaB pathway in human cytomegalovirus-infected cells is necessary for efficient transactivation of the major immediate-early promoter. J Virol. 2004;78(9):4498–507. doi: 10.1128/JVI.78.9.4498-4507.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayden MS, West AP, Ghosh S. NF-kappaB and the immune response. Oncogene. 2006;25(51):6758–80. doi: 10.1038/sj.onc.1209943. [DOI] [PubMed] [Google Scholar]
- 34.Tao N, Gao GP, Parr M, Johnston J, Baradet T, Wilson JM, et al. Sequestration of adenoviral vector by Kupffer cells leads to a nonlinear dose response of transduction in liver. Mol Ther. 2001;3(1):28–35. doi: 10.1006/mthe.2000.0227. [DOI] [PubMed] [Google Scholar]