Abstract
A healthy pregnancy requires strict coordination of genetic, physiologic, and environmental factors. The relatively common incidence of infertility and pregnancy complications has resulted in increased interest in understanding the mechanisms that underlie normal versus abnormal pregnancy. The peptide hormone adrenomedullin has recently been the focus of some exciting breakthroughs in the pregnancy field. Supported by mechanistic studies in genetic animal models, there continues to be a growing body of evidence demonstrating the importance of adrenomedullin protein levels in a variety of human pregnancy complications. With more extensive mechanistic studies and improved consistency in clinical measurements of adrenomedullin, there is great potential for the development of adrenomedullin as a clinically-relevant biomarker in pregnancy and pregnancy complications.
The calcitonin gene-related peptide (CGRP) family
There are a multitude of genetic, physiologic, and environmental factors that must all work in perfect harmony throughout pregnancy to produce the so-called “miracle” that is a healthy, full-term baby. Any aberration in this process may result in pregnancy complications, which can include implantation failure, miscarriage, fetal growth restriction, gestational diabetes, preeclampsia (PE), and preterm birth. Given this complexity, there is a currently major interest and effort in the field, to expand our understanding of the factors that contribute to healthy versus unhealthy pregnancies.
One of these active areas of study is the calcitonin gene-related peptide (CGRP) family and the critical roles these peptides play in female reproductive biology. The CGRP family is composed of five known peptides: CGRP, adrenomedullin (AM), calcitonin (CT), amylin (AMY), and intermedin/adrenomedullin 2 (IMD), that share a similar molecular structure and overlapping biological functions. The critical role of the CGRP family in sustaining life is suggested by the fact that these peptides are highly conserved throughout vertebrate evolution, with CGRP family genes dating as far back as the evolutionarily-distant teleost fish species [1]. The peptides of this family have little sequence homology but share similar secondary structures consisting of an amino acid ring structure formed by a single disulfide bond and a carboxyl terminus amidation [2–4]. CGRP family peptides are widely expressed in both peripheral tissues as well as the central nervous system and they are involved in diverse physiological functions, including vasodilation (AM, CGRP, IMD), angiogenesis (AM, CGRP), pain perception (CGRP), glucose metabolism (AMY), and bone mineral metabolism (CT) [5]. In addition to these previously known roles, emerging research implicates the CGRP family as having multiple essential roles in the establishment and maintenance of the healthy pregnancy. This review will focus on the CGRP family member adrenomedullin and the many well-characterized and emerging roles it has in reproduction.
Adrenomedullin was first identified in 1993 from human pheochromocytoma tissue extracts and is perhaps best known for its potent vasodilatory action [6]. The AM signaling paradigm is a unique one, in which AM binds its G protein-coupled receptor, calcitonin receptor-like receptor (CLR), when the receptor is associated with receptor activity modifying protein 2 or 3 (RAMP2 or 3). The RAMPs dictate ligand binding specificity, so when CLR associates with RAMP1 this complex forms a receptor for the peptide CGRP rather than AM [7]. Other CGRP family members utilize different receptor and RAMP combinations. CT binds the calcitonin receptor without a RAMP present, but when RAMPs1, 2, or 3 associate with the calcitonin receptor it forms a receptor for AMY [2]. The receptor for IMD is not well characterized and is perhaps as yet unknown. This signaling paradigm adds a layer of complexity to interpreting experimental findings related to AM. The fact that CLR binds both CGRP and AM as ligands means that changes in CLR cannot always be extrapolated to indicate changes in AM signaling. Similarly, the RAMP family members interact with other receptors besides CLR [8] so RAMP alterations are also not necessarily specific to AM. Since its discovery, AM signaling has been implicated in numerous biological functions including cellular growth, regulation of blood pressure, protection from vascular hypertrophy and inflammation, inhibition of left ventricular hypertrophy and remodeling, stimulation of diuresis and natriuresis, and promotion of both angiogenesis and lymphangiogenesis [9]. However, recent studies using genetic animal models add complimentary evidence of critical roles for AM in reproductive biology.
Adrenomedullin and normal pregnancy
1. Expression of Adrenomedullin
AM and its receptor components are highly expressed in reproductive tissues, including the uterine endometrium [10], fetal membranes [11], placenta [12], stromal macrophages [13], and trophoblast cells [14–17]. AM expression is regulated by multiple factors involved in the physiology of reproduction. Hypoxia, via hypoxia-inducible factor 1 alpha (HIF-1α), potently upregulates AM expression in multiple tissue types in culture, including placental cytotrophoblast cells [18–20]. The regulation of AM by HIF-1α in hypoxia is of particular relevance to pregnancy because physiological hypoxia in the first trimester placenta is essential for normal trophoblast invasion and proper placental and embryonic development [21]. In contrast, the low oxygen tension that is necessary for first trimester development would be considered pathological in later pregnancy, and late pregnancy hypoxia is associated with complications including preeclampsia and intrauterine growth restriction [22]. Therefore, it is likely that both normal and abnormal levels of oxygen tension during pregnancy directly contribute to the expression and secretion of AM from reproductive tissues. However, the downstream physiological effects of hypoxia-induced AM expression in placental tissues have yet to be resolved.
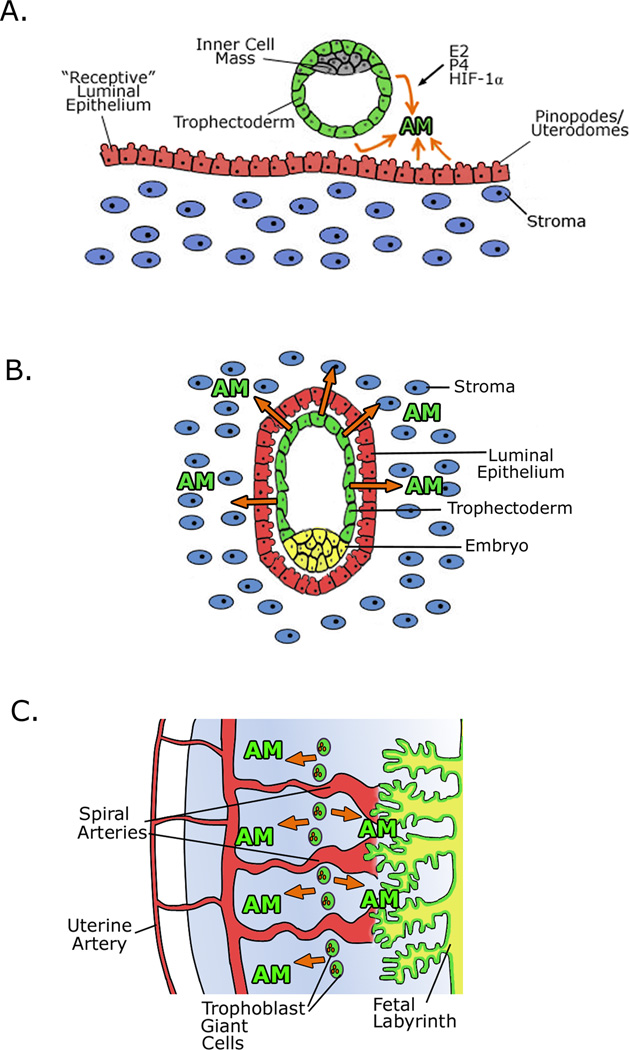
Figure 1 summarizes the pattern of AM expression in multiple stages of pregnancy. Watanabe et. al. administered estrogen to mice and found that with estrogen treatment AM and one of its receptor components, RAMP3, are transcriptionally upregulated in the endometrial stroma, by estrogen receptor alpha (ERα) (Figure 1a) [23]. ERα is expressed in the blastocyst trophectoderm and the uterine luminal epithelium in the peri-implantation period [24–25]. Estradiol levels reach their peak at ovulation, which prepares the uterus for implantation by stimulating cellular proliferation and differentiation of the luminal and glandular epithelium, and by induction of progesterone receptor expression in the endometrial stroma and myometrium. This process, as well as the upregulation of AM and RAMP3 gene expression, fails in mice lacking ERα, resulting in implantation failure and infertility [25]. By genome-wide microarray analysis, RAMP3 was identified as one of the most potently estrogen-induced genes in the uterus [26]; thus it is likely that AM signaling through a CLR/RAMP3 complex plays an important function in regulating some of the estrogenic effects of uterine receptivity and implantation.
Figure 1. Adrenomedullin expression in multiple stages of pregnancy.
A) AM expression is regulated by estrogen (E2), progesterone, and hypoxia inducible factor 1α (HIF-1a). At the pre-implantation stage, AM is expressed by both the trophectoderm cells and the luminal epithelium, promoting uterine receptivity. The maternal components are colored blue and red while the fetal components are depicted in green and gray. B) At the site of implantation, AM continues to be expressed from the trophectoderm cells and from the luminal epithelium, which is important for successful implantation. C) In the developed placenta, AM is most highly expressed by trophoblast giant cells (mice) or extravillous cytotrophoblasts (humans) and may contribute to the maintenance of placental vascular tone. For both B and C, maternal components are colored blue and red and the fetal components are depicted in green and yellow.
The dynamic regulation of AM expression in female reproductive tissues results in significant changes in plasma AM levels during the course of human gestation. During normal pregnancy, plasma AM concentration increases steadily, reaching levels four to five times higher than the pre-pregnancy state by the third trimester [12, 27–31]. Plasma AM levels rapidly drop back to pre-pregnancy values within 24 hours after delivery [12], which supports the notion that maternal plasma AM is derived largely from the placenta.
2. Adrenomedullin in fertility and implantation
An important role of AM in fertility and implantation has come from well-characterized animal models. Recent findings have implicated AM in even the earliest stages of pregnancy. Lei et. al. showed that in a rat model, ovarian AM expression increases from small antral follicles to large antral follicles to the formation of the corpus luteum, and AM appears to be involved in the regulation of progesterone production from the corpus luteum [32]. AM also increases ciliary beat frequency and reduces contraction in the rat oviduct, pointing to a role for AM-mediated regulation of embryo transport to the uterus [33]. Expression of AM and its receptor components are induced in the luminal epithelium of the murine uterus as early as pregnancy day 0.5. By the peri-implantation period, AM is expressed both by the blastocyst trophectoderm and the uterine luminal epithelium and stroma at the implantation site (Figure 1a–b) [34]. Therefore, the peptide is abundantly expressed throughout the female reproductive tract from the earliest stages of pregnancy.
Homozygous deletion of Adm results in embryonic lethality with abnormal development of the heart and lymphatic vascular system [35]. However, female mice heterozygous for Adm (50% AM expression) survive and are a very useful model for the study of haploinsufficiency of AM during pregnancy. Adm+/− female mice have a significantly reduced pregnancy success rate compared to wild type females, despite the fact that ovulation and fertilization occurs normally in these mice. This reduced pregnancy rate persists even when wild type blastocysts are transferred to the Adm+/− female, indicating that reduced maternal AM is responsible for the uterine receptivity defects in this model [36].
Furthermore, Adm+/− female mice have reduced numbers of uterine pinopodes (referred to as uterodomes in humans), which are plasma membrane extravasations of the uterine luminal epithelium that faithfully mark the window of uterine receptivity [34]. Even when implantation is successful in the Adm+/− female, fertility defects persist. The litter sizes of Adm+/− female mice are reduced when mated to wild type males, while normal litter sizes are born to wild type females mated to Adm+/− males. The implantation sites in pregnant Adm+/− females are abnormally spaced and overcrowded, resulting in increased rates of embryo loss and remarkable subfertility [36]. Therefore, even a modest 50% reduction in maternal expression of AM is sufficient to cause major implantation and fertility complications in genetic mouse models.
This strong genetic and physiological evidence from rodent models is beginning to be translated to clinical medicine as well. For example, Marinoni et. al. have recently found that elevated AM levels in follicular fluid is associated with negative outcomes in in vitro fertilization patients [37].
3. AM in placentation and maternal-fetal circulation
One of the most essential maternal responses to pregnancy is the vascular remodeling of uterine spiral arteries, which ensures adequate blood flow to the developing fetus. The development of the placenta is central to this process. The earliest stages of placental development in humans and rodents occur during implantation, when trophectoderm cells from the blastocyst attach and invade into the wall of the receptive uterus. These trophectoderm cells differentiate into multinucleate trophoblast cells termed extravillous cytotrophoblasts in humans and giant trophoblast cells in rats and mice, which invade the uterine lining and establish the vascular connection between fetal placental tissue and the maternal blood supply [38]. High AM expression is present in the trophectoderm cells and persists in trophoblast giant cells in the mouse [17, 36] while AM expression in the extravillous cytotrophoblast lineage has been shown in the normal term placenta in humans (Figure 1c) [14–15, 39]. Support for a role for AM in trophoblast invasion has come from in vitro studies. Zhang et. al. showed that AM induces proliferation and invasion in JAr cells, a choriocarcinoma cell line, and in HTR-8/SV neo cells, a first-trimester cytotrophoblast cell line [40]. In isolated fetoplacental vascular beds and stem villous arteries previously constricted with a thromboxane sympathomimetic, AM infusion induces a dose-dependent vasodilation, suggesting that AM may help maintain low placental vascular resistance [41–42]. Recently, Ross et. al. found that AM treatment in rats induces relaxation of the uterine artery and this effect is enhanced in pregnancy or with estradiol treatment, providing further evidence for a functional role for AM in maintaining vascular tone in pregnancy [43]. In women with unexplained recurrent pregnancy loss, high plasma AM was associated with increased uterine artery pulsatily index and an increased number of previous miscarriages, from which the authors suggest that increased AM may be acting in a compensatory role [44].
Adrenomedullin in pregnancy complications
Defects in the ability of trophectoderm cells to fully invade the maternal uterine wall and remodel vessels are thought to underlie many serious reproductive conditions [45–46]. Given that female mice heterozygous for Adm exhibit marked subfertility, and that homozygous deletion of Adm causes embryonic lethality, it is not surprising that altered AM expression has been associated with several of these pregnancy complications. Table 1 summarizes many of the clinical studies that have measured AM in pregnancy complications.
Table 1. AM levels in human pregnancy complications.
Studies investigating AM levels in intrauterine growth restriction, gestational diabetes, preeclampsia, and preterm labor vary widely based on the source of the sample, method of AM measurement, and the magnitude and direction of change in AM levels. Bold text indicates increased AM levels and itaclic text represents reduced AM levels. This wide variation underscores the need for more reliable techniques for sample collection and quantification of AM levels.
| Pregnancy Complication |
Sample Source |
Sample Type |
Assay | Change in Adrenomedullin Levels |
Reference |
|---|---|---|---|---|---|
| Intrauterine Growth Restriction |
umbilical plasma |
AM protein |
commercial radioimmunoassay |
↑ 1.67X in IUGR |
45 |
| Intrauterine Growth Restriction |
maternal and cord venous blood |
AM protein |
"home brewed" radioimmunoassay |
No change |
48 |
| Reduced Fetal Growth |
amniotic fluid | AM protein |
"home brewed" radioimmunoassay |
AM levels inversely correlate with birth weight and height |
46 |
| Intrauterine Growth Restriction |
umbilical and maternal plasma |
AM protein |
HPLC |
No change |
47 |
| Gestational Diabetes |
maternal plasma |
AM protein |
commercial radioimmunoassay |
No change |
49 |
| Gestational Diabetes and Pregnant Women with Type I Diabetes |
maternal and fetal plasma, amniotic fluid |
AM protein |
commercial radioimmunoassay |
↑ 1.36X in amniotic fluid of diabetic pregnancies; no change in maternal or fetal plasma |
50 |
| Pregnant Women with Type I Diabetes |
maternal plasma |
AM protein |
"home brewed" radioimmunoassay |
No change |
51 |
| Gestational Diabetes |
maternal plasma |
AM protein |
"home brewed" radioimmunoassay |
No change |
52 |
| Preeclampsia | maternal plasma |
AM protein |
commercial radioimmunoassay |
↓1.35X in PE |
54 |
| Preeclampsia | amniotic fluid, maternal and umbilical plasma |
AM protein |
commercial radioimmunoassay |
↑2.25X in amniotic fluid and ↑2.13X in umbilical plasma in PE; no change in maternal plasma |
58 |
| Preeclampsia | maternal plasma |
AM protein |
"home-brewed" radioimmunoassay |
No change |
11 |
| Preeclampsia | maternal plasma |
AM protein |
commercial radioimmunoassay |
↑2.5X in PE |
59 |
| Preeclampsia | maternal plasma |
AM protein |
"home-brewed" radioimmunoassay |
No change |
38 |
| Preeclampsia | placental villi | AM mRNA |
qRT-PCR |
↓1.83X expression in PE |
56 |
| Preeclampsia | purified cytotrophoblast cultures |
AM protein and mRNA |
commercial radioimmunoassay, Northern blot |
↓2.91X mRNA and ↓5X protein in PE |
57 |
| Preeclampsia | choriodecudia, amnion, and placental tissue |
AM protein and mRNA |
commercial radioimmunoassay, Northern blot |
↑1.66–2.9X in choriodecidua, ↑1.63–2.26X in amnion; no change in placental tissue |
60 |
| Preeclampsia | maternal plasma |
AM protein |
ELISA |
↑1.3X in PE |
29 |
| Preeclampsia | maternal plasma |
AM protein |
HPLC |
↓1.92X in PE |
63 |
| Preeclampsia | placental tissue | AM mRNA | qRT-PCR |
↓1.18X mRNA in PE |
61 |
| Preeclampsia | maternal plasma |
AM protein |
ELISA |
↑1.07X in PE |
62 |
| Preterm Labor / Premature Rupture of Membranes |
amniotic fluid | AM protein |
commercial radioimmunoassay |
↑1.75X in PROM |
64 |
| Preterm Labor | maternal and fetal plasma, amniotic fluid |
AM protein |
commercial radioimmunoassay |
↑2.95X in amniotic fluid in preterm labor; no change in maternal or fetal plasma |
65 |
| Preterm Delivery | amniotic fluid | AM protein |
"home-brewed" radioimmunoassay |
↑ 1.40X in preterm delivery |
46 |
| Preterm Labor | amniotic fluid | AM protein |
ELISA |
No change |
67 |
IUGR = Intrauterine Growth Restriction, PE = Preeclampsia, RIA= radioimmunoassay, T1DM = type 1 diabetes mellitus, qRT-PCR = Real-Time Quantitative Reverse Transcription PCR, ELISA = Enzyme-linked immunosorbent assay, HPLC = High-performance liquid chromatography
1. Intrauterine growth restriction
Numerous rodent models have provided evidence for the necessity of AM in normal fetal growth. Yallampalli’s laboratory found that antagonism of AM during pregnancy resulted in intrauterine growth restriction (IUGR), abnormal placental vascularization, and increased fetal resorption, in the rat [47–48]. Similar studies in the mouse have shown that Adm+/− mothers have a high rate of fetal growth restriction, which occurs in all fetal genotypes. The incidence of fetal growth restriction was highest among Adm−/− embryos, indicating that both maternal and fetal AM may contribute to normal fetal growth [36].
Results from human studies have not been as consistent. Two early studies found that elevated AM levels in the umbilical plasma and amniotic fluid, respectively, were associated with reduced fetal growth [49–50], which could be a compensatory effect. However, a 2001 study by Yamashiro et. al. and a more recent study by Akturk et. al. showed no difference in AM concentrations between small for gestational age and appropriate for gestational age infants [51–52]. Based on animal studies, it is likely that altered AM levels may contribute to either the development of IUGR or the resulting adaptive compensation to other primary causes of IUGR. However, the inconsistencies between studies in the human population points to the necessity of further studies to determine with certainty how changes in AM levels may be involved in the pathogenesis of growth restricted pregnancies.
2. Adrenomedullin in gestational diabetes
To date, there is relatively limited data available on whether AM levels are altered in gestational diabetes. Martinez et. al. showed no change in plasma AM levels in pregnant women with gestational diabetes, compared to pregnant women without gestational diabetes [53]. Di Iorio et. al. also found that AM levels were not changed in the maternal circulation, but found higher AM levels in the amniotic fluid of pregnant diabetic women [54]. Plasma AM was found to be unchanged in pregnant women with type I diabetes mellitus (T1DM) [55], and in a subsequent study AM was found to be unchanged in the plasma of women with gestational diabetes [56]. These data suggest that circulating AM may not be altered in pregnant women with gestational diabetes, but fetal or placental AM production may be elevated, potentially resulting in the observed increased amniotic fluid AM concentration.
3. Adrenomedullin in preeclampsia
Based on the known roles for AM in trophoblast invasion and vascular adaptation to pregnancy, there is significant interest in determining whether changes in AM peptide or expression levels contributes to the pathogenesis of preeclampsia (PE). Results from an experimental rat model of maternal hypertension further piqued this interest. In this model, whereby administration of the inhibitor of nitric oxide synthases L-NAME (nitro-L-arginine methyl ester) during gestation, results in hypertension and pup mortality, maternal infusion of AM attenuated the hypertensive phenotype [57].
However, results from human studies have been highly variable and controversial. One of the earliest studies examining AM levels in PE, by Hata et. al., found that circulating AM levels were reduced in women with PE [58]. But a multitude of other studies have found markedly variable results. For example, studies have shown either decreased [59–61] or increased [14] AM mRNA levels in placental tissues of preeclamptic patients. Attempts to look at AM protein levels have been equally variable. AM peptide production from purified cytotrophoblast cultures of preeclamptic patients was shown to be decreased [62], and the majority of studies looking at plasma levels of AM have been inconsistent, showing both increased [31, 63–65], decreased [66], and unchanged [12, 42] AM levels in PE. Al-Ghafra et. al. attempted to clarify the role of AM in PE by limiting their study to patients with severe PE and by separating patients by term versus preterm delivery; they found that AM protein levels were increased in fetal membranes in PE patients with both term and preterm gestation, and AM mRNA levels were also increased in preterm choriodecidual tissue in PE patients [67]. Though there is strong evidence for altered AM levels being either a cause or a secondary effect in PE, it is clear that more controlled experiments to address the direction of the change and the exact role of AM in PE are needed.
4. Adrenomedullin and preterm birth
It has long been thought that altered AM levels may be present in cases of preterm birth. Human studies dating back over 10 years ago have suggested that AM protein may be elevated in patients with preterm birth. The Di Iorio laboratory has published several studies on this subject, finding increased amniotic AM levels in cases of preterm premature rupture of membranes (PPROM) [68], and increased amniotic fluid AM in patients with preterm labor [69]. Elevated second trimester amniotic fluid levels of AM have also been reported in patients that go on to preterm deliveries [50]. Glucocorticoids may be involved in the regulation of AM levels in pregnancy, as administration of betamethasone to stimulate fetal lung maturity in patients at risk for preterm birth resulted in significantly increased maternal and fetal plasma AM levels [70]. However, a 2009 study by Iavazzo et. al. found that there was no change in AM levels in spontaneous preterm delivery or PPROM [71], suggesting that further studies may still be needed in this area.
In addition to genetic and environmental causes, it is interesting to speculate as to whether AM may play a role in another major cause of preterm birth – ascending intrauterine bacterial infections, which are thought to account for 25–40% of preterm births [72]. The spread of microorganisms to the amniotic cavity causes the activation of toll-like receptors and the release of proinflammatory cytokines, leading to increased prostaglandins and the stimulation of premature uterine contractions and degradation of fetal membranes [73–74]. There are multiple characteristics of AM that implicate it as a regulator of innate immunity and host defense [75]. These include its six-member cysteine ring structure which is a characteristic of both human and murine beta-defensins [76] and which allows these molecules to penetrate bacterial cell membranes, and the known anti-inflammatory effects of AM [77]. The potent bactericidal properties of AM [78] and its high level of expression in the skin, oral cavity cervical mucosa, fetal membranes, and breast milk [11, 79] also support its role as a possible mediator of host defense. Future studies exploring a potential role for AM in providing antimicrobial effects in relation to preterm birth have the potential to be highly informative.
5. Caveats to current AM detection methods and MR-proADM
The broad expression pattern of AM, its variety of biological activities during pregnancy, and the variation in clinical criteria between studies are complicating factors that may be responsible for some of the inconsistencies in characterizing AM levels in abnormal pregnancies (Table 1). However, the assay systems used to measure AM are also likely culprits in this controversy. The accurate measurement of AM is technically challenging, due in large part to the short half life of AM peptide (22 min) [80], and that it is highly unstable and “sticky”. Issues regarding antibody specificity and species differences in detection methods can also be confounding factors. However, the precursor prohormone to AM, the midregional fragment of pro-adrenomedullin (MR-proADM) has been identified as a stable circulating peptide [81]. MR-proADM is an excellent proxy for measuring AM levels as it is highly stable, in contrast to the mature form of AM [82]. Measurement of MR-proADM via a sandwich fluoroimmunoassay has been described [82–83] and has been used to measure AM in patients with sepsis [84], community-acquired pneumonia [85], and heart failure [86–87] and demonstrates excellent clinical utility for MR-proADM as a diagnostic and prognostic biomarker.
Application of this new assay has been limited in the reproductive and pregnancy field so far, but to date two groups have measured MR-proADM levels in neonates. Miguel et. al. established reference values for MR-proADM in umbilical cord blood of newborn infants [88], while Cao et. al. found that serum levels of MR-proADM were significantly elevated by more than two-fold in newborns with severe infection, compared to those with mild or no infection [89]. Though this study was performed in newborns, it provides an interesting correlation with the hypothesis that AM may act as a regulator in host defense preventing infections during pregnancy, and suggests that this AM precursor could potentially be used as a laboratory marker of bacterial infection in neonates. Further studies utilizing the MR-proADM assay in the study of pregnancy have the potential to yield newly accurate and exciting results, improving our understanding of how AM levels change in healthy versus complicated pregnancies in humans and perhaps even validating MR-proAM as a potential biomarker for pregnancy-related diseases.
6. Adrenomedullin system polymorphisms
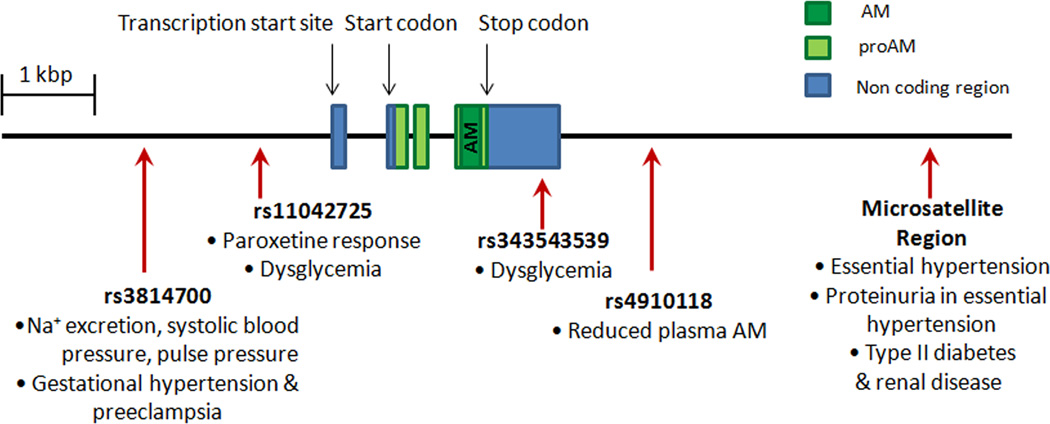
Though the intriguing results from human studies characterizing AM levels in pregnancy conditions are still relatively inconclusive when taken together, the potential clinicial significance of altered levels of AM and its receptor components is bolstered by the discovery of polymorphisms in the human AM system genes. Figure 2 depicts the structure of the ADM gene and the location of clinically relevant polymorphisms. The ADM gene is flanked by a 3’ microsatellite cytosine adenine (CA) repeat region, and in a Japanese patient population, an ADM allele with 19 CA repeats was found with significantly increased frequency in individuals with essential hypertension (EH) compared to normotensive controls [90]. This same polymorphism has been associated with predisposition to proteinuria in EH and is found with greater frequency in type II diabetic patients in renal failure compared to controls or diabetic patients without nephropathy [91–92]. Specific single nucleotide polymorphisms (SNPs) in AM have also been associated with reduced systolic blood pressure, lower pulse pressure, and reduced urinary sodium excretion in a Chinese population [93], increased risk of developing dysglycemia in a Chinese population [94], and altered response to the antidepressant paroxetine [95]. A particular SNP in the AM receptor gene, CALCRL was found with increased frequency specifically among women with EH in a Japanese population [96], and other studies have found CLR SNPs associated with severe periodontitis [97] and acute primary angle closure glaucoma [98]. Recently, different ADM haplotypes have been shown to correlate with changes in plasma AM levels [99], making the connection between polymorphisms in the ADM gene and level of expression.
Figure 2. Clinically relevant AM polymorphisms.
The structure of the adrenomedullin gene and the location of clinically relevant AM polymorphisms are shown. The dark green box represents AM, light green represents proAM, the prohormone for both AM and proadrenomedullin N-terminal 20 peptide (PAMP). and blue represents noncoding regions of the AM gene. Polymorphisms in AM have been associated with changes in plasma AM levels, drug responses, blood pressure, proteinuria, renal disease, dysglycemia, and preeclampsia.
With relation to the important role of AM signaling in female reproduction, a study by Wang et. al. tested SNPs from 41 genes associated with fetal growth. Interestingly, a SNP in the AM receptor CALCRL, was one of just six SNPs in five genes that were significantly associated with birth weight in a population of African-American women [100], indicating that CLR levels (and by extension, AM signaling) may be important for fetal growth in human subjects. Boc-Zalewska et. al. have now shown that ADM gene polymorhpisms are associated with changes in AM mRNA expression and increased incidence of gestational hypertension and preeclampsia [61, 65], providing additional evidence for a role for AM in the pathogenesis of this condition. Further characterization of ADM polymorphisms and whether they are associated with pregnancy complications is an emerging translational research target with great potential.
Concluding Remarks
The importance of CGRP family peptide AM in the establishment and maintenance of the healthy pregnancy is clearly supported by many studies spanning the past 15 years. Our understanding of the functions of AM in normal and in complicated pregnancies has advanced significantly in the past five years in large part to studies using genetic animal models. However, unanswered questions remain. Inconsistent results from human studies attempting to characterize changes in AM levels in pregnancy-related diseases have left us with few definitive conclusions. A newly available method to detect AM with accuracy holds great promise in clarifying these variable findings. Furthermore, additional in vitro and in vivo methods should be employed to gain a greater understanding of the mechanisms by which AM mediates its effects in reproductive biology, with the intention of linking the biological functions of the peptide to physiologically relevant paradigms such as host defense, vasodilation, angiogenesis or regulation of innate immunity. While there is still much to learn, given the essential roles of AM in reproduction, there is potential for the development of AM as a clinically-relevant biomarker in pregnancy and pregnancy complications.
Outstanding Questions.
What are the downstream physiological effects of hypoxia-induced AM expression in placental tissue?
What role does AM signaling through the CLR/RAMP3 complex play in regulating estrogenic effects in the uterus?
Are AM levels altered in human pregnancy complications such as intrauterine growth restriction, gestational diabetes, preeclampsia, or preterm birth?
If AM levels are altered in human pregnancy complications, are the changes in AM involved in the pathogenesis of the conditions or are they a compensatory effect?
What role does the antimicrobial properties of AM play in relation to normal reproductive physiology and preterm birth?
Can the application of newer detection methods for AM (i.e. the MR-proADM assay) be used to clarify conflicting clinical findings regarding changes in AM in pregnancy-related conditions?
Do polymorphisms in the ADM gene have clinically significant effects in human pregnancy?
What are the downstream molecular mechanisms by which AM exerts its effects during pregnancy?
Can AM be developed as a biomarker or therapeutic target in pregnancy and pregnancy complications?
Acknowledgements
The Authors would like to thank past and current members of the Caron Laboratory for their helpful comments and discussions. This work was supported by NIH/NICHD HD060860, The March of Dimes Birth Defects Foundation and The Burrouhgs Wellcome Fund grants to KMC and NIH/NHLBI F30 Fellowship HL104778 to PML.
Glossary
- Adrenomedullin (AM=peptide, ADM=gene)
a multifunctional 52-amino acid peptide hormone that belongs to the calcitonin gene-related peptide family. In humans, AM is highly expressed in endothelial cells and vascular smooth muscle cells and thus is produced at high levels in highly vascularized tissues including the placenta, lung, heart, and kidney. AM acts as a potent vasodilator, angiogenic factor, growth factor, natriuretic factor, immune modulator, and antimicrobial factor.
- Amylin (AMY)
a 37-amino acid peptide hormone that belongs to the calcitonin gene-related peptide family. AMY is co-secreted from pancreatic islet cells along with insulin. It acts to inhibit gastric emptying and gastric acid secretion resulting in reduced nutrient intake.
- Calcitonin (CT)
a 32-amino acid peptide hormone that belongs to the calcitonin gene-related peptide family. In humans, it is produced by the parafollicular cells of the thyroid. It acts to reduce blood calcium levels by opposing the effects of parathyroid hormone.
- Calcitonin gene-related peptide (CGRP)
a 37-amino acid peptide that belongs to the calcitonin gene-related peptide family. CGRP is highly expressed in central and peripheral neurons and functions in pain perception. It is a potent vasodilator and can influence uterine contractility.
- Calcitonin gene-related peptide family
a family of peptides composed of five known members: calcitonin gene-related peptide (CGRP), adrenomedullin (AM) calcitonin (CT), amylin (AMY), and intermedin/adrenomedullin 2 (IMD). These peptides share a similar molecular structure and overlapping biological functions.
- Calcitonin receptor-like receptor (CLR=peptide, CALCRL=gene)
a G protein-coupled receptor that is related to the calcitonin receptor. CLR functionally dimerizes to one of three single transmembrane domain receptor activity-modifying proteins (RAMPs) that are essential for its binding to AM and CGRP ligands.
- Intermedin (IMD)/Adrenomedullin 2
a 47-amino acid peptide that belongs to the calcitonin gene-related peptide family. Intermedin is highly expressed in the pituitary and gastrointestinal tract. In mice, it inhibits gastric emptying and food intake and acts as a vasodilator.
- Receptor Activity Modifying Protein (RAMP1, -2, -3)
a family of chaperone proteins that modulate G protein-coupled receptor trafficking, ligand binding and G protein-coupling efficiency consisting of three members, RAMP1, 2, and 3. RAMPs were first identified in association with the calcitonin receptor-like receptor (CLR). Association of CLR with RAMP1 forms a receptor that binds to CGRP, while association of RAMP2 or RAMP3 with CLR forms a receptor that binds to AM. Since their discovery, RAMPs have been shown to partner with a variety of different G protein-coupled receptors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ogoshi M, et al. Evolutionary history of the calcitonin gene-related peptide family in vertebrates revealed by comparative genomic analyses. Peptides. 2006;27:3154–3164. doi: 10.1016/j.peptides.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Poyner DR, et al. International Union of Pharmacology. XXXII. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol Rev. 2002;54:233–246. doi: 10.1124/pr.54.2.233. [DOI] [PubMed] [Google Scholar]
- 3.Takei Y, et al. Identification of novel adrenomedullin in mammals: a potent cardiovascular and renal regulator. FEBS Lett. 2004;556:53–58. doi: 10.1016/s0014-5793(03)01368-1. [DOI] [PubMed] [Google Scholar]
- 4.Roh J, et al. Intermedin is a calcitonin/calcitonin gene-related peptide family peptide acting through the calcitonin receptor-like receptor/receptor activity-modifying protein receptor complexes. J Biol Chem. 2004;279:7264–7274. doi: 10.1074/jbc.M305332200. [DOI] [PubMed] [Google Scholar]
- 5.Muff R, et al. Biological importance of the peptides of the calcitonin family as revealed by disruption and transfer of corresponding genes. Peptides. 2004;25:2027–2038. doi: 10.1016/j.peptides.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Kitamura K, et al. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun. 1993;192:553–560. doi: 10.1006/bbrc.1993.1451. [DOI] [PubMed] [Google Scholar]
- 7.McLatchie LM, et al. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- 8.Christopoulos A, et al. Novel receptor partners and function of receptor activity-modifying proteins. J Biol Chem. 2003;278:3293–3297. doi: 10.1074/jbc.C200629200. [DOI] [PubMed] [Google Scholar]
- 9.Kuwasako K, et al. Shared and separate functions of the RAMP-based adrenomedullin receptors. Peptides. 2011;32:1540–1550. doi: 10.1016/j.peptides.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 10.Hague S, et al. Expression of the hypoxically regulated angiogenic factor adrenomedullin correlates with uterine leiomyoma vascular density. Clin Cancer Res. 2000;6:2808–2814. [PubMed] [Google Scholar]
- 11.Trollmann R, et al. Adrenomedullin gene expression in human placental tIssue and leukocytes: a potential marker of severe tIssue hypoxia in neonates with birth asphyxia. Eur J Endocrinol. 2002;147:711–716. doi: 10.1530/eje.0.1470711. [DOI] [PubMed] [Google Scholar]
- 12.Minegishi T, et al. Adrenomedullin and atrial natriuretic peptide concentrations in normal pregnancy and pre-eclampsia. Mol Hum Reprod. 1999;5:767–770. doi: 10.1093/molehr/5.8.767. [DOI] [PubMed] [Google Scholar]
- 13.Zhao Y, et al. PCR display identifies tamoxifen induction of the novel angiogenic factor adrenomedullin by a non estrogenic mechanism in the human endometrium. Oncogene. 1998;16:409–415. doi: 10.1038/sj.onc.1201768. [DOI] [PubMed] [Google Scholar]
- 14.Gratton RJ, et al. Adrenomedullin messenger ribonucleic acid expression in the placentae of normal and preeclamptic pregnancies. J Clin Endocrinol Metab. 2003;88:6048–6055. doi: 10.1210/jc.2003-030323. [DOI] [PubMed] [Google Scholar]
- 15.Marinoni E, et al. Immunoreactive adrenomedullin in human fetoplacental tissues. Am J Obstet Gynecol. 1998;179:784–787. doi: 10.1016/s0002-9378(98)70083-3. [DOI] [PubMed] [Google Scholar]
- 16.Montuenga LM, et al. Expression of adrenomedullin and its receptor during embryogenesis suggests autocrine or paracrine modes of action. Endocrinology. 1997;138:440–451. doi: 10.1210/endo.138.1.4881. [DOI] [PubMed] [Google Scholar]
- 17.Yotsumoto S, et al. Expression of adrenomedullin, a hypotensive peptide, in the trophoblast giant cells at the embryo implantation site in mouse. Dev Biol. 1998;203:264–275. doi: 10.1006/dbio.1998.9073. [DOI] [PubMed] [Google Scholar]
- 18.Nakayama M, et al. Induction of adrenomedullin by hypoxia and cobalt chloride in human colorectal carcinoma cells. Biochem Biophys Res Commun. 1998;243:514–517. doi: 10.1006/bbrc.1998.8131. [DOI] [PubMed] [Google Scholar]
- 19.Cormier-Regard S, et al. Adrenomedullin gene expression is developmentally regulated and induced by hypoxia in rat ventricular cardiac myocytes. J Biol Chem. 1998;273:17787–17792. doi: 10.1074/jbc.273.28.17787. [DOI] [PubMed] [Google Scholar]
- 20.Marinoni E, et al. Regulation by hypoxia of adrenomedullin output and expression in human trophoblast cells. Eur J Obstet Gynecol Reprod Biol. 2011;154:146–150. doi: 10.1016/j.ejogrb.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 21.Patel J, et al. Regulation of hypoxia inducible factors (HIF) in hypoxia and normoxia during placental development. Placenta. 2010;31:951–957. doi: 10.1016/j.placenta.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Schneider H. Oxygenation of the placental-fetal unit in humans. Respir Physiol Neurobiol. 2011;178:51–58. doi: 10.1016/j.resp.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe H, et al. The estrogen-responsive adrenomedullin and receptor-modifying protein 3 gene identified by DNA microarray analysis are directly regulated by estrogen receptor. J Mol Endocrinol. 2006;36:81–89. doi: 10.1677/jme.1.01825. [DOI] [PubMed] [Google Scholar]
- 24.Hou Q, et al. Immunolocalization of estrogen receptor protein in the mouse blastocyst during normal and delayed implantation. Proc Natl Acad Sci U S A. 1996;93:2376–2381. doi: 10.1073/pnas.93.6.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 26.Hewitt SC, et al. Global uterine genomics in vivo: microarray evaluation of the estrogen receptor alpha-growth factor cross-talk mechanism. Mol Endocrinol. 2005;19:657–668. doi: 10.1210/me.2004-0142. [DOI] [PubMed] [Google Scholar]
- 27.Hayashi Y, et al. Circulating mature adrenomedullin is related to blood volume in full-term pregnancy. Anesth Analg. 2005;101:1816–1820. doi: 10.1213/01.ANE.0000182329.02880.83. [DOI] [PubMed] [Google Scholar]
- 28.Di Iorio R, et al. Adrenomedullin in pregnancy. Lancet. 1997;349:328. doi: 10.1016/s0140-6736(05)62827-9. [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi K, et al. Immunoreactive adrenomedullin (AM) concentration in maternal plasma during human pregnancy and AM expression in placenta. Eur J Endocrinol. 2000;142:683–687. doi: 10.1530/eje.0.1420683. [DOI] [PubMed] [Google Scholar]
- 30.Hoshimoto K, et al. Mature adrenomedullin concentrations in plasma during pregnancy. J Matern Fetal Neonatal Med. 2002;11:126–129. doi: 10.1080/jmf.11.2.126.129. [DOI] [PubMed] [Google Scholar]
- 31.Senna AA, et al. Study of plasma adrenomedullin level in normal pregnancy and preclampsia. Medscape J Med. 2008;10:29. [PMC free article] [PubMed] [Google Scholar]
- 32.Li L, et al. Adrenomedullin in rat follicles and corpora lutea: expression, functions and interaction with endothelin-1. Reprod Biol Endocrinol. 2011;9:111. doi: 10.1186/1477-7827-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liao SB, et al. Adrenomedullin increases ciliary beat frequency and decreases muscular contraction in the rat oviduct. Reproduction. 2011;141:367–372. doi: 10.1530/REP-10-0230. [DOI] [PubMed] [Google Scholar]
- 34.Li M, et al. Haploinsufficiency for Adrenomedullin Reduces Pinopodes and Diminishes Uterine Receptivity in Mice. Biol Reprod. 2008 doi: 10.1095/biolreprod.108.069336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fritz-Six KL, et al. Adrenomedullin signaling is necessary for murine lymphatic vascular development. J Clin Invest. 2008;118:40–50. doi: 10.1172/JCI33302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li M, et al. Reduced maternal expression of adrenomedullin disrupts fertility, placentation, and fetal growth in mice. J Clin Invest. 2006;116:2653–2662. doi: 10.1172/JCI28462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marinoni E, et al. Intrafollicular concentration of adrenomedullin is associated with IVF outcome. Gynecol Endocrinol. 2010;26:435–439. doi: 10.3109/09513591003632076. [DOI] [PubMed] [Google Scholar]
- 38.Lee KY, DeMayo FJ. Animal models of implantation. Reproduction. 2004;128:679–695. doi: 10.1530/rep.1.00340. [DOI] [PubMed] [Google Scholar]
- 39.Nikitenko LL, et al. Differential and cell-specific expression of calcitonin receptor-like receptor and receptor activity modifying proteins in the human uterus. Mol Hum Reprod. 2001;7:655–664. doi: 10.1093/molehr/7.7.655. [DOI] [PubMed] [Google Scholar]
- 40.Zhang X, et al. Adrenomedullin enhances invasion by trophoblast cell lines. Biol Reprod. 2005;73:619–626. doi: 10.1095/biolreprod.105.040436. [DOI] [PubMed] [Google Scholar]
- 41.Hoeldtke NJ, et al. Vasodilatory response of fetoplacental vasculature to adrenomedullin after constriction with the thromboxane sympathomimetic U46619. Am J Obstet Gynecol. 2000;183:1573–1578. doi: 10.1067/mob.2000.108071. [DOI] [PubMed] [Google Scholar]
- 42.Jerat S, et al. Effect of adrenomedullin on placental arteries in normal and preeclamptic pregnancies. Hypertension. 2001;37:227–231. doi: 10.1161/01.hyp.37.2.227. [DOI] [PubMed] [Google Scholar]
- 43.Ross GR, et al. Adrenomedullin relaxes rat uterine artery: mechanisms and influence of pregnancy and estradiol. Endocrinology. 2010;151:4485–4493. doi: 10.1210/en.2010-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.El-mashad AI, et al. Role of uterine artery Doppler velocimetry indices and plasma adrenomedullin level in women with unexplained recurrent pregnancy loss. J Obstet Gynaecol Res. 2011;37:51–57. doi: 10.1111/j.1447-0756.2010.01318.x. [DOI] [PubMed] [Google Scholar]
- 45.Chaddha V, et al. Developmental biology of the placenta and the origins of placental insufficiency. Semin Fetal Neonatal Med. 2004;9:357–369. doi: 10.1016/j.siny.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 46.Lala PK, Chakraborty C. Factors regulating trophoblast migration and invasiveness: possible derangements contributing to pre-eclampsia and fetal injury. Placenta. 2003;24:575–587. doi: 10.1016/s0143-4004(03)00063-8. [DOI] [PubMed] [Google Scholar]
- 47.Witlin AG, et al. Placental and fetal growth and development in late rat gestation is dependent on adrenomedullin. Biol Reprod. 2002;67:1025–1031. doi: 10.1095/biolreprod.101.002196. [DOI] [PubMed] [Google Scholar]
- 48.Penchalaneni J, et al. Adrenomedullin antagonist treatment during early gestation in rats causes fetoplacental growth restriction through apoptosis. Biol Reprod. 2004;71:1475–1483. doi: 10.1095/biolreprod.104.032086. [DOI] [PubMed] [Google Scholar]
- 49.Di Iorio R, et al. Adrenomedullin is increased in the fetoplacental circulation in intrauterine growth restriction with abnormal umbilical artery waveforms. Am J Obstet Gynecol. 2000;182:650–654. doi: 10.1067/mob.2000.103944. [DOI] [PubMed] [Google Scholar]
- 50.Yamashiro C, et al. Adrenomedullin concentrations in early 2nd-trimester amniotic fluid: relation to preterm delivery and fetal growth at birth. Gynecol Obstet Invest. 2002;54:99–104. doi: 10.1159/000067720. [DOI] [PubMed] [Google Scholar]
- 51.Akturk A, et al. Maternal and umbilical venous adrenomedullin and nitric oxide levels in intrauterine growth restriction. J Matern Fetal Neonatal Med. 2007;20:521–525. doi: 10.1080/14767050701412263. [DOI] [PubMed] [Google Scholar]
- 52.Yamashiro C, et al. Plasma adrenomedullin levels in pregnancies with appropriate for gestational age and small for gestational age infants. J Perinat Med. 2001;29:513–518. doi: 10.1515/JPM.2001.071. [DOI] [PubMed] [Google Scholar]
- 53.Martinez A, et al. Is adrenomedullin a causal agent in some cases of type 2 diabetes? Peptides. 1999;20:1471–1478. doi: 10.1016/s0196-9781(99)00158-8. [DOI] [PubMed] [Google Scholar]
- 54.Di Iorio R, et al. Fetomaternal adrenomedullin levels in diabetic pregnancy. Horm Metab Res. 2001;33:486–490. doi: 10.1055/s-2001-16942. [DOI] [PubMed] [Google Scholar]
- 55.Loukovaara S, et al. Vasoactive mediators and retinopathy during type 1 diabetic pregnancy. Acta Ophthalmol Scand. 2005;83:57–62. doi: 10.1111/j.1600-0420.2005.00384.x. [DOI] [PubMed] [Google Scholar]
- 56.Poyhonen-Alho M, et al. Imbalance of the autonomic nervous system at night in women with gestational diabetes. Diabet Med. 2010;27:988–994. doi: 10.1111/j.1464-5491.2010.03062.x. [DOI] [PubMed] [Google Scholar]
- 57.Makino I, et al. Adrenomedullin attenuates the hypertension in hypertensive pregnant rats induced by N(G)-nitro-L-arginine methyl ester. Eur J Pharmacol. 1999;371:159–167. doi: 10.1016/s0014-2999(99)00151-x. [DOI] [PubMed] [Google Scholar]
- 58.Hata T, et al. Decreased circulating adrenomedullin in pre-eclampsia. Lancet. 1997;350:1600. doi: 10.1016/S0140-6736(05)64016-0. [DOI] [PubMed] [Google Scholar]
- 59.Kanenishi K, et al. Immunohistochemical adrenomedullin expression is decreased in the placenta from pregnancies with pre-eclampsia. Pathol Int. 2000;50:536–540. doi: 10.1046/j.1440-1827.2000.01085.x. [DOI] [PubMed] [Google Scholar]
- 60.Knerr I, et al. Adrenomedullin, calcitonin gene-related peptide and their receptors: evidence for a decreased placental mRNA content in preeclampsia and HELLP syndrome. Eur J Obstet Gynecol Reprod Biol. 2002;101:47–53. doi: 10.1016/s0301-2115(01)00519-x. [DOI] [PubMed] [Google Scholar]
- 61.Boc-Zalewska A, et al. Adrenomedullin mRNA expression in placenta of preeclamptic women. Ginekol Pol. 2011;82:585–591. [PubMed] [Google Scholar]
- 62.Li H, et al. Adrenomedullin is decreased in preeclampsia because of failed response to epidermal growth factor and impaired syncytialization. Hypertension. 2003;42:895–900. doi: 10.1161/01.HYP.0000095613.41961.6E. [DOI] [PubMed] [Google Scholar]
- 63.Di Iorio R, et al. Adrenomedullin, a new vasoactive peptide, is increased in preeclampsia. Hypertension. 1998;32:758–763. doi: 10.1161/01.hyp.32.4.758. [DOI] [PubMed] [Google Scholar]
- 64.Lauria MR, et al. Adrenomedullin levels in normal and preeclamptic pregnancy at term. J Soc Gynecol Investig. 1999;6:318–321. doi: 10.1016/s1071-5576(99)00041-6. [DOI] [PubMed] [Google Scholar]
- 65.Boc-Zalewska A, et al. The possible role of adrenomedullin in the etiology of gestational hypertension and preeclampsia. Ginekol Pol. 2011;82:178–184. [PubMed] [Google Scholar]
- 66.Dikensoy E, et al. The Changes of Plasma Malondialdehyde, Nitric Oxide, and Adrenomedullin Levels in Patients with Preeclampsia. Hypertens Pregnancy. 2009:1–7. doi: 10.3109/10641950802629634. [DOI] [PubMed] [Google Scholar]
- 67.Al-Ghafra A, et al. Increased adrenomedullin protein content and mRNA expression in human fetal membranes but not placental tissue in pre-eclampsia. Mol Hum Reprod. 2006;12:181–186. doi: 10.1093/molehr/gal016. [DOI] [PubMed] [Google Scholar]
- 68.Marinoni E, et al. Amniotic fluid concentrations of adrenomedullin in preterm labor. Obstet Gynecol. 1999;93:964–967. doi: 10.1016/s0029-7844(98)00551-1. [DOI] [PubMed] [Google Scholar]
- 69.Di Iorio R, et al. Influence of labor on fetoplacental adrenomedullin concentrations. Am J Obstet Gynecol. 2001;185:697–702. doi: 10.1067/mob.2001.117189. [DOI] [PubMed] [Google Scholar]
- 70.Marinoni E, et al. Effect of betamethasone in vivo on placental adrenomedullin in human pregnancy. J Soc Gynecol Investig. 2006;13:418–424. doi: 10.1016/j.jsgi.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 71.Iavazzo C, et al. Adrenomedullin concentration in second trimester amniotic fluid cannot be used as a predictor of preterm delivery. In Vivo. 2009;23:1021–1026. [PubMed] [Google Scholar]
- 72.Goldenberg RL, et al. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Challis JR, et al. Inflammation and pregnancy. Reprod Sci. 2009;16:206–215. doi: 10.1177/1933719108329095. [DOI] [PubMed] [Google Scholar]
- 74.Romero R, et al. Infection and prematurity and the role of preventive strategies. Semin Neonatol. 2002;7:259–274. doi: 10.1016/s1084-2756(02)90121-1. [DOI] [PubMed] [Google Scholar]
- 75.Zudaire E, et al. The central role of adrenomedullin in host defense. J Leukoc Biol. 2006;80:237–244. doi: 10.1189/jlb.0206123. [DOI] [PubMed] [Google Scholar]
- 76.Bauer F, et al. Structure determination of human and murine beta-defensins reveals structural conservation in the absence of significant sequence similarity. Protein Sci. 2001;10:2470–2479. doi: 10.1110/ps.24401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Christiaens I, et al. Inflammatory processes in preterm and term parturition. J Reprod Immunol. 2008;79:50–57. doi: 10.1016/j.jri.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 78.Allaker RP, et al. Mechanisms of adrenomedullin antimicrobial action. Peptides. 2006;27:661–666. doi: 10.1016/j.peptides.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 79.Allaker RP, et al. An investigation into the antimicrobial effects of adrenomedullin on members of the skin, oral, respiratory tract and gut microflora. FEMS Immunol Med Microbiol. 1999;23:289–293. doi: 10.1111/j.1574-695X.1999.tb01250.x. [DOI] [PubMed] [Google Scholar]
- 80.Meeran K, et al. Circulating adrenomedullin does not regulate systemic blood pressure but increases plasma prolactin after intravenous infusion in humans: a pharmacokinetic study. J Clin Endocrinol Metab. 1997;82:95–100. doi: 10.1210/jcem.82.1.3656. [DOI] [PubMed] [Google Scholar]
- 81.Struck J, et al. Identification of an Adrenomedullin precursor fragment in plasma of sepsis patients. Peptides. 2004;25:1369–1372. doi: 10.1016/j.peptides.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 82.Morgenthaler NG, et al. Measurement of midregional proadrenomedullin in plasma with an immunoluminometric assay. Clin Chem. 2005;51:1823–1829. doi: 10.1373/clinchem.2005.051110. [DOI] [PubMed] [Google Scholar]
- 83.Caruhel P, et al. Homogeneous time-resolved fluoroimmunoassay for the measurement of midregional proadrenomedullin in plasma on the fully automated system B.R.A.H.M.S KRYPTOR. Clin Biochem. 2009;42:725–728. doi: 10.1016/j.clinbiochem.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 84.Christ-Crain M, et al. Mid-regional pro-adrenomedullin as a prognostic marker in sepsis: an observational study. Crit Care. 2005;9:R816–R824. doi: 10.1186/cc3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Christ-Crain M, et al. Pro-adrenomedullin to predict severity and outcome in community-acquired pneumonia [ISRCTN04176397] Crit Care. 2006;10:R96. doi: 10.1186/cc4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maisel A, et al. Midregion prohormone adrenomedullin and prognosis in patients presenting with acute dyspnea: results from the BACH (Biomarkers in Acute Heart Failure) trial. J Am Coll Cardiol. 2011;58:1057–1067. doi: 10.1016/j.jacc.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 87.Klip IT, et al. Prognostic value of mid-regional pro-adrenomedullin in patients with heart failure after an acute myocardial infarction. Heart. 2011;97:892–898. doi: 10.1136/hrt.2010.210948. [DOI] [PubMed] [Google Scholar]
- 88.Miguel D, et al. Cord blood plasma reference intervals for potential sepsis markers: pro-adrenomedullin, pro-endothelin, and pro-atrial natriuretic peptide. Clin Biochem. 2011;44:337–341. doi: 10.1016/j.clinbiochem.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 89.Cao Y, et al. Precursors of adrenomedullin, endothelin, and atrial natriuretic peptide as diagnostic markers of neonatal infection. Acta Paediatr. 2011 doi: 10.1111/j.1651-2227.2011.02511.x. [DOI] [PubMed] [Google Scholar]
- 90.Ishimitsu T, et al. Microsatellite DNA polymorphism of human adrenomedullin gene in normotensive subjects and patients with essential hypertension. Hypertension. 2001;38:9–12. doi: 10.1161/01.hyp.38.1.9. [DOI] [PubMed] [Google Scholar]
- 91.Kobayashi Y, et al. Haplotype-based case-control study revealing an association between the adrenomedullin gene and proteinuria in subjects with essential hypertension. Hypertens Res. 2005;28:229–236. doi: 10.1291/hypres.28.229. [DOI] [PubMed] [Google Scholar]
- 92.Ishimitsu T, et al. Microsatellite DNA polymorphism of human adrenomedullin gene in type 2 diabetic patients with renal failure. Kidney Int. 2003;63:2230–2235. doi: 10.1046/j.1523-1755.2003.00020.x. [DOI] [PubMed] [Google Scholar]
- 93.Li Y, et al. Blood pressure and urinary sodium excretion in relation to the A-1984G adrenomedullin polymorphism in a Chinese population. Kidney Int. 2006;69:1153–1158. doi: 10.1038/sj.ki.5000213. [DOI] [PubMed] [Google Scholar]
- 94.Ong KL, et al. A genetic variant in the gene encoding adrenomedullin predicts the development of dysglycemia over 6.4 years in Chinese. Clin Chim Acta. 2011;412:353–357. doi: 10.1016/j.cca.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 95.Glubb DM, et al. Association of a functional polymorphism in the adrenomedullin gene (ADM) with response to paroxetine. Pharmacogenomics J. 2010;10:126–133. doi: 10.1038/tpj.2009.33. [DOI] [PubMed] [Google Scholar]
- 96.Sano M, et al. Association study of calcitonin-receptor-like receptor gene in essential hypertension. Am J Hypertens. 2005;18:403–408. doi: 10.1016/j.amjhyper.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 97.Suzuki A, et al. Large-scale investigation of genomic markers for severe periodontitis. Odontology. 2004;92:43–47. doi: 10.1007/s10266-004-0035-4. [DOI] [PubMed] [Google Scholar]
- 98.Cao D, et al. Investigation of the association between CALCRL polymorphisms and primary angle closure glaucoma. Mol Vis. 2009;15:2202–2208. [PMC free article] [PubMed] [Google Scholar]
- 99.Cheung BM, et al. Plasma adrenomedullin level is related to a single nucleotide polymorphism in the adrenomedullin gene. Eur J Endocrinol. 2011;165:571–577. doi: 10.1530/EJE-11-0513. [DOI] [PubMed] [Google Scholar]
- 100.Wang L, et al. Polymorphism in maternal LRP8 gene is associated with fetal growth. Am J Hum Genet. 2006;78:770–777. doi: 10.1086/503712. [DOI] [PMC free article] [PubMed] [Google Scholar]