Abstract
Background
Recent clinical studies that evaluated the effects of supplemental omega-3 polyunsaturated fatty acids (n-3 PUFAs) on sudden cardiac death have yielded conflicting results. Our aim was to clarify this issue using an established and clinical relevant canine model of sudden cardiac death.
Methods and Results
Susceptibility to ventricular fibrillation (VF) was evaluated using a 2 minute left circumflex artery occlusion during the last minute of an exercise test in 76 dogs (from two independent studies) with healed myocardial infarctions (MI); 44 developed VF (susceptible, VF+) while 32 did not (resistant, VF−). These dogs were then randomly assigned to either placebo (1 g/day, corn oil; 15 VF+, 11 VF−) or n-3 PUFA (1–4 g/day, docosahexaenoic acid + eicosapentaenoic acid ethyl esters, 29 VF+, 21 VF−) groups. Seven sham (no-MI) dogs were also treated with n-3 PUFA (4 g/day). After treatment (3 months), the exercise + ischemia test was repeated. Dietary n-3 PUFAs produced significant (P<0.01) increases in red blood cell and left ventricular n-3 PUFA levels. Nine post MI (5 placebo vs. 4 n-3 PUFA) and 2 sham dogs died suddenly during the 3-month treatment period. The n-3 PUFA treatment failed to prevent arrhythmias in VF+ dogs (decreased in 27% placebo vs. 24% n-3 PUFA, P=0.5646) but induced VT/VF in VF− animals (n-3 PUFA 33% vs. placebo 0%, P=0.0442).
Conclusions
Despite large increases in cardiac tissue n-3 PUFA content, dietary n-3 PUFAs did not prevent ischemia-induced VF and actually increased arrhythmia susceptibility in both non-infarcted and low risk post-MI dogs.
Keywords: omega-3 polyunsaturated fatty acids, fish oil, ventricular fibrillation, myocardial ischemia, myocardial infarction
Introduction
The cardiovascular benefits of dietary omega-3 polyunsaturated fatty acids (n-3 PUFAs) have been actively investigated for nearly 40 years (1). Epidemiological data provide strong evidence for an inverse relationship between fatty fish consumption and cardiac mortality (2). In contrast to these observational studies, interventional studies using n-3 PUFAs for the secondary prevention of adverse cardiovascular events in patients recovering from myocardial infarction (MI) have yielded conflicting results (Table 1) and are a current area of active debate (14). Nevertheless, the American Heart Association first recommended fish oils for secondary prevention in post-MI patients in 2003 (15). Based in part upon these recommendations, consumer demand for n-3 PUFA products has exploded. It has been estimated that 5–10% of the adult U.S. population use a fish oil supplement and sales are projected to exceed 7 billion dollars by the end of 2011 (www.marketresearch.com).
Table 1.
| A. Effect of omega-3 fatty acids on mortality and sudden cardiac death: Results of secondary prevention trials | ||||
|---|---|---|---|---|
| Study | Patient population | Treatment | Mortality | Sudden Cardiac Death |
| DART, 1989 [3] | Post MI Fish advice n = 1018 No advice n = 1015 |
Advised to eat 100 g portion fatty fish at least twice per week |
↓ | Not Reported |
| Nilsen et al. 2001 [4] |
Post MI n-3 PUFA n = 150 placebo n = 150 |
n-3 PUFA 4 g/day (~ 1.2 g EPA + ~ 2.4 g DHA per day) |
↓ | No Change |
| GISSI- Prevenzione, 2002 [5] |
Post MI n-3 PUFA n = 5666 Placebo n = 5658 |
n-3 PUFA 1 g/day (300 mg EPA + 600 mg DHA per day) |
↓ | ↓ |
| Burr et al. 2003 [6] |
Stable angina Fish advice/n-3 PUFA n = 1571 Placebo n = 1543 |
Advice to eat 2 oily fish portions/week or to take fish oil capsules (540 mg EPA + 360 mg DHA per day) |
↑ |
↑ |
| JELIS 2007 [7] | Hypercholesterolemic on statins (30 % had prior MI) n-3 PUFA n = 9326 Placebo n = 9319 |
EPA 1.8 g/day | No Change | No Change |
| GISSI-HF 2008 [8] |
Chronic heart failure ~ 42 had prior MI n-3 PUFA n = 3494 Placebo n = 3481 |
n-3 PUFA 1 g/day (~ 300 mg EPA + ~ 600 mg DHA per day) |
↓ |
No Change |
| OMEGA-trial 2009 [9] |
Post MI n-3 PUFA n = 1919 Placebo n = 1885 |
n-3 PUFA 1 g/day (300 mg EPA + 600 mg DHA per day) |
No Change | No Change |
| Alpha-Omega trial [10] |
Post MI n-3 PUFA + ALA n = 1212 n-3 PUFA n = 1192 ALA n = 1197 Placebo n = 1236 |
226 mg EPA + 150 mg DHA per day or 1.9 g/day ALA or Both |
No Change | No Change |
| B. Effect of omega-3 fatty acids on cardiac events in ICD patients | ||||
|---|---|---|---|---|
| Study | Patients | Treatment | Time to first event or death |
Comment |
| Leaf et al. 2005 [11] |
50% EF ≤ 30% ~78% CHD ~ 47% VT on entry MI not reported n-3 PUFA n = 200 Placebo n = 202 |
EPA 18.2 mg + DHA 2.4 g/day |
↑ |
|
| Raitt et al. 2005 [12] |
~ 46% EF ≤ 30% ~ 73% CHD ~ 64% VT on entry ~ 55% MI n-3 PUFA n = 100 Placebo n = 100 |
756 mg EPA + 540 DHA mg per day |
No change | Increased VT in subgroup analysis |
| Brouwer et al. 2006 [13] |
~ 33% EF ≤ 30% ~ 76% CHD ~ 75% VT on entry ~ 68% MI n-3 PUFA n = 273 Placebo n = 273 |
464 mg EPA + 335 mg DHA per day |
No Change | |
We previously demonstrated that the acute intravenous infusion of an emulsion of either fish oil or highly purified n-3 PUFAs prevented ventricular fibrillation (VF) in a well-established and clinically relevant canine model of sudden cardiac death (16, 17). However, the effects of long-term ingestion (the most common route of administration) of n-3 PUFA have not been evaluated in this model. Recently, the acute and chronic administration of n-3 PUFAs was found to exert profoundly different electrophysiological actions (18–20). Furthermore, dietary n-3 PUFAs increased rather than decreased susceptibility to arrhythmias induced during regional myocardial ischemia (21). Accordingly, we evaluated the actions of the long-term ingestion of n-3 PUFAs on cardiac rhythm in animals at both a high and a low risk for arrhythmia development
Methods
This report includes the results obtained from two independent laboratories that coincidentally explored the same issue using the identical canine model. All the animal procedures were approved by the University of Oklahoma Health Sciences Center (study 1) or the Ohio State University (study 2) Institutional Animal Care and Use Committee and conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health
Study 1 (the University of Oklahoma Protocol)
Surgical Preparation and Sudden Death Testing
Thirty-eight animals heartworm free mix-breed male dogs (20–25 kg) were instrumented and had a MI induced by the two stage ligation of the left anterior descending artery as previously described (22, 23); 11 (29%) dogs died within 48–72 hours and 3 could not be classified due to malfunction of the left circumflex coronary artery occluder.
The susceptibility to VF was evaluated 3–4 weeks following MI in the remaining 24 dogs using an exercise plus ischemia test as previously described (22, 23). This test induced VF in 10 dogs (VF+) but not in the remaining 14 (VF−). Two of the VF+ dogs were not successfully resuscitated. The VF− dogs were not used to evaluate n-3 PUFA treatment in Study 1. The remaining VF+ dogs (n=8) were then treated with n-3 PUFAs (1 g/day for 8 weeks). The dogs were given supplements similar to those used in the GISSI-Prevenzione study (5, 8). Each 1 g capsule contained 465 mg ethyl eicosapentaenoate, EPA, and 375 mg ethyl docosahexaenoate, DHA (GlaxoSmithKline, Research Triangle Park, NC). The exercise plus ischemia test was repeated at the end of the 8-week n-3 PUFA treatment period. Blood samples were collected at baseline and at the end of the treatment period to assess n-3 PUFA plasma levels
Study 2 (The Ohio State University Protocol)
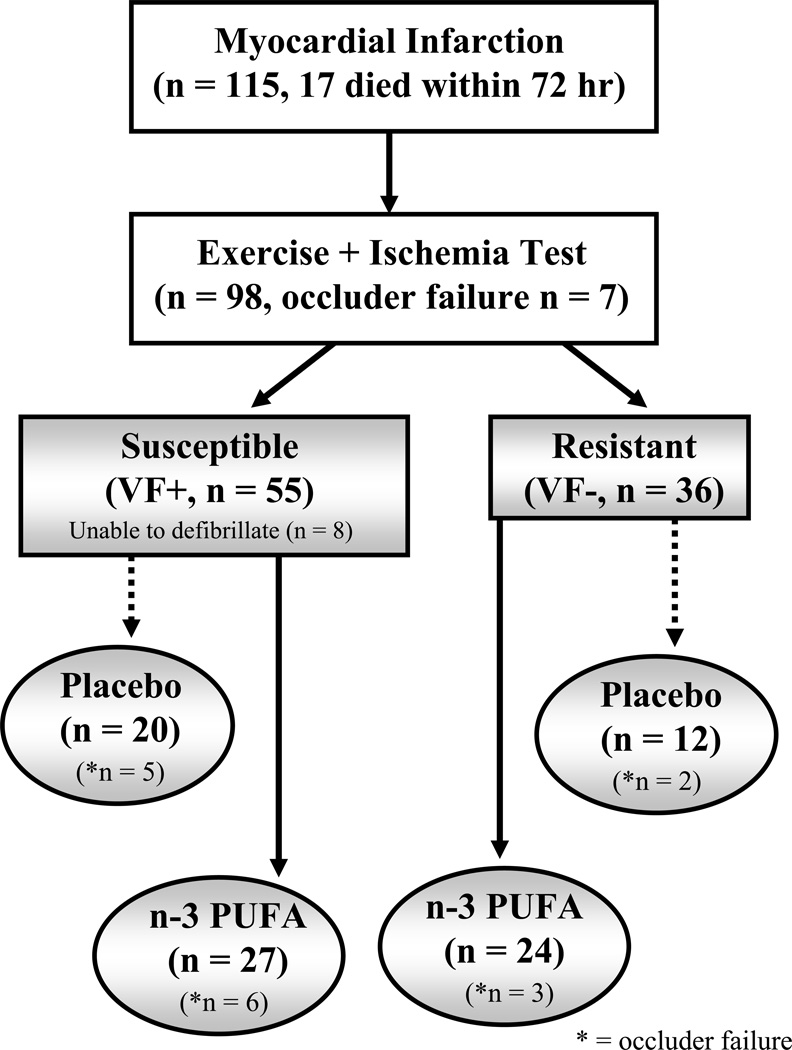
The surgical preparation and the classification as to the susceptibility to VF for the animals in study 2 were identical to that used in study 1. Heartworm-free mixed breed dogs (n = 115, 19 males, 76 females, 2–3 y old) weighing 19.4±0.2 kg were used in this study (figure 1). Of the 115 animals that underwent surgery, 24 animals could not be tested either due to death within 72-hrs of the MI (n=17, 15%) or occluder failure (n=7). Thus, the exercise plus ischemia test was performed on 91 of the original 115 post MI dogs. Fifty-five dogs developed VF (VF+, susceptible) while the remaining 36 did not (VF−, resistant). Eight VF+ animals were not successfully defibrillated. Seven sham operated (i.e., left anterior descending artery was isolated but not ligated) dogs were also classified using this exercise plus ischemia test (2 VF+, 5 VF−).
Figure 1.
A flow chart of the post-MI animals used in Study 2. The exercise + ischemia test could not be repeated at the end of treatment in some animals due to occluder failure [11 VF+ (5 placebo, 6 n-3 PUFA) and 5 VF− (2 placebo, 3 n-3 PUFA)] or spontaneous death (VF+: 5 placebo, 4 n-3 PUFA). VF = ventricular fibrillation; n-3 PUFA = omega-3 polyunsaturated fatty acids.
The n-3 PUFA treatment protocol used in study 2 lasted 3 months and included doses of 1, 2, and 4 g/day. The dogs were maintained on a diet that did not contain any n-3 PUFAs (Harlan Teklad, Harlan Laboratories, Inc, Indianapolis, IN) beginning one week prior to the instrumentation surgery (total duration ~4 months). After the pre-treatment data (3–4 weeks after the surgery) had been collected, 47 susceptible and 36 resistant dogs were assigned to the following groups: Placebo (20 VF+, 12 VF−) and n-3 PUFA (1–4 g/day; 27 VF+, 24 VF−). Seven sham (no MI) dogs received n-3 PUFA treatment (4 g/day). The n-3 PUFA group received the same n-3 PUFA formulation as described for study 1 (1–4 capsules/day). The placebo was corn oil (1 g/day, 58% linoleic acid + 28% oleic acid). The capsules were given per os prior to the daily feeding (between 8:00 and 10:00 AM each day, 7 days per week). The exercise plus ischemia test was repeated after treatment with either the placebo or the n-3 PUFAs for both the post-MI and the sham-infarcted dogs except for animals that either died (9 post-MI, 2 sham) or the occluder failed (n=11) prior to completion of the treatment period (Figure 1).
Red Blood Cell and Cardiac Tissue Fatty Acid Analysis
Fasting blood samples (5 ml) were drawn into EDTA tubes from a cephalic vein between 8:00 and 9:00 AM at the following time points: one day prior to treatment and when tissue was harvested at the end of the study (~14 weeks of treatment). Right atrial and left ventricular tissue were obtained when the hearts were harvested; the tissue and red blood cells (RBC) were flash frozen in liquid nitrogen and stored at −80° C for subsequent analysis.
RBC and phospholipids from cardiac tissue were analyzed for fatty acid composition (24, 25) by gas chromatography using a GC2010-FID (Shimadzu Corporation, Columbia, MD) equipped with a 100mm capillary column (SP-2560, Supelco, Bellefonte, PA). Fatty acids of interest were identified by comparison with known standards and expressed as a percent of total fatty acids. The coefficient of variation for the RBC EPA+DHA assays was <5%
Data Analysis
The lipid analysis data are reported as mean ( SEM. The ECG data were digitized (1 kHz) and recorded using a Biopac MP-100 data acquisition system (Biopac Systems, Inc., Goleta, CA). Red blood cell and cardiac tissue lipid compositions were compared using a two factor (dose, pre-post) ANOVA with repeated measures on one factor (pre-post) or a one factor ANOVA, respectively (NCSS statistical software, Kaysville, UT). Post-hoc comparisons were made using the Tukey-Kramer Multiple-Comparison Test. The effects of the interventions (placebo vs. n-3 PUFA) on arrhythmias/mortality were evaluated using Fisher’s Exact test. Arrhythmias severity was quantified by using the Lambeth Convention (26) criteria for arrhythmia score: 0 = no arrhythmias, 1 = premature ventricular complexes; 2 = ventricular tachycardia (of at least 4 beats duration); 3 = ventricular fibrillation; 4 = spontaneous death.
Results
Study 1
Dietary n-3 PUFA treatment (8 weeks) significantly (P=0.0340) increased plasma omega-3 index (EPA + DHA, from 2.5±0.4 to 4.1±0.4%) levels. No animals died spontaneously during the course of this study. At the end of the treatment with 1 g/day, the exercise plus ischemia test was repeated and VF recurred in 7 of the 8 animals tested (87.4%, pre-treatment vs. post-treatment, P=0.5000).
Study 2
Dietary n-3 PUFA treatment, dose-dependently increased RBC membrane and cardiac tissue n-3 PUFA content while the lipid composition did not change in the placebo treated animals (online supplement Tables 1 and 2). As all three n-3 PUFA doses had similar effects on the susceptibility to VF, the results for all the post-MI dogs (study 2) treated with n-3 PUFAs were combined for all subsequent analyses and are displayed in figures 2 (VF+) and 3 (VF−).
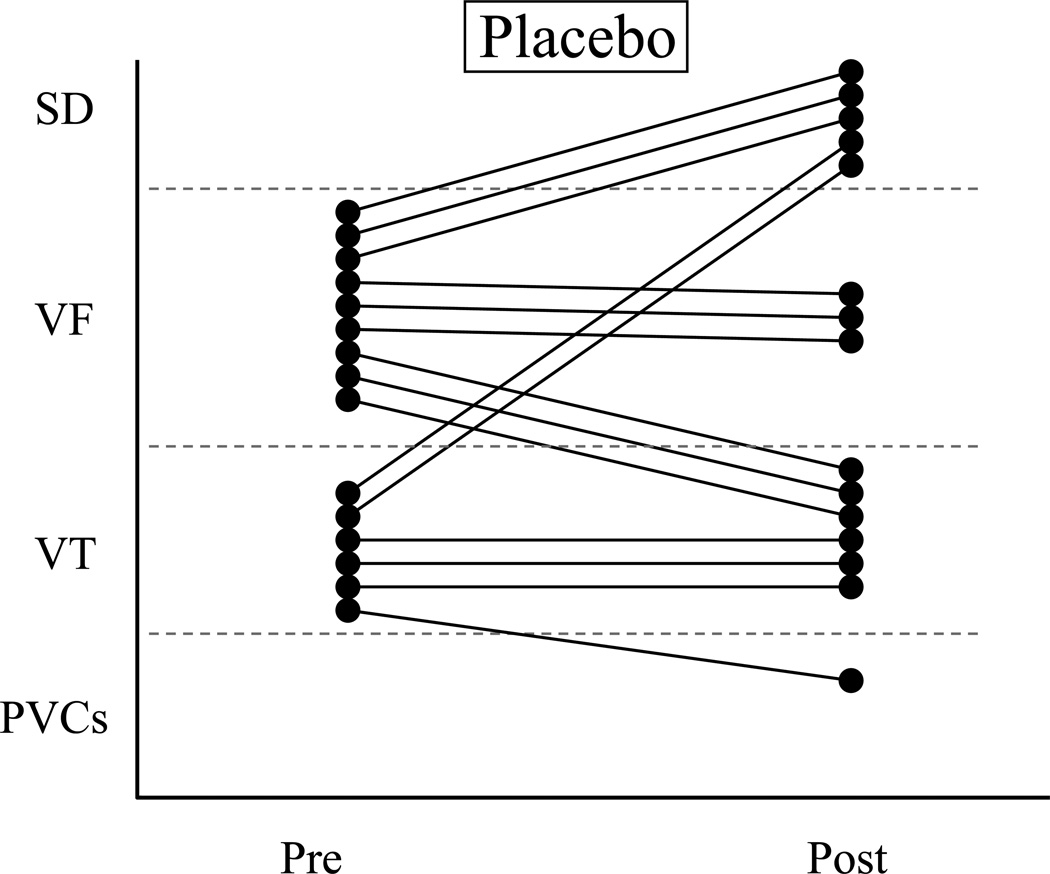
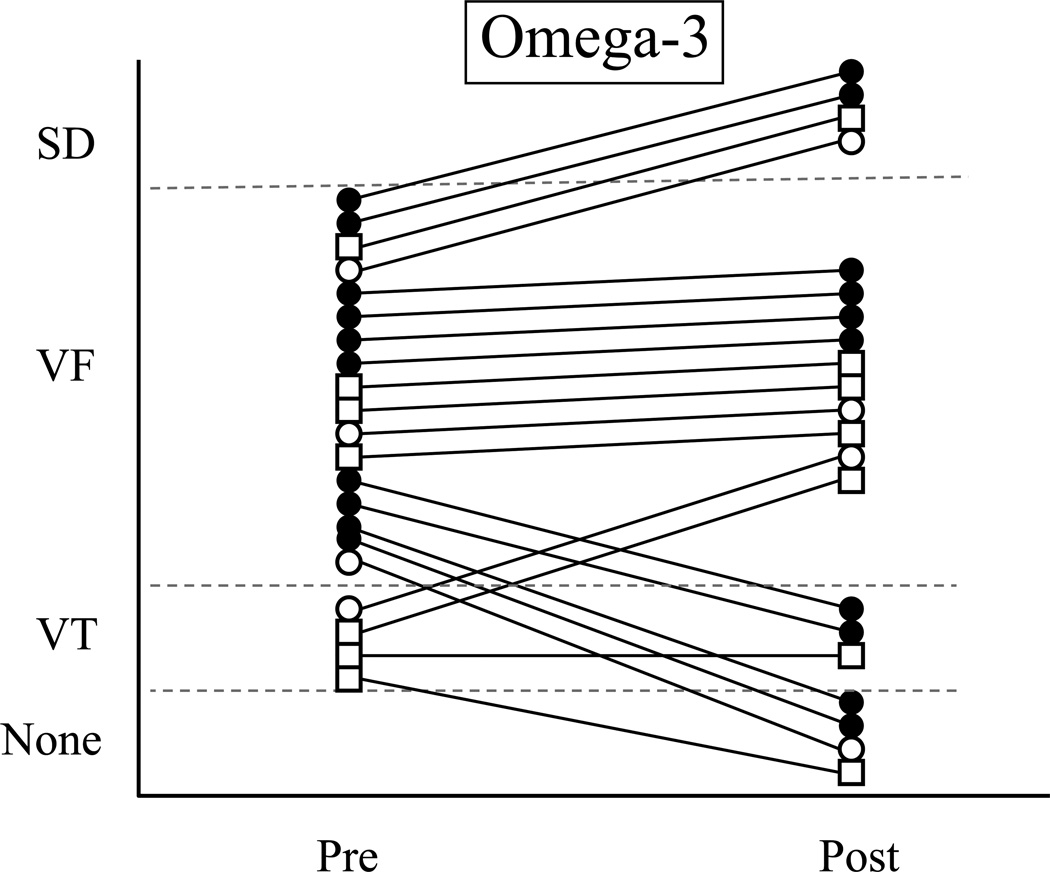
Figure 2.
The response of each susceptible (VF+) dog before and at the end of a 3 month treatment period with either placebo (Fig 2a., 1 g/day corn oil, n=15) or n-3 PUFA (Fig 2b., 1-4 g/day, n=21). Only the results of the post-MI animals used in the Study 2 are shown. Note that 9 dogs died spontaneously (SD; 5 placebo, 4 n-3 PUFA) and could not receive a post-treatment exercise + ischemia test. The n-3 PUFA treatment did not prevent ventricular tachyarrhythmias for any n-3 PUFA dose (1 g/day P=0.7278; 2 g/day, P=0.4769; 4 g/day P=0.5159). VF = ventricular fibrillation; PVCs = premature ventricular complexes; Pre = before the onset of treatment; Post = after 3-months of treatment. Open circle = 1 g/day; open square = 2 g/day, and closed circle = 4 g/day.
VF+ animals
9 dogs died spontaneously during the 3-month feeding study: 5 of 20 placebo (10, 32, 65, 72, and 79 days after treatment onset) and 4 of 27 [1 (84 days, witnessed VF) with 1 g/day; 1 (52 days) with 2 g/day and 2 (40 and 59 days after treatment onset) with 4 g/day] n-3 PUFA (P=0.3055). With one notable exception (witnessed collapse and subsequent VF confirmation), the cause for the spontaneous deaths could not be determined but, consistent with clinical practice, were assumed to result from arrhythmias. The arrhythmia score was not affected by either the placebo (pre-treatment 2.5±0.1 vs. post-treatment 2.8±0.3) or n-3 PUFA treatment (pre-treatment 2.8±0.1 vs. post-treatment 2.5±0.3). Similar reductions (placebo 4 of 15, 26.7% vs. n-3 PUFA 6 of 21, 28.6%; P=0.6023) and increases (placebo 5 of 15 vs. n-3 PUFA 6 of 21, P=0.5209) in the occurrence of ventricular tachyarrhythmias were noted after treatment in both groups.
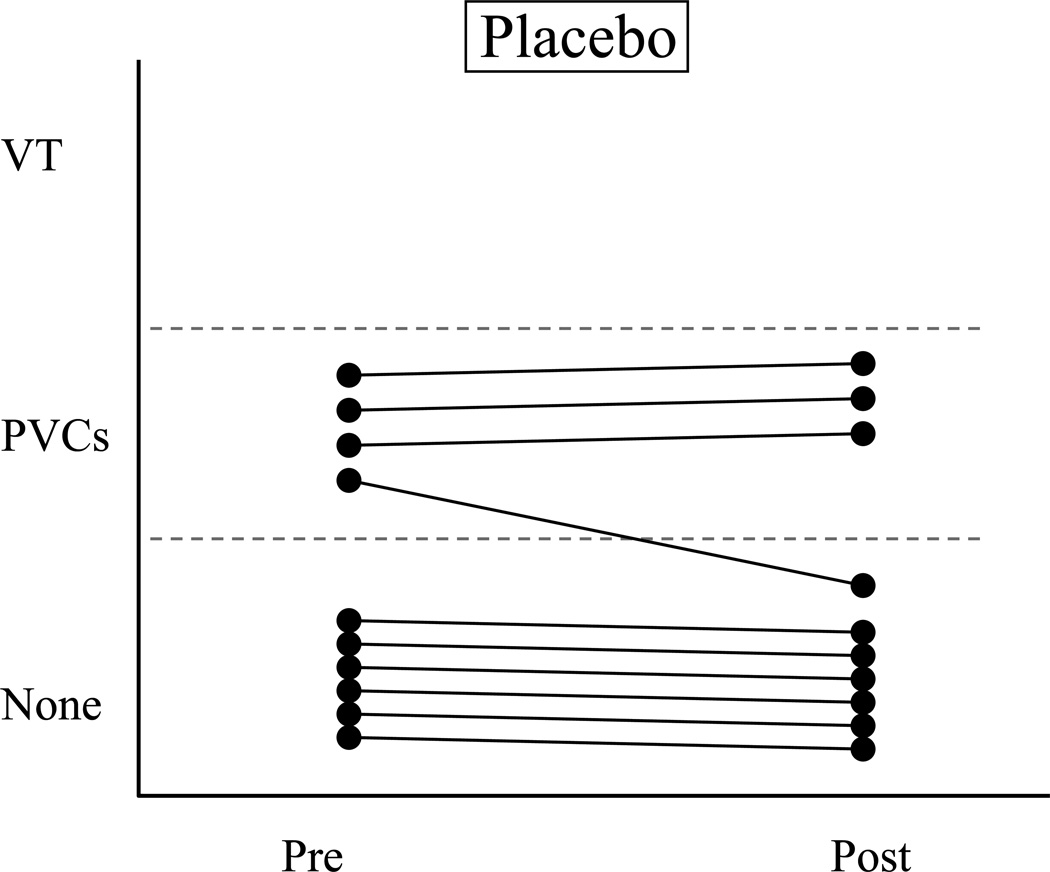
VF− dogs
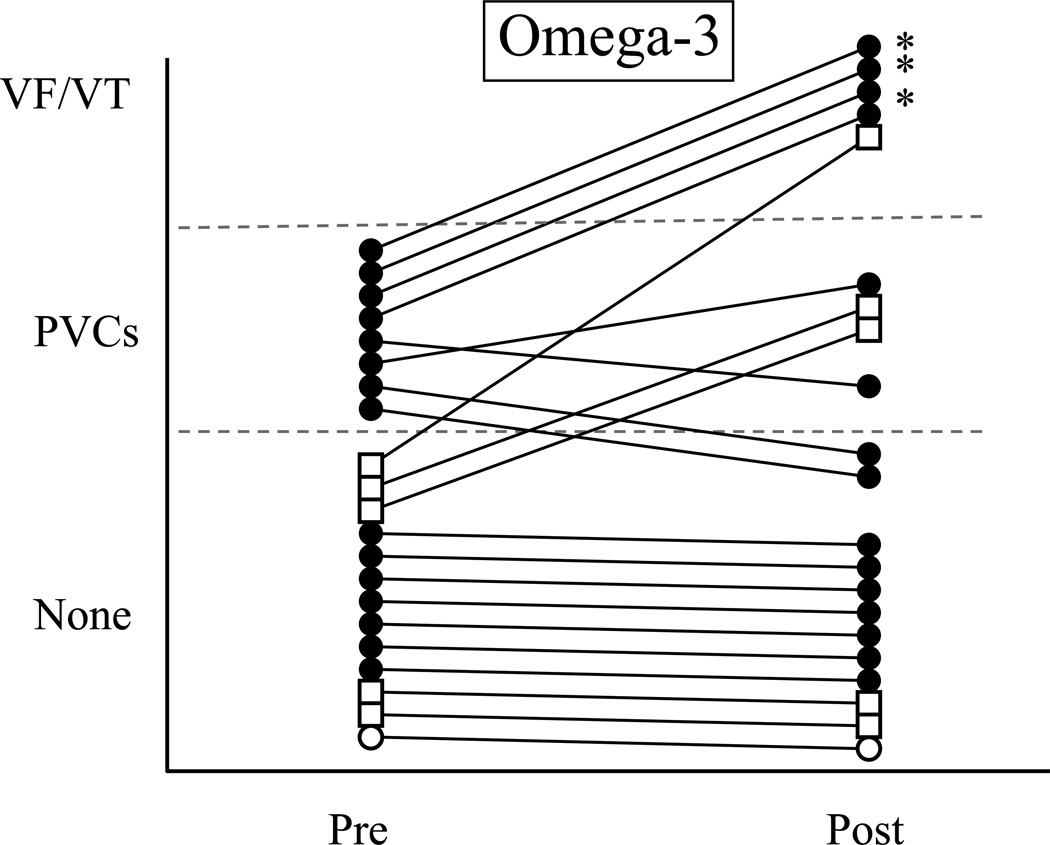
no VF− dogs died spontaneously in either the placebo (Figure 3a) or n-3 PUFA treated groups (Figure 3b). However, n-3 PUFA treatment significantly (P=0.0442) increased the incidence of ventricular tachyarrhythmias. The exercise plus ischemia test did not alter arrhythmias in the 10 placebo treated dogs (Figure 3a) but induced arrhythmias in 7 of 21 (2 of 5 with 2 g/day and 4 of 15 with 4 g/day) n-3 PUFA (Figure 3b) treated animals, including the induction of VF in 3 dogs. The arrhythmia score doubled following n-3 PUFA treatment (pre-treatment 0.4±0.1 vs. post-treatment 0.8±0.2) but did not change in the placebo group (pre-treatment 0.4±0.1 vs. post-treatment 0.3±0.3).
Figure 3.
The response of each resistant (VF−) dog before and at the end of a 3 month treatment period with either placebo (Fig 3a., 1 g/day corn oil, n=10) or n-3 PUFA (Fig 3b., 1–4 g/day, n=21). Only the results of the post-MI animals used in the Study 2 are shown. Note that the n-3 PUFA treatment increased (P=0.442) the severity of arrhythmias in one third (7 of 21; 3 of 5 with 2 g/day & 4 of 15 with 4 g/day) of these dogs while the placebo treatment did not induce arrhythmias in any animal. * indicates an episode of ventricular fibrillation (VF). VT = ventricular tachycardia; PVCs = premature ventricular complexes; none = no arrhythmias; Pre = before the onset of treatment; Post = after 3-months of treatment. Open circle = 1 g/day; open square = 2 g/day, and closed circle = 4 g/day.
Sham (no MI) dogs
Seven sham dogs (2 VF+, 5 VF−) were also tested with 4 g/day n-3 PUFA, two (1 VF+ 35 days, 1 VF− 74 days post-treatment, 28.6%) of these dogs died spontaneously during the 3-month n-3 PUFA treatment while there was no change in the arrhythmias in the remaining 5 dogs. There were no placebo treated sham dogs. However, 0 of 195 non-infarcted dogs died spontaneously following thoracic surgery in previous studies (n-3 PUFA treated vs. historical data, P=0.0010)
Discussion
There were two major findings from this study that have important clinical implications. First, despite large increases in cardiac tissue n-3 PUFA concentration, n-3 PUFA treatment did not reduce life-threatening ventricular arrhythmias in post-MI dogs at high risk for ventricular fibrillation. Second, and contrary to both current views and to our initial expectations, n-3 PUFA treatment significantly increased the susceptibility to malignant arrhythmias in low risk dogs (both dogs with and without MI). Long-term dietary n-3 PUFA treatment induced ventricular tachyarrhythmias in one third of the VF− post MI dogs while two non-infarcted dogs died spontaneously during the 3-month n-3 PUFA treatment. This latter observation is particularly noteworthy, as these non-infarcted dogs would normally exhibit a negligible risk for sudden death. In fact, these are the only non-infarcted dogs that have died suddenly following thoracic surgery in our laboratories during the last 35 years (n=195). Furthermore, it should also be emphasized that it is difficult to induce ventricular tachyarrhythmias in VF− dogs (23, 27); repetition of the exercise plus ischemia test never induced ventricular tachyarrhythmias in our combined experience with over 220 post-MI dogs initially classified as VF−. When considered together, these data strongly suggest that dietary n-3 PUFAs not only lack significant antiarrhythmic benefits in this model but also actually enhances the risk for severe ventricular tachyarrhythmias in some settings
Interventional studies using n-3 PUFAs for the secondary prevention of adverse cardiovascular events in patients recovering from MI have yielded conflicting results (Table 1a). The DART (3) and GISSI-Prevenzione (5) trials reported 10–20% reductions in all-cause mortality with up to a 45% reduction in sudden death (5). In marked contrast, Burr et al. (6) reported that n-3 PUFAs increased, rather than decreased, all cause mortality (15% increase over 9 year follow-up period, with a 54% increase in sudden death), while the JELIS trial found that EPA supplements did not alter either sudden death or fatal MI despite decreasing non-fatal coronary events (7). Most recently, both the OMEGA (9) and the Alpha Omega (10) trials reported that n-3 PUFA supplements failed to alter either total mortality or sudden death rates in post-MI patients. Similar inconsistent findings have been obtained from meta-analysis of these trials (28–31). Two analyses failed to find a relationship between fish or fish oil consumption and a reduction in cardiac mortality (28, 29), a third analysis found an inverse relationship between the incidence of sudden death in MI patients and n-3 PUFA consumption, but an increased risk for adverse cardiac events in patients with angina (30), and a fourth study reported a significant dose-independent reduction in cardiac mortality but not in sudden death or in all-cause mortality (31)
Omega-3 PUFA supplements have also been given to patients with a demonstrated risk for ventricular arrhythmias (i.e., those with implanted cardioverter/defibrillators, ICDs) yielding similar mixed results (Table 1b). Leaf and co-workers (11) reported that fish oil supplements did not reduce death rates but they found a trend toward benefit in the combined endpoint of time to ICD discharge and all-cause mortality. In contrast, Raitt and co-workers (12) reported that fish oil supplements not only did not reduce ICD events or mortality but also increased arrhythmic events in the subgroup of patients (67%) who received an ICD with ventricular tachycardia as an indication. Heart failure patients (NYHA class II and III) with the highest RBC n-3 PUFA levels also exhibited an increased risk for ventricular arrhythmias that required anti-tachycardic therapy (32). However, the largest ICD study to date, found that n-3 PUFA treatment had no effect on adverse cardiac events (13). Accordingly, meta-analysis of these ICD trials found that n-3 PUFA treatment was neither anti-arrhythmic nor pro-arrhythmic in this patient population (33, 34)
Variable results have also been reported in animal studies (21, 35–37). For example, Coronel et al. (21) found that dietary n-3 PUFA increased the incidence of VF during regional ischemia in isolated pig heart preparations. Conversely, tuna oil supplements prevented ventricular tachyarrhythmias during ischemia and reperfusion in isolated rat hearts (35) and increased the current necessary to induce VF in non-human primate hearts (36). A meta-analysis of 27 (23 feeding, 4 acute intravenous infusion) animal studies found that n-3 PUFA (fish oil, EPA or DHA, but not ALA) treatment attenuated ischemia-induced ventricular arrhythmias but was ineffective in ischemia-reperfusion models (37). Thus, as emphasized in a recent review (14), the effects of n-3 PUFA on sudden death – whether harmful or beneficial – have yet to be convincingly demonstrated
Strengths and Limitations of the study
A major strength of our findings lies in the very high translational value of our experimental model. In order to provide an accurate assessment of the factors responsible for sudden cardiac death, a model must mimic, as closely as possible, the underlying pathological conditions associated with a high risk of sudden death in patients. Clinical studies indicate that among the most important factors associated with a high risk of sudden death are the following: acute myocardial ischemia (38), previous myocardial ischemic injury (39), and alterations cardiac autonomic regulation (40). Nearly thirty year ago, we developed a canine model of sudden cardiac death that fulfils these criteria. Over 50 peer-reviewed publications have resulted from these studies including a recent comprehensive review (23). Most importantly, the results first obtained in our model have been subsequently validated in large human studies (41). For example, autonomic markers for sudden death (both baroreflex sensitivity and HR variability) first identified in our model (23, 42, 43) were confirmed in large prospective clinical studies in post-MI patients (41). Furthermore, the efficacy of anti-arrhythmic interventions as determined in our model was subsequently confirmed in major clinical trials (27). Specifically, azimilide (10%, 1 of 10), d-sotolol (11.1%, 2 of 18), and dofedilide (14.3%, 1 of 7), (used without success in the clinical trials ALIVE, SWORD, and DIAMOND) each failed to provide protection from VF while clinically effective agents, beta-adrenoceptor blockers (68.4%↓, 63 of 92) and amiodarone (94.2%↓, 33 of 35), successfully reduced VF in our model (23, 27, 40). While great care is always necessary when attempting to draw clinical implications from experimental studies, we believe that our preparation for sudden death has proven to be reliable over time.
Defining human-equivalent doses of n-3 PUFA for animal studies is challenging. In study 2, the average n-3 PUFA dose (adjusted for body surface area) was equivalent to about 4 g/day in human subjects. As such, this dose is higher than those used in the most of the interventional studies in Table 1. However, it is equivalent to the dose of prescription n-3 PUFA (Lovaza®, GlaxoSmithKline) used to treat hypertriglyceridemia (44) and this dose was ineffective in the treatment of paroxysmal atrial fibrillation (45). Furthermore, the doses used in the present study yielded RBC membrane EPA + DHA levels that were associated with a significant reduction the risk for sudden death in epidemiological studies (46, 47). A mean RBC n-3-PUFA concentration of 6.9% was associated with a 90% reduction in the risk for sudden death (47), a value that compares favorably to that obtained the present study (after 3 months at 4 g/day, mean RBC concentration was ~6.8%, range 4.3 – 10.7%).
There also were no placebo-treated non-infarcted dogs and, as such, it is difficult to access the role n-3 PUFA treatment played in the deaths noted in this group. However, historically, no non-infarcted dog (n = 195) has died spontaneously following thoracic surgery. It, therefore, seems likely that the deaths in these dogs could be attributed to the n-3 PUFA treatment. With one notable exception (witnessed collapse and confirmed VF), the cause of the spontaneous death was not determined but, consistent with clinical studies, was assumed to result from arrhythmias.
Finally, although the potential pro-arrhythmic effects of n-3 PUFA ingestion were assessed by the inclusion of low-risk animals, the present study was not designed to investigate arrhymthogenic mechanisms if this hypothesis were to be confirmed. As a consequence, it was not possible to determine the electrophysiological basis for the apparent pro-arrhythmic action of the n-3 PUFA treatment. However, there are at least two mechanisms by which n-3 PUFA might enhance the risk for arrhythmias in our model: alterations in repolarization and/or myocyte calcium dysregulation. We previously demonstrated that myocardial infarction provokes reductions in repolarization reserve in both VF− and VF+ dogs, with the largest reductions noted in the VF+ animals (48, 49). As dietary n-3 PUFA treatment has been shown to prolong myocyte action potential duration via inhibition of outward potassium currents (18–20), these fatty acids could provoke further reductions in repolarization reserve, that lead to marked regional differences in repolarization during myocardial ischemia. Indeed, dietary n-3 PUFA enhanced arrhythmia formation in isolated porcine hearts during ischemia but not during normoxic conditions (21). These regional differences in repolarization would be difficult to detect with a body surface ECG but should be more obvious with multi-electrode mapping and refractory period studies. Thus, changes in repolarizing currents could explain both the lack of beneficial actions in the VF+ dogs and the induction of tachyarrhythmias in some of the VF− dogs (by “exhausting” the repolarization reserve, tipping the balance in favor arrhythmia formation). In a similar manner, cardiomyocyte calcium dysregulation also appears to contribute to arrhythmia formation in VF+ animals. Myocytes from these animals exhibit greater spontaneous calcium release and calcium alternans, phenomena that could be eliminated by reducing agents and replicated in control myocytes by oxidant stress (50). It is possible that n-3 PUFA treatment could further disrupt the regulation of sarcoplasmic reticular calcium release particularly during the oxidant stress associated with ischemia and/or in response to sympathetic nerve activation (β-adrenoceptor stimulation) in dogs previously shown to be resistant to VF. The relative contribution, if any, of n-3 PUFA mediated changes in calcium regulation and repolarization reserve to the pro-arrhythmia noted in the present study remain to be determined.
Summary and Conclusions
In the present study, despite large increases in cardiac tissue n-3 PUFA content, dietary n-3 PUFAs not only did not prevent ischemia-induced VF but actually induced arrhythmias in about one-third of the dogs that were previously resistant to malignant arrhythmias. Given the inconsistent benefits reported in clinical and experimental studies and the potential adverse actions on cardiac rhythm noted in the present study, recommendations (15) to use n-3 PUFA in the post-MI patient need to be reconsidered
Supplementary Material
Acknowledgements
The authors also wish to thank GlaxoSmithKline for generously providing the n-3 PUFA ethyl ester and placebo capsules for study 2 and SPA for performing the plasma lipid analysis for study 1.
Funding Sources: This work was supported by National Heart, Lung and Blood Institute Grant HL086700 (study 2 to G.E.B] and a research grant from SPA Company, Milan Italy (study 1 to P.J.S)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: W.S.H. is a scientific advisor to Omthera, Aker Biomarine, and Amarin, is a speaker for GlaxoSmithKline, and is the owner of OmegaQuant Analytics, a company offering blood omega-3 testing.
References
- 1.Dyerberg J, Bang HO, Stoffersen E, Moncada S, Vane JR. Eicosapentaenoic acid and prevention of thrombosis and atherosclerosis? Lancet. 1978;2:117–119. doi: 10.1016/s0140-6736(78)91505-2. [DOI] [PubMed] [Google Scholar]
- 2.Kromhout D, Bosschieter EB, de Lezenne CC. The inverse relation between fish consumption and 20-year mortality from coronary heart disease. N Engl J Med. 1985;312:1205–1209. doi: 10.1056/NEJM198505093121901. [DOI] [PubMed] [Google Scholar]
- 3.Burr ML, Gilbert JF, Holliday RM, Elwood PC, Fehily AM, Rogers S, Sweetnam PM, Deadman NM. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART) Lancet. 1989;2:757–761. doi: 10.1016/s0140-6736(89)90828-3. [DOI] [PubMed] [Google Scholar]
- 4.Nilsen DWT, Albrektsen G, Landmark K, Moen S, Aarsland T, Woie Effects of a high-dose concentrate on n-3 fatty acids or corn oil introduced early after acute myocardial infarction on serum triacylglyerol and HDL cholesterol. Am J Clin Nutr. 2001;74:50–56. doi: 10.1093/ajcn/74.1.50. [DOI] [PubMed] [Google Scholar]
- 5.Marchioli R, Barzi F, Bomba E, Chieffo C, Di Gregorio D, Di Mascio R, Franzosi MG, Geraci E, Levantesi G, Maggioni AP, Mantini L, Marfisi RM, Mastrogiuseppi G, Mininni N, Nicolisi GL, Santini M, Schweiger C, Tavazzi L, Tognoni G, Tucci C, Valagussa F. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI)-Prevenzione. Circulation. 2002;105:1897–1903. doi: 10.1161/01.cir.0000014682.14181.f2. [DOI] [PubMed] [Google Scholar]
- 6.Burr ML, Ashfield-Watt PA, Dunstan FD, Fehily AM, Breay P, Ashton T, Zotos PC, Haboubi NAA, Elwood PC. Lack of benefit of dietary advice to men with angina: results of controlled trial. Eur J Clin Nutr. 2003;57:193–200. doi: 10.1038/sj.ejcn.1601539. [DOI] [PubMed] [Google Scholar]
- 7.Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K Japan EPA lipid intervention study (JELIS) Investigators. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolemic (JELIS): a randomized open-label, blinded endpoint analysis. Lancet. 2007;369:1090–1098. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 8.GISSI-HF investigators. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomized, double-blind, placebo-controlled trial. Lancet. 2008;372:1223–1230. doi: 10.1016/S0140-6736(08)61239-8. [DOI] [PubMed] [Google Scholar]
- 9.Rauch B, Schiele R, Schneider S, Diller F, Victor N, Gohlke H, Gottwik M, Steinbeck G, Del Castillo U, Sack R, Worth H, Katus H, Spitzer W, Sabin G, Senges J for the OMEGA Study Group. OMEGA, a randomized, placebo-controlled trial to test the effect of highly purified omega-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarction. Circulation. 2010;122:2152–2159. doi: 10.1161/CIRCULATIONAHA.110.948562. [DOI] [PubMed] [Google Scholar]
- 10.Kromhout D, Giltay EJ, Geleijnse JM for the Alpha Omega Trial Group. n-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 2010;363:2015–2026. doi: 10.1056/NEJMoa1003603. [DOI] [PubMed] [Google Scholar]
- 11.Leaf A, Albert CM, Josephson M, Steinhaus D, Kluger J, Kang JX, Cox B, Zhang H, Schoenfeld D. Prevention of fatal arrhythmias in high-risk subjects by fish oil n-3 fatty acid intake. Circulation. 2005;112:2762–2768. doi: 10.1161/CIRCULATIONAHA.105.549527. [DOI] [PubMed] [Google Scholar]
- 12.Raitt MH, Connor WE, Morris C, Kron J, Halpren B, Chugh SS, McClelland J, Cook J, MacMurdy K, Swenson R, Conner SL, Gerhard G, Kraemer DF, Oseran D, Marchant C, Calhoun D, Shnider R, McAnulty J. Fish oil supplementation and risk of ventricular tachycardia and ventricular fibrillation in patients with implantable defibrillators: a randomized controlled trial. JAMA. 2005;293:2884–2891. doi: 10.1001/jama.293.23.2884. [DOI] [PubMed] [Google Scholar]
- 13.Brouwer IA, Zock PL, Camm AJ, Boecker D, Hauer RN, Wever EF, Dullemeijer C, Ronden JE, Katan MB, Lubinski A, Buschler H, Schouten EG SOFA Study Group. Effect of fish oil on ventricular tachyarrhythmia and death in patients with implantable cardioverter defibrillators: the study on omega-3 fatty acids and ventricular arrhythmias (SOFA) JAMA. 2006;295:2613–2619. doi: 10.1001/jama.295.22.2613. [DOI] [PubMed] [Google Scholar]
- 14.De Caterina R. n-3 Fatty acids in cardiovascular disease. N Engl J Med. 2011;364:2439–2450. doi: 10.1056/NEJMra1008153. [DOI] [PubMed] [Google Scholar]
- 15.Kris-Etherton PM, Harris WS Appel LJ for the AHA Nutrition Committee. Omega-3 fatty acids and cardiovascular disease: New recommendations from the American Heart Association. Arterioscler Thromb Vasc Biol. 2003;23:151–152. doi: 10.1161/01.atv.0000057393.97337.ae. [DOI] [PubMed] [Google Scholar]
- 16.Billman GE, Hallaq H, Leaf A. Prevention of ischemia-induced ventricular fibrillation by omega-3 fatty acids. Proc Natl Acad Sci USA. 1994;91:4427–4430. doi: 10.1073/pnas.91.10.4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Billman GE, Kang JX, Leaf A. Prevention of sudden death by dietary pure n-3 polyunsaturated fatty acids in dogs. Circulation. 1999;99 doi: 10.1161/01.cir.99.18.2452. 2542-2457. [DOI] [PubMed] [Google Scholar]
- 18.Anders BP, Weber AR, Rampersad PP, Gilchrist JSC, Pierce GN, Lukas A. Dietary flax seed protects against ventricular fibrillation induced by ischemia –reperfusion in normal and hypercholestrolemic rabbits. J Nutr. 2004;134:3250–3256. doi: 10.1093/jn/134.12.3250. [DOI] [PubMed] [Google Scholar]
- 19.Dhein S, Michaelis B, Mohr FW. Antiarrhythmic and electrophysioloical effects of long-chain omega-3 polyunsaturated fatty acids. Naunyn-Schmiedeberg’s Arch Pharmacol. 2005;371:202–211. doi: 10.1007/s00210-005-1024-z. [DOI] [PubMed] [Google Scholar]
- 20.Den Ruijter HM, Bercki G, Opthof T, Verkerk AO, Zock PL, Coronel R. Pro- and anti-arrhythmic properties of diet rich in fish oil. Cardiovasc Res. 2007;73:316–325. doi: 10.1016/j.cardiores.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 21.Coronel R, Wilms-Schopman FJG, Den Ruijter HM, Beltermean CN, Schumacher CA, Opthof T, Hovenier R, Lemmens AG, Terpstra AHM, Katan MB, Zock P. Dietary n-3 fatty acids promote arrhythmias during acute regional myocardial ischemia in isolated pig hearts. Cardiovasc Res. 2007;73:386–394. doi: 10.1016/j.cardiores.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz PJ, Billman GE, Stone HL. Autonomic mechanisms in ventricular fibrillation due to acute myocardial ischemia during exercise in dogs with healed myocardial infarction: an experimental model for sudden cardiac death. Circulation. 1984;69:790–800. doi: 10.1161/01.cir.69.4.790. [DOI] [PubMed] [Google Scholar]
- 23.Billman GE. A comprehensive review and analysis of 25 years of data from an in vivo canine model of sudden cardiac death: Implications for future anti-arrhythmic drug development. Pharmacol Therap. 2006;111:808–835. doi: 10.1016/j.pharmthera.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Bligh EG, Dyer WJ. A rapid method for total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 25.Morrison WR, Smith LM. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride-methanol. J Lipid Res. 1964;5:600–608. [PubMed] [Google Scholar]
- 26.Walker MJ, Curtis MJ, Hearse DJ, Campbell RW, Janse MJ, Yellon DM, Cobbe SM, Coker SJ, Harness JB, Harron DW, Higgins AJ, Julian DG, Lab MJ, Manning AS, Northover BJ, Parratt JR, Riemersma RA. The Lambeth conventions: guidelines for the study of arrhythmias in ischaemia, infarction, and reperfusion. Cardiovasc Res. 1988;22:447–455. doi: 10.1093/cvr/22.7.447. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz PJ. Do animal models have clinical value? Am J Cardiol. 1998;81:14D–20D. doi: 10.1016/s0002-9149(98)00148-9. [DOI] [PubMed] [Google Scholar]
- 28.Hooper L, Thompson RL, Harrison RA, Summerbell CD, Moore H, Worthington HV, Durrington PN, Ness AR, Capps NE, Davey-Smith G, Riemersma RA, Ebrahim SB. Omega-3 fatty acids for prevention and treatment of cardiovascular disease. Cochrane Database Syst Rev. 2004;4:CD003177. doi: 10.1002/14651858.CD003177.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Filion KB, El Khoury F, Bielinski M, Schiller I, Dendukuri N, Brophy JM. Omega-3 fatty acids in high-risk cardiovascular patients: a meta-analysis of randomized controlled trials. BMC Cardiovasc Discord. 2010;10:24. doi: 10.1186/1471-2261-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao YT, Chen Q, Sun YX, Li XB, Zhang P, Xu Y, J-H G. Prevention of sudden cardiac death with omega-3 fatty acids in patients with coronary heart disease: meta-analysis of randomized controlled trials. Ann Med. 2009;41:301–310. doi: 10.1080/07853890802698834. [DOI] [PubMed] [Google Scholar]
- 31.Leon H, Shibata MC, Sivakumaran, Dorgan M, Chatterley T, Tsuyuki RT. Effect of fish oil on arrhythmias and mortality: systematic review. Brit Med J. 2009;338:a2931. doi: 10.1136/bmj.a2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilhelm M, Tobis R, Asskali F, Kraehner R, Kuly S, Klinghammer L, Boehles H, Daniel WG. Red blood cell omega-3 fatty acids and the risk of ventricular arrhythmias in patients with heart failure. Am Heart J. 2008;155:971–977. doi: 10.1016/j.ahj.2007.11.045. [DOI] [PubMed] [Google Scholar]
- 33.Brouwer IA, Riatt MH, Dullemeijer C, Kraemer DF, Zock PL, Morris C, Katan MB, Connor WE, Camm JA, Schouten EG, McAnulty J. Effect of fish oil on ventricular tachyarrhythmia in three studies in patients with implantable cardioverter defibrillators. Eur Heart J. 2009;30:820–826. doi: 10.1093/eurheartj/ehp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jenkins DJ, Josse AR, Beyene J, Dorian P, Burr ML, LaBelle R, Kendall CW, Cunnane SC. Fish-oil supplementation in patients with implantable cardioverter defibrillators: a meta-analysis. Can Med Assc J. 2008;178:157–164. doi: 10.1503/cmaj.070261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McLennan PL, Abeywardena MY, Charnock JS. Dietary fish oil prevents ventricular fibrillation following coronary artery occlusion and reperfusion. Am Heart J. 1988;116:709–717. doi: 10.1016/0002-8703(88)90328-6. [DOI] [PubMed] [Google Scholar]
- 36.McLennan PL, Bridle TM, Abeywardena MY, Charnock JS. Dietary lipid modulation of ventricular fibrillation threshold in the marmoset monkey. Am Heart J. 1992;123:1555–1561. doi: 10.1016/0002-8703(92)90809-a. [DOI] [PubMed] [Google Scholar]
- 37.Matthan NR, Jordan H, Chung M, Lichtenstein AH, Lathrop DA, Lau J. A systematic review and meta-analysis of the impact of ω-3 fatty acids on selected arrhythmia outcomes in animal models. Metabol Clin Exp. 2005;54:1557–1565. doi: 10.1016/j.metabol.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 38.Abildstrom SZ, Kobler L, Torp-Pedersen C. Epidemiology of arrhythmic and sudden death in the chronic phase of ischemic heart disease. Cardiac Electrophysiol Rev. 1999;3:177–179. doi: 10.1023/a:1017935732134. [DOI] [PubMed] [Google Scholar]
- 39.Schuster EH, Bulkley BH. Ischemia at a distant site after myocardial infarction: a cause of early postinfarction angina. Circulation. 1980;62:509–515. doi: 10.1161/01.cir.62.3.509. [DOI] [PubMed] [Google Scholar]
- 40.Billman GE. Cardiac autonomic neural “remodeling” and susceptibility to sudden cardiac death: effect of endurance exercise training. Am J Physiol Heart Circ Physiol. 2009;297:H1171–H1193. doi: 10.1152/ajpheart.00534.2009. [DOI] [PubMed] [Google Scholar]
- 41.La Rovere MT, Bigger JT, Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet. 1998;351:478–484. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 42.Billman GE, Schwartz PJ, Stone HL. Baroreceptor reflex control of heart rate: A predictor of sudden death. Circulation. 1982;66:874–880. doi: 10.1161/01.cir.66.4.874. [DOI] [PubMed] [Google Scholar]
- 43.Schwartz PJ, Vanoli E, Stramba-Badiale M, DeFerrari GM, Billman GE, Foreman RD. Autonomic mechanisms and sudden death, new insight from the analysis of baroreceptor reflexes in conscious dogs with and without a myocardial infarction. Circulation. 1988;78:969–979. doi: 10.1161/01.cir.78.4.969. [DOI] [PubMed] [Google Scholar]
- 44.Von Schacky C. A review of omega-3 ethyl esters for the cardiovascular prevention and treatment of increased blood triglyceride levels. Vasc Health Risk Manag. 2006;2:251–262. doi: 10.2147/vhrm.2006.2.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kowey PR, Reiffel JA, Ellenbogen KA, Naccarelli GV, Pratt CM. Efficacy and safety of prescription omega-3 fatty acids for the prevention of recurrent symptomatic atrial fibrillation: a randomized controlled trial. JAMA. 2010;304:E1–E10. doi: 10.1001/jama.2010.1735. [DOI] [PubMed] [Google Scholar]
- 46.Siscovick DS, Raghunathan TE, King I, Weinmann S, Wicklund KG, Albright J, Bouberg V, Arbogast P, Smith H, Kushi LH, Cobb LA, Copass MK, Psaty B, Lemaitre R, Retzlaff B, Childs M, Knopp RH. Dietary intake and cell membrane levels of long-chain n-3 polyunsaturated fatty acids and the risk of primary cardiac arrest. JAMA. 1995;274:1363–1367. doi: 10.1001/jama.1995.03530170043030. [DOI] [PubMed] [Google Scholar]
- 47.Albert CM, Campos H, Stampfer MJ, Ridker PM, Mason JE, Willet WC, Ma J. Blood levels of long-chain n-3 fatty acids and the risk of sudden death. N Engl J Med. 2002;346:1113–1118. doi: 10.1056/NEJMoa012918. [DOI] [PubMed] [Google Scholar]
- 48.Sridhar A, Nishijima Y, Terentyev D, Terentyeva R, Uelman R, Kukielka M, Bonilla IM, Robertson GA, Gyorke S, Billman GE, Carnes CA. Repolarization abnormalities and afterdepolarizations in a canine model of sudden cardiac death. Am J Physiol Reg Integ Physiol. 2008;295:R1463–R1472. doi: 10.1152/ajpregu.90583.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Swann MH, Nakagawa H, Vanoli E, Lazzara R, Schwartz PJ, Adamson PB. Heterogeneous regional endocardial repolarization is associated with increased risk fro ischemia-dependent ventricular fibrillation after myocardial infarction. J Cardiovasc Elctrophysiol. 2003;14:873–879. doi: 10.1046/j.1540-8167.2003.03100.x. [DOI] [PubMed] [Google Scholar]
- 50.Belevych A, Terentyev D, Sridhar A, Nishijima Y, Wilson LD, Cardounel AJ, Laurita K, Carnes CA, Billman GE, Gyorke S. Redox-modification of ryanodine receptors underlies sarcoplasmic reticulum luminal calcium dependent cardiac alternans in a canine model of sudden cardiac death. Cardiovasc Res. 2009;84:387–395. doi: 10.1093/cvr/cvp246. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.