Abstract
Background
Electromechanical coupling, a well-described phenomenon in systolic dysfunction, has not been well studied in diastole. We hypothesized that the ECG T-peak to T-end (TpTe) interval, representing transmural dispersion of repolarization, is associated with echocardiographic markers of diastolic dysfunction (DD).
Methods and Results
We performed a prospective, cross-sectional study of the association between TpTe and markers of DD in 84 consecutive, unselected patients referred for exercise echocardiography. We systematically measured TpTe on the resting electrocardiogram (ECG), and we performed comprehensive assessment of DD at rest and at peak stress. ECGs and echocardiograms were analyzed independently, blinded to each other and to all clinical data. By univariable analysis, increased TpTe was associated with older age, increased E/e’ ratio and DD (P<0.05 for all associations after correcting for multiple comparisons). Increased TpTe was inversely associated with reduced tissue Doppler e’ velocity, a marker of DD (R=−0.66, P<0.0001). This association persisted after adjusting for age, QTc, exercise-induced wall motion abnormalities, and left ventricular mass index (β=−0.41 [95% CI −0.70 to −0.12] cm/s per 10-ms increase in TpTe; P=0.006). Baseline TpTe was also independently associated with resting DD (adjusted OR=3.9 [95% CI 1.4 to 10.7]; P=0.009), and peak exercise E/e’ ratio (P<0.0001).
Conclusions
Increased TpTe is associated with both resting and exercise-induced DD. Electromechanical coupling may represent a pathophysiologic link between electrical transmural dispersion of repolarization and abnormal myocardial relaxation, and may be a novel therapeutic target.
Keywords: diastole, ECG, echocardiography, repolarization, tissue Doppler imaging
Introduction
Diastolic dysfunction (DD) identified by comprehensive echocardiography is common, with a prevalence of up to 30% in the community, and both the presence and progression of DD have been associated with adverse outcomes, including progression to overt heart failure (HF).1–3 Although much is known about the pathogenesis of DD and its relation to HF syndromes, the underlying myocardial electrophysiologic properties associated with DD have been less well studied. Characterization of the electrophysiologic substrate underlying DD may allow for the development of novel therapies for DD and may also provide insight into the high prevalence of sudden cardiac death observed in patients with HF with preserved ejection fraction (HFpEF; also known as diastolic HF).4
While inherited long QT syndrome (LQTS) has historically been considered a purely electrical disease, echocardiographic studies over the past two decades have demonstrated a crude but replicable relationship between a prolonged QT interval and abnormal mechanical function.5–9 Observational data paired with animal studies propose the theory that electrical transmural dispersion of repolarization manifest on the surface electrocardiogram (ECG) can be associated with mechanical dispersion of left ventricular relaxation observed using comprehensive echocardiography.10–15 We recently reported an association between ECG QTc interval and DD among patients undergoing echocardiography for suspected HF, none of whom had known LQTS.16 We also validated this finding in an unselected cohort of patients referred for echocardiography.16 These findings extended prior results showing that electrical repolarization correlates with mechanical relaxation in a broad sample of patients.17
Although these prior studies demonstrate an association between QTc and DD, the QTc interval encompasses both depolarization (systole) and repolarization (diastole). Animal models have demonstrated that the ECG T-peak to T-end (TpTe) interval is more representative of transmural dispersion of repolarization than QTc.18 We therefore sought to evaluate the relationship between TpTe and DD. We hypothesized that TpTe would be independently associated with DD even after adjusting for QTc, and would be more closely related to tissue Doppler e’ velocity than QTc.
Methods
Study design
We performed a prospective, cross-sectional study of the association between TpTe interval and DD in 84 patients at the Bluhm Cardiovascular Institute of Northwestern Memorial Hospital (Chicago, Illinois). We initially recruited 107 consecutive unselected patients who presented for full Doppler exercise echocardiography from June 30, 2008 to August 21, 2008. We excluded 17 patients due to poor echocardiographic image quality or poor quality tissue Doppler tracings, 1 due to ventricular paced rhythm, and 5 due to atrial arrhythmia, resulting in 84 patients for the final analysis. The study protocol was approved by the institutional review board at Northwestern University Feinberg School of Medicine, and all study participants provided written informed consent.
Clinical characteristics
We collected and analyzed the following clinical characteristics of the study participants: demographics, comorbidities, medications, body-mass index, and laboratory data (which included serum potassium and renal function). Estimated glomerular filtration rate (eGFR) was calculated using the Modified Diet in Renal Disease equation.
Hypertension was defined by systolic blood pressure >140 mmHg or diastolic blood pressure >90 mmHg, physician documented history of hypertension, or use of antihypertensive medications. Diabetes mellitus was defined by the presence of physician-documented history of diabetes or use of oral hypoglycemic agents or insulin for the treatment of hyperglycemia. Coronary artery disease (CAD) was defined by the presence of physician documented history of CAD, known coronary stenosis > 50%, prior history of myocardial infarction, percutaneous intervention, coronary artery bypass grafting, or abnormal stress test consistent with myocardial ischemia. Obesity was defined by a body mass index > 30 kg/m2.
Electrocardiography
All subjects underwent baseline 12-lead ECG recording (Marquette MAC 5000 Resting ECG System; GE Healthcare; Boston, MA) at the time of baseline echocardiogram. ECGs were analyzed by a single trained reader blinded to the echocardiographic findings. Each ECG was analyzed according to a systematic protocol which included measurement of the PR interval, QRS duration, QT interval, and QTc interval as described in a prior study of ours.16 We excluded patients with atrial arrhythmias, premature atrial or ventricular beats, irregular R-R intervals, or paced rhythms due to their influence on transmural dispersion of repolarization as well as difficulty measuring QTc and TpTe in these cases.19 We analyzed the T-wave and measured TpTe interval based on methods described in previous studies.12 We employed a manual analysis of standard 12-lead ECG tracings at 25-mm/s speed and 10-mm/mV amplitude. Since previous studies have postulated that precordial leads best reflect transmural dispersion of repolarization, while limb leads best reflect apical-basal or global spatial dispersion, TpTe was measured from the peak of the T-wave to the end of the T-wave using the best available T-wave in lead V5, a method which has been described previously.20–22 In cases in which V5 was not suitable for analysis leads, V4 and V6 (in that order) were measured.21 Since both animal and human models have previously demonstrated that transmural dispersion of repolarization typically manifest as late T-wave abnormalities in downslope or morphology, the offset of the descending limb of the T-wave was defined as the intersection of the tangent to the terminal downslope of the T-wave and the isoelectric line (Figure 1).21, 23, 24 For purposes of maintaining precision, leads were excluded from analysis if there was a prominent U-wave or if the T-wave amplitude was less than 1.5 mV. For the assessment of interobserver variability of TpTe measurements, a second trained investigator independently measured the TpTe interval in 15 randomly selected patients, blinded to all other clinical, ECG, and echocardiographic data.
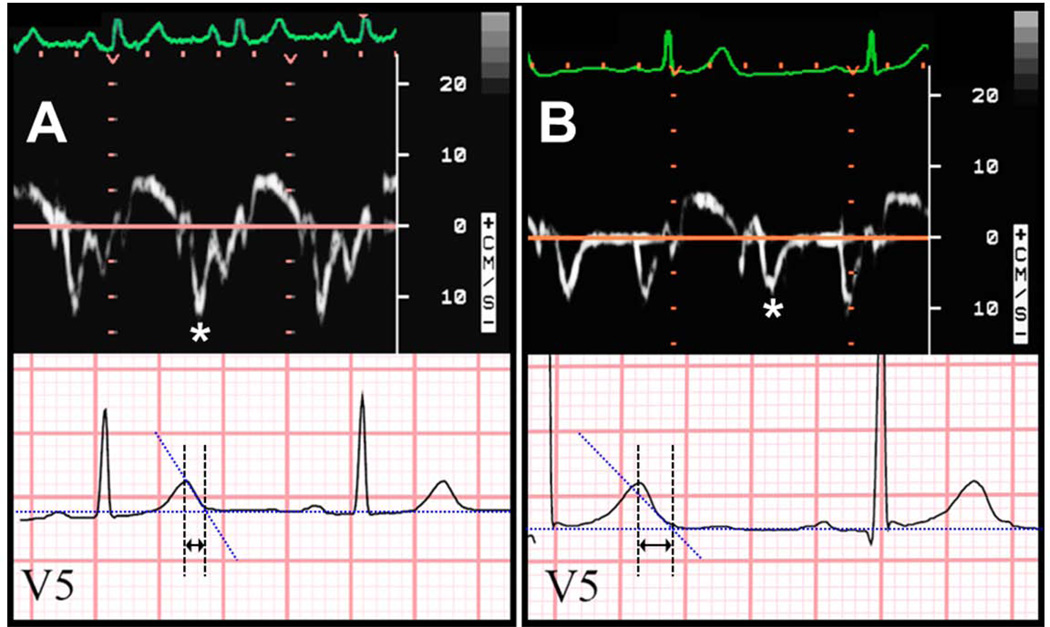
Figure 1.
Examples of the relation between tissue Doppler e’ velocity and electrocardiographic T-peak to T-end interval. Panel A: Normal e’ velocity (12.1 cm/s) and short TpTe interval (65 ms). Panel B: Abnormally reduced e’ velocity (7.8 cm/s) and long TpTe interval (115 ms). Asterisks denote e’ wave on tissue Doppler tracings. Arrows denote TpTe interval on electrocardiography tracings.
Echocardiography
All subjects underwent a complete M-mode, two-dimensional, Doppler and tissue Doppler echocardiographic examination using a Sonos 7500 or iE33 system (Philips Medical Systems, Andover, MA) with additional dedicated imaging of mitral inflow using pulse wave Doppler echocardiography and pulse wave tissue Doppler echocardiography of the septal mitral annulus according to the published guidelines.25, 26 Echocardiograms were reviewed by a single trained reader blinded to the ECG and clinical data. Diastolic function was diagnosed and graded as normal, mild dysfunction (grade 1), or moderate or greater dysfunction (grade 2 or 3) based on mitral inflow velocities (i.e., ratio of early [E] to late [A] mitral inflow velocity [E/A ratio]), tissue Doppler e’ velocities, and E/e’ ratio.3
Exercise testing and exercise echocardiography
Exercise stress testing (including diastolic stress echocardiography) was performed using a protocol consistent with prior published studies.27, 28 All patients underwent treadmill exercise according to the Bruce protocol with physician supervision to encourage maximally tolerated exercise. Immediately following peak exercise, regional wall motion was assessed using standardized two-dimensional views. Shortly after two-dimensional image acquisition, mitral inflow and septal tissue Doppler velocities were obtained within 5 minutes of cessation of exercise at the earliest time to sufficiently allow separation of the E and A velocities permitting appropriate measurement (typically when heart rate decreased to < 90 bpm).
Statistical analysis
For the evaluation of interobserver variability of TpTe measurements, the agreement between the measurements obtained by 2 independent investigators was determined as the mean bias and 95% limits of agreement (1.96 SD). Interobserver reliability also was evaluated using the intraclass correlation coefficient29 and Bland-Altman analysis.
For descriptive purposes, we dichotomized study participants into 2 groups by median TpTe interval (TpTe < 75 ms vs. TpTe ≥ 75 ms). Clinical characteristics, laboratory data, resting ECG parameters, resting echocardiographic parameters, and stress test data (exercise duration and capacity, peak stress wall motion, and peak stress diastolic parameters) were compared between groups. Continuous data, displayed as mean ± standard deviation, were compared using t-tests (all continuous data were normally distributed). Chi-squared tests (or Fisher’s exact test when appropriate) were used to compare categorical variables between groups. To account for multiple comparisons (N=54 statistical tests in Tables 1–4), we used 3 approaches: Bonferroni, Šidák, and false discovery rate (FDR). Results using the 3 methods were similar, except for a significant difference in peak heart rate detected using the FDR method but not after correcting for multiple comparisons using the other 2 methods (see Table 3).
Table 1.
Clinical Characteristics by Median TpTe Interval
| Characteristic | All patients (N=84) |
TpTe < 75 ms (N=41) |
TpTe ≥ 75 ms (N=43) |
P value |
|---|---|---|---|---|
| T peak – T end interval (ms) | 75±17 | 61±8 | 88±11 | |
| Age (years) | 52±14 | 45±12 | 58±13 | <0.0001* |
| Female (n, %) | 40 (47) | 24 (58) | 16 (37) | 0.05 |
| Body-mass index (kg/m2) | 26±5 | 25±4 | 27±5 | 0.02 |
| Comorbidities (n, %) | ||||
| Hypertension | 28 (34) | 12 (30) | 16 (37) | 0.48 |
| Coronary artery disease | 20 (24) | 7 (17) | 13 (30) | 0.17 |
| Diabetes mellitus | 5 (6) | 2 (5) | 3 (7) | 0.70 |
| Chronic kidney disease | 5 (6) | 3 (7) | 2 (5) | 0.58 |
| Dyslipidemia | 37 (44) | 15 (37) | 22 (51) | 0.21 |
| Obesity | 17 (20) | 5 (12) | 12 (28) | 0.10 |
| Medications (n, %) | ||||
| Beta-blocker | 31 (37) | 14 (35) | 17 (40) | 0.61 |
| ACE-I / ARB | 24 (29) | 8 (20) | 16 (38) | 0.08 |
| Aspirin | 32 (38) | 11 (27) | 21 (49) | 0.046 |
| Statin | 31 (38) | 13 (32) | 18 (43) | 0.33 |
| Diuretic | 10 (12) | 5 (12) | 5 (12) | 0.93 |
| Laboratory data | ||||
| Serum potassium (mEq/L) | 4.1±0.5 | 4.2±0.4 | 4.1±0.5 | 0.72 |
| Estimated GFR (ml/min/1.73m2) | 77±22 | 78±24 | 75±21 | 0.58 |
ACE-I / ARB = angiotensin converting enzyme inhibitor or angiotensin receptor blocker
GFR = glomerular filtration rate
Remains significant after correction for multiple comparisons
Table 4.
Peak Stress Echocardiographic Characteristics by Mean TpTe Interval.
| Characteristic | All patients (N=84) |
TpTe < 75 ms (N=41) |
TpTe ≥ 75 ms (N=43) |
P value |
|---|---|---|---|---|
| T peak – T end interval (ms) | 75±17 | 61±8 | 88±11 | |
| Regional wall motion abnormality (n, %) |
6 (7) | 2 (5) | 4 (9) | 0.67 |
| Early mitral inflow (E) velocity (cm/s) | 111±25 | 113±24 | 108±26 | 0.44 |
| Late mitral inflow (A) velocity (cm/s) | 87±30 | 85±27 | 88±32 | 0.69 |
| E/A ratio | 1.3±0.5 | 1.3±0.5 | 1.2±0.5 | 0.27 |
| Mitral E deceleration time (ms) | 195±84 | 194±86 | 195±83 | 0.97 |
| Septal e' tissue velocity (cm/s) | 12.9±4.9 | 14.7±5.2 | 11.2±4.1 | <0.0001** |
| E/e’ ratio | 9.4±3.1 | 8.3±2.4 | 10.5±3.2 | <0.0001** |
| E/e’ ratio > 13* (n, %) | 11 (13) | 1 (2) | 10 (23) | 0.01 |
E/e’ > 13 represents a positive diastolic stress test
Remains significant after correction for multiple comparisons
Table 3.
Exercise Treadmill Testing Results by Median TpTe Interval
| Characteristic | All patients (N=84) |
TpTe < 75 ms (N=41) |
TpTe ≥ 75 ms (N=43) |
P value |
|---|---|---|---|---|
| T peak – T end interval (ms) | 75±17 | 61±8 | 88±11 | |
| Resting HR (bpm) | 80±13 | 83±10 | 76±15 | 0.02 |
| Peak HR (bpm) | 156±23 | 164±21 | 149±23 | 0.002* |
| Percent predicted peak HR (%) | 92±10 | 94±11 | 91±10 | 0.12 |
| Resting systolic BP (mmHg) | 120±16 | 117±18 | 122±14 | 0.14 |
| Resting diastolic BP (mmHg) | 77±11 | 75±11 | 77±11 | 0.37 |
| Resting pulse pressure (mmHg) | 43±14 | 41±16 | 45±12 | 0.31 |
| Peak systolic BP (mmHg) | 157±20 | 158±22 | 157±18 | 0.73 |
| Peak diastolic BP (mmHg) | 70±13 | 68±12 | 72±13 | 0.22 |
| Peak pulse pressure (mmHg) | 88±20 | 90±21 | 85±19 | 0.26 |
| Exercise time (minutes) | 10.1±3.1 | 10.7±3.1 | 9.5±3.0 | 0.07 |
| Exercise capacity (METs) | 11.8±3.5 | 12.6±3.4 | 11.1±3.5 | 0.049 |
HR = heart rate, BP = blood pressure, METs = metabolic equivalents
Remains significant after correction for multiple comparisons using the false discovery rate (FDR) method (FDR-corrected P=0.015) but not by Bonferroni and Šidák methods (P=0.11 and P=0.10, respectively).
Next, we performed univariable and multivariable linear regression analyses to determine whether TpTe (independent variable) was associated with tissue Doppler e’ velocity (dependent variable). We similarly performed univariable and multivariable logistic regression to determine whether TpTe (independent variable) was associated with the presence of DD (dependent variable). Due to the relatively small sample size of our study, we used parsimonious models to avoid over-adjustment. Candidate covariates were selected for inclusion into our multivariable models if they were previously known to be associated with DD and therefore thought to be potential confounders. We excluded collinearity with TpTe for all covariates prior to inclusion into our multivariable models. All analyses were performed using Stata v.10.1 (StataCorp, College Station, TX).
Results
In the sample of 84 consecutive outpatients referred for stress echocardiography, the mean age was 52±14 years, 47% were female, and comorbidities such as hypertension, CAD, obesity, and dyslipidemia were common. The majority of subjects had normal cardiac chamber dimensions and wall thickness. Left ventricular ejection fraction was normal (> 55%) in all subjects. Of the 84 patients, 63% had normal diastolic function, 13% had mild (grade 1) DD, and 24% had moderate or greater (grade 2 or 3) DD.
The mean TpTe was 75±17 ms, and the mean QTc was 423±27 ms. There was good agreement in measurements of TpTe between 2 blinded investigators. Correlation between measurements made by the 2 independent investigators was high (R=0.94, P<0.0001), and differences in TpTe values measured by the 2 independent investigators were not statistically significant (P=0.83 by paired t-test). Mean bias and 95% limits of agreement for the TpTe measurement were −0.33 ms and −12.01 to 11.35 ms, respectively (see Supplemental Figure S1 for Bland-Altman plot). The intraclass correlation coefficient was 0.94 (95% confidence interval 0.89–0.99).
Table 1 displays the baseline clinical characteristics by TpTe group (below vs. above median TpTe). Those with higher TpTe values were older, but otherwise were similar to those with lower TpTe values with a similar prevalence of comorbidities between the two groups. Table 2 summarizes the resting ECG and echocardiographic characteristics by median TpTe. Subjects with an increased TpTe had a more leftward QRS axis, longer PR interval, longer QT interval, and longer QTc interval but none of these differences remained significant after accounting for multiple comparisons; QRS duration was similar among the two groups. There were several echocardiographic differences among TpTe groups: in those in the TpTe ≥ 75 ms group, septal wall thickness and posterior wall thickness were higher and early mitral inflow (E) velocity and E/A ratio were lower. By tissue Doppler imaging, subjects in the TpTe ≥ 75 ms group had a much lower septal e’ velocity when compared to those with lower TpTe (7.7±2.2 cm/s vs. 10.9±2.3 cm/s, P<0.0001). Left ventricular filling pressure (estimated non-invasively by the E/e’ ratio) was also higher among patients in the TpTe ≥ 75 ms group (10.7±3.2 vs. 8.3±2.2, P=0.0001). Although there were several echocardiographic differences among the TpTe groups, the only differences which remained statistically significant after multiple comparison testing were e’ velocity, E/e’ ratio, and DD.
Table 2.
Resting Electrocardiographic and Echocardiographic Parameters by Median TpTe Interval
| Parameter | All patients (N=84) |
TpTe < 75 ms (N=41) |
TpTe ≥ 75 ms (N=43) |
P value |
|---|---|---|---|---|
| T peak – T end interval (ms) | 75±17 | 61±8 | 88±11 | |
| Electrocardiography: | ||||
| PR interval (ms) | 160±28 | 153±23 | 167±31 | 0.03 |
| QRS interval (ms) | 90±14 | 87±9 | 93±17 | 0.06 |
| QT interval (ms) | 401±41 | 390±30 | 413±46 | 0.01 |
| QTc interval (ms) | 423±27 | 416±22 | 430±29 | 0.02 |
| QRS axis (degrees) | 36±38 | 46±29 | 27±42 | 0.02 |
| Echocardiography: | ||||
| LV end-diastolic dimension (cm) | 4.4±0.6 | 4.3±0.6 | 4.4±0.5 | 0.69 |
| LV end-systolic dimension (cm) | 2.9±0.5 | 2.9±0.5 | 2.9±0.5 | 0.91 |
| LV posterior wall thickness (cm) | 1.0±0.2 | 0.9±0.1 | 1.0±0.2 | 0.02 |
| LV septal wall thickness (cm) | 1.0±0.2 | 0.9±0.1 | 1.0±0.2 | 0.02 |
| LV mass index (g/m2) | 78±20 | 75±20 | 80±20 | 0.22 |
| LV ejection fraction (%) | 60±4 | 59±3 | 60±5 | 0.24 |
| Left atrial dimension (cm) | 3.3±0.4 | 3.2±0.5 | 3.4±0.4 | 0.12 |
| Early mitral inflow (E) velocity (cm/s) | 83±19 | 88±20 | 80±17 | 0.04 |
| Late mitral inflow (A) velocity (cm/s) | 69±17 | 67±17 | 70±16 | 0.44 |
| E/A ratio | 1.3±0.5 | 1.4±0.6 | 1.2±0.3 | 0.03 |
| Mitral E deceleration time (ms) | 204±70 | 198±70 | 210±71 | 0.42 |
| Septal e' tissue velocity (cm/s) | 9.3±2.8 | 10.9±2.3 | 7.7±2.2 | <0.0001* |
| E/e' ratio | 9.5±3.0 | 8.3±2.2 | 10.7±3.2 | 0.0001* |
| Diastolic function (n, %) | <0.0001* | |||
| Normal diastolic function | 53 (63) | 40 (98) | 13 (30) | |
| Grade 1 diastolic dysfunction | 11(13) | 1(2) | 10 (23) | |
| Grade 2 or 3 diastolic dysfunction | 20 (24) | 0 (0) | 20 (47) | |
Remains significant after correction for multiple comparisons
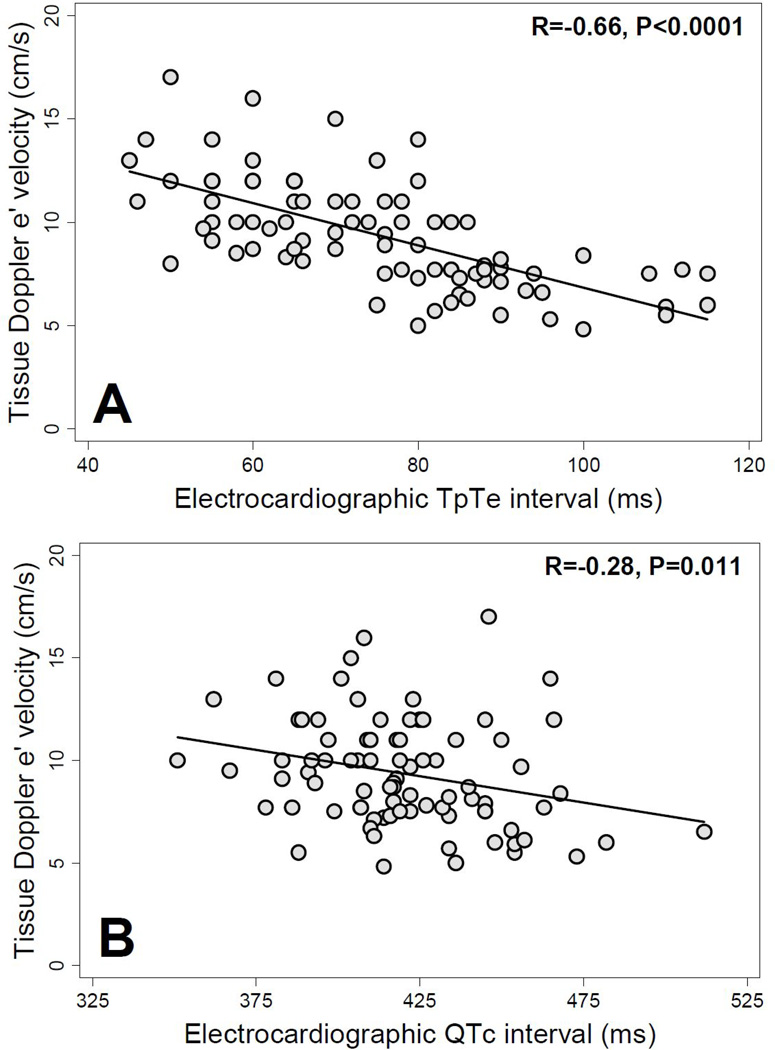
Figure 1 displays representative examples of tissue Doppler images and corresponding lead V5 ECG tracings in a patient with short TpTe (and normal e’ velocity) and a patient with long TpTe (and reduced e’ velocity). Figure 2 demonstrates the inverse relationship between TpTe interval and e’ velocity as well as the inverse relationship between QTc interval and e’ velocity. The correlation between TpTe and e’ velocity (R=−0.66, P<0.0001) was much stronger than the correlation between QTc and e’ velocity (R=−0.28, P=0.011).
Figure 2.
Scatterplots of tissue Doppler e’ velocity versus T-peak to T-end interval and QTc interval. TpTe = T-peak to T-end interval; (A) e’ velocity vs. TpTe interval; (B) e’ velocity vs. QTc interval.
Tables 3 and 4 display the exercise test characteristics by median TpTe. Resting heart rate was similar among groups; however, patients with increased TpTe demonstrated lower heart rate at peak exercise and reduced exercise capacity. There were no significant differences in regional wall motion abnormalities, mitral inflow velocities, or mitral E deceleration time at peak stress. However, higher TpTe was associated with a decreased peak-exercise tissue Doppler septal e’ velocity and an increased peak-exercise E/e’ ratio. In addition, the number of subjects with an abnormal diastolic stress test (i.e., peak exercise E/e’ > 13) was much higher in the TpTe ≥ 75 ms group compared to the TpTe < 75 ms group (23% vs. 2%, P=0.007). Only peak heart rate, peak exercise e’ velocity, and and peak exercise E/e’ ratio remained significantly different among the TpTe groups after accounting for multiple comparisons.
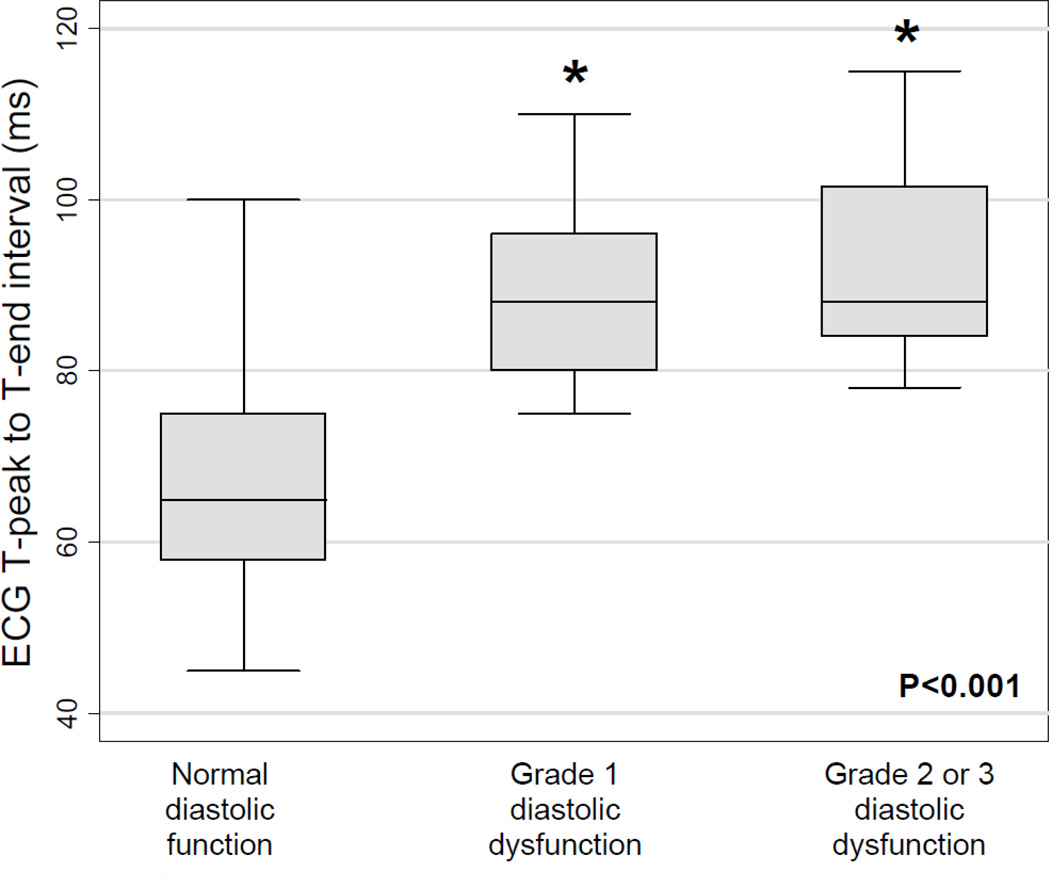
Table 5 summarizes univariable and multivariable regression analyses for the association between TpTe and septal e’ velocity, and for the association between TpTe and DD. These results demonstrate the independent inverse association between TpTe and e’ velocity even after adjusting for potential confounders, including age, QTc, left ventricular mass index, and exercise-induced wall motion abnormalities. In unadjusted analyses, each 10-ms increase in TpTe was associated with a 1.02 cm/s decrease in e’ velocity (P<0.001). In the final multivariable model, each 10-ms increase in TpTe was associated with a 0.41 cm/s decrease in e’ velocity (P=0.006), and QTc was no longer associated with e’ velocity (P=0.08). TpTe was also independently associated with worse DD (Figure 3 and Table 5).
Table 5.
Univariable and Multivariable Regression Analyses for the Association of TpTe Interval with Resting Tissue Doppler e’ Velocity and Diastolic Dysfunction
| Tissue Doppler e’ velocity | Diastolic dysfunction | |||||
|---|---|---|---|---|---|---|
| Model | β-Coefficient* | 95% CI | P value | Odds Ratio* | 95% CI | P value |
| Unadjusted | −1.02 | −1.28, −0.77 | < 0.001 | 8.3 | 3.1, 22.3 | < 0.001 |
| Model I | −0.60 | −0.86, −0.34 | < 0.001 | 5.6 | 1.9, 16.4 | 0.002 |
| Model II | −0.50 | −0.77, −0.22 | 0.001 | 4.7 | 1.6, 13.8 | 0.005 |
| Model III | −0.41 | −0.70, −0.12 | 0.006 | 3.9 | 1.4, 10.7 | 0.009 |
Per standard deviation 10-ms increase in TpTe
Model I: adjusted for age
Model II: adjusted for age and QTc interval
Model III: adjusted for age, QTc interval, left ventricular mass index, and the presence of wall motion abnormality on stress echocardiography
Figure 3.
Box-and-whisker plot of T-peak to T-end interval versus left ventricular diastolic dysfunction grade. P-value across all 3 groups calculated using one-way analysis of variance. Individual diastolic dysfunction groups were compared to normal diastolic function using t tests. Asterisk denotes P<0.0001 for comparison versus normal diastolic function group.
Discussion
In a sample of 84 consecutive outpatients referred for exercise echocardiography, we found a significant inverse linear association between the TpTe interval and tissue Doppler septal e’ velocity. This association persisted after adjustment for several important potential confounders, including age, QTc interval, and left ventricular wall thickness. Moreover, TpTe remained associated with DD independent of exercise-induced regional wall motion abnormalities, suggesting TpTe relates directly to DD rather than representing a surrogate of ischemia. After adjusting for TpTe and other clinical and echocardiographic covariates, QTc was not independently associated with septal e’ velocity. These findings suggest that TpTe prolongation explains the QTc prolongation previously associated with DD16 and may better represent the electrical manifestations associated with abnormal relaxation and DD.
Ours is the first study to our knowledge to demonstrate the inverse relationship between ECG TpTe and tissue Doppler septal e’ in unselected patients without LQTS. However, electromechanical coupling in the setting of prolonged QTc is not new. It was first demonstrated as an unsuspected late systolic left ventricular thickening seen on M-mode in patients with genetic LQTS without structural abnormalities.5 These findings were replicated using a 12-segment M-mode measurement of thickening time in cases of LQTS versus controls, suggesting not only electrical but also mechanical dispersion of the left ventricle in patients with LQTS.7 The advent of tissue Doppler imaging has allowed for further demonstration of abnormalities in left ventricular systolic and diastolic function in LQTS patients when compared to controls.8 A larger case-control study using tissue Doppler imaging demonstrated that patients with inherited LQTS have a longer contraction duration when compared to controls and increased duration of contraction was more predictive of cardiac events than QTc.9 Mechanical implications of electrical heterogeneity have also been studied and validated in animal models.13
Multiple recent studies have demonstrated the role of T-wave analysis, and TpTe in particular, as a potential ECG biomarker of dispersion of repolarization.18, 20, 30–33 In addition, some experiments have begun to investigate the ability of pharmacologically reversing dispersion of repolarization measured by TpTe as a potential therapeutic mechanism for reducing proarrhythmic substrate.34 Nevertheless, transmural dispersion of repolarization has only recently been demonstrated as a potential mechanism contributing to mechanical dysfunction in patients with overt HF.35
Our results should not be interpreted to suggest that TpTe measured on surface ECG can be used to screen for DD or exercise-induced DD on echocardiography. Rather, these findings add to the growing literature of evidence suggesting a possible contribution of electromechanical coupling of dispersion of repolarization as a mechanism of DD. The novel inverse association of TpTe with septal e’ among unselected patients should prompt further investigations into both the electrical and mechanical markers of dispersion and an improved understanding of the mechanism behind electromechanical coupling.
The following limitations should be considered when interpreting the results of our study. We performed measurement of TpTe manually (and not by automated, computerized analysis), which may have introduced slight errors into the TpTe measurement; however, inaccuracy in TpTe would only decrease the association between TpTe and DD, and our evaluation of interobserver variability showed that there was good agreement in TpTe measurements among independent observers. Nevertheless, TpTe may be a crude measure of dispersion compared to more sophisticated software analysis of T-wave itself; particular markers such as T-wave slope, roundness, area and morphology may better reflect transmural dispersion of repolarization after further validation.36 Moreover, we cannot account for the effect or the significance of excluding prominent U-waves from our analysis since the cellular basis for the origin of the U-wave remains inadequately defined.37–39 Finally, the tissue Doppler measurement of e’ velocity, though a marker of DD, may not directly reflect the transmural dispersion of mechanical myocardial relaxation.
In conclusion, there is an inverse, linear correlation between TpTe and tissue Doppler e’ velocity, an indicator of left ventricular myocardial relaxation and diastolic function. Furthermore, increased TpTe is associated with both resting DD and exercise-induced DD. Electromechanical coupling may represent a link between transmural dispersion of repolarization and abnormal mechanical myocardial relaxation. Further studies are warranted to investigate the mechanism underlying diastolic electromechanical coupling of dispersion of repolarization since such a mechanism may be a novel target for therapy in patients with HF syndromes.
Supplementary Material
Acknowledgments
Funding Sources: This work was supported by grants from the American Heart Association (Scientist Development Grant #0835488N) and the National Institutes of Health (R01 HL107557) (both to S.J.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: None.
References
- 1.Abhayaratna WP, Marwick TH, Smith WT, Becker NG. Characteristics of left ventricular diastolic dysfunction in the community: An echocardiographic survey. Heart. 2006;92:1259–1264. doi: 10.1136/hrt.2005.080150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kane GC, Karon BL, Mahoney DW, Redfield MM, Roger VL, Burnett JC, Jr, Jacobsen SJ, Rodeheffer RJ. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA. 2011;306:856–863. doi: 10.1001/jama.2011.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: Appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 4.Zile MR, Gaasch WH, Anand IS, Haass M, Little WC, Miller AB, Lopez-Sendon J, Teerlink JR, White M, McMurray JJ, Komajda M, McKelvie R, Ptaszynska A, Hetzel SJ, Massie BM, Carson PE. Mode of death in patients with heart failure and a preserved ejection fraction: Results from the Irbesartan in Heart Failure with Preserved Ejection Fraction Study (I-PRESERVE) trial. Circulation. 2010;121:1393–1405. doi: 10.1161/CIRCULATIONAHA.109.909614. [DOI] [PubMed] [Google Scholar]
- 5.Nador F, Beria G, De Ferrari G, Stramba-Badiale M, Locati E, Lotto A, Schwartz P. Unsuspected echocardiographic abnormality in the long QT syndrome. Diagnostic, prognostic, and pathogenetic implications. Circulation. 1991;84:1530–1542. doi: 10.1161/01.cir.84.4.1530. [DOI] [PubMed] [Google Scholar]
- 6.De Ferrari G, Nador F, Beria G, Sala S, Lotto A, Schwartz P. Effect of calcium channel block on the wall motion abnormality of the idiopathic long QT syndrome. Circulation. 1994;89:2126–2132. doi: 10.1161/01.cir.89.5.2126. [DOI] [PubMed] [Google Scholar]
- 7.Nakayama K, Yamanari H, Otsuka F, Fukushima K, Saito H, Fujimoto Y, Emori T, Matsubara H, Uchida S, Ohe T. Dispersion of regional wall motion abnormality in patients with long QT syndrome. Heart. 1998;80:245–250. doi: 10.1136/hrt.80.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Savoye C, Klug D, Denjoy I, Ennezat PV, Le Tourneau T, Guicheney P, Kacet S. Tissue Doppler echocardiography in patients with long QT syndrome. Eur J Echocardiogr. 2003;4:209–213. doi: 10.1016/s1525-2167(03)00011-8. [DOI] [PubMed] [Google Scholar]
- 9.Haugaa KH, Edvardsen T, Leren TP, Gran JM, Smiseth OA, Amlie JP. Left ventricular mechanical dispersion by tissue Doppler imaging: A novel approach for identifying high-risk individuals with long QT syndrome. Eur Heart J. 2009;30:330–337. doi: 10.1093/eurheartj/ehn466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belardinelli L, Dhalla A, Shryock J. Abnormal left ventricular relaxation in patients with long QT syndrome. Eur Heart J. 2009;30:2813–2814. doi: 10.1093/eurheartj/ehp444. [DOI] [PubMed] [Google Scholar]
- 11.Rosenbaum DS. Is long QT syndrome a disease of abnormal mechanical contraction? Circulation. 2010;122:1353–1354. doi: 10.1161/CIRCULATIONAHA.110.980706. [DOI] [PubMed] [Google Scholar]
- 12.Antzelevitch C. Role of spatial dispersion of repolarization in inherited and acquired sudden cardiac death syndromes. Am J Physiol Heart Circ Physiol. 2007;293:H2024–H2038. doi: 10.1152/ajpheart.00355.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cordeiro JM, Greene L, Heilmann C, Antzelevitch D, Antzelevitch C. Transmural heterogeneity of calcium activity and mechanical function in the canine left ventricle. Am J Physiol Heart Circ Physiol. 2004;286:H1471–H1479. doi: 10.1152/ajpheart.00748.2003. [DOI] [PubMed] [Google Scholar]
- 14.Provost J, Lee W-N, Fujikura K, Konofagou EE. Imaging the electromechanical activity of the heart in vivo. Proc Natl Acad Sci U S A. 2011;108:8565–8570. doi: 10.1073/pnas.1011688108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laurita KR, Katra R, Wible B, Wan X, Koo MH. Transmural heterogeneity of calcium handling in canine. Circ Res. 2003;92:668–675. doi: 10.1161/01.RES.0000062468.25308.27. [DOI] [PubMed] [Google Scholar]
- 16.Wilcox JE, Rosenberg J, Vallakati A, Gheorghiade M, Shah SJ. Usefulness of electrocardiographic QT interval to predict left ventricular diastolic dysfunction. Am J Cardiol. 2011;108:1760–1766. doi: 10.1016/j.amjcard.2011.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alessandrini RS, McPherson DD, Kadish AH, Kane BJ, Goldberger JJ. Cardiac memory: A mechanical and electrical phenomenon. Am J Physiol Heart Circ Physiol. 1997;272:H1952–H1959. doi: 10.1152/ajpheart.1997.272.4.H1952. [DOI] [PubMed] [Google Scholar]
- 18.Antzelevitch C. T peak-T end interval as an index of transmural dispersion of repolarization. Eur J Clin Invest. 2001;31:555–557. doi: 10.1046/j.1365-2362.2001.00849.x. [DOI] [PubMed] [Google Scholar]
- 19.Hinterseer M, Thomsen MB, Beckmann BM, Pfeufer A, Schimpf R, Wichmann HE, Steinbeck G, Vos MA, Kaab S. Beat-to-beat variability of QT intervals is increased in patients with drug-induced long-QT syndrome: A case control pilot study. Eur Heart J. 2008;29:185–190. doi: 10.1093/eurheartj/ehm586. [DOI] [PubMed] [Google Scholar]
- 20.Antzelevitch C, Sicouri S, Di Diego JM, Burashnikov A, Viskin S, Shimizu W, Yan G-X, Kowey P, Zhang L. Does T peak–T end provide an index of transmural dispersion of repolarization? Heart Rhythm. 2007;4:1114–1116. doi: 10.1016/j.hrthm.2007.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panikkath R, Reinier K, Uy-Evanado A, Teodorescu C, Hattenhauer J, Mariani R, Gunson K, Jui J, Chugh SS. Prolonged T peak-to-T end interval on the resting ECG is associated with increased risk of sudden cardiac death. Circ Arrhythm Electrophysiol. 2011;4:441–447. doi: 10.1161/CIRCEP.110.960658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamaguchi M, Shimizu M, Ino H, Terai H, Uchiyama K, Oe K, Mabuchi T, Konno T, Kaneda T, Mabuchi H. T wave peak-to-end interval and QT dispersion in acquired long QT syndrome: A new index for arrhythmogenicity. Clin Sci (Lond) 2003;105:671–676. doi: 10.1042/CS20030010. [DOI] [PubMed] [Google Scholar]
- 23.Okin PM, Devereux RB, Fabsitz RR, Lee ET, Galloway JM, Howard BV. Principal component analysis of the T wave and prediction of cardiovascular mortality in American Indians. Circulation. 2002;105:714–719. doi: 10.1161/hc0602.103585. [DOI] [PubMed] [Google Scholar]
- 24.Yan GX, Antzelevitch C. Cellular basis for the normal T wave and the electrocardiographic manifestations of the long-QT syndrome. Circulation. 1998;98:1928–1936. doi: 10.1161/01.cir.98.18.1928. [DOI] [PubMed] [Google Scholar]
- 25.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, St John Sutton M, Stewart WJ. Recommendations for chamber quantification: A report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–133. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 27.Burgess MI, Jenkins C, Sharman JE, Marwick TH. Diastolic stress echocardiography: Hemodynamic validation and clinical significance of estimation of ventricular filling pressure with exercise. J Am Coll Cardiol. 2006;47:1891–1900. doi: 10.1016/j.jacc.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 28.Grewal J, McCully RB, Kane GC, Lam C, Pellikka PA. Left ventricular function and exercise capacity. JAMA. 2009;301:286–294. doi: 10.1001/jama.2008.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGraw K, Wong S. Forming inferences about some intraclass correlation coefficients. Psychol Methods. 1996;1:30–46. [Google Scholar]
- 30.Andersen MP, Xue JQ, Graff C, Hardahl TB, Toft E, Kanters JK, Christiansen M, Jensen HK, Struijk JJ. A robust method for quantification of IKr-related T-wave morphology abnormalities. Comput Cardiol. 2007;34:341–344. [Google Scholar]
- 31.Antzelevitch C. Arrhythmogenic mechanisms of QT prolonging drugs: Is QT prolongation really the problem? J Electrocardiol. 2004;37:15–24. doi: 10.1016/j.jelectrocard.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 32.Couderc JP, McNitt S, Hyrien O, Vaglio M, Xia X, Polonsky S, Moss AJ, Zareba W. Improving the detection of subtle I(Kr)-inhibition: Assessing electrocardiographic abnormalities of repolarization induced by moxifloxacin. Drug Saf. 2008;31:249–260. doi: 10.2165/00002018-200831030-00006. [DOI] [PubMed] [Google Scholar]
- 33.Couderc JP, Vaglio M, Xia X, McNitt S, Wicker P, Sarapa N, Moss AJ, Zareba W. Impaired T-amplitude adaptation to heart rate characterizes I(Kr) inhibition in the congenital and acquired forms of the long QT syndrome. J Cardiovasc Electrophysiol. 2007;18:1299–1305. doi: 10.1111/j.1540-8167.2007.00960.x. [DOI] [PubMed] [Google Scholar]
- 34.Jia S, Lian J, Guo D, Xue X, Patel C, Yang L, Yuan Z, Ma A, Yan G-X. Modulation of the late sodium current by ATX-II and ranolazine affects the reverse use-dependence and proarrhythmic liability of IKr blockade. Br J Pharmacol. 2011;164:308–316. doi: 10.1111/j.1476-5381.2010.01181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lou Q, Fedorov VV, Glukhov AV, Moazami N, Fast VG, Efimov IR. Transmural heterogeneity and remodeling of ventricular excitation-contraction coupling in human heart failure. Circulation. 2011;123:1881–1890. doi: 10.1161/CIRCULATIONAHA.110.989707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Couderc J-P, Xia X, Peterson DR, McNitt S, Zhao H, Polonsky S, Moss AJ, Zareba W. Twave morphology abnormalities in benign, potent, and arrhythmogenic IKr inhibition. Heart Rhythm. 2011;8:1036–1043. doi: 10.1016/j.hrthm.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Antzelevitch C. Cellular basis for the repolarization waves of the ECG. Ann N Y Acad Sci. 2006;1080:268–281. doi: 10.1196/annals.1380.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hopenfeld B, Ashikaga H. Origin of the electrocardiographic U wave: Effects of M cells and dynamic gap junction coupling. Ann Biomed Eng. 2010;38:1060–1070. doi: 10.1007/s10439-010-9941-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ritsema van Eck HJ, Kors JA, van Herpen G. The U wave in the electrocardiogram: A solution for a 100-year-old riddle. Cardiovasc Res. 2005;67:256–262. doi: 10.1016/j.cardiores.2005.04.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.