Abstract
Background
Blockade of inward-rectifier K+ channels by chloroquine terminates reentry in cholinergic atrial fibrillation (AF). However, it is unknown whether inward-rectifier K+ channels and reentry are also important in maintaining stretch-induced AF (SAF). We surmised that reentry underlies SAF, and that abolishing reentry with chloroquine terminates SAF more effectively than traditional Na+-channel blockade by flecainide.
Methods and Results
Thirty Langendorff-perfused sheep hearts were exposed to acute and continuous atrial stretch, and mapped optically and electrically. AF dynamics were studied under control and during perfusion of either chloroquine (4 μM, N=7) or flecainide (2–4 μM, N=5). Chloroquine increased rotor core size and decreased reentry frequency from 10.6±0.7 Hz in control to 6.3±0.7 Hz (p<0.005) just before restoring sinus rhythm (7/7). Flecainide had lesser effects on core size and reentry frequency than chloroquine and did not restore sinus rhythm (0/5). Specific IKr-blockade by E-4031 (N=7) did not terminate AF when frequency values were >8Hz. During pacing (N=11) flecainide reversibly reduced conduction velocity (~30% at cycle length 300, 250 and 200 ms, p<0.05) to a larger extent than chloroquine (11% to 19%, cycle length 300, 250 and 200 ms, p<0.05). Significant action potential duration prolongation was demonstrable only for chloroquine at CL 300 (12%) and 250 ms (9%) (p<0.05).
Conclusions
Chloroquine is more effective than flecainide in terminating SAF in isolated-sheep hearts by significantly increasing core size and decreasing reentry frequency. Chloroquine’s effectiveness may be explained by its inward-rectifier K+ channel blockade profile and suggest that reentry is important to maintain acute SAF.
Keywords: arrhythmia (mechanisms), atrial fibrillation, drug therapy, electrophysiology mapping
Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia in clinical practice.1 Although the mechanisms that sustain AF are incompletely understood, strong evidence in humans2 and in animal models3, 4 supports the hypothesis that high-frequency reentrant sources (rotors) are essential to maintain AF. It is possible to demonstrate discrete sites of high frequency periodic activity during AF, along with frequency gradients between left and right atria.3 Occasionally, a long-lasting rotor is identified within the area of maximum dominant frequency (DFmax).5
In the human heart, atrial dilatation and stretch predisposes to AF with DF values directly related to left atrial (LA) pressure.6 Similarly, in a sheep model of stretch-induced AF (SAF), the posterior left atrium (PLA) plays a major role in the maintenance of the arrhythmia.4
Less clear is the role of rotors in SAF, where focal discharges (FDs) and reentrant sources interact in such a way that FDs can destabilize and terminate rotors but also can give raise to new wavebreaks and rotor formation.5 The mechanism underlying FDs is uncertain with either triggered activity or reentry being possible; a more depolarized resting membrane potential (RMP) and the activation of stretch-activated non selective-cationic channels (SACs) enable the generation of after depolarizations7 that might explain FDs (breakthroughs) in the optically-mapped areas. However, FDs might also be the surface reflection of intramural reentrant sources.8
Inward-rectifier K+ currents (IK1, IK-Ach and IK-ATP) play important roles in controlling rotor dynamics.2, 9, 10 Recently, Noujaim et al have demonstrated that chloroquine blocks the pore-forming subunits Kir2.1, Kir3.1 and Kir6.2 responsible for the inward-rectifier K+ current (IK1), the acetylcholine-sensitive K+ current (IK-Ach) and the ATP-sensitive K+ current (IK-ATP), respectively.11 Interestingly, chloroquine depolarizes the RMP and increases automaticity, which can be explained by its blocking effects on IK1.12 The latter might increase FDs underlying a triggered activity mechanism.
We hypothesized that, if reentry plays a critical role sustaining SAF, then chloroquine should effectively restore sinus rhythm (SR) based on its ability to preferentially block inward-rectifier K+ currents. We therefore compared the effects of chloroquine with those of flecainide on SAF and excitation properties using optical mapping in Langendorff-perfused sheep hearts. The rationale for such a comparison was based on the fact that, similar to amiodarone13 and the most recently introduced drugs dronedarone14 and vernakalant,15 flecainide16 does not block IK1 within the therapeutic range of concentrations. Flecainide is more effective than amiodarone in early reversion of recent-onset AF17 (<48 h) and it is highly recommended in patients with no underlying structural heart disease.18 However our data demonstrate that chloroquine is more effective than flecainide in restoring SR in this model. Taken together, our results support the hypothesis that rotor activity underlies SAF and that blockade of inward-rectifier K+ currents may be a viable approach for its termination.
Methods
Experimental set up
All procedures were approved by the University of Michigan Committee on Use and Care of Animals (UCUCA) and complied with National Institutes of Health guidelines. Thirty 6 month-old sheep (weight: ~35 kg) were included in the study. Anesthesia was induced with 4–6 mg/Kg of propofol and 60–100 mg/Kg of sodium pentobarbital. Hearts were removed via thoracotomy and connected to a Langendorff perfusion system with re-circulating oxygenated (95% O2, 5% CO2) Tyrode’s solution at constant flow rate of 240–270 ml/min, pH 7.4 and 35.5–37.5 °C. The Tyrode’s composition (in mM) was: NaCl 130, KCl 4.0, MgCl2 1, CaCl2 1.8, NaHCO3 24, NaH2PO41.2, Glucose 5.6, and Albumin 0.04 g/L. Blebbistatin 10 μM was used to reduce the contractile force.
After atrial trans-septal puncture the intra-atrial pressure was increased to 14 cm H2O to induce continuous atrial stretch. Bipolar electrograms from each of the pulmonary veins (PVs), top and roof of the left atrial appendage (LAA) and right atrial appendage (RAA) were obtained (sampling rate, 1.0 kHz). All vein orifices were sealed except for the inferior vena cava, which was cannulated and used for controlling the level of the intra-atrial pressure.
Optical mapping movies (5 sec) were obtained using a Little Joe CCD camera (80×80 pixels, 500–1000 frames per second). After a bolus injection of 5 to 10 ml Di-4-ANEPPS (10 mg/mL) (Sigma-Aldrich) voltage-sensitive fluorescence was acquired from the RAA and LAA (area: ~14 cm2). In seven experiments epicardial mapping from the LAA was complemented with endocardial mapping of the PLA (area: ~3.7 cm2) using a dual-channel rigid borescope (see supplemental Methods and supplementary Figure 1).
Experimental Protocols
Baseline action potential duration at 70% repolarization (APD70) was optically measured on both atrial appendages (n=5) at progressively shorter pacing cycle lengths (CLs; 300–250–200 ms). The pacing electrode was placed on the top of the LAA. Mean APD70 was obtained for RAA and LAA surfaces by averaging APD70 from all pixels. AF was then induced via burst pacing (12 Hz) and allowed to continue for 15 min of baseline control. Thereafter, the antifibrillatory effects of either 4 μM chloroquine diphosphate (N=7) or 2 μM flecainide (N=5) (Sigma-Aldrich) dissolved in Tyrode’s solution were investigated. The concentrations were based on the highest therapeutic level for flecainide19 and chloroquine.20 Drug perfusion was maintained for 20 min, after which the concentration was doubled and maintained for 30 additional min, as long as SR was not already restored. AF was terminated by DC shock in those hearts in which AF persisted for 50 min. Burst pacing was subsequently applied after AF termination to re-induce AF. Finally, AF re-induction was attempted again after a washout period of 30 and 45 min for flecainide and chloroquine, respectively.
To better delineate the specific roles of IK1 versus IKr we conducted experiments (N=7) using the same stretch-induced AF model to study the antifibrillatory effects of specific IKr-blockade. After 15 min of baseline AF, 0.5–1 μM E-4031 was perfused for 20 and 30 min as above.21
An additional set of experiments was carried out to investigate the electrophysiological effects of 4 μM chloroquine (N=5) and 4 μM flecainide (N=6). Pacing protocols were completed under basal conditions, after 15 min of either chloroquine or flecainide, and after washout. No AF was induced in these hearts. Activation times were determined at 50% of action potential amplitude and conduction velocity (CV) was calculated.22 Supplemental Figures 2A and 2B illustrate the time-line of all experimental protocols.
Frequency analysis
Dominant frequency (DF) maps were obtained for each optical movie in AF after applying a fast Fourier transform (FFT) of the fluorescence signal recorded at each pixel.23 Bipolar electrograms recorded during AF were high-pass filtered at 3Hz and low-pass filtered at 35 Hz. FFT was also applied to the 5-sec bipolar signals synchronized with the optical movies.
Atrial fibrillation dynamics
Phase movies were constructed using the Hilbert transformation.24 In each movie, a rotor was defined as a point of phase-convergence (singularity point; SP) lasting more than one rotation. A breakthrough was defined as a wave appearing inside the field of view and propagating outward (supplemental Figure 3). During AF, 5-sec movies were acquired and analyzed frame by frame. Rotor analysis was carried out on the PLA and LAA by measuring the total number of rotations in 5 sec, regardless of the life-span of individual rotors, but considering their frequency of rotation. The number of breakthroughs in 5 sec was also quantified on the PLA, since PVs represent the region where FDs are identified in some cases of AF.25
Reentry meandering around SPs was analyzed further to quantify the respective effects of chloroquine and flecainide on the unexcited core size. A detailed description of the method is described in Figures 4–6 of the online supplement.
Statistical Analyses
Results are reported as mean±SEM. DF data were verified (Shapiro-Wilk) and other continuous measurements were assumed to distribute normally. One-way or two-way repeated measures ANOVA or a mixed model ANOVA was used for continuous measurements as appropriate. Fisher’s exact test was used to demonstrate significant differences on AF termination and re-inducibility. Post hoc comparisons were employed following a Bonferroni correction. P<0.05 was considered statistically significant.
Results
Stretch-induced AF in the control
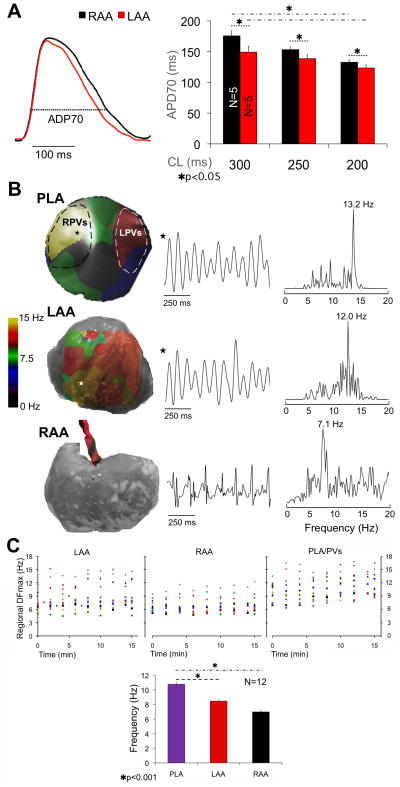
Figure 1A (left) shows examples of simultaneously obtained single pixel recordings from the LAA (red) and RAA (black) at 300 ms CL. As summarized graphically on the right, APD70 was significantly shorter in LAA than RAA (n=5) at three different CLs (300, 149.3±9.3 vs 175.7±8.2 ms; 250, 138.7±6.0 vs 153.3±4.5 ms; 200, 123.5±5.0 vs 132.8±3.6 ms, p<0.05).
Figure 1.
A, left, single pixel AP from the LAA (red) and RAA (black). Right, APD70 at varying pacing CLs is shorter in LAA than RAA. APD70 significantly shortens in both appendages from 300 to 200 ms CL. B, left column, DF maps of the PLA and LAA, and electrode location on the RAA. Center column; single pixel activations (PLA, LAA) and bipolar electrograms (RAA). Right Column, power spectra showing DFmax at each location. C, top, LAA, RAA and PLA/PVs DFmax during 15 min of control AF. Bottom, bar graph of mean±SEM DFmax during control AF shows significant DF gradient between PLA and LAA/RAA.
Once SAF stabilized, the DFmax locations were identified across the mapped regions. Figure 1B shows DF maps, sample local activations and corresponding power spectra from the PLA, LAA and RAA after 14 min of control AF in a representative heart. The DFmax was localized at the right PVs with a frequency gradient from PLA (13.2Hz) to LAA (12.0Hz) and RAA (7.1 Hz).
The regional DFmax was consistently localized either at one of the PVs or somewhere on the PLA (Figure 1C, top). As expected,4 a statistically significant frequency gradient was observed from PLA to LAA and RAA (Figure 1C, bottom) (10.8±0.3, 8.5±0.2 and 7.0±0.1 Hz, respectively. N=12, p<0.001).
Chloroquine terminates SAF
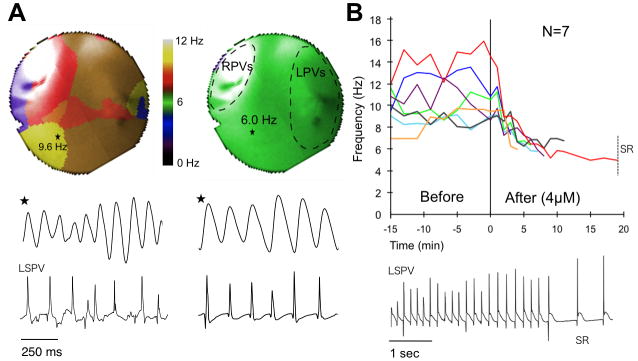
To test the hypothesis that reentry is essential to maintain SAF we determined the effect of 4 μM chloroquine in terminating AF, based on its ability to block the inward-rectifier K+ currents.11 As shown in Figure 2, 4 μM chloroquine reduced AF frequency and restored SR after 4 to 19 min (N=7). Figure 2A shows representative PLA DF maps in control AF (DFmax 9.6 Hz) and just before AF termination (DFmax 6 Hz). Single pixel optical activations from the DFmax region and electrograms from the left superior PV (LSPV) are shown below the maps. Figure 2B shows the time-course of the DFmax location in control AF and after chloroquine. The DFmax sharply decreased from 10.6±0.7 to 6.3±0.2 Hz (N=7) in the first few min after chloroquine onset.
Figure 2.
Chloroquine terminates AF. A, top: DF maps of PLA during control AF and before termination; middle and bottom: single pixel recordings and electrograms from LSPV. B, top: time course of DFmax, before and during chloroquine; bottom: electrograms from LSPV at the moment of termination.
Figure 4.
A, left, sustained AF/AT is re-inducible during flecainide but not during chloroquine perfusion. Right, after washout sustained AF/AT is re-inducible in both groups. B, representative traces; rapid pacing at 12 Hz did not capture or re-induce non-sustained AF during chloroquine (top); at the same pacing frequency, sustained AF was readily induced during flecainide (bottom). C, time-course of DFmax, SR is restored when chloroquine was perfused after flecainide washout.
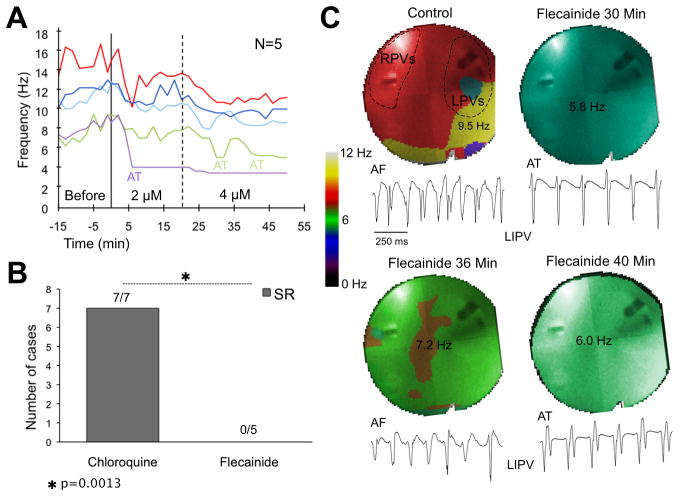
Five additional experiments were conducted using 1–2 μM chloroquine to increase the selectivity of IK1-blockade.12 At 1 μM, chloroquine terminated AF in 4 out of 5 AF episodes after 3.5 to 9.5 min. Increasing the concentration to 2 μM restored SR in the fifth AF episode after 14 min. The DFmax decreased from 10.5±0.8 Hz in control to 6.7±0.2 Hz before resumption of SR (supplementary Figure 8).
Flecainide does not terminate SAF
Na+-channel blockade may affect reentrant activity and rotor dynamics leading to AF termination.26 As illustrated in Figure 3A, after 15 min of control AF, perfusion with 2 μM flecainide for 20 min and 4 μM for 30 additional min significantly decreased the DFmax from 11.1±1.3 to 8.1±1.3 Hz (p=0.0014) but did not restore SR (N=5). As shown in Figure 3B, flecainide was significantly inferior to chloroquine in its ability to restore SR (0/5 vs. 7/7, respectively, p=0.0013). In two hearts the initial DFmax was slower than in the other three cases (8.0±0.1 vs. 13.1±1.8 Hz) and AF converted to atrial tachycardia (AT). Figure 3C shows DF maps and bipolar recordings from the PLA during control AF and after 4 μM flecainide. Interestingly, after 40 min flecainide, AF converted to AT, which was sustained by a long meandering rotor located in the roof/PLA-LAA junction (supplemental Movie 1).
Figure 3.
A, time-course of DFmax before and during flecainide. B, flecainide is significantly inferior to chloroquine in resuming SR. C, DF maps of the PLA and bipolar electrograms from the left inferior PV (LIPV) show control AF and conversion to AT during flecainide.
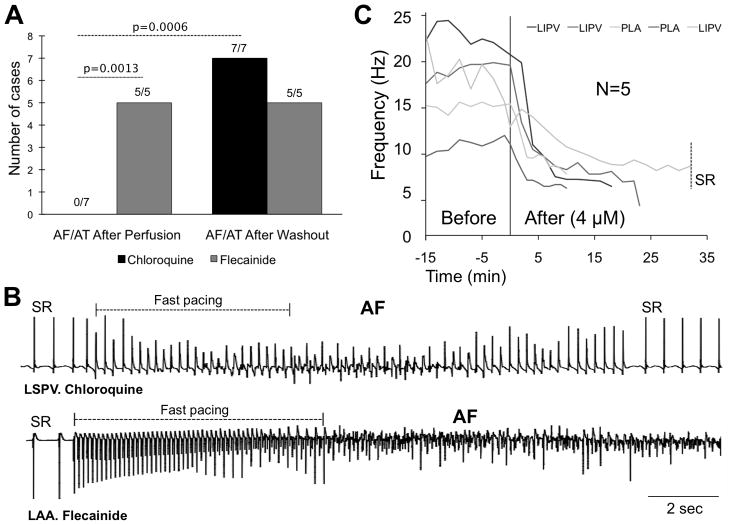
Sustained AF is inducible in the presence of flecainide but not chloroquine
As shown in Figure 4A, only non-sustained AF or AT was induced in the presence of chloroquine (N=7). Conversely, upon cardioversion, AF (N=4) or AT (N=1) was re-induced and sustained for 15 min in the presence of flecainide. After the washout period, AF (N=6, chloroquine. N=5, flecainide) or AT (N=1, chloroquine) was re-induced and sustained in both groups. In Figure 4B, the bipolar electrograms illustrate how initial rapid pacing in the presence of chloroquine was followed by a very brief AF episode, in contrast to sustained AF under flecainide.
Chloroquine terminates AF where flecainide does not
We determined the effects of chloroquine in the 5 experiments in which flecainide was unable to restore SR. Thus, AF was re-induced after 30 min of flecainide washout. Fifteen min of control AF were registered and optically mapped. Figure 4C shows the evolution of the DFmax on the PLA. DFmax values were significantly higher than at the beginning of the protocol (16.9±2.0 vs 11.1±1.3 Hz, p=0.018), which might be due to a decrease in optimal conditions of the heart after 3 hours of artificial perfusion. Higher DFmax may also imply a role of other inward-rectifiers K+ currents, possibly IK,ATP. Nevertheless, 4 μM chloroquine effectively terminated AF (N=5). Time to AF termination was longer (10–29 min) relative to the first application of chloroquine (4–19 min, Figure 2B).
Chloroquine’s effects on reentry and breakthroughs
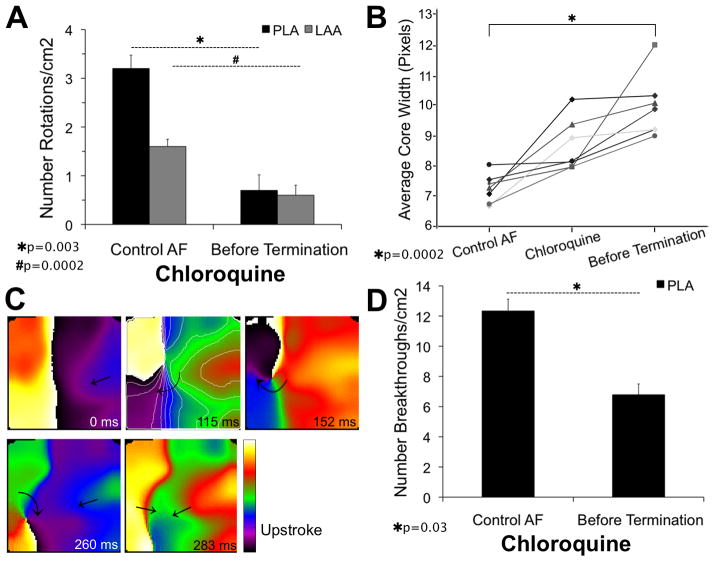
To determine the basis for chloroquine superiority we studied the effects of each drug on AF patterns of excitation. In Figure 5A, the number of identifiable rotations was quantified on the PLA and LAA every 2 min in control AF and every min after chloroquine. Data were normalized as number of rotations/cm2. In control AF, the PLA (N=2; n=16; where N is the number of animals and n is the number of episodes analyzed) harbored twice as many identifiable rotations/cm2 compared to LAA (N=7; n=56) (3.2±0.3 vs.1.6±0.1). On chloroquine, the last 2 min before termination showed a drastic reduction in the number of rotations in both PLA (3.2±0.3 to 0.7±0.3, p=0.003) and LAA (1.6±0.1 to 0.6±0.2, p=0.0002) (see also supplemental Figure 7A). Supplemental movies 2 and 3 show, respectively, PLA movies of short-lasting rotors (1–2 rotations) in control AF and during a short time before termination. Quantification of the core width using the new method illustrated in supplemental Figures 4–6, showed a significant increase before AF termination under chloroquine (Figure 5B). A larger core due to preferential IK1-blockade may reduce the probability of stable rotor initiation as shown in Figure 5C, where a rotor around a SP is observed after a wavebreak and curling of the wavefront. However the rotating front is annihilated by a new wavefront coming from the PLA.
Figure 5.
Chloroquine effects on reentry and breakthroughs (5-sec movies). A, number of rotations/cm2 in the PLA/LAA in control and just before chloroquine-induced AF termination. B, average core width significantly increases in size during chloroquine. C, phase maps show a wavebreak and the initiation of a rotor that cannot complete an entire rotation during chloroquine. D, number of breakthroughs/cm2 in control and during chloroquine.
In the stretched atrium, chloroquine’s effects on IK112 might increase automaticity and FDs, which may underlie breakthroughs in the field of view. However, the number of breakthroughs/cm2 decreased (12.3±0.8 to 6.8±0.7, p=0.03. Figure 5D) suggesting that such breakthroughs represented intramural reentrant activity. AF terminated despite the fact that just before termination the number of breakthroughs/cm2 was higher than the number of identifiable rotations/cm2 in control AF (6.8±0.7 vs. 3.2±0.3, respectively), which supports the essential role of reentrant activity to sustain SAF.
Reentry and breakthroughs under flecainide
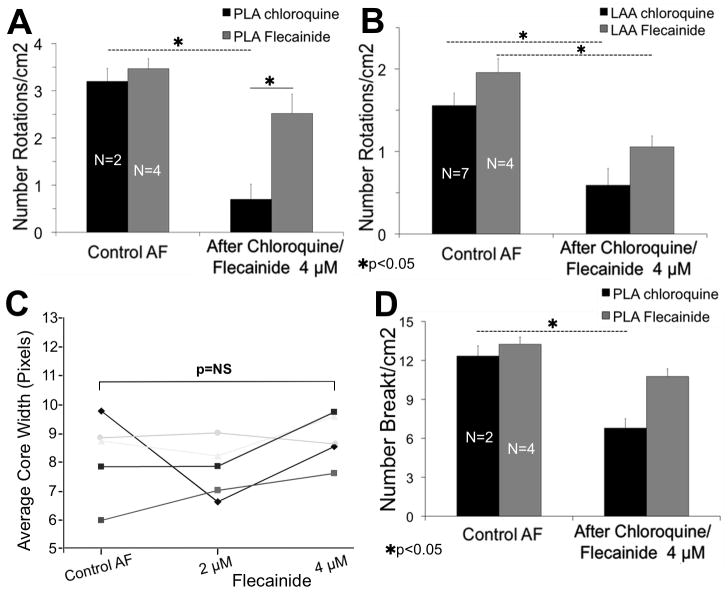
As shown in Figure 6A, B, the number of identifiable rotations decreased after 4 μM flecainide (N=4), both in the PLA (3.7±0.2 to 2.7±0.4, p=NS) and LAA (2.0± 0.2 to 1.1± 0.1, p<0.05) (see also supplemental Figure 7B and supplemental Movies 4, 5). However, only chloroquine significantly decreased the number of rotations and breakthroughs at the PLA (3.2±0.3 to 0.7±0.3 and 12.3±0.8 to 6.8±0.7, respectively, p<0.05. Figures 6A, D). The core size showed no significant increase after 4 μM flecainide (Figure 6C).
Figure 6.
Comparing chloroquine to flecainide. A and B, although flecainide reduces the number of rotations/cm2 at the PLA and LAA (5-sec movies), only chloroquine significantly decrease the number of rotations/cm2 at the PLA. C, the core size does not significantly increase during flecainide. D, Similarly, only chloroquine significantly decreases the number breakthroughs/cm2 at the PLA.
Electrophysiological effects of chloroquine and Flecainide
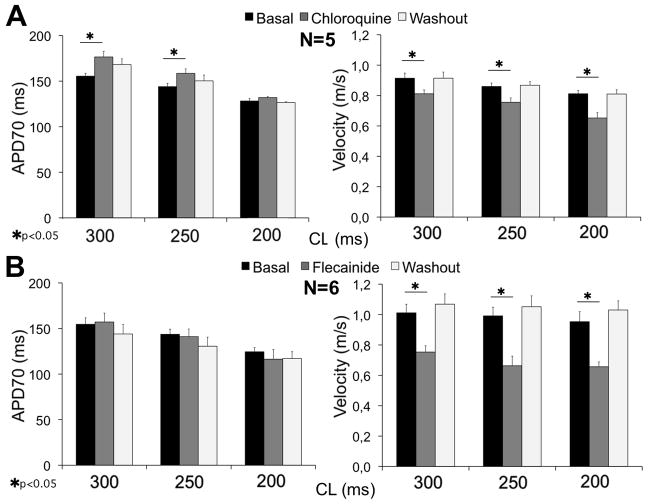
As summarized on the left side of Figure 7A, B, 4 μM chloroquine significantly increased the APD70 at 300 and 250 ms CL (155±3.1 to 176±6.0 and 144±3.4 to 158±5.0 ms, respectively, p<0.05) compared to 4 μM flecainide, which had no significant effect at any pacing CL (supplemental Figure 9). On the right side of Figure 7A, B, both chloroquine and flecainide decreased the CV at all CLs. However, flecainide’s effects were substantially greater than chloroquine; 33% reduction at 250 ms CL compared to 12% reduction, respectively.
Figure 7.
A, left, mean APD70 for control, chloroquine and washout at 300, 250, and 250 ms CL; right, mean CV for control, chloroquine and washout; 300, 250, and 250 ms CL. B, left: flecainide does not significantly affect the APD70 at any CL; right, flecainide strongly reduces velocity in a reversible manner.
Role of IKr in acute SAF
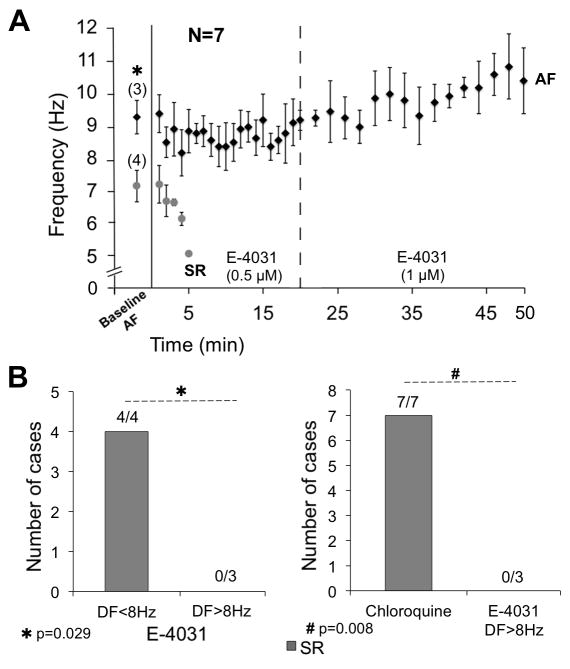
Chloroquine, while nominally an IK1 blocker, also blocks other currents, including IKr. An additional series of experiments (N=7) using the same AF model showed that IKr-blockade after 0.5–1 μM E-4031 restored SR in 4 out of 7 animals (Figure 8A). When we classified those experiments based on DFmax values at baseline (Figure 8B, left); E-4031 restored SR in those cases in which the AF DFmax was <8Hz (4/4), but not in cases with DFmax>8Hz (0/3) (7.1±0.4 vs 9.2±0.5 Hz, respectively, p=0.029). Unlike E-4031, chloroquine effectively terminated SAF when DFmax was >8 Hz (7/7 vs 0/3, p=0.008. Figure 8B, right).
Figure 8.
A, time-course of DFmax after E-4031 0.5–1 μM. B, E-4031 restored SR in those cases with DFmax<8 Hz. C, chloroquine was more effective than E-4031 to terminate SAF with DFmax>8 Hz.
Discussion
The present study demonstrates that chloroquine, but not flecainide, effectively converted SAF to SR in a sheep heart model that approximates AF in the human heart with pressure overload and atrial dilatation.6 SAF termination by chloroquine is preceded by a significant increase in rotor core size, which correlates with a reduction in AF frequency and number of breakthroughs. Altogether these data support the idea that reentry and fibrillatory conduction underlie the overall dynamics of SAF in this model. The Na+-channel blocker, flecainide converted AF to AT but did not restore SR. Preferential blockade of the inward-rectifier K+ currents by chloroquine11 terminated fast reentrant sources persisting after flecainide washout. In addition, unlike E-4031 chloroquine terminated AF episodes whose DFmax was >8Hz. Thus, even though E-4031 terminated some of the episodes, the above results demonstrate the superiority of chloroquine over E-4031 in terminating SAF. Nevertheless, chloroquine being a “dirty drug”,12 its antifibrillatory effect is likely not due solely to IK1-blockade but to some combination of IK1 and IKr-blockade, which would vary depending on the dose being used.
Since IK-ATP plays a prominent role in ischemia27 and IK,ACh underlies vagally-mediated AF,11 it seems reasonable to consider the role of IK1 in the present SAF model. Rotor acceleration and stabilization of the reentry have been associated with IK1 overexpression.9 In humans, gain of function of IK1 due to mutation in KCNJ2 is also associated with familial AF28.
Why is Chloroquine more effective than Flecainide in SAF?
The greater effectiveness of chloroquine over flecainide in terminating SAF in this model may be related to the fact that atrial dilatation decreases the CV and increases the degree of spatial heterogeneities in conduction.29 Therefore, under conditions of stretch, anatomical and functional obstacles may become prominent and facilitate initiation and maintenance of reentrant activity. Flecainide further decreases CV, which increases core size and promotes attachment to and rotation around an obstacle. It therefore results in slower rotating activity. This would explain the decrease in DF values and conversion to AT in the presence of flecainide. Under certain critical conditions, unexcitable obstacles may destabilize propagation, causing the formation of self-sustained vortices. Wavefront instabilities have been demonstrated after blockade of Na+-channel conductance leading to detachment of the wavefront from the edge of the functional/anatomical barrier and initiation of reentry.30
Controversies in SAF
The mechanisms sustaining acute SAF are controversial. Stretch induces depolarization of the RMP and generation of after depolarizations.7 Stretch promotes the occurrence of breakthroughs in the LAA,5 which might be interpreted as being produced either by local triggered activity or intramural reentry. In the present study, a higher number of breakthroughs compared to reentrant activity were also identified at the endocardial side of the PLA. However after either chloroquine or flecainide the number of breakthroughs decreased concomitantly with reentry, suggesting the possibility that the breakthroughs represent intramural reentry. More interesting is the role of chloroquine, which upon preferential blockade of the inward-rectifier K+ channels strongly affects reentry and terminates AF. Patterns of activation on the PLA before AF termination also show that the number of breakthroughs is higher than the number of rotors in control AF but the arrhythmia terminates. The latter suggests that reentrant activity is essential to sustain SAF and that intramural reentry in the PLA was responsible for the majority of those breakthroughs.
Clinical Perspective
Atrial dilatation and stretch predispose to AF with DF values directly related to LA pressure.6 Similar to the human heart,2 SAF in the sheep heart results in DF gradients, where DF at PLA > LAA > RAA. We demonstrate that a reentry-based strategy of blocking the inward-rectifier K+ channels effectively terminates SAF. Conversely, Na+-channel blockade by flecainide was unable to restore SR during atrial stretch, similarly to the loss of antifibrillatory effect that is observed also in patients with atrial dilatation.31
Although attractive, IK1-blockade is currently not a clinical strategy to treat AF because of the lack of anti-arrhythmic drugs having this property at therapeutic concentrations, along with the fear that reducing IK1 would cause diastolic depolarization and might subsequently increase the propensity for triggered arrhythmias.32 However, such a fear may be reduced by considering that heterozygous Andersen syndrome patients, who lack one allele of the KCNJ2 gene with loss-of-function of Kir2.1 channels, have mild QTc prolongation and seldom undergo sudden cardiac death.33 In addition, chloroquine does not induce early after depolarizations in single myocytes under current-clamp conditions, even at 10 μM.12 Finally, Noujaim et al demonstrated that rather than being pro-arrhythmic, IK1-blockade is antiarrhythmic as evidenced by chloroquine’s ability to terminate reentry in cholinergic induced-AF.11 Here we have used a well-established model,4 in which if at all present, the pro-arrhythmic effect of blocking IK1 should be more evident.5, 7 Clinical AF cases reported in the 50’s also demonstrated high rates of AF termination after high doses of chloroquine.34
Based on the expression profile of the Kir2.X subfamily, Kir2.3 transcripts are more concentrated in the human atrium than in the ventricles.35 Protein crystallization, molecular modeling and interactions with modulators (e.g. PIP2)36 might lead to new pharmacological strategies focusing on selective blockade of Kir2.3 subunits. The latter becomes especially relevant in the setting of congestive heart failure, in which IK1 downregulation may be associated with increased susceptibility to ventricular arrhythmias.37
Limitations
Although the chloroquine concentrations we have used preferentially block the inward-rectifier K+ channels, termination of AF might also be mediated in part through its less prominent effects on IKr, INa, and ICaL. Additional experiments with 1 μM chloroquine (IK1-blockade, 74%; IKr, 38%; INa,14%)12 and E-4031 support the role of preferential Ik1-blockade to terminate SAF. Further experiments would be required to rule out the possibility that the effectiveness of chloroquine in terminating SAF depends at least in part on a drug action on SACs.38 Stretch-activated K+ channels (TREK-1) might also be involved in the absence of APD prolongation after flecainide.
The antiarrhythmic effects of chloroquine need to be tested in persistent AF, in which IK1 up-regulation might be involved. In addition, the role of the autonomic system is difficult to mimic in isolated hearts. However in previous experiments using the same model and adreno-cholinergic stimulation, reentrant activity played a major role in the sustainability of the arrhythmia after abolishing triggered activity.
Conclusions
Chloroquine effectively terminates SAF in the sheep heart, and is more effective than flecainide in restoring SR, which may be explained by a preferential blockade of the inward-rectifier k+ channels and reentry termination. The results may open novel therapeutic strategies for clinical AF.
Supplementary Material
Acknowledgments
We thank Sherry Morgenstern and Iván Filgueiras-Rama for their help in the illustration of supplemental Figures.
Funding Sources: This work was supported in part by National Heart, Lung, and Blood Institute Grants [P01-HL039707 and P01-HL087226 to JJ and OB, and K99HL105574 to SN], the Leducq Foundation and CNIC (JJ and OB), a Spanish Society of Cardiology Fellowship, the Pedro Barrié de la Maza and Alfonso Martín Escudero Foundations (DFR), the Fédération Française de Cardiologie (RPM).
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: Population-based estimates. Am J Cardiol. 1998;82:2N–9N. doi: 10.1016/s0002-9149(98)00583-9. [DOI] [PubMed] [Google Scholar]
- 2.Atienza F, Almendral J, Moreno J, Vaidyanathan R, Talkachou A, Kalifa J, Arenal A, Villacastin JP, Torrecilla EG, Sanchez A, Ploutz-Snyder R, Jalife J, Berenfeld O. Activation of inward rectifier potassium channels accelerates atrial fibrillation in humans: Evidence for a reentrant mechanism. Circulation. 2006;114:2434–2442. doi: 10.1161/CIRCULATIONAHA.106.633735. [DOI] [PubMed] [Google Scholar]
- 3.Mandapati R, Skanes A, Chen J, Berenfeld O, Jalife J. Stable microreentrant sources as a mechanism of atrial fibrillation in the isolated sheep heart. Circulation. 2000;101:194–199. doi: 10.1161/01.cir.101.2.194. [DOI] [PubMed] [Google Scholar]
- 4.Kalifa J, Jalife J, Zaitsev AV, Bagwe S, Warren M, Moreno J, Berenfeld O, Nattel S. Intra-atrial pressure increases rate and organization of waves emanating from the superior pulmonary veins during atrial fibrillation. Circulation. 2003;108:668–671. doi: 10.1161/01.CIR.0000086979.39843.7B. [DOI] [PubMed] [Google Scholar]
- 5.Yamazaki M, Vaquero LM, Hou L, Campbell K, Zlochiver S, Klos M, Mironov S, Berenfeld O, Honjo H, Kodama I, Jalife J, Kalifa J. Mechanisms of stretch-induced atrial fibrillation in the presence and the absence of adrenocholinergic stimulation: Interplay between rotors and focal discharges. Heart Rhythm. 2009;6:1009–1017. doi: 10.1016/j.hrthm.2009.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshida K, Ulfarsson M, Oral H, Crawford T, Good E, Jongnarangsin K, Bogun F, Pelosi F, Jalife J, Morady F, Chugh A. Left atrial pressure and dominant frequency of atrial fibrillation in humans. Heart Rhythm. 2011;8:181–187. doi: 10.1016/j.hrthm.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamkin A, Kiseleva I, Wagner KD, Bohm J, Theres H, Gunther J, Scholz H. Characterization of stretch-activated ion currents in isolated atrial myocytes from human hearts. Pflugers Arch. 2003;446:339–346. doi: 10.1007/s00424-002-0948-0. [DOI] [PubMed] [Google Scholar]
- 8.Wellner M, Berenfeld O, Jalife J, Pertsov AM. Minimal principle for rotor filaments. Proc Natl Acad Sci U S A. 2002;99:8015–8018. doi: 10.1073/pnas.112026199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noujaim SF, Pandit SV, Berenfeld O, Vikstrom K, Cerrone M, Mironov S, Zugermayr M, Lopatin AN, Jalife J. Up-regulation of the inward rectifier k+ current (i k1) in the mouse heart accelerates and stabilizes rotors. J Physiol. 2007;578:315–326. doi: 10.1113/jphysiol.2006.121475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uchida T, Yashima M, Gotoh M, Qu Z, Garfinkel A, Weiss JN, Fishbein MC, Mandel WJ, Chen PS, Karagueuzian HS. Mechanism of acceleration of functional reentry in the ventricle: Effects of atp-sensitive potassium channel opener. Circulation. 1999;99:704–712. doi: 10.1161/01.cir.99.5.704. [DOI] [PubMed] [Google Scholar]
- 11.Noujaim SF, Stuckey JA, Ponce-Balbuena D, Ferrer-Villada T, Lopez-Izquierdo A, Pandit S, Calvo CJ, Grzeda KR, Berenfeld O, Chapula JA, Jalife J. Specific residues of the cytoplasmic domains of cardiac inward rectifier potassium channels are effective antifibrillatory targets. FASEB J. 2010;24:4302–4312. doi: 10.1096/fj.10-163246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanchez-Chapula JA, Salinas-Stefanon E, Torres-Jacome J, Benavides-Haro DE, Navarro-Polanco RA. Blockade of currents by the antimalarial drug chloroquine in feline ventricular myocytes. J Pharmacol Exp Ther. 2001;297:437–445. [PubMed] [Google Scholar]
- 13.Sato R, Koumi S, Singer DH, Hisatome I, Jia H, Eager S, Wasserstrom JA. Amiodarone blocks the inward rectifier potassium channel in isolated guinea pig ventricular cells. J Pharmacol Exp Ther. 1994;269:1213–1219. [PubMed] [Google Scholar]
- 14.Patel C, Yan GX, Kowey PR. Dronedarone. Circulation. 2009;120:636–644. doi: 10.1161/CIRCULATIONAHA.109.858027. [DOI] [PubMed] [Google Scholar]
- 15.Fedida D. Vernakalant (rsd1235): A novel, atrial-selective antifibrillatory agent. Expert Opin Investig Drugs. 2007;16:519–532. doi: 10.1517/13543784.16.4.519. [DOI] [PubMed] [Google Scholar]
- 16.Caballero R, Dolz-Gaiton P, Gomez R, Amoros I, Barana A, Gonzalez de la Fuente M, Osuna L, Duarte J, Lopez-Izquierdo A, Moraleda I, Galvez E, Sanchez-Chapula JA, Tamargo J, Delpon E. Flecainide increases kir2.1 currents by interacting with cysteine 311, decreasing the polyamine-induced rectification. Proc Natl Acad Sci U S A. 2010;107:15631–15636. doi: 10.1073/pnas.1004021107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donovan KD, Power BM, Hockings BE, Dobb GJ, Lee KY. Intravenous flecainide versus amiodarone for recent-onset atrial fibrillation. Am J Cardiol. 1995;75:693–697. doi: 10.1016/s0002-9149(99)80655-9. [DOI] [PubMed] [Google Scholar]
- 18.Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, Van Gelder IC, Al-Attar N, Hindricks G, Prendergast B, Heidbuchel H, Alfieri O, Angelini A, Atar D, Colonna P, De Caterina R, De Sutter J, Goette A, Gorenek B, Heldal M, Hohloser SH, Kolh P, Le Heuzey JY, Ponikowski P, Rutten FH, Vahanian A, Auricchio A, Bax J, Ceconi C, Dean V, Filippatos G, Funck-Brentano C, Hobbs R, Kearney P, McDonagh T, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Vardas PE, Widimsky P, Agladze V, Aliot E, Balabanski T, Blomstrom-Lundqvist C, Capucci A, Crijns H, Dahlof B, Folliguet T, Glikson M, Goethals M, Gulba DC, Ho SY, Klautz RJ, Kose S, McMurray J, Perrone Filardi P, Raatikainen P, Salvador MJ, Schalij MJ, Shpektor A, Sousa J, Stepinska J, Uuetoa H, Zamorano JL, Zupan I. Guidelines for the management of atrial fibrillation: The task force for the management of atrial fibrillation of the european society of cardiology (esc) Europace. 2010;12:1360–1420. doi: 10.1093/europace/euq350. [DOI] [PubMed] [Google Scholar]
- 19.Roden DM, Woosley RL. Drug therapy. Flecainide. N Engl J Med. 1986;315:36–41. doi: 10.1056/NEJM198607033150106. [DOI] [PubMed] [Google Scholar]
- 20.Frisk-Holmberg M, Bergqvist Y, Englund U. Chloroquine intoxication. Br J Clin Pharmacol. 1983;15:502–503. doi: 10.1111/j.1365-2125.1983.tb01540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spector PS, Curran ME, Keating MT, Sanguinetti MC. Class iii antiarrhythmic drugs block herg, a human cardiac delayed rectifier k+ channel. Open-channel block by methanesulfonanilides. Circ Res. 1996;78:499–503. doi: 10.1161/01.res.78.3.499. [DOI] [PubMed] [Google Scholar]
- 22.Bayly PV, KenKnight BH, Rogers JM, Hillsley RE, Ideker RE, Smith WM. Estimation of conduction velocity vector fields from epicardial mapping data. IEEE Trans Biomed Eng. 1998;45:563–571. doi: 10.1109/10.668746. [DOI] [PubMed] [Google Scholar]
- 23.Samie FH, Berenfeld O, Anumonwo J, Mironov SF, Udassi S, Beaumont J, Taffet S, Pertsov AM, Jalife J. Rectification of the background potassium current: A determinant of rotor dynamics in ventricular fibrillation. Circ Res. 2001;89:1216–1223. doi: 10.1161/hh2401.100818. [DOI] [PubMed] [Google Scholar]
- 24.Warren M, Guha PK, Berenfeld O, Zaitsev A, Anumonwo JM, Dhamoon AS, Bagwe S, Taffet SM, Jalife J. Blockade of the inward rectifying potassium current terminates ventricular fibrillation in the guinea pig heart. J Cardiovasc Electrophysiol. 2003;14:621–631. doi: 10.1046/j.1540-8167.2003.03006.x. [DOI] [PubMed] [Google Scholar]
- 25.Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Metayer P, Clementy J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 26.Kneller J, Kalifa J, Zou R, Zaitsev AV, Warren M, Berenfeld O, Vigmond EJ, Leon LJ, Nattel S, Jalife J. Mechanisms of atrial fibrillation termination by pure sodium channel blockade in an ionically-realistic mathematical model. Circ Res. 2005;96:e35–47. doi: 10.1161/01.RES.0000160709.49633.2b. [DOI] [PubMed] [Google Scholar]
- 27.Noma A. Atp-regulated k+ channels in cardiac muscle. Nature. 1983;305:147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- 28.Xia M, Jin Q, Bendahhou S, He Y, Larroque MM, Chen Y, Zhou Q, Yang Y, Liu Y, Liu B, Zhu Q, Zhou Y, Lin J, Liang B, Li L, Dong X, Pan Z, Wang R, Wan H, Qiu W, Xu W, Eurlings P, Barhanin J. A kir2.1 gain-of-function mutation underlies familial atrial fibrillation. Biochem Biophys Res Commun. 2005;332:1012–1019. doi: 10.1016/j.bbrc.2005.05.054. [DOI] [PubMed] [Google Scholar]
- 29.Eijsbouts SC, Houben RP, Blaauw Y, Schotten U, Allessie MA. Synergistic action of atrial dilation and sodium channel blockade on conduction in rabbit atria. J Cardiovasc Electrophysiol. 2004;15:1453–1461. doi: 10.1046/j.1540-8167.2004.04326.x. [DOI] [PubMed] [Google Scholar]
- 30.Cabo C, Pertsov AM, Davidenko JM, Baxter WT, Gray RA, Jalife J. Vortex shedding as a precursor of turbulent electrical activity in cardiac muscle. Biophys J. 1996;70:1105–1111. doi: 10.1016/S0006-3495(96)79691-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bollmann A, Binias KH, Toepffer I, Molling J, Geller C, Klein HU. Importance of left atrial diameter and atrial fibrillatory frequency for conversion of persistent atrial fibrillation with oral flecainide. Am J Cardiol. 2002;90:1011–1014. doi: 10.1016/s0002-9149(02)02690-5. [DOI] [PubMed] [Google Scholar]
- 32.Opthof T. Ik1 blockade is unlikely to be a useful antiarrhythmic mechanism. Cardiovasc Res. 1994;28:420. doi: 10.1093/cvr/28.3.420. [DOI] [PubMed] [Google Scholar]
- 33.Tristani-Firouzi M, Jensen JL, Donaldson MR, Sansone V, Meola G, Hahn A, Bendahhou S, Kwiecinski H, Fidzianska A, Plaster N, Fu YH, Ptacek LJ, Tawil R. Functional and clinical characterization of kcnj2 mutations associated with lqt7 (andersen syndrome) J Clin Invest. 2002;110:381–388. doi: 10.1172/JCI15183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burrell ZL, Jr, Martinez AC. Chloroquine and hydroxychloroquine in the treatment of cardiac arrhythmias. N Engl J Med. 1958;258:798–800. doi: 10.1056/NEJM195804172581608. [DOI] [PubMed] [Google Scholar]
- 35.Schram G, Pourrier M, Melnyk P, Nattel S. Differential distribution of cardiac ion channel expression as a basis for regional specialization in electrical function. Circ Res. 2002;90:939–950. doi: 10.1161/01.res.0000018627.89528.6f. [DOI] [PubMed] [Google Scholar]
- 36.Du X, Zhang H, Lopes C, Mirshahi T, Rohacs T, Logothetis DE. Characteristic interactions with phosphatidylinositol 4,5-bisphosphate determine regulation of kir channels by diverse modulators. J Biol Chem. 2004;279:37271–37281. doi: 10.1074/jbc.M403413200. [DOI] [PubMed] [Google Scholar]
- 37.Pogwizd SM, Schlotthauer K, Li L, Yuan W, Bers DM. Arrhythmogenesis and contractile dysfunction in heart failure: Roles of sodium-calcium exchange, inward rectifier potassium current, and residual beta-adrenergic responsiveness. Circ Res. 2001;88:1159–1167. doi: 10.1161/hh1101.091193. [DOI] [PubMed] [Google Scholar]
- 38.Kuijpers NH, Potse M, van Dam PM, ten Eikelder HM, Verheule S, Prinzen FW, Schotten U. Mechanoelectrical coupling enhances initiation and affects perpetuation of atrial fibrillation during acute atrial dilation. Heart rhythm. 2011;8:429–436. doi: 10.1016/j.hrthm.2010.11.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.