Abstract
Multiple myeloma (MM) is an incurable malignancy of plasma secreting B-cells disseminated in the bone marrow. Successful utilization of oncolytic virotherapy for myeloma treatment requires a systemically administered virus that selectively destroys disseminated myeloma cells in an immune-competent host. Vesicular stomatitis virus (VSV) expressing Interferon-β (IFNβ) is a promising new oncolytic agent that exploits tumor-associated defects in innate immune signaling pathways to specifically destroy cancer cells. We demonstrate here that a single, intravenous dose of VSV-IFNβ specifically destroys subcutaneous and disseminated 5TGM1 myeloma in an immune competent myeloma model. VSV-IFN treatment significantly prolonged survival in mice bearing orthotopic myeloma. Viral murine IFNβ expression further delayed myeloma progression and significantly enhanced survival compared to VSV expressing human IFNβ. Evaluation of VSV-IFNβ oncolytic activity in human myeloma cell lines and primary patient samples confirmed myeloma specific oncolytic activity but revealed variable susceptibility to VSV-IFNβ oncolysis. The results indicate that VSV-IFNβ is a potent, safe oncolytic agent that can be systemically administered to effectively target and destroy disseminated myeloma in immune competent mice. IFNβ expression improves cancer specificity and enhances VSV therapeutic efficacy against disseminated myeloma. These data show VSV-IFNβ to be a promising vector for further development as a potential therapy for treatment of Multiple myeloma.
Keywords: Oncolytic, virotherapy, myeloma, Vesicular stomatitis virus, systemic
Introduction
Multiple myeloma (MM) is a disseminated malignancy of terminally differentiated plasma cells residing primarily in the bone marrow. The disease is associated with bone destruction, anemia and compromised immune function(1). Current treatments for MM, including stem cell transplantation and high-dose chemotherapy, have significantly prolonged median survival to 6 years, but still fall short as a cure. There is a clear need for the development and testing of novel treatment options that can be delivered intravenously to reach and destroy disseminated disease sites(2).
Vesicular stomatitis virus (VSV) is a non-segmented, negative strand RNA virus of the Rhabdoviridae family that selectively kills malignant cells and has demonstrated oncolytic activity in various preclinical cancer models (3). VSV infections normally occur in livestock, causing only mild symptoms during naturally occurring human infections (4). Lethal infection of neural tissue in animal models prompted the use of viral engineering to attenuate VSV and minimize toxicity (5, 6). Detection of viral infection in cells results in activation of Interferon regulatory factors (IRFs), and expression of Type I Interferons (IFN) that can bind the α/β interferon receptor (IFNAR). IFNAR activation induces cellular innate immune responses by stimulating production of proteins that facilitate viral clearance by degrading viral intermediates, inhibiting translation, inducing apoptosis and activating release of pro-inflammatory cytokines(7, 8). The Type I Interferons include several IFNα subtypes encoded by 14 IFNα genes and four IFNα pseudogenes, and IFNβ, which is encoded by a single gene(9). While there have been differences described in receptor activation by IFNα and IFNβ(10), IFNβ has been described to be expressed early upon viral detection, inducing IRF7 expression that activates further expression of the various IFNα genes(9). Of the five genes encoded by the VSV genome, the VSV Matrix protein (M) facilitates evasion of innate immunity by blocking nucleocytoplasmic transport of mRNA preventing synthesis of IFN and other antiviral or pro-inflammatory proteins (11, 12). Aberrations leading to tumorigenesis also diminish innate immune response pathways thereby rendering cancer cells susceptible to VSV replication and oncolysis(8). VSV attenuation can be achieved by deleting (or mutating) residue 51 of the M protein (VSV-MΔ51) to reverse viral suppression of IFN synthesis(13) or by incorporation of the IFNβ gene into the viral genome(14), inducing IFNβ expression and activation of downstream antiviral genes including IFNα, to promote viral clearance from non-cancerous tissues. Cancer cells that are weakly responsive to IFN remain permissive to viral propagation and oncolysis.
The potential of VSV as a novel myeloma therapy was assessed in the immune competent 5TGM1 model of MM. The 5TGM1 murine myeloma cell-line can be implanted in C57Bl/KaLwRij syngeneic mice to form rapidly growing subcutaneous tumors or injected intravenously to induce orthotopic myeloma that can be monitored by measuring IgG2b paraprotein secreted by myeloma cells (15). This allows novel MM therapies to be tested in model that closely resembles disseminated myeloma in patients in the presence of intact anti-viral innate and adaptive immune responses.
While VSV-MΔ51 has enhanced tumor specificity by inducing IFN production, viral replication is significantly compromised even in IFN-resistant cells (16). Having previously demonstrated that recombinant VSV-MΔ51 coding for the sodium iodide symporter (NIS) has weak anti-myeloma activity(17), we hypothesized VSV coding for Interferon-β (VSV-IFNβ) would specifically destroy cancer cells in vivo while maintaining viral potency. IFNα has historically been used as therapy against myeloma, albeit with weak and variable efficacy and has reported to have various anti-tumor properties including direct pro-apoptotic activity, promotion of long-lasting anti-tumor immunity and inhibition of tumor blood vessel formation (18–20). IFNβ however, has been utilized extensively in various oncolytic viral vectors including adenovirus(21), vaccinia(22) and measles viruses (23), demonstrating that viral vectors expressing IFNβ can be safely and successfully utilized for the treatment of cancer. VSV expressing IFNβ has also previously shown antitumor efficacy in preclinical cancer models including renal cell carcinoma(14) and malignant pleural mesothelioma(24), while safety studies indicates viral IFNβ expression successfully alleviates VSV neurotoxicity(5), making VSV-IFNβ a potentially potent and safe vector for systemic treatment of Multiple myeloma.
Here we report the potent oncolytic activity of intravenously administered VSV-IFNβ in an immune competent model of MM. VSV is able to specifically target tumor sites in vivo inducing destruction of myeloma cells in subcutaneous tumors and within the bone marrow. Destruction of disseminated orthotopic myeloma resulted in transiently reduced and subsequently delayed disease burden and prolonged survival. Therapeutic efficacy was achieved in the presence of robust antiviral antibody response with no detectable toxicity. We further demonstrate that VSV expressing murine IFNβ shows significantly enhanced therapy in mice bearing disseminated myeloma making VSV-IFNβ a strong candidate as a potential new therapeutic vector for the treatment of myeloma.
Results
VSV expressing IFNβ has enhanced cancer specificity without viral attenuation
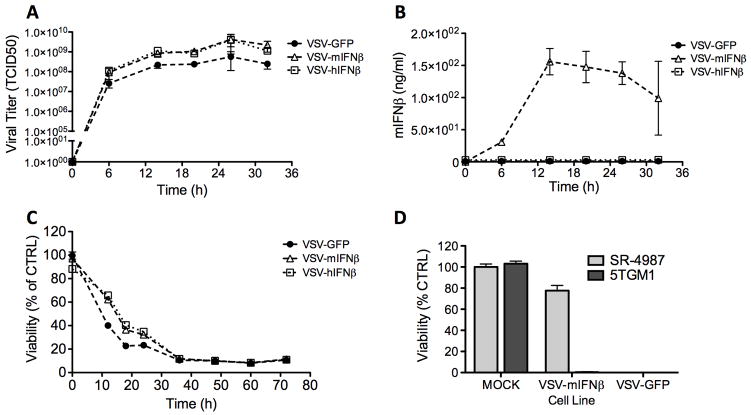
Recombinant VSV coding for IFNβ (VSV-IFNβ) was generated by incorporation of the IFNβ gene cDNA into the previously constructed pVSV-XN2 plasmid containing the full-length positive strand VSV antigenome (14). PCR generated IFNβ cNDA was inserted into a unique restriction site between the viral G and L genes coding for the viral surface glycoprotein and polymerase protein respectively. Functional virus was recovered as previously described(25). Newly generated VSV-IFNβ vectors, containing transgenes coding for either murine IFNβ (VSV-mIFNβ) or human IFNβ (VSV-hIFNβ), had similar growth characteristics to VSV-GFP (VSV expressing GFP) in BHK (baby hamster kidney) cells, a cell line that is not responsive to either murine or human IFNβ (not shown). VSV oncolytic efficacy against myeloma was initially assessed in vitro indicating VSV-GFP, VSV-mIFNβ and VSV-hIFNβ viruses replicate rapidly in 5TGM1 murine myeloma cells following infection at multiplicity of infection (MOI) 3.0 (Fig 1A). VSV-mIFNβ infection induced expression of murine IFNβ (Fig 1B), which did not impede VSV oncolysis of 5TGM1 cells compared to VSV-hIFNβ and VSV-GFP (Fig 1C). Evaluation of VSV-IFNβ specificity indicated VSV-mIFNβ kills 5TGM1 myeloma cells but has diminished cytotoxicity in SR-4987 immortalized bone marrow stromal cells. VSV-GFP however kills both cell types effectively in vitro (Fig 1D). Overall these results demonstrate ability of VSV-IFNβ to target and kill 5TGM1 myeloma cells while exhibiting enhanced specificity in vitro with diminished potency in noncancerous murine bone marrow stromal cells.
Figure 1. Characterization of recombinant VSV-IFN in vitro.
5TGM1 cells were infected at with a Vesicular stomatitis virus (VSV) coding for GFP, murine IFNβ (VSV-mIFN) or human IFNβ (VSV-hIFN) at multiplicity of infection (MOI) 3.0. Cells were analyzed at specific time points to measure (A) viral replication by measuring viral titer in supernatant of infected cells (B) Viral murine IFNβ expression by ELISA analysis of cell supernatant, and (C) cell viability by MTT assay. (D) VSV-IFN specificity was evaluated by comparing viability of 5TGM1 versus SR4987 murine bone marrow stromal cells at 48h following mock infection or infection with VSV-mIFNβ or VSV-GFP (MOI 3.0).
VSV-IFNβ has potent therapeutic efficacy against MM in immune competent mice
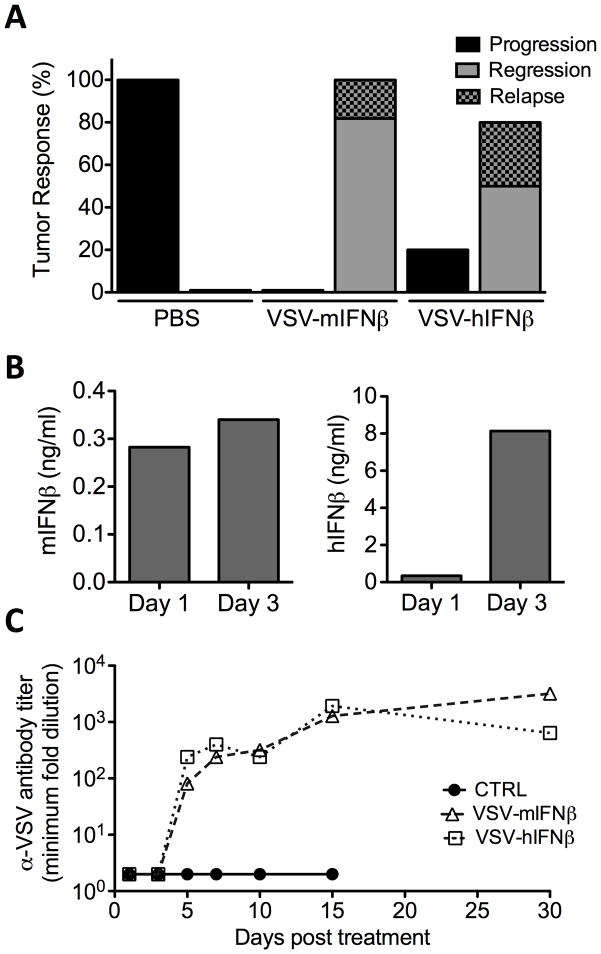
Safety studies with VSV-IFNβ have previously demonstrated that viral expression of host-specific IFNβ diminished toxicity in preclinical cancer models (26). VSV-IFNβ oncolytic efficacy against MM was assessed in vivo in C57Bl/KaLwRij bearing syngeneic 5TGM1 subcutaneous myeloma tumors. Mice were administered with a single, intravenous dose of 1×108 TCID50 VSV-mIFNβ, VSV-hIFNβ or 100ul PBS via tail vein injection. Tumor responses were monitored and categorized into tumor progression, tumor regression or regression followed by relapse as shown (Fig 2A). All mice treated with PBS had rapid tumor progression and were sacrificed. 100% of tumors regressed in response to VSV-mIFNβ treatment, while 80% of tumors regressed following VSV-hIFNβ treatment, with no associated symptoms of neurotoxicity. These tumor responses correlated with significantly prolonged survival of mice treated with VSV-mIFNβ (**P=0.0018) or VSV-hIFN (*P=0.04) compared to PBS treated mice using log-rank survival comparisons (data not shown). In vivo production of murine or human IFNβ was confirmed by analysis of serum from treated mice at days 1 and 3 post VSV administration by ELISA (Fig 2B). A small proportion of mice with regressing tumors relapsed and mice were eventually sacrificed due to tumor burden (Fig 2A).
Figure 2. VSV-IFN oncolytic activity against 5TGM1 myeloma tumors in syngeneic immune competent mice.
Female, 6–8 week old C57Bl/KaLwRij mice bearing subcutaneous syngeneic 5TGM1 myeloma tumors (~200mm3) were treated with single intravenous (IV) dose of 100ul PBS, or 1×108TCID50 VSV-mIFNβ or VSV-hIFNβ. (A) Tumor responses following IV treatment was monitored by serial caliper measurements and categorized into tumor progression (black), regression (grey), or regression with relapse (shaded grey). Tumor responses are shown as percentage of mice in each treatment group. (B) In vivo IFNβ expression following treatment with VSV-mIFNβ (red) or VSV-hIFNβ (blue) was measured in serum harvested at day 1 and 3 post treatment assayed by ELISA for murine IFNβ or human IFNβ respectively. (C) α-VSV neutralizing antibodies in serum of mice treated with PBS (black), VSV-mIFNβ (red) or VSV-hIFNβ (blue). Serial 2-fold dilutions of heat-inactivated serum pre-incubated with VSV-GFP and subsequently used to infect Vero cells. Measured antibody titer is the minimum serum dilution that fails to protect against VSV induced CPE. Serum was harvested from n=2 mice per groups at each time point, except where only one mouse was surviving in the treatment group.
Adaptive immune responses against systemically administered virus in the form of neutralizing antibodies are a significant barrier to effective oncolytic virotherapy (27, 28). VSV especially is a highly immunogenic virus (29). Neutralizing antibodies were detected in serum by day 5 post-treatment with no significant difference in antibody titer generated between VSV-mIFNβ or VSV-hIFNβ treated mice (Fig 2C). No anti-VSV neutralizing antibodies were detected in PBS treated mice. Overall these data indicate that systemically administered VSV-IFNβ has potent and specific activity against myeloma tumors in vivo in immune competent mice. At this dose level, VSV-IFNβ demonstrates oncolytic efficacy with no associated toxicity, regardless of murine IFNβ expression. The transient expression of detectable IFNβ and rapid generation of neutralizing antibodies against VSV-IFNβ suggests that tumor regression occurs in response to a limited period of viral activity before antibody mediated viral clearance, prompting further analysis of early tumor specific viral activity.
VSV-mIFNβ specifically and rapidly destroys myeloma tumors
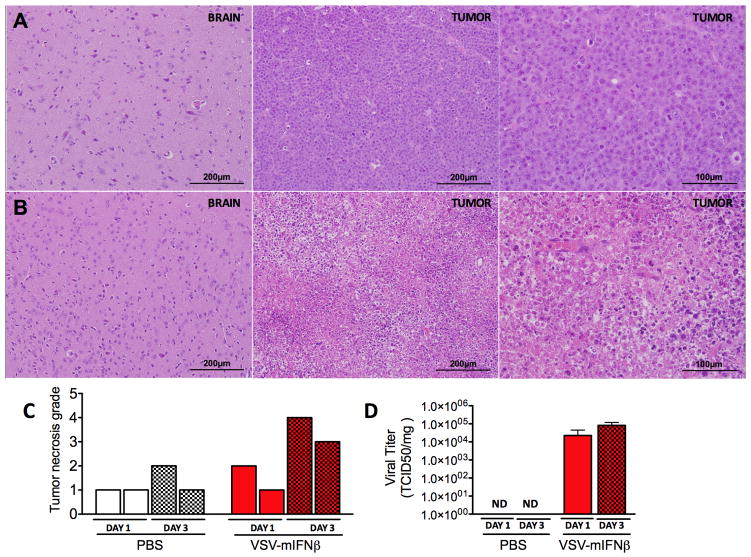
VSV-IFNβ activity in vivo was further evaluated by analysis of tumors and brains harvested from mice following intravenous virus treatment. Brain tissues were analyzed to monitor potential VSV toxicity, which proceeds by infection of neural tissues (6, 30). Portions of tumors and brains from PBS and VSV-mIFNβ treated mice were sectioned and stained using hematoxylin and eosin (H&E). Histopathological analysis of harvested tumors showed extensive necrosis in tumors from VSV-mIFNβ treated mice, with low to no necrosis in PBS treated mice (Fig 3A–B). Degree of tumor necrosis was quantified indicating increased tumor cell death at day 3 post VSV-mIFNβ treatment (Fig 3C). To confirm tumor destruction was associated with viral oncolysis, supernatants from processed tumors were titered showing viral load of approximately 1×105 TCID50 per mg of tissue in VSV-mIFNβ treated mice and 1×103 TCID50 per mg of tissue in VSV-hIFNβ treated mice (Fig 2C). While the differences are not significant, tumor specific viral burden and degree of tissue necrosis increased from day 1 to day 3 post viral administration suggesting that VSV is able to effectively undergo both viral replication and by 72h induce cell death in >50% of tumor cells. There were no indications of necrosis or inflammation, or virus recovered from brains of VSV-IFNβ treated mice (Fig 3A and data not shown).
Figure 3. Tumor necrosis and virus recovery following systemic delivery of VSV-mIFNβ.
Representative appearance of brain and tumor tissues harvested from mice at day 3 post IV treatment with (A) PBS or (B) 1×108 TCID50 VSV-mIFNβ. Tissues were harvested, paraffin-embedded and processed for H&E. Images are shown at 200x or 400x magnification (C) Quantification of tumor necrosis from H&E tumor sections from n=2 mice culled at day 1 (unshaded) or day 3 (shaded) following IV treatment with PBS (white) or VSV-mIFNβ (red). Degree of necrosis was measured using 4-point scale 1: <25% tissue necrosis 2: 25–50 tissue necrosis, 3: 50–75% tissue necrosis, 4: >75% tissue necrosis. (D) Viral load in tumor harvested at day 1 (unshaded) and day 3 (shaded) post IV PBS or VSV-mIFNβ treatment. Tumor specific viral load was measured by maceration of tumors and measurement of viral titer in supernatant of tumor suspensions. Results are plotted as viral titer per mg of tissue.
Of note, histopathological analysis revealed minimal to negligible infiltration of immune cells at the site of tumor at days 1 and 3 post treatment suggesting neither the presence of virus nor viral destruction of tumor cells stimulated early infiltration of inflammatory immune cells. Analysis of tumors at day 15 post PBS, VSV-mIFNβ or VSV-hIFNβ administration indicated slight mixed inflammatory infiltrate including macrophages and lymphocytes in tumors treated with VSV-mIFNβ (Table 1). This suggests murine IFNβ expression may enhance late infiltration of immune cells at tumor site. These data collectively indicate that systemically administered VSV-IFNβ localizes to site of tumor to induce tumor destruction. There is no observed toxicity and no detectable virus in brain demonstrating VSV-IFNβ is can be safely administered intravenously at this dose level.
Table 1. Tumor inflammation in response to systemic VSV-IFN treatment.
Inflammatory infiltrate in tumors processed for H&E 15 days post IV PBS, VSV-mIFNβ or VSV-hIFNβ administration. Inflammatory infiltrate are categorized as none, minimal or slight for mixed infiltrate including fibroblasts, macrophages and lymphocytes
| Treatment | Days post treatment | Inflammatory Infiltrate | |
|---|---|---|---|
| PBS | 15 | M18 | No significant inf. |
| VSV-mlFNβ | 15 | M10 | Slight mixed inf. |
| M45 | Slight mixed inf. | ||
| VSV-hlFNβ | 15 | M37 | No significant inf. |
| M40 | No significant inf. | ||
VSV-IFNβ has potent therapeutic efficacy against disseminated myeloma
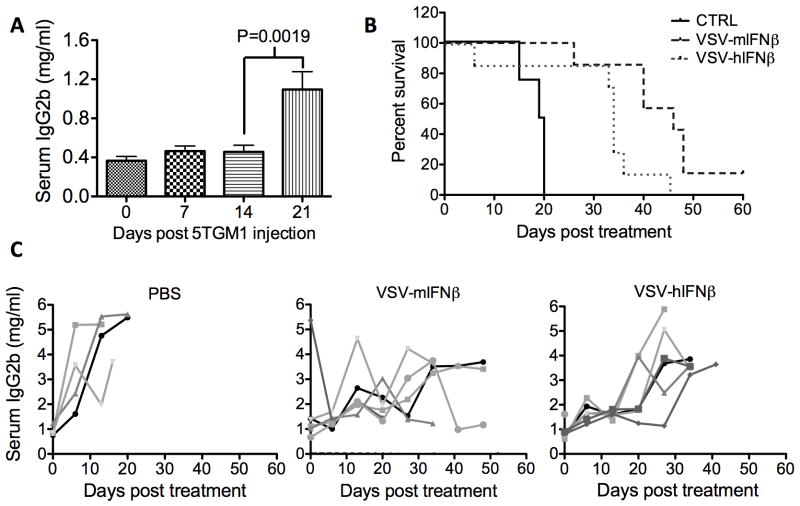
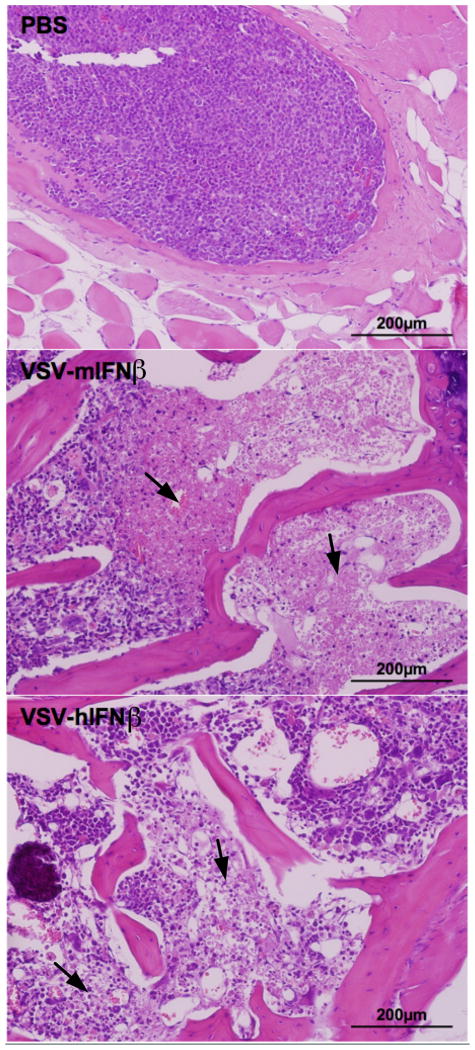
The potent efficacy of systemically administered VSV-IFNβ against subcutaneous myeloma tumors prompted studies to evaluate ability of VSV-IFNβ to reach and destroy disseminated myeloma. Orthotopic myeloma was established in mice by intravenous implantation of 5TGM1 cells. 5TGM1 myeloma cells, like myeloma in patients, secrete monoclonal antibodies, specifically an IgG2b serum paraprotein. Myeloma burden was assessed by IgG2b ELISA as described previously (17). ELISA quantification of serum IgG2b showed a significant increase by day 21-post 5TGM1 injection indicative of increasing myeloma burden (Fig 4A, **P=0.0019). Myeloma burden and survival were monitored following PBS, VSV-mIFNβ or VSV-hIFNβ treatment. PBS treated mice had rapidly progressing myeloma and were sacrificed due to weight loss or hind limb paralysis. A single intravenous dose of 5×107 TCID50 VSV-mIFNβ or VSV-hIFNβ delayed myeloma progression and significantly prolonged survival of myeloma bearing mice (Fig 4B, C). Analysis of spine and femur sections harvested from myeloma bearing mice at 48h post treatment showed foci of myeloma cells packed in the bone marrows of PBS treated mice, while bone marrows of VSV-IFNβ treated mice contained regions of necrosis indicating that VSV-IFNβ could reach and destroy disseminated foci of myeloma within the bone marrow (Fig 5). There was no appreciable inflammatory infiltrate within the bone marrow of either PBS, VSV-mIFNβ or VSV-hIFNβ treated mice at 48h post treatment. We further observed that systemic VSV-mIFNβ treatment significantly prolonged survival of mice bearing disseminated myeloma compared to VSV-hIFNβ treated mice, with one mouse being completely cured of systemic disease (*P=0.021). The basis of IFNβ induced enhancement of oncolytic efficacy in mice with disseminated myeloma is as yet unknown though hypotheses include direct anti-myeloma activity, IFNβ effects on the bone marrow microenvironment and potentially IFNβ induced anti-tumor immune responses.
Figure 4. VSV-IFN therapeutic efficacy in mice bearing orthotopic myeloma.
5TGM1 cells were implanted IV in syngeneic C57Bl/KalWrij mice. (A) Mean serum IgG2b concentration was quantified at 7 day intervals (n=26 mice). Mean serum IgG2b was measured by ELISA and compared using a t-test indicating a significant change in serum IgG2b from day 14 to day 21 post 5TGM1 implantation (*P=0.0019) (B) Survival response in myeloma bearing mice following a single IV dose of PBS, or 1×108 VSV-mIFNβ or VSV-hIFNβ. Survival was compared by log-rank analysis. VSV-mIFNβ and VSV-hIFNβ treatment significantly prolonged survival compared to PBS (P=0.0008*** and P=0.017* respectively), and survival of mice treated with VSV-mIFNβ is significantly prolonged compared to VSV-hIFNβ treated mice (P=0.021*) (C) Myeloma burden in response to IV treatment with PBS, VSV-mIFNβ or VSV-hIFNβ measured by serum IgG2b ELISA.
Figure 5. VSV-IFN oncolysis of orthotopic 5TGM1 myeloma.
Bone marrows harvested 48h post IV PBS, VSV-mIFNβ or VSV-hIFNβ administration processed for H&E (magnification 200x). Arrows indicate regions of cellular necrosis.
VSV coding for IFNβ has variable oncolytic activity in myeloma cells
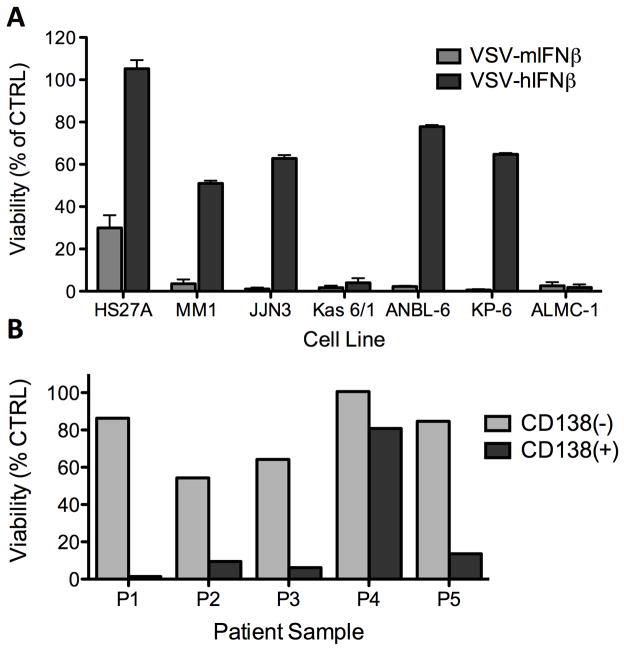
Systemically administered VSV-IFNβ has demonstrated potent in vivo efficacy against both subcutaneous and disseminated myeloma indicating VSV-IFNβ is a promising oncolytic candidate for myeloma therapy. These studies, however, have been limited to evaluation of viral oncolytic activity in 5TGM1 cells and in mice bearing 5TGM1 myeloma. The use of VSV-IFNβ as a potential anti-myeloma therapy will depend the ability of VSV-IFNβ to exert oncolytic activity on various types of myeloma. A panel of human myeloma cell lines was infected with VSV-mIFNβ or VSV-hIFNβ and cell viability was measured 72h later. This data shows firstly, that VSV-mIFNβ is potently cytopathic against all myeloma cell lines in the selected panel including HS27A, a human bone marrow stromal cell line (Fig 6A). HS27A cells however were relatively resistant to VSV-hIFNβ oncolysis indicating viral expression of human IFNβ suppresses VSV induced cell death in responsive human bone marrow stromal cells. Human IFNβ production also diminished VSV oncolytic activity in four of the six cell lines in the myeloma panel indicating these cells were responsive to IFNβ production albeit weakly when compared to non-cancerous bone marrow stromal cells (Fig 6A). These data indicate oncolytic activity of VSV-IFNβ may be diminished in myeloma cells that retain responsiveness to IFN. VSV-IFNβ antimyeloma activity was also assessed in primary myeloma cell lines. Viral oncolytic activity and specificity are gauged by measuring ability of VSV-hIFNβ to kill five primary patients samples sorted into CD138+ myeloma cells and CD138- non-myeloma cells. Cell death following infection was measured by staining cells with a dead cell marker and monitored using flow cytometry. In four out of five samples, >80% of CD138(+) myeloma cells were killed. In comparison, CD138(−) cells were resistant to VSV-hIFNβ oncolysis (Fig 6B). These results demonstrate the specific activity of VSV-hIFNβ against myeloma cell lines and primary myeloma cells. Viral human IFNβ expression enhances specificity of VSV oncolytic activity demonstrated by the diminished ability of VSV-hIFNβ to kill human bone marrow stromal cells. Human IFNβ expression in human myeloma cells may however potentially attenuate VSV-IFNβ oncolytic activity depending on how significantly innate immune pathways are impaired in cancer cells and resultant responsiveness to IFN induction. Overall the results support the hypothesis that IFNβ expression enhances specificity VSV, exerting cytopathic activity against various types of human myeloma cell lines and in primary myeloma cells, with diminished activity in non-cancerous bone marrow stromal cells and CD138 (−) cells.
Figure 6. In vitro activity of recombinant VSV-IFN in human myeloma cell lines.
(A) Variable cytopathic activity of VSV-mIFNβ vs. VSV-hIFNβ in a panel of human myeloma cell lines. Oncolytic activity was determined by MTT assay at 72h post infection of myeloma cell lines at MOI 1.0. Results are plotted as percentage of mock-infected cells (B) VSV-hIFNβ cytopathic activity in primary myeloma samples from 5 separate patients. Patient bone marrow aspirates sorted into CD138(+) myeloma (grey) and CD138(−) non myeloma cells (shaded) infected with VSV-hIFNβ (MOI 1.0). Cell viability was assessed at 48h post infection by flow cytometry analysis.
Discussion
Viral attenuation is utilized to decrease potential toxicity of oncolytic viral cancer therapies(8, 31). Clinical studies testing oncolytic viruses have demonstrated limited therapeutic efficacy of systemically administered viral treatments indicating viral engineering utilized to enhance tumor specificity also debilitate viral replication and oncolysis(32). The use of VSV-IFNβ as a potential anticancer agent allows utilization of a viral vector with intact and functional viral genes that produces exogenous IFNβ. The results demonstrate VSV-IFNβ retains robust viral replication and oncolytic potency in 5TGM1 myeloma cells while exhibiting diminished ability to kill non-cancerous cells.
There are various in vivo barriers to effective systemic oncolytic virotherapy. These include off-target sequestration, complement inactivation, cellular innate immunity, neutralizing antibodies, and physical barriers such as tumor stroma that prevent effective viral propagation and spread (7, 33–35). In our studies, therapeutic efficacy is achieved following a single intravenous dose of VSV-IFNβ in an immune competent myeloma mouse model. This data indicate that sufficient virions of systemically administered VSV-IFNβ reach the tumor site, selectively propagate within and kill myeloma cells to exert potent and specific antitumor activity. The absence of neurotoxicity indicates insubstantial off-target replication at this dose of VSV-IFNβ, though previous studies have demonstrated that IFNβ expression alleviates viral toxicity at high doses (26). The potent tumor specific activity of intravenous VSV-IFNβ against myeloma tumors warrants further investigation into the processes and roles of viral extravasation, propagation and virus induced cell death in mediating successful tumor destruction.
Our results demonstrate that VSV expressing murine IFNβ induction delays myeloma progression and improves survival compared to VSV expressing human IFNβ that is not functional in mouse tissues. This indicates that while IFNβ can alleviate potential VSV neurotoxicity, it also enhances VSV antitumor efficacy in myeloma bearing immune competent mice. It will be important to further understand the basis of IFNβ induced enhancement of therapeutic efficacy in mice with disseminated myeloma. IFN has been used as a therapy for treatment of myeloma and tested against other malignancies with limited success (18) and viral IFNβ expression may exert direct antitumor effects on myeloma cells or the bone marrow microenvironment. Tumor histology indicates murine IFNβ expression does not affect early but may promote late infiltration of immune cells within 5TGM1 myeloma tumors suggesting IFNβ could enhance the antitumor immune response (36). Further investigations are underway to delineate specific mechanisms by which IFNβ induction enhances VSV oncolytic efficacy against disseminated myeloma.
Currently used myeloma therapies have extended the life expectancy of patients diagnosed with myeloma but are not curative. Refractory or relapsed myeloma evolve mechanisms to resist chemotherapies(37). The studies described here demonstrate the ability of VSV-IFNβ to target and destroy disseminated myeloma to delay myeloma progression and improve survival in mice. VSV-IFNβ destroys myeloma cells based on systematic weaknesses in cellular innate immunity that arise from various tumorigenic alterations (38). In addition, VSV-IFNβ infection results in (variably) reduced viability of human myeloma cell lines and primary patient samples. The potential ability of VSV-IFNβ to kill different types of myeloma cells and the rapidity of VSV-IFNβ oncolysis (>50% tumor destruction by 72h post treatment) reduces the likelihood that myeloma cells can escape or evolve to resist viral oncolysis, both features that favor the proposed utilization of VSV-IFNβ as a potential myeloma therapy.
Viral IFNβ induction can potentially either enhance VSV therapeutic efficacy against disseminated myeloma or attenuate viral propagation in target cells that respond to IFNβ. It will be important in future studies to test VSV-IFNβ efficacy in different immune competent models of Multiple myeloma, firstly to validate that viral efficacy is not limited to this specific model of MM and additionally to shed further light into the role of viral IFNβ expression in successful tumor oncolysis. The data demonstrate overall, that VSV-IFNβ is a potent oncolytic candidate for clinical use for myeloma treatment and a promising platform for further engineering to improve viral targeting, monitoring and therapy.
Materials and Methods
Cells
5TGM1 murine myeloma cell line, obtained from Dr. Babatunde O. Oyajobi (University of Texas Health Sciences Center, San Antonio, TX) was grown in Iscove-modified Dulbecco Medium (IMDM) supplemented with 10% fetal bovine serum, 100 U.ml penicillin and 100mg/ml streptomycin. These cells were not authenticated, however confirmation of secretion of the specific IgG2b paraprotein (by IgG2b ELISA, see below) and tumor formation in the specific C57Bl/KalwRij syngeneic mouse strain (and not in C57Bl mouse strain) provided evidence of correct myeloma cell identity. BHK-21 cells were grown in Dulbecco modified eagles medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and penicillin, streptomycin antibiotics. Human myeloma cell lines were obtained at Mayo Clinic courtesy of Dr. Diane Jelinek (Kas 6/1, ALMC-1, JJN-3, KP-6, and ANBL-6, Rochester, MN) or Dr. Rafael Fonseca (MM-1, Scottsdale, AZ). These cell lines were not authenticated. Human and murine bone marrow stromal cell lines, HS27A and SR4987 was obtained from American Type Culture Collection. HS27A, JJN-3 and MM-1 cells were grown in RPMI 1640 supplemented with 10% FBS, and penicillin and streptomycin antibiotics; KAS 6/1 and ANBL-6 cells were supplemented with 1ng/ml interleukin-6 (IL-6). All cell lines were tested negative for mycoplama contamination. Primary cells were obtained from bone marrow samples from patients with advanced multiple myeloma at the Mayo Clinic. These cells were sorted in CD138+ myeloma cells and CD138− normal bone marrow progenitor cells. Primary myeloma cells are grown in RPMI 1640 supplemented with 10% FBS, and penicillin and streptomycin antibiotics and IL-6 (1ng/ml).
Viruses
VSV coding for murine interferon-β (IFN) and human IFNβ were generated in the lab of Dr. Glen N. Barber (University of Miami School of Medicine, Miami FL) as described previously(14). The VSV-IFNβ gene contains the IFNβ gene at Xho1/NheI restriction sites generated in between the G and L viral genes. VSV-GFP (Indiana strain) was also provided by Dr. Glen N. Barber. VSV vectors were amplified using BHK-21 cells. BHK-21 cells plated in flasks were allowed to grow to ~80% confluency. Cells were infected at a multiplicity of infection (MOI) of 0.01 for 1 hour in serum free DMEM. Virus was then removed and cells incubated at 37°C, in 5% CO2 incubator. Complete cytopathic effect (CPE) was seen by ~48h post infection. Culture medium was harvested, frozen in liquid nitrogen and thawed at 37°C, subjected to low-speed centrifugation and filtered through a 0.2-uM filter. The supernatant was loaded on 10% w/v sucrose and centrifuged at 27,000g for 2 hours. Pelleted particles were resuspended in phosphate buffered saline (PBS), aliquoted and stored at −80°C. Virus stock titers were measured by infection of BHK-21 cells plated in 96-well plates (7×103 cells/well/0.05ml) with serial dilutions of virus stock. Tissue culture infective dose (TCID50) values are determined by the Spearman and karber equation.
In vitro viral activity
Viral replication was compared by infection of BHK-21 cells plated in 24-well plates (1×105 cells/well/ml). These cells were incubated overnight mock-infected or infected with VSV-GFP, VSV-mIFNβ and VSV-hIFNβ (MOI 3.0, in opti-mem at 37°C for 1h). Cell supernatant was harvested at specified time points and viral titer was measured using the previously described method and calculated using the Spearman and karber equation.
Viral growth characteristics were measured in myeloma suspension cells mock infected or infected with VSV viruses in opti-mem. Cell supernatant was harvested at specified time points to measure viral titer (using the titer measurement protocol described above) and secreted IFNβ by using an ELISA kit designed to specifically detect murine IFNβ (PBL InterferonSource 42400-1). Cells were harvested to measure viability by flow cytometry using the LIVE/DEAD fixable dead cell stain kit (Invitrogen Molecular probes). Assays to measure viable cell proliferation were carried out on myeloma cells and measured using the MTT [3-(4,5-dimethylthiazlyl-2)-2,-5-diphenyltetrozolium bromide] assay (ATCC 30–1010K). Cells were mock infected or infected with VSV-GFP, VSV-mIFNβ or VSV-hIFNβ (MOI 1.0 in opti-mem, 1h at 37°C). Virus was washed out and cells were seeded into 96-well plates (1×104 cells/well/100ul) in their respective media. Plates were incubated for 72h. 0.01ml MTT reagent was added to each well and incubated at 37°C for 3h. 0.1ml detergent was added to cells to extract formazen and color intensity was measured using a microplate reader (570nm). Experiments were performed in triplicate and results tabulated as a percentage of mock-infected cells.
In vivo studies
4–6 week old female C57Bl/KaLwRij mice were obtained from Harlan, Netherlands. Tumors were implanted in mice by a subcutaneous injection of 5×106 cells in 100ul PBS. Subcutaneous tumors were established by day 14-post 5TGM1 implantation. Tumor burden was monitored by serial caliper measurements to measure tumor volume. Mice were randomized and treated with a single intravenous (IV) dose of 100ul PBS (control treatment), VSV-mIFNβ or VSV-hIFNβ at a dose of 1×108 TCID50/mouse. Mice reached sac criterion if tumor burden exceeded 10% of body weight, if mice were unable to access food or water or if tumors had indications of ulceration. Mice were culled at day 1 and 3 post VSV administration (n=2/day). Tumor, brain and mouse serum was harvested. Serum was further harvested at day 5, 7, 10, 15 and 30 post-VSV administration (2 mice or surviving mice at each time point). Harvested tissues were processed and analyzed as described below. Orthotopic 5TGM1 myeloma was established in syngeneic C57Bl/KalwRij mice by IV administration of 5×106 5TGM1 cells. Mice were administered with a single IV dose of 100ul PBS, or VSV-mIFNβ or VSV-hIFNβ (5×107 TCID50/mouse) at day 21 post-injection. Myeloma burden was monitored by measurement of serum IgG2b using Mouse IgG2b ELISA Quantitation kit (Bethyl laboratories, E90–109). Mice were monitored for symptoms of myeloma including weight loss, hind limb paralysis or the growth of subscapular plasmacytomas and sacrificed according to the previously described criterion.
Tissue analysis
Serum collected at day 1 and 3 was analyzed for murine IFNα and IFNβ using IFNα and IFNβ ELISA kits (PBL InterferonSource). Serum was also used to measure generation of anti-VSV neutralizing antibodies. Serum was pre-incubated for 30 min at 56°C to inactivate complement. 2-fold dilutions of inactivated serum were incubated with 500 TCID50 VSV-GFP for 1h at 37°C. The mixture of serum and virus was then plated onto vero cells in 96-well plates. Anti-VSV antibody titer was quantified as the minimum dilution of serum that failed to protect vero cells from VSV induced CPE. Harvested organs, tumor and brain were segmented into two portions, one portion flash-frozen and another portion stored in 10% formalin. Frozen tumor and brain sections were weighed, macerated to form tissue suspension. Tissue suspension was subjected to two rounds of freeze/thaw (in liquid nitrogen) and centrifuged to collect tissue supernatant. Tissue supernatant was titered as previously described to measure viral recovery per mg of tissue. Tissues stored in formalin were embedded in paraffin, sectioned and subject to staining with hematoxylin and eosin (H&E). Tissue sections were subject to independent histopathological analysis by Dr. Ronald Marler, Mayo Clinic, Scottsdale AZ.
Acknowledgments
This work was supported by the Mayo Foundation and NCI grant awards, R01CA100634 and R0129966, the Richard M Schulze Family Foundation and a gift from Al and Mary Agnes McQuinn. The authors thank Dr. Ron Marler from the Mayo Clinic Histology Core, Scottsdale AZ.
References
- 1.Raab MS, Podar K, Breitkreutz I, Richardson PG, Anderson KC. Multiple myeloma. Lancet. 2009;374:324–39. doi: 10.1016/S0140-6736(09)60221-X. [DOI] [PubMed] [Google Scholar]
- 2.Laubach JP, Mahindra A, Mitsiades CS, et al. The use of novel agents in the treatment of relapsed and refractory multiple myeloma. Leukemia. 2009;23:2222–32. doi: 10.1038/leu.2009.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barber GN. Vesicular stomatitis virus as an oncolytic vector. Viral Immunol. 2004;17:516–27. doi: 10.1089/vim.2004.17.516. [DOI] [PubMed] [Google Scholar]
- 4.Roberts A, Buonocore L, Price R, Forman J, Rose JK. Attenuated vesicular stomatitis viruses as vaccine vectors. Journal of virology. 1999;73:3723–32. doi: 10.1128/jvi.73.5.3723-3732.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jenks N, Myers R, Greiner SM, et al. Safety studies on intrahepatic or intratumoral injection of oncolytic vesicular stomatitis virus expressing interferon-beta in rodents and nonhuman primates. Human gene therapy. 2010;21:451–62. doi: 10.1089/hum.2009.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bi Z, Barna M, Komatsu T, Reiss CS. Vesicular stomatitis virus infection of the central nervous system activates both innate and acquired immunity. Journal of virology. 1995;69:6466–72. doi: 10.1128/jvi.69.10.6466-6472.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiocca EA. The host response to cancer virotherapy. Curr Opin Mol Ther. 2008;10:38–45. [PubMed] [Google Scholar]
- 8.Naik S, Russell SJ. Engineering oncolytic viruses to exploit tumor specific defects in innate immune signaling pathways. Expert Opin Biol Ther. 2009;9:1163–76. doi: 10.1517/14712590903170653. [DOI] [PubMed] [Google Scholar]
- 9.Conzelmann KK. Transcriptional activation of alpha/beta interferon genes: interference by nonsegmented negative-strand RNA viruses. Journal of virology. 2005;79:5241–8. doi: 10.1128/JVI.79.9.5241-5248.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Platanias LC, Uddin S, Domanski P, Colamonici OR. Differences in interferon alpha and beta signaling. Interferon beta selectively induces the interaction of the alpha and betaL subunits of the type I interferon receptor. J Biol Chem. 1996;271:23630–3. doi: 10.1074/jbc.271.39.23630. [DOI] [PubMed] [Google Scholar]
- 11.von Kobbe C, van Deursen JM, Rodrigues JP, et al. Vesicular stomatitis virus matrix protein inhibits host cell gene expression by targeting the nucleoporin Nup98. Molecular cell. 2000;6:1243–52. doi: 10.1016/s1097-2765(00)00120-9. [DOI] [PubMed] [Google Scholar]
- 12.Petersen JM, Her LS, Varvel V, Lund E, Dahlberg JE. The matrix protein of vesicular stomatitis virus inhibits nucleocytoplasmic transport when it is in the nucleus and associated with nuclear pore complexes. Mol Cell Biol. 2000;20:8590–601. doi: 10.1128/mcb.20.22.8590-8601.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stojdl DF, Lichty BD, tenOever BR, et al. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell. 2003;4:263–75. doi: 10.1016/s1535-6108(03)00241-1. [DOI] [PubMed] [Google Scholar]
- 14.Obuchi M, Fernandez M, Barber GN. Development of recombinant vesicular stomatitis viruses that exploit defects in host defense to augment specific oncolytic activity. J Virol. 2003;77:8843–56. doi: 10.1128/JVI.77.16.8843-8856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oyajobi BO, Munoz S, Kakonen R, et al. Detection of myeloma in skeleton of mice by whole-body optical fluorescence imaging. Mol Cancer Ther. 2007;6:1701–8. doi: 10.1158/1535-7163.MCT-07-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu L, Huang TG, Meseck M, et al. rVSV(M Delta 51)-M3 is an effective and safe oncolytic virus for cancer therapy. Human gene therapy. 2008;19:635–47. doi: 10.1089/hum.2007.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goel A, Carlson SK, Classic KL, et al. Radioiodide imaging and radiovirotherapy of multiple myeloma using VSV(Delta51)-NIS, an attenuated vesicular stomatitis virus encoding the sodium iodide symporter gene. Blood. 2007;110:2342–50. doi: 10.1182/blood-2007-01-065573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrantini M, Capone I, Belardelli F. Interferon-alpha and cancer: mechanisms of action and new perspectives of clinical use. Biochimie. 2007;89:884–93. doi: 10.1016/j.biochi.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Alexanian R, Weber D. Whither interferon for myeloma and other hematologic malignancies? Ann Intern Med. 1996;124:264–5. doi: 10.7326/0003-4819-124-2-199601150-00012. [DOI] [PubMed] [Google Scholar]
- 20.Aman MJ, Keller U, Derigs G, Mohamadzadeh M, Huber C, Peschel C. Regulation of cytokine expression by interferon-alpha in human bone marrow stromal cells: inhibition of hematopoietic growth factors and induction of interleukin-1 receptor antagonist. Blood. 1994;84:4142–50. [PubMed] [Google Scholar]
- 21.Odaka M, Sterman DH, Wiewrodt R, et al. Eradication of intraperitoneal and distant tumor by adenovirus-mediated interferon-beta gene therapy is attributable to induction of systemic immunity. Cancer research. 2001;61:6201–12. [PubMed] [Google Scholar]
- 22.Kirn DH, Wang Y, Le Boeuf F, Bell J, Thorne SH. Targeting of interferon-beta to produce a specific, multi-mechanistic oncolytic vaccinia virus. PLoS Med. 2007;4:e353. doi: 10.1371/journal.pmed.0040353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li H, Peng KW, Dingli D, Kratzke RA, Russell SJ. Oncolytic measles viruses encoding interferon beta and the thyroidal sodium iodide symporter gene for mesothelioma virotherapy. Cancer Gene Ther. 17:550–8. doi: 10.1038/cgt.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Willmon CL, Saloura V, Fridlender ZG, et al. Expression of IFN-beta enhances both efficacy and safety of oncolytic vesicular stomatitis virus for therapy of mesothelioma. Cancer research. 2009;69:7713–20. doi: 10.1158/0008-5472.CAN-09-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whelan SP, Ball LA, Barr JN, Wertz GT. Efficient recovery of infectious vesicular stomatitis virus entirely from cDNA clones. Proc Natl Acad Sci U S A. 1995;92:8388–92. doi: 10.1073/pnas.92.18.8388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jenks N, Myers R, Greiner SM, et al. Safety studies on intrahepatic or intratumoral injection of oncolytic vesicular stomatitis virus expressing interferon-beta in rodents and nonhuman primates. Human gene therapy. 21:451–62. doi: 10.1089/hum.2009.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gobet R, Cerny A, Ruedi E, Hengartner H, Zinkernagel RM. The role of antibodies in natural and acquired resistance of mice to vesicular stomatitis virus. Experimental cell biology. 1988;56:175–80. doi: 10.1159/000163477. [DOI] [PubMed] [Google Scholar]
- 28.Power AT, Wang J, Falls TJ, et al. Carrier cell-based delivery of an oncolytic virus circumvents antiviral immunity. Mol Ther. 2007;15:123–30. doi: 10.1038/sj.mt.6300039. [DOI] [PubMed] [Google Scholar]
- 29.Cobleigh MA, Buonocore L, Uprichard SL, Rose JK, Robek MD. A vesicular stomatitis virus-based hepatitis B virus vaccine vector provides protection against challenge in a single dose. Journal of virology. 84:7513–22. doi: 10.1128/JVI.00200-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huneycutt BS, Bi Z, Aoki CJ, Reiss CS. Central neuropathogenesis of vesicular stomatitis virus infection of immunodeficient mice. J Virol. 1993;67:6698–706. doi: 10.1128/jvi.67.11.6698-6706.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang S, Guo ZS, O’Malley ME, Yin X, Zeh HJ, Bartlett DL. A new recombinant vaccinia with targeted deletion of three viral genes: its safety and efficacy as an oncolytic virus. Gene Ther. 2007;14:638–47. doi: 10.1038/sj.gt.3302914. [DOI] [PubMed] [Google Scholar]
- 32.Liu TC, Kirn D. Systemic efficacy with oncolytic virus therapeutics: clinical proof-of-concept and future directions. Cancer research. 2007;67:429–32. doi: 10.1158/0008-5472.CAN-06-2871. [DOI] [PubMed] [Google Scholar]
- 33.Russell SJ, Peng KW. Viruses as anticancer drugs. Trends Pharmacol Sci. 2007;28:326–33. doi: 10.1016/j.tips.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaur B, Cripe TP, Chiocca EA. “Buy one get one free”: armed viruses for the treatment of cancer cells and their microenvironment. Curr Gene Ther. 2009;9:341–55. doi: 10.2174/156652309789753329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ochsenbein AF, Fehr T, Lutz C, et al. Control of early viral and bacterial distribution and disease by natural antibodies. Science. 1999;286:2156–9. doi: 10.1126/science.286.5447.2156. [DOI] [PubMed] [Google Scholar]
- 36.Li J, O’Malley M, Urban J, et al. Chemokine expression from oncolytic vaccinia virus enhances vaccine therapies of cancer. Mol Ther. 19:650–7. doi: 10.1038/mt.2010.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hideshima T, Anderson KC. Molecular mechanisms of novel therapeutic approaches for multiple myeloma. Nat Rev Cancer. 2002;2:927–37. doi: 10.1038/nrc952. [DOI] [PubMed] [Google Scholar]
- 38.Balachandran S, Porosnicu M, Barber GN. Oncolytic activity of vesicular stomatitis virus is effective against tumors exhibiting aberrant p53, Ras, or myc function and involves the induction of apoptosis. Journal of virology. 2001;75:3474–9. doi: 10.1128/JVI.75.7.3474-3479.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]