Summary
Genotoxic activity of hexavalent chromium (chromate) results from its reductive activation inside the cell. Cr(VI) metabolism in vivo is primarily driven by ascorbate (Asc) but in cultured cells by glutathione (GSH). Given the common use of cultured cells for mechanistic studies, it is important to establish whether Cr(VI) activated by Asc and GSH displays the same genotoxic properties. Using 2’,7’ dichlorofluoroscin (DCFH) as a redox sensitive probe, we found that Asc-dependent reduction of Cr(VI) in vitro under physiological conditions generated 25–80 times lower yields of oxidants compared to GSH. When both reducers were present, Asc dominated Cr(VI) metabolism and inhibited DCFH oxidation. Consistent with the findings in defined chemical reactions, restoration of physiological levels of Asc in human lung H460 cells led to the loss of their hypersensitivity to clonogenic killing by Cr(VI) in the presence of methoxyamine, which inhibits base excision repair of oxidative DNA damage. Despite suppressed oxidative damage, Asc-containing cells formed a large number of DNA double-strand breaks after exposure to a dose of Cr(VI) corresponding to the drinking water standard of 100 ppb. Our results indicate that Asc-driven metabolism of Cr(VI) shifts its genotoxicity toward nonoxidative mechanisms.
Keywords: chromium, chromate, vitamin C, ascorbate, glutathione, oxidative damage, reduction
Introduction
Cr(VI)-containing compounds are firmly established human carcinogens [1]. Inhalation of Cr(VI) in occupational settings is a strong risk factor for lung cancer [2]. Environmental exposure is caused by ambient pollution and ingestion of drinking water contaminated with natural and anthropogenic Cr(VI) [3]. Cr(VI) is unreactive toward DNA and other macromolecules and requires reductive activation inside the cell to cause toxic effects. Studies with tissue extracts [4,5] and the measurements of reaction rates with individual reducers [6] have provided strong evidence that cellular reduction of Cr(VI) is primarily nonenzymatic. Ascorbate (Asc) was estimated to account for 80–95% of all Cr(VI) metabolism, with glutathione (GSH) largely being responsible for the remaining activity. The final product of all Cr(VI) reduction reactions is Cr(III), which generates DNA-protein crosslinks [7] and several forms of small Cr-DNA adducts [8–10]. Direct reactivity of Cr(V,IV) intermediates and their ability to catalyze Fenton-like reactions with H2O2 contribute to the oxidative component of Cr(VI) genotoxicity [11,12].
Reactions of Cr(VI) with Asc and GSH exhibit dramatically different rates and the yields of Cr intermediates. At physiological levels, Asc is more than 10-times faster at reducing Cr(VI) than GSH [6]. GSH-driven reactions generate Cr(V) intermediate [13], which was undetectable for the two-electron reducer Asc under biologically relevant conditions [14,15]. Cultured human and nonhepatic rodent cells contain very low Asc levels [10,16–18], reflecting the absence of vitamin C in nutrient mixtures and a typical supplementation of growth media with only 10–15% serum. This raises a concern whether mechanistic studies with cultured cells provide a faithful assessment of the toxicological properties of Cr(VI), particularly its oxidative mechanisms. Here, we examined the yield of oxidizing species during Cr(VI) metabolism by Asc and GSH and the impact of restored Asc levels on the extent of oxidative DNA damage by Cr(VI) in human lung cells.
Materials and Methods
Materials
L-ascorbic acid (99.9% pure), dehydro-L-(+)-ascorbic acid dimer (DHA) and glutathione (>98% pure) were from Sigma. GSH and Asc solutions were always freshly prepared and kept on ice. Potassium chromate (A.C.S. reagent, >99% purity) was from Aldrich.
Cells and treatments
Human lung epithelial H460 cells were grown in RPMI1640 medium containing 10% serum. For Asc loading, cells were incubated for 2 hr with 0.5 mM DHA in complete media. Stock solutions of 10 mM DHA were freshly prepared in the Krebs-HEPES buffer. Cellular concentrations of Asc were measured by a fluorescent detection of its conjugate with 1,2-diamino-4,5-dimethoxybenzene dihydrochloride [18]. All treatments with K2CrO4 [Cr(VI)] were in complete media.
Clonogenic survival
Cells were seeded onto 60 mm dishes (400 cells per dish), allowed to attach overnight and treated with Cr(VI) for 1 or 3 hr. To block repair of abasic sites, 6 mM methoxyamine was added for 2 hr before Cr(VI) and was present during and after treatments for 48 hr. Cells were grown without replating and stained with Giemsa solution.
Cr(VI) reduction and detection of oxidants with 2’,7’ dichlorofluoroscin (DCFH)
Reduction of Cr(VI) was monitored by absorbance measurements at 372 nm. Reaction mixtures contained 50 mM buffer (pH 7.0), 100 mM NaCl, 0.2 mM diethylenetriaminepentaacetic acid (DTPA), 0.1 mM Cr(VI) and 2–5 mM Asc and/or GSH. Production of oxidizing species during Cr(VI) reduction was monitored by oxidation of DCFH into fluorescent 2,’7’ dichlorofluoroscein (DCF). DCFH-acetate was activated by incubation in 10 mM NaOH at 37°C for 30 min. Reaction mixtures contained 10 µM DCFH, 50 mM buffer (pH 7.0), 100 mM NaCl, 0.2 mM DTPA, Cr(VI) were incubated for 1 hr at 37°C. DCF fluorescence was recorded with 485 nm excitation and 535 nm emission wavelength settings.
Immunostaining
Cells were permeabilized for 10 min on ice in PBS-0.5% Triton X-100 and then fixed with 4% paraformaldehyde for 15 min at room temperature. Nonspecific binding was blocked with 3% BSA for 1 hr before addition of primary antibodies. 53BP1 antibodies were from Novus Biologicals and γH2AX antibodies from Millipore. Nuclei were stained with DAPI. Images were obtained with the Zeiss LSM 710 confocal microscope.
Results and Discussion
Formation of oxidants during in vitro Cr(VI) reduction
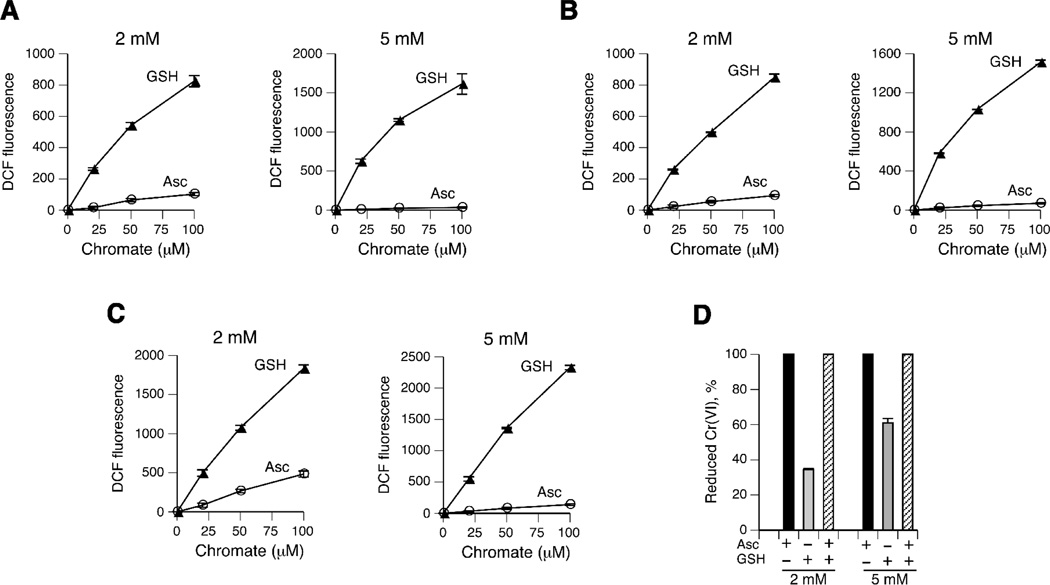
To compare the overall production of oxidizing species during Cr(VI) reduction by Asc and GSH, we employ a widely used redox-sensitive probe DCFH. Oxidation of DCFH can monitor the presence of both reactive oxygen species and reactive Cr intermediates [19]. The formation of DCF, the oxidized product of DCFH, has been previously found to occur during Cr(VI) reduction in cells [20] and in vitro with cysteine [21,22] and Asc [6,23]. We performed Cr(VI) reduction reactions at physiologically relevant conditions (ionic strength, pH and temperature) and included the iron chelator DTPA to block contributions from any potential contamination with this redox active metal. The inclusion of DTPA has been previously determined to exert no effect on kinetics of Cr(VI) reduction by GSH [24]. Fig. 1A shows that 1 hr-long reactions of Cr(VI) with 2 mM GSH in MOPS buffer resulted in an extensive, dose-dependent formation of DCFH-oxidizing species. Parallel reactions of Cr(VI) with 2 mM Asc also generated fluorescent DCF but in much smaller amounts. The differences in slopes revealed 9.1-times higher production of oxidants in 2 mM GSH versus 2 mM Asc samples. Reactions with 5 mM concentrations of the reducers exhibited even more dramatic differences in the yield of oxidizing species between GSH and Asc (48-fold difference in slopes). Although MOPS has been found to exhibit the least significant interactions with DNA-reactive Cr forms [25], we next tested other buffers to exclude a possible reaction system bias. Similar to MOPS-based reactions, we found that reduction of Cr(VI) with 2 mM reducers in HEPES buffer generated a 9.2-times higher yield of oxidants in the presence of GSH relative to Asc (Fig. 1B). In reactions with 5 mM reducers, DCFH oxidation in HEPES was on average 22-times higher with GSH than Asc. Lastly, we measured the production of oxidants in phosphate buffer and observed the same trend for much stronger DCF fluorescence in GSH-containing reactions for both 2 and 5 mM concentrations of the reducers (Fig. 1C). The differences in the generation of oxidants between two main Cr(VI) reducers are even more striking considering that unlike Asc, GSH-based reactions showed only a partial reduction of Cr(VI) (Fig. 1D). After normalization for the amount of reduced Cr(VI) in MOPS-based reactions, 2 mM and 5 mM GSH generated 26.5- and 79.1-times more oxidants than corresponding samples with Asc. For MOPS-based reactions that were run in parallel, we found that the yield of DCF with 5 mM GSH was 1.9-times higher than with 2 mM GSH, which closely corresponds to a 1.8-times higher amount of reduced Cr(VI) at 5 mM GSH (Fig. 1D). The DCF ratios of 5 mM/2 mM GSH for HEPES and phosphate-based reactions were 2.0 and 2.1, respectively. Thus, the production of oxidants in GSH reactions is related to the amount of reduced Cr(VI), not GSH concentration itself. For Asc reactions, which all exhibited 100% Cr(VI) reduction (Fig. 1D), the yield of DCF at 5 mM was lower than at 2 mM Asc for all three buffers on average by 1.4-fold. A similar trend for suppression of DCFH oxidation by Cr(VI) at higher Asc concentrations was observed earlier in a comparison of 0.2 and 1 mM Asc [23].
Figure 1.
Oxidation of DCFH during Cr(VI) reduction by GSH and Asc in (A) MOPS, (B) HEPES and (C) phosphate buffers. DCF fluorescence is expressed in arbitrary units. Data are means±SD (n=4). Where not seen, error bars were smaller than symbols. (D) Cr(VI) reduction after incubation for 1 hr in MOPS buffer (means±SD, n=8).
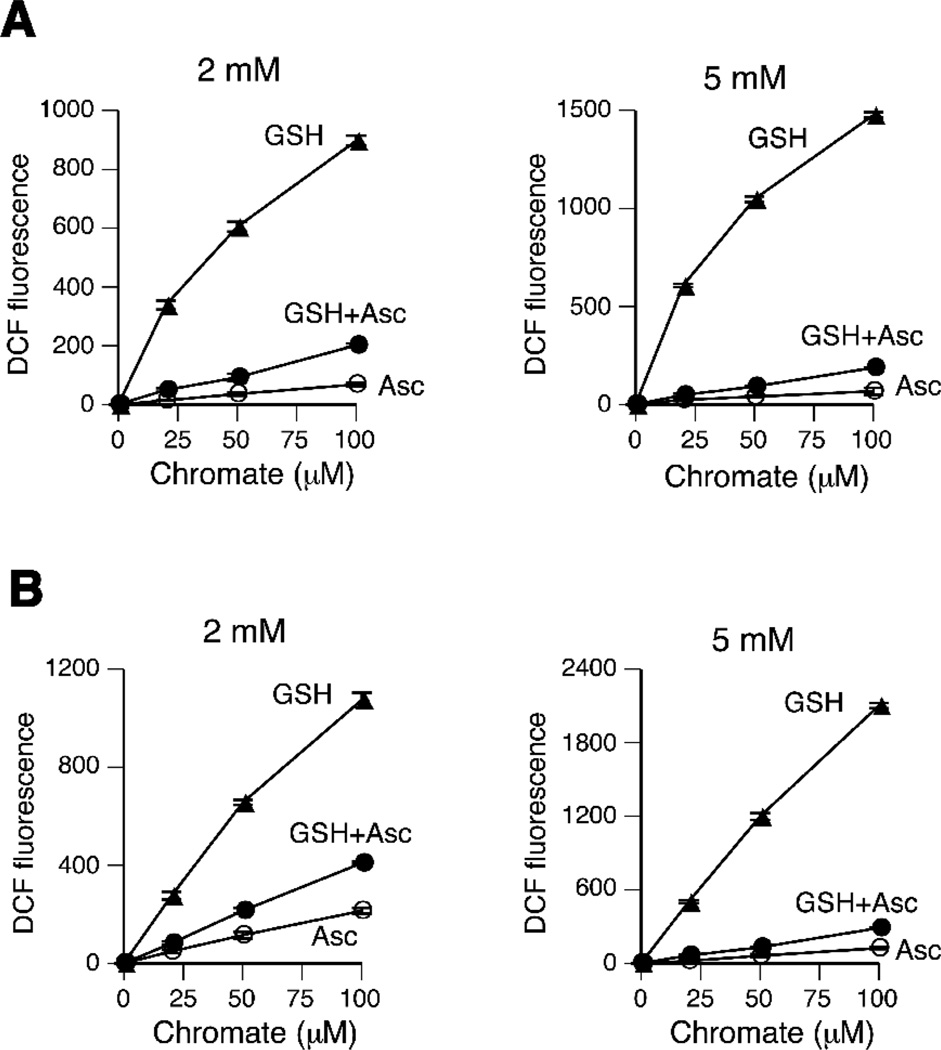
In a final series of DCFH experiments, we examined the production of oxidants in Cr(VI) reactions with single reducers versus their mixtures (Fig. 2). We found that Asc exerted a dominant effect in the mixed reducer reactions, as evidenced by much lower yields of DCFH-oxidizing species during Cr(VI) reduction in the presence of Asc and GSH than GSH alone. This result was clearly observed in two buffers (MOPS and phosphate) and both 2 and 5 mM concentrations of the reducers. The oxidant-suppressive effects of Asc are even more striking considering that mixed reactions metabolized 100% Cr(VI) as compared to only 34.4% and 61% in 2 mM and 5 mM GSH single reducer reactions, respectively (Fig. 1D). Although Asc-dependent metabolism was clearly dominant, a moderately higher DCFH oxidation in mixed reactions (average for two buffers: 2.1- and 2.7-fold for 2 and 5 mM reducers, respectively) compared to Asc alone indicates a remaining minor contribution of GSH. Based on the DCF yields, we estimated that Asc was responsible for approximately 95% of Cr(VI) reduction in mixed Asc+GSH reactions, which closely corresponds to the calculated contribution of Asc to Cr(VI) reduction in the lung tissue [5].
Figure 2.
Production of oxidants during Cr(VI) reduction by mixtures of GSH and Asc. (A) MOPS-based reactions. (B) Phosphate-based reactions. Data are means±SD (n=4). Where not seen, error bars were smaller than symbols.
Cr(VI) genotoxicity in Asc-restored human cells
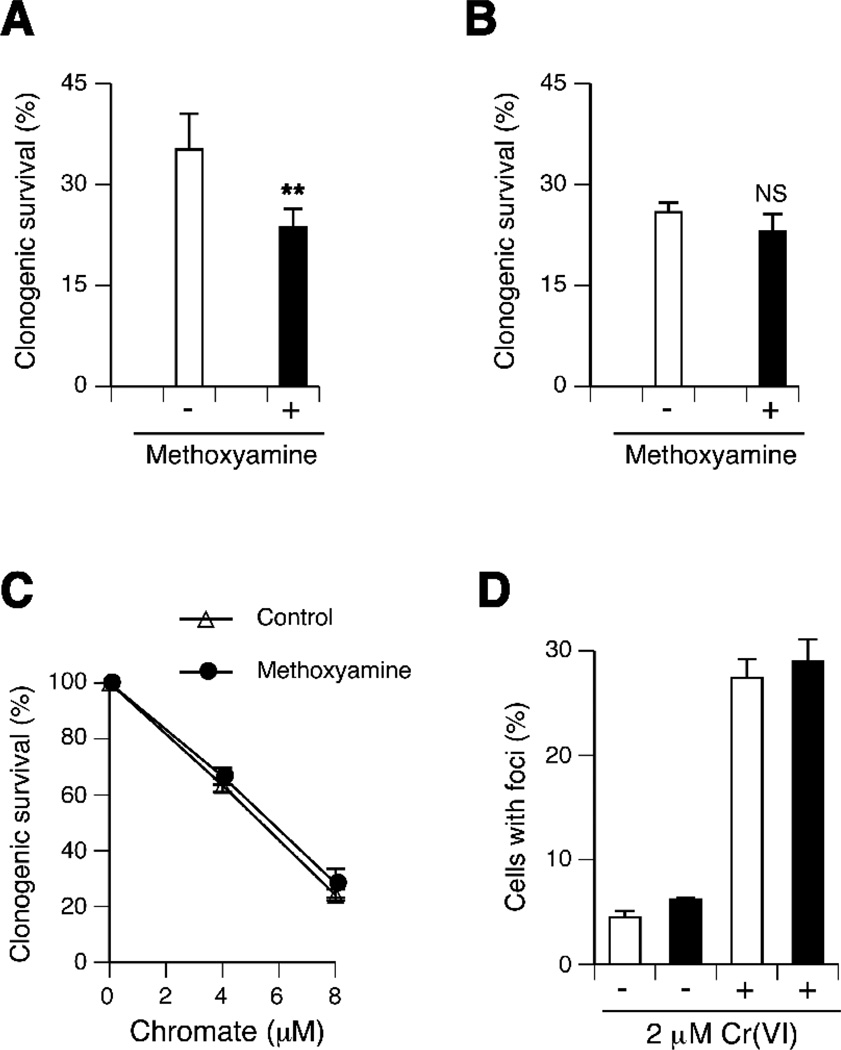
The suppressed formation of DCFH-oxidizing species during Cr(VI) reduction by Asc suggested that a GSH-based metabolism of Cr(VI) in standard cultures of Asc-deficient cells [10,16–18] could exacerbate its DNA-oxidizing potential. To test the formation of toxicologically important levels of oxidative DNA damage, we examined clonogenic survival of H460 cells in which repair of oxidized bases and abasic sites was blocked by the addition of methoxyamine. Methoxyamine forms covalent adducts with abasic sites produced directly or as a result of base removal by glycosylases, which blocks subsequent steps in base excision repair. The use of this inhibitor has already been found to increase clonogenic toxicity of Cr(VI) in CHO cells [26]. We also observed higher clonogenic toxicity of Cr(VI) in H460 cells in the presence of methoxyamine (Fig. 3A). However, when Asc levels in H460 cells were elevated from <0.01 mM in standard cultures to 1.2 mM, methoxyamine lost its ability to enhance cytotoxicity of Cr(VI) (Fig. 3B,C). Human lungs contain on average 1.3 mM Asc [27]. Although oxidative DNA damage was suppressed in Asc-restored H460 cells, treatments with a low dose of 2 µM Cr(VI) for 3 hr resulted in a large increase in the frequency of cells containing nuclear foci of 53BP1 and γH2AX (Fig. 3D), which have been validated as reliable biochemical markers of DNA double-strand breaks in Cr-treated cells [28]. Importantly, 2 µM Cr(VI) (100 ppb Cr) corresponds to a long-maintained maximum contaminant level for chromium in drinking water set by the US-EPA [3]. These results point to the importance of nonoxidative mechanisms in chromosome-breaking activity of Cr(VI), which is consistent with replication-blocking and mutagenic properties of Cr-DNA adducts detected in shuttle-vector studies [6,23,29,30].
Figure 3.
Genotoxicity of Cr(VI) in H460 cells. (A) Decreased clonogenic survival of H460 cells treated with 25 µM Cr(VI) for 1 hr in the presence of 6 mM methoxyamine. Means±SD for 5 independently treated dishes, **- p=0.002. (B) Methoxyamine does not affect clonogenic toxicity of Cr(VI) in H460 cells with restored Asc levels. Means±SD for 4 independently treated dishes (30 µM Cr for 1 hr), NS – nonsignificant differences (C) Clonogenic survival of Asc-restored H460 treated with Cr(VI) for 3 hr. Means±SD for 4 independently treated dishes. (D) Induction of DNA-double strand breaks in Asc-restored H460 cells treated with 2 µM Cr(VI) for 3 hr and fixed at 4 hr post-exposure. Open bars - 53BP1 foci-positive cells, black bars - γH2AX-positive cells. Means±SD for 3 slides with >100 cells scored per slide.
Conclusions
Reductive metabolism of Cr(VI) by Asc generates low amounts of oxidants as compared to reactions with GSH. Restoration of physiological levels of Asc in cultured human lung H460 cells suppressed the formation of oxidative DNA damage by Cr(VI) as evidenced by the absence of survival effects from the inhibition of base excision repair. These results taken together with recent findings on the loss of hypersensitivity of XRCC1-deficient cells to toxic effects of Cr(VI) under Asc-normalized conditions [31] indicate that Asc deficiency of cultured cells leads to oxidant-prone metabolism of Cr(VI) by the secondary reducer GSH.
Acknowledgements
This work was supported by grants R01 ES008786 and P42 ES013660 from the National Institute of Environmental Health Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Costa M, Klein CB. Toxicity and carcinogenicity of chromium compounds in humans. Crit. Rev. Toxicol. 2006;36:155–163. doi: 10.1080/10408440500534032. [DOI] [PubMed] [Google Scholar]
- 2.Park RM, Bena JF, Stayner LT, Smith RJ, Gibb HJ, Lees PS. Hexavalent chromium and lung cancer in the chromate industry: a quantitative risk assessment. Risk Anal. 2004;24:1099–1108. doi: 10.1111/j.0272-4332.2004.00512.x. [DOI] [PubMed] [Google Scholar]
- 3.Zhitkovich A. Chromium in drinking water: sources, metabolism and cancer risks. Chem. Res. Toxicol. 2011;24:1617–1629. doi: 10.1021/tx200251t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Standeven AM, Wetterhahn KE. Ascorbate is the principal reductant of chromium (VI) in rat liver and kidney ultrafiltrates. Carcinogenesis. 1991;12:1733–1737. doi: 10.1093/carcin/12.9.1733. [DOI] [PubMed] [Google Scholar]
- 5.Standeven AM, Wetterhahn KE. Ascorbate is the principal reductant of chromium(VI) in rat lung ultrafiltrates and cytosols, and mediates chromium-DNA binding in vitro. Carcinogenesis. 1992;13:1319–1324. doi: 10.1093/carcin/13.8.1319. [DOI] [PubMed] [Google Scholar]
- 6.Quievryn G, Peterson E, Messer J, Zhitkovich A. Genotoxicity and mutagenicity of chromium(VI)/ascorbate-generated DNA adducts in human and bacterial cells. Biochemistry. 2003;42:1062–1070. doi: 10.1021/bi0271547. [DOI] [PubMed] [Google Scholar]
- 7.Macfie A, Hagan E, Zhitkovich A. Mechanism of DNA-protein cross-linking by chromium. Chem. Res. Toxicol. 2010;23:341–347. doi: 10.1021/tx9003402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhitkovich A, Voitkun V, Costa M. Glutathione and free amino acids form stable adducts with DNA following exposure of intact mammalian cells to chromate. Carcinogenesis. 1995;16:907–913. doi: 10.1093/carcin/16.4.907. [DOI] [PubMed] [Google Scholar]
- 9.Zhitkovich A, Voitkun V, Costa M. Formation of the amino acid-DNA complexes by hexavalent and trivalent chromium in vitro: importance of trivalent chromium and the phosphate group. Biochemistry. 1996;35:7275–7282. doi: 10.1021/bi960147w. [DOI] [PubMed] [Google Scholar]
- 10.Quievryn G, Messer J, Zhitkovich A. Carcinogenic chromium(VI) induces cross-linking of vitamin C to DNA in vitro and in human lung A549 cells. Biochemistry. 2002;41:3156–3167. doi: 10.1021/bi011942z. [DOI] [PubMed] [Google Scholar]
- 11.Sugden KD, Stearns DM. The role of chromium(V) in the mechanism of chromate-induced oxidative DNA damage and cancer. J. Environ. Pathol. Toxicol. Oncol. 2000;19:215–230. [PubMed] [Google Scholar]
- 12.Yao H, Guo L, Jiang BH, Luo J, Shi X. Oxidative stress and chromium(VI) carcinogenesis. J. Environ. Pathol. Toxicol. Oncol. 2008;27:77–88. doi: 10.1615/jenvironpatholtoxicoloncol.v27.i2.10. [DOI] [PubMed] [Google Scholar]
- 13.Bose RN, Moghaddas S, Gelerinter E. Long-lived chromium(IV), chromium(V) metabolites in the chromium(VI)-glutathione reaction: NMR, ESR, HPLC, and kinetic characterization. Inorg. Chem. 1992;31:1987–1994. [Google Scholar]
- 14.Stearns DM, Wetterhahn KE. Reaction of Cr(VI) with ascorbate produces chromium(V), chromium(IV), and carbon-based radicals. Chem. Res. Toxicol. 1994;7:219–230. doi: 10.1021/tx00038a016. [DOI] [PubMed] [Google Scholar]
- 15.Lay PA, Levina A. Activation of molecular oxygen during the reactions of chromium(VI/V/IV) with biological reductants: implications for chromium-induced genotoxicities. J. Am. Chem. Soc. 1998;120:6704–6714. [Google Scholar]
- 16.Karaczyn A, Ivanov S, Reynolds M, Zhitkovich A, Kasprzak KS, Salnikow K. Ascorbate depletion mediates up-regulation of hypoxia-associated proteins by cell density and nickel. J. Cell. Biochem. 2006;97:1025–1035. doi: 10.1002/jcb.20705. [DOI] [PubMed] [Google Scholar]
- 17.Reynolds M, Stoddard L, Bespalov I, Zhitkovich A. Ascorbate acts as a highly potent inducer of chromate mutagenesis and clastogenesis: linkage to DNA breaks in G2 phase by mismatch repair. Nucleic Acids Res. 2007;35:465–476. doi: 10.1093/nar/gkl1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reynolds M, Zhitkovich A. Cellular vitamin C increases chromate toxicity via a death program requiring mismatch repair but not p53. Carcinogenesis. 2007;28:1613–1620. doi: 10.1093/carcin/bgm031. [DOI] [PubMed] [Google Scholar]
- 19.Martin BD, Schoenhard JA, Sugden KD. Hypervalent chromium mimics reactive oxygen species as measured by the oxidant-sensitive dyes 2',7'-dichlorofluorescin and dihydrorhodamine. Chem. Res. Toxicol. 1998;11:1402–1410. doi: 10.1021/tx9801559. [DOI] [PubMed] [Google Scholar]
- 20.Martin BD, Schoenhard JA, Hwang JM, Sugden KD. Ascorbate is a pro-oxidant in chromium-treated human lung cells. Mutat. Res. 2006;610:74–84. doi: 10.1016/j.mrgentox.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 21.Zhitkovich A, Song Y, Quievryn G, Voitkun V. Non-oxidative mechanisms are responsible for the induction of mutagenesis by reduction of Cr(VI) with cysteine: role of ternary DNA adducts in Cr(III)-dependent mutagenesis. Biochemistry. 2001;40:549–560. doi: 10.1021/bi0015459. [DOI] [PubMed] [Google Scholar]
- 22.Quievryn G, Goulart M, Messer J, Zhitkovich A. Reduction of Cr (VI) by cysteine: significance in human lymphocytes and formation of DNA damage in reactions with variable reduction rates. Mol. Cell. Biochem. 2001;222:107–118. [PubMed] [Google Scholar]
- 23.Quievryn G, Messer J, Zhitkovich A. Lower mutagenicity but higher stability of Cr-DNA adducts formed during gradual chromate activation with ascorbate. Carcinogenesis. 2006;27:2316–2321. doi: 10.1093/carcin/bgl076. [DOI] [PubMed] [Google Scholar]
- 24.Messer J, Reynolds M, Stoddard L, Zhitkovich A. Causes of DNA single-strand breaks during reduction of chromate by glutathione in vitro and in cells. Free Radic. Biol. Med. 2006;40:1981–1992. doi: 10.1016/j.freeradbiomed.2006.01.028. [DOI] [PubMed] [Google Scholar]
- 25.Zhitkovich A, Shrager S, Messer J. Reductive metabolism of Cr(VI) by cysteine leads to the formation of binary and ternary Cr-DNA adducts in the absence of oxidative DNA damage. Chem. Res. Toxicol. 2000;13:1114–1124. doi: 10.1021/tx0001169. [DOI] [PubMed] [Google Scholar]
- 26.Brooks B, O'Brien TJ, Ceryak S, Wise JP, Sr, Wise SS, Wise JP, Jr, Defabo E, Patierno SR. Excision repair is required for genotoxin-induced mutagenesis in mammalian cells. Carcinogenesis. 2008;29:1064–1069. doi: 10.1093/carcin/bgn058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slade R, Stead AG, Graham JA, Hatch GE. Comparison of lung antioxidant levels in humans and laboratory animals. Am. Rev. Respir. Dis. 1985;131:742–746. doi: 10.1164/arrd.1985.131.5.742. [DOI] [PubMed] [Google Scholar]
- 28.Reynolds MF, Peterson-Roth EC, Bespalov IA, Johnston T, Gurel VM, Menard HL, Zhitkovich A. Rapid DNA double-strand breaks resulting from processing of Cr-DNA cross-links by both MutS dimers. Cancer Res. 2009;69:1071–1079. doi: 10.1158/0008-5472.CAN-08-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Voitkun V, Zhitkovich A, Costa M. Cr(III)-mediated crosslinks of glutathione or amino acids to the DNA phosphate backbone are mutagenic in human cells. Nucleic Acids Res. 1998;26:2024–2030. doi: 10.1093/nar/26.8.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhitkovich A, Quievryn G, Messer J, Motylevich Z. Reductive activation with cysteine represents a chromium(III)-dependent pathway in the induction of genotoxicity by carcinogenic chromium(VI) Environ. Health Perspect. 2002;110(Suppl 5):729–731. doi: 10.1289/ehp.02110s5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reynolds M, Armknecht S, Johnston T, Zhitkovich A. Undetectable role of oxidative DNA damage in cell cycle, cytotoxic and clastogenic effects of Cr(VI) in human lung cells with restored ascorbate levels. Mutagenesis. 2012 doi: 10.1093/mutage/ger095. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]