Abstract
Caspases have been extensively studied as critical initiators and executioners of cell death pathways. However, caspases also take part in non-apoptotic signalling events such as the regulation of innate immunity and activation of nuclear factor-κB (NF-κB). How caspases are activated under these conditions and process a selective set of substrates to allow NF-κB signalling without killing the cell remains largely unknown. Here, we show that stimulation of the Drosophila pattern recognition protein PGRP-LCx induces DIAP2-dependent polyubiquitylation of the initiator caspase DREDD. Signal-dependent ubiquitylation of DREDD is required for full processing of IMD, NF-κB/Relish and expression of antimicrobial peptide genes in response to infection with Gram-negative bacteria. Our results identify a mechanism that positively controls NF-κB signalling via ubiquitin-mediated activation of DREDD. The direct involvement of ubiquitylation in caspase activation represents a novel mechanism for non-apoptotic caspase-mediated signalling.
Keywords: caspase, Drosophila , IAP, innate immunity, ubiquitin
Introduction
The conjugation and deconjugation of ubiquitin (Ub) to target proteins influence diverse biological processes that can contribute to tumour formation when deregulated (Hoeller and Dikic, 2009). The conjugation of Ub, thereby, can stimulate the assembly of transient signalling hubs in which the Ub adduct forms part of a new docking site for proteins with specialized binding motifs that help to build reversible, short-lived signalling centres (Grabbe and Dikic, 2009). Ub-mediated signalling is particularly important for activation of nuclear factor-κB (NF-κB) during innate immune responses (Skaug et al, 2009). Deregulated activation of NF-κB is recognized as one of the major underlying causes for chronic, cancer-related inflammation that drives tumour development, and is seen in most tumour types including leukaemia, lymphomas and solid tumours (Karin and Greten, 2005; Grivennikov et al, 2010; Nathan and Ding, 2010). Hence, a better understanding of the processes that regulate NF-κB and innate immunity is critically important.
Metazoans live in close contact with a multitude of microbes with which they establish complex reciprocal interactions. Drosophila relies on innate immunity responses to combat microbial challenges (Ferrandon et al, 2007; Lemaitre and Hoffmann, 2007). Depending on the invading microorganism, Drosophila activate either the Toll or immune deficiency (imd) pathway (Lemaitre et al, 1995, 1996). Infection with fungi or certain Gram-positive bacteria activates the Toll pathway, which initiates an intracellular signalling cascade that culminates in the nuclear translocation of the NF-κB-like transcription factors Dif and Dorsal (Lemaitre et al, 1996; Manfruelli et al, 1999; Meng et al, 1999; Rutschmann et al, 2000a, 2002). On the other hand, the IMD pathway is activated by Gram-negative bacteria, which in turn stimulates activation of the NF-κB-like transcription factor Relish (also known as IRD4) (Hedengren et al, 1999). Activation of both pathways induces the expression of a plethora of NF-κB responsive antimicrobial peptide (AMP) genes, which are important for fending off invading microorganisms (Tzou et al, 2000).
The IMD signal transduction pathway shows striking similarities to the ones stimulated by members of the mammalian TNF-receptor super-family (Tanji and Ip, 2005). These pathways are Ub-dependent signal transduction cascades in which Ub ligases, Ub receptors and deubiquitylating enzymes (DUBs) build up, recognize and remove Ub signals, allowing the temporally controlled assembly of protein complexes that lead to the activation of kinases that regulate NF-κB. Ub, thereby, functions as a binding surface for proteins with Ub-binding domains (UBD), also referred to as Ub receptors (Dikic et al, 2009). The conjugation of Ub requires a stepwise process that involves Ub-activating enzymes (E1), Ub-conjugating enzymes (E2) and Ub protein ligases (E3) (Dikic et al, 2009). E3s confer substrate specificity and enable the formation of an isopeptide linkage between the carboxyl-terminus of Ub (glycine (G)76) and the amino group of a reactive side chain of the substrate. Ub can be conjugated either as a single moiety or as chains of variable length (Komander, 2009). Further complexity is provided by different linkage types, as Ub moieties can be conjugated to one another via different lysine (K) residues within Ub. At least eight different types of Ub chains exist. The different types of Ub chain linkages exert distinct functional outcomes (Hoeller and Dikic, 2009). This is predominantly due to the fact that the different chain types adopt distinct topologies, which in turn are recognized by specific Ub receptors (Komander, 2009).
Signalling through the IMD pathway is activated by the Gram-negative cell wall component diaminopymelic-type peptidoglycan (DAP-PGN). DAP-PGN is recognized by the pattern recognition protein PGRP-LE and the transmembrane receptor PGRP-LCx (Choe et al, 2002; Gottar et al, 2002; Ramet et al, 2002; Leulier et al, 2003; Kaneko et al, 2004, 2006). Binding of DAP-PGN to PGRP-LCx causes receptor oligomerization and triggers recruitment of the adaptor protein IMD (Choe et al, 2005), which carries a C-terminal death domain (DD) that is similar to the one of the mammalian adaptor protein RIP1 (Georgel et al, 2001). Through its DD, IMD subsequently recruits dFADD (also known as BG4), which in turn binds and presumably activates the Drosophila caspase-8 orthologue Death-related ced-3/Nedd2-like protein (DREDD; also known as DCP2) (Leulier et al, 2000, 2002; Naitza et al, 2002). Following activation, DREDD cleaves off the amino (N)-terminal portion of IMD, thereby exposing an evolutionarily conserved inhibitor of apoptosis (IAP)-binding motif (IBM) at the neo-N-terminus of IMD (Paquette et al, 2010). This IBM is recognized by the E3 ligase Drosophila IAP protein 2 (DIAP2), which brings it into position for IMD ubiquitylation. In conjunction with the E2s Effete (UBC5) and UEV1a/Bendless (UEV1a/UBC13), DIAP2 targets IMD for K63-linked polyubiquitylation (Zhou et al, 2005; Paquette et al, 2010). According to the current model, the attached Ub chains function as scaffolds for the recruitment of the Drosophila MAP kinase kinase kinase dTAK1/TAB2 and the Relish kinase complex IRD5/Kenny (IKKβ/IKKγ) (Ferrandon et al, 2007). Ub-dependent recruitment of dTAK1/TAB2 and IRD5/Kenny is thought to be mediated by their respective Ub receptors TAB2 and Kenny (Rutschmann et al, 2000b; Silverman et al, 2000; Lu et al, 2001; Vidal et al, 2001; Silverman et al, 2003; Kanayama et al, 2004; Kleino et al, 2005; Zhuang et al, 2006). Ub-dependent complex formation is presumed to result in activation of dTAK1, which in turn phosphorylates and activates IRD5/Kenny and MKK4/7 (Silverman et al, 2003; Geuking et al, 2009). While MKK4/7 promotes JNK activation, IRD5/Kenny phosphorylate Relish (Rutschmann et al, 2000b; Silverman et al, 2000; Lu et al, 2001; Vidal et al, 2001; Geuking et al, 2009), which activates its transcriptional activity (Erturk-Hasdemir et al, 2009). In addition to IRD5/Kenny-mediated phosphorylation of Relish, activation also requires DREDD-dependent proteolytic processing of Relish (Elrod-Erickson et al, 2000; Leulier et al, 2000; Stoven et al, 2000, 2003; Erturk-Hasdemir et al, 2009). Activated DREDD cleaves off an inhibitory C-terminal ankyrin repeat domain of Relish, thereby allowing the translocation of the N-terminal portion to the nucleus where it induces expression of AMP genes (Silverman et al, 2000; Stoven et al, 2000, 2003; Erturk-Hasdemir et al, 2009). Loss-of-function mutations in most of the components of the IMD signalling cascade results in an immune deficiency phenotype, in which animals become acutely susceptible to infection by Gram-negative bacteria. Common to all these mutants is their failure to induce expression of antibacterial peptide genes and, therefore, to fend off bacterial infection (Ferrandon et al, 2007; Lemaitre and Hoffmann, 2007). While E3 ligases promote IMD signalling via ubiquitylation, pathway activation can be suppressed via Ub deconjugation by dUSP36 and dCYLD (Tsichritzis et al, 2007; Thevenon et al, 2009), reinforcing the importance of Ub in the regulation of innate immunity.
At present, DIAP2 is the sole E3 ligase implicated in Ub-mediated IMD signalling (Gesellchen et al, 2005; Kleino et al, 2005; Leulier et al, 2006; Huh et al, 2007). DIAP2 is a member of the evolutionarily conserved IAP protein family, whose members are best known for their ability to regulate caspases and apoptosis (Gyrd-Hansen and Meier, 2010). The defining feature of an IAP protein is the presence of the baculovirus IAP repeat (BIR) domain(s), a zinc-binding fold of ∼70 amino-acid residues that mediates protein interactions. Most IAPs harbour additional domains such as the C-terminal RING finger domain that provides them with E3 Ub ligase activity by mediating the transfer of Ub from E2s to target substrate.
Although it is clear that DIAP2 is required for Rel/NF-κB activation (Gesellchen et al, 2005; Kleino et al, 2005; Leulier et al, 2006; Huh et al, 2007), the precise mechanism through which DIAP2 mediates Ub-dependent activation of NF-κB remains ill defined. At present, the only known targets for DIAP2-induced ubiquitylation are the adaptor protein IMD and DIAP2 itself (Paquette et al, 2010). To gain a better understanding of Ub-dependent NF-κB activation, and to explore the possibility that DIAP2 mediates its effect through modulating caspases, we studied the molecular mechanism through which DIAP2 triggers Ub-dependent signalling. Here, we show that DIAP2 interacts with the initiator caspase DREDD and that DIAP2 targets this caspase for non-degradative ubiquitylation in a signal-dependent manner. Further, we find that impaired ubiquitylation of DREDD blocks IMD and Relish cleavage and renders mutant flies acutely sensitive to Gram-negative bacterial infection. Thus, the Ub chains on DREDD might function as anchor points for Ub receptors that help dimerization and full activation of DREDD. The mechanism of DREDD activation might parallel the one of caspase-8 in mammals, where the Ub receptor p62 reportedly promotes aggregation, activation and processing of CUL3-modified caspase-8 (Jin et al, 2009). Together, our data indicate that DIAP2-mediated ubiquitylation of DREDD is required for innate immune signalling.
Results
DIAP2 binds to DREDD and targets it for polyubiquitylation in a RING finger-dependent manner
To determine the molecular mechanism through which DIAP2 controls innate immunity, we first determined the contribution of individual domains of DIAP2 that are required for IMD signalling. To this end, we used UAS-driven transgenes encoding specific DIAP2 mutants and assessed their ability to rescue the lethality of Diap2 mutant flies following septic injury by Erwinia carotovora carotovora 15 (Ecc15), a Gram-negative bacteria. Transgenes were expressed ubiquitously under the control of daughterless (Da)-GAL4. While expression of wild-type (WT) DIAP2 rescued the lethality of the Diap2 mutant flies (Diap27c) (Leulier et al, 2006) in response to Ecc15 septic injury, reconstitution with an E3-defective DIAP2 mutant (DIAP2RINGmut) completely failed to protect Diap2 mutant animals (Figure 1A), which is in agreement with an earlier report (Huh et al, 2007). Of note, DIAP2RINGmut, which carries a mutation that removes the zinc-coordinating cysteine required for the proper folding of the RING domain, is as stable as WT DIAP2 (Ribeiro et al, 2007). To evaluate the contribution of individual BIR domain of DIAP2, we generated transgenic flies using germ-line-specific phiC31 integration into pre-defined landing sites, which allows accurate comparison between mutants (Bischof et al, 2007). DIAP2 transgenes encoding DIAP2 mutants with amino-acid (aa) substitutions in either the BIR2 (D163A) or BIR3 (D263A) domain (Ribeiro et al, 2007) efficiently rescued the sensitivity to Ecc15 infection seen in Diap27c mutant flies. In contrast, double mutants carrying a substitution in BIR2 and BIR3 (D163A/D263A) failed to provide protection from Ecc15 septic injury (Figure 1B). This is consistent with the observation that either BIR2 or BIR3 can mediate IMD binding (Paquette et al, 2010). Given that expression of DIAP2RINGmut fails to rescue Ecc15-mediated lethality of diap27c mutant animals, this indicates that DIAP2-mediated ubiquitylation of IMD pathway components is indispensable for IMD signalling.
Figure 1.
DIAP2 binds and ubiquitylates the initiator caspase DREDD. (A, B) DIAP2’s RING finger and both BIR domains are required to resist Ecc15 bacterial infection. Diap27c mutant animals were reconstituted with rescue constructs expressing the indicated DIAP2 proteins. To confirm the requirement of DIAP2 E3 ligase activity for the IMD pathway (A), DIAP2 constructs were ubiquitously expressed in transgenic flies under the control of daughterless (Da)-GAL4. Animals were pricked with a needle previously dipped into Ecc15 and the survival rates monitored. To evaluate the contribution of individual BIR domain of DIAP2 (B), transgenic flies were generated using germ-line-specific phiC31 integration (Bischof et al, 2007). Transgenes were expressed using the fat-body-specific c564-Gal4 driver line. Animals were injected with 13.8 nl of Ecc15 bacterial solution (optical density of 300) and the survival rates monitored. The experiments were performed using at least 20 flies for each genotype. Shown is a representative experiment of at least three repeats. C466Y represents a RING mutant of DIAP2, which lacks E3 ligase activity (Ribeiro et al, 2007), D163 and D263A disrupts the BIR pocket of BIR2 and BIR3, respectively. (C, D) DIAP2 binds to DREDD. Reciprocal binding assays with DIAP2 and DREDD. The indicated constructs were transfected into S2 cells and GST-tagged DIAP2 (C) and V5-tagged DREDD (D) were purified with Glutathione resin and α-V5 antibodies, respectively. The expression and the presence of the co-purified proteins were assessed by immunoblotting with the indicated antibodies. (E) DIAP2 ubiquitylates DREDD in a RING finger-dependent manner. HA–Ub was co-expressed with DREDD–V5 in S2 cells together with vector control, DIAP2WT or the DIAP2 RING mutants DIAP2C466Y and DIAP2F469A. Cells were lysed under denaturing conditions and ubiquitylated proteins isolated using α-HA antibodies. The presence and ubiquitylation of DREDD and DIAP2 was assessed with the indicated antibodies. (F) DREDD is transiently ubiquitylated in a signal-dependent manner. S2 cells were transfected with DREDD–V5. Subsequently, cells were treated with 1 μg/ml DAP-PGN for the indicated time points before being harvested. DREDD was immunoprecipitated using α-V5 resin and ubiquitylated DREDD analysed using α-Ub antibodies. (G) DIAP2 promotes the conjugation of K63-linked Ub chains on DREDD. The indicated constructs were transfected into S2 cells, and HA-tagged DREDD and IMD were purified under denaturing conditions. The presence of the purified proteins and identity of the respective Ub chains were detected by immunoblotting using the indicated antibodies (see Materials and methods for details).
To identify components of the IMD pathway that are targeted by DIAP2, we first performed binding assays with DIAP2 and known members of this pathway. We co-expressed DIAP2 with tagged components of the IMD pathway in S2 cells and analysed their ability to interact with each another. The interaction between DIAP2 and PGRP-LCx was performed in the presence or absence of DAP-PGN to assess whether receptor activation is required for binding to DIAP2. PGRP-LCx failed to interact with DIAP2 under these conditions (data not shown). Cleaved IMD, which is truncated at aspartic acid residue 30 following pathway activation and exposes a bona fide IBM at the neo-amino-terminus of the C-terminal portion of IMD, readily bound to DIAP2 (data not shown; Paquette et al, 2010). While DIAP2 failed to associate with other IMD pathway components such as dFADD, IRD5, dTAB2 and dTAK1 (data not shown), DIAP2 tightly bound to DREDD (Figure 1C). DIAP2 also bound to the heterodimeric E2 UEV1A/Bendless that promotes the conjugation of K63-linked polyubiquitin chains, as previously reported (data not shown; Zhou et al, 2005; Paquette et al, 2010). Reciprocal pulldown experiments confirmed the interaction between DREDD and DIAP2 (Figure 1D).
To assess whether DIAP2 can target DREDD for ubiquitylation, we next expressed non-tagged DIAP2 in S2 cells together with HA-tagged Ub and V5-tagged DREDD. Ubiquitylated proteins were affinity purified under denaturing conditions using α-HA columns (see Materials and methods for details), and the presence of ubiquitylated proteins was assessed by immunoblotting the eluate. Under these conditions, DIAP2 efficiently ubiquitylated DREDD (Figure 1E) and IMD (data not shown; Paquette et al, 2010). Ubiquitylation of DREDD was dependent on DIAP2’s E3 ligase activity, as the RING mutants DIAP2F469A and DIAP2C466Y, which abrogate the Ub-E3 activity of IAPs (Ribeiro et al, 2007; Ditzel et al, 2008), failed to ubiquitylate DREDD (Figure 1E, top panel, compare lane 3 with lanes 4 and 5). Together, these data indicate that DIAP2 can bind to DREDD and promote its polyubiquitylation.
Next, we determined whether ubiquitylation of DREDD occurred in a signal-dependent manner. For this purpose, we induced the IMD pathway in Drosophila S2 cells using bacterial-derived DAP-PGN. For this assay, we relied on endogenous DIAP2, but stably expressed DREDD–V5, because no antibody is currently available that detects endogenous DREDD. Interestingly, while ectopically expressed DREDD remained unmodified under these conditions, treatment with DAP-PGN induced prominent and transient ubiquitylation of DREDD (Figure 1F). DREDD ubiquitylation occurred as early as 1 min and was no longer detectable 40–60 min after DAP-PGN treatment. Consistent with the notion that DIAP2 can bind to the heterodimeric E2 UEV1 A/Bendless, we found that DIAP2 promoted the conjugation of K63-linked Ub chains on DREDD (Figure 1G). Likewise, DIAP2 also triggered K63-ubiquitylation of IMD. Given that DIAP2 binds and ubiquitylates DREDD, and that DIAP2 is the key E3 ligase of the IMD pathway (Gesellchen et al, 2005; Kleino et al, 2005; Leulier et al, 2006; Huh et al, 2007), these data suggest that activation of the IMD pathway results in transient, DIAP2-mediated ubiquitylation of DREDD.
DIAP2 binds to the DED1 of DREDD
DIAP2 is the first IAP found to interact with a death effector domain (DED)-containing initiator caspase. To better understand the nature of this interaction, we generated various deletion mutants of DREDD and DIAP2 (Figure 2A) and determined the domains that are required for their association. Similar to caspase-8, DREDD contains a long pro-domain with two DEDs in tandem (Chen et al, 1998). This is followed by the large (p20) and small (p10) caspase subunits. Co-immunoprecipitation assays revealed that DIAP2 readily bound to the prodomain of DREDD (DED1/2, aa 1–264) (Figure 2B). In contrast, DIAP2 failed to associate with the C-terminal caspase domains (p20/p10, aa 265–517) of DREDD. Further, the binding of DIAP2 to DREDD appeared to depend on the presence of the DED1 because DIAP2 only weakly bound to DREDD–ΔDED1 (aa 143–517) that lacks DED1. Consistently, DED1 in isolation co-purified with DIAP2 (Figure 2C), while the DED2 did not interact with DIAP2. This indicates that the first DED is necessary and sufficient for DREDD to bind to DIAP2. Intriguingly, we found that the adaptor protein dFADD also selectively bound to DED1 (Figure 2D), which is in contrast to an earlier report (Hu and Yang, 2000). Although dFADD and DIAP2 both bound to DED1, they did not compete with one another for the binding to DREDD (Figure 2E). This is evident because co-expression of dFADD did not abrogate the interaction of DIAP2 with DREDD. Together, our data indicate that DIAP2 and dFADD bind to the DED1 of DREDD, forming a trimeric complex.
Figure 2.
DIAP2 binds to the DED1 of DREDD. (A) Schematic representation of the DIAP2 and DREDD constructs used in this study. (B) DIAP2 binds to the pro-domain of DREDD. Co-IP assays with DIAP2 and various fragments of DREDD. S2 cells were co-transfected with HA-tagged DIAP2 and V5-tagged DREDD. Expression and the presence of co-purified proteins were analysed by immunoblotting with the indicated antibodies. (C) DIAP2 associates with DED1 of DREDD. Co-IPs were performed with DIAP2 and individual DEDs or the prodomain of DREDD as in (B). (D) dFADD also binds to the DED1 of DREDD. Co-IPs were performed with dFADD and individual DEDs or the prodomain of DREDD as in (B). (E) Competition assay with DIAP2, dFADD and DREDD indicates that DIAP2 and dFADD can bind to DREDD simultaneously. Co-IPs were performed with dFADD, DIAP2 and DREDD. Expression and the presence of co-purified proteins were analysed as in (B). (F) The N-terminal portion of DIAP2 interacts with DREDD. Co-IPs were performed with DREDD and the indicated fragments of DIAP2. Expression and the presence of co-purified proteins were analysed as in (B). (G) The BIR2 and BIR3 domains mediate binding to DREDD. Co-IPs were performed with DREDD and individual BIR domains of DIAP2. Expression and the presence of co-purified proteins were analysed as in (B). An asterisk marks a cleavage product of BIR3. (H) The interaction between DIAP2 and DREDD does not require the hydrophobic pocket of the BIR2 and BIR3. Co-IPs were performed with DREDD and the indicated WT or mutant BIR domains. Expression and the presence of co-purified proteins were analysed as in (B). (I) In contrast, the binding of DIAP2 to cleaved IMD critically depends on the hydrophobic pockets of the BIR2 and BIR3. IMD corresponds to the DREDD-cleaved form of IMD (aa 31–273) and was expressed using the Ub-fusion technique (Varshavsky, 2000; Tenev et al, 2005). Co-IPs with DREDD and the indicated WT or mutant BIR domains were performed as in (H). BIR1/2/3 encompasses DIAP2’s N-terminal segment that carries the BIR1, BIR2 and BIR3 domains.
To identify the region of DIAP2 that interacts with DREDD, we first examined the requirement of the BIR domains, since BIRs generally function as protein interaction motifs (Eckelman et al, 2008). We found that the BIR domain-containing amino-terminal portion of DIAP2 (DIAP2-BIR1/2/3, aa 1–338) bound to DREDD as efficiently as WT DIAP2. In contrast, the carboxy-terminal portion of DIAP2 (DIAP2281–498), which lacks the BIR domains, failed to associate with DREDD (Figure 2F). Based on the presence of a deep peptide-binding groove, BIR domains can roughly be grouped into type-I and type-II domains (Gyrd-Hansen and Meier, 2010). Type-I BIR domains lack a peptide-binding groove, or possess a shallow pocket only, whereas type-II BIRs carry a distinctive hydrophobic cleft through which they can bind to IBMs. According to this classification, DIAP2’s BIR1 represents a type-I, whereas the BIR2 and BIR3 domains are type-II domains. Co-immunoprecipitation assays indicated that DIAP2’s BIR1 domain in isolation did not interact with DREDD (Figure 2G). Under the same conditions, DREDD readily associated with the BIR2 and BIR3 domains. To determine whether DREDD interacted with these BIRs through their respective peptide-binding grooves, we examined the ability of DREDD to bind to DIAP2 proteins that carry substitution mutations of the Zn2+-coordinating Cysteine residue in the BIR2 (C149G) and/or BIR3 (C249G) (Ribeiro et al, 2007). Intriguingly, single or double mutations in the BIR2 and/or BIR3 did not abrogate the binding of DIAP2 to DREDD (Figure 2H). This is in stark contrast to the interaction between DIAP2 and IMD, which was lost when the peptide-binding groove of both BIR2 and BIR3 were mutated (Figure 2I) (Paquette et al, 2010). The observation that DIAP2C149/C249 retained its ability to interact with DREDD indicates that the DREDD-binding surface of DIAP2 does not coincide with the one required for binding to the neo-amino-terminus of IMD. It also indicates that the interaction between DIAP2 and DREDD does not rely on an IBM-like motif in DREDD.
The loss-of-function DREDD allele D44 carries a mutation that disrupts DIAP2-mediated ubiquitylation
Loss-of-function mutations in DREDD occur throughout the primary sequence of DREDD (Leulier et al, 2000). The alleles B118, D55 and F64 generate either premature stop codons or frameshift changes in the DREDD prodomain. In contrast, the loss-of-function dredd allele D44 carries a missense mutation (G120R) in the sequence encoding the DED1 (Figure 3A). Importantly, D44 blocks diptericin expression to the same degree as B118, D55 and F64 (Leulier et al, 2000). The strong phenotype associated with the G120R mutation of D44 indicates that the DED1 is essential for DREDD’s function in immunity. Given that DIAP2 and dFADD both interact with the DED1 of DREDD, we examined the functional consequence of the G120R mutation.
Figure 3.
The G120R loss-of-function mutation of DREDD disrupts DIAP2-mediated ubiquitylation. (A) Schematic diagram of the domain architecture of DREDD. Residue G120 maps to the DED1 of DREDD. (B) Ribbon representation of the prodomain structure of DREDD (residues 40–235). The 3D structure of DREDD’s prodomain was modelled using the X-ray structure of the DEDs of the viral-FLIP MC159 (pdb 2bbz) as a template. The six-helical bundle structure of the death fold of DED1 is indicated. The position of G120 is highlighted in red. (C) Molecular surfaces of the same residues as shown in (B). G120 and G120R of WT DREDD (left panel) and DREDDD44 (right panel) are highlighted in red. DED1 is indicated in green, while the DED2 is depicted in grey. (D) DREDDD44 binds to DIAP2 like WT DREDD. Co-IPs were performed with DIAP2 and DREDDWT and DREDDD44. Expression and the presence of co-purified proteins were analysed by immunoblotting with the indicated antibodies. Of note, DIAP2 only co-purifies full-length DREDD, but not processed DREDD (p20-10) that lacks the pro-domain. (E) The G120R mutation does not affect the ability of DREDD to bind to dFADD. Co-IPs with dFADD and DREDDWT or DREDDD44 were performed as in (D). (F, G) DREDDD44 is not impaired in its ability to hetero- or homodimerize with WT and mutant DREDDD44. Co-IPs with DREDDWT and DREDDD44 were performed as in (D). (H) The G120R mutation does not affect the inherent catalytic activity of DREDD, since DREDDD44 can cleave Relish similar to DREDDWT upon overexpression. S2 cells were co-transfected with FLAG-tagged Relish and empty vector, V5-tagged WT, catalytically inactive (C408A) or G120R mutant DREDD. Relish cleavage was assessed by immunoblotting using α-FLAG antibodies. (I) The G120R mutation does not affect DREDD’s protein stability. Cycloheximide (CHX) chase experiments to compare the protein stability of DREDDWT and DREDDD44. Equal loading was assessed with α-actin antibodies.
The 3D structure of DREDD’s prodomain was modelled using the X-ray structure of the DEDs of the viral-FLIP MC159 (pdb 2bbz) as a template (Yang et al, 2005). The predicted tandem DEDs of DREDD adopt a dumbbell-shaped arrangement, with the two DEDs at two opposing ends (Figure 3B and C). According to the structural modelling, DED1 (residues 18–242) adopts a classical death fold with a six-helical bundle structure. Glycine 120, thereby, is positioned in a loop that connects helix α5a with α6a (Figure 3B). The small side chain of G120 faces outwards and does not contribute to the overall fold of the death motif. Consequently, substitution of G120 to R120 results in DREDD with a positively charged side chain protruding from DED1 (Figure 3C, right panel). Even though the G120R is a loss-of-function mutation, DREDDD44 was fully competent in binding to dFADD and DIAP2 (Figure 3D and E). This is evident, because DREDDD44 bound to DIAP2 (Figure 3D) and dFADD (Figure 3E) like WT DREDD. The G120R mutation also did not affect auto-processing of DREDD following its overexpression, as evidenced by the conversion of full-length DREDD to processed DREDD (Figure 3D). Since initiator caspases are activated following dimerization-induced conformational changes, this suggests that the G120R mutation does not affect DREDD dimerization. Accordingly, DREDDD44 readily homo-dimerized with WT or mutant DREDD (Figure 3F and G). Further, upon overexpression, mutant DREDD cleaved Relish like its WT counterpart, in both Drosophila S2 cells (Figure 3H) and mammalian 293T cells (data not shown). While this overexpression experiment does not address the physiological mechanism of DREDD activation, it demonstrates that the G120R mutation neither influences the inherent catalytic activity of DREDD, for example by distorting the catalytically active centre, nor interferes with substrate entry. We also assessed the protein stability of DREDDD44 following cycloheximide treatment, and found that the protein half-life of DREDDD44 was comparable with the one of WT DREDD (Figure 3I).
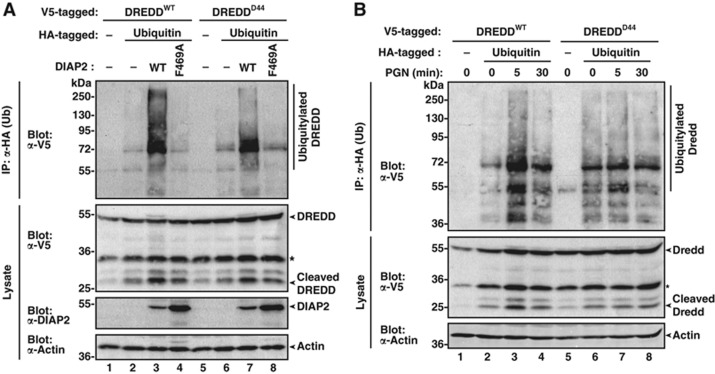
Next, we examined whether the G120R mutation affects DIAP2-mediated ubiquitylation of DREDD. To this end, we performed ubiquitylation assays with DIAP2 and WT or mutant DREDD. Intriguingly, DREDDD44 was significantly less ubiquitylated by DIAP2 than WT DREDD (Figure 4A). This is unexpected, as DIAP2 binds to DREDDD44 as efficiently as to WT DREDD (Figure 3D). Furthermore, while treatment with DAP-PGN induced prominent and transient ubiquitylation of WT DREDD, DREDDD44 failed to be ubiquitylated in a signal-dependent manner (Figure 4B). Of note, the base-line ubiquitylation status of DREDDD44 was higher than WT DREDD, indicating that DREDDD44 can be modified under steady-state conditions. However, signal-dependent ubiquitylation of DREDDD44 did not occur upon treatment with DAP-PGN. This demonstrates that the G120R mutation affects the ability of DREDD to be ubiquitylated in a signal-dependent manner. Most likely, the protruding positively charged side chain of R120 (Figure 3C) prevents DIAP2-mediated ubiquitylation allosterically, possibly by re-positioning DREDD in respect to DIAP2’s E2s, which moves the Ub-acceptor lysines of DREDD out of reach for the E2, making them no longer available for Ub transfer.
Figure 4.
The G120R mutation impairs DREDD ubiquitylation. (A) DIAP2 ubiquitylates DREDDD44 less efficiently than WT DREDD. HA–Ub was co-expressed with DREDDWT and DREDDD44 in S2 cells together with vector control, DIAP2WT or the DIAP2 RING mutant DIAP2F496A. Cells were lysed under denaturing conditions and ubiquitylated proteins isolated using α-HA antibodies. The presence of DREDD and DIAP2 and the DREDD ubiquitylation was assessed with the indicated antibodies. (B) DREDDD44 fails to be ubiquitylated in a signal-dependent manner. S2 cells were co-transfected with HA–Ub and DREDDWT or DREDDD44. Subsequently, cells were treated with 1 μg/ml DAP-PGN for the indicated time points before being harvested under denaturing conditions. Ubiquitylated DREDD was purified and detected as described in (A). An asterisk marks a cross-reactive band.
Impaired ubiquitylation of DREDD abrogates expression of AMP genes following septic injury
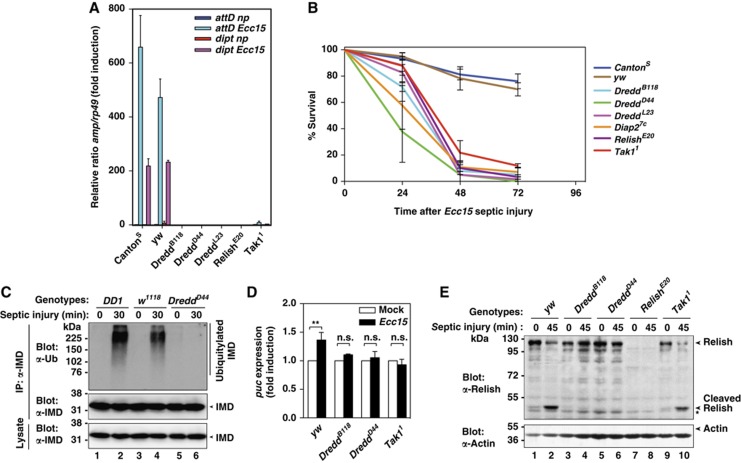
The observation that DREDDD44 fails to be ubiquitylated in a signal-dependent manner is consistent with a model in which ubiquitylation of DREDD is required for innate immunity. To investigate the role of DREDD ubiquitylation in NF-κB-mediated induction of AMPs, we first analysed the expression levels of attacin D and diptericin following septic injury with the Gram-negative bacteria Ecc15. Of note, attacin D and diptericin are AMP genes that are controlled by the IMD pathway (Lemaitre et al, 1997). While WT flies readily induced expression of attacin D and diptericin, flies with mutations in Relish (RelishE20) or Tak1 (Tak11), failed to mount such an IMD response (Figure 5A) (Leulier et al, 2006; Huh et al, 2007). Likewise, homozygous DreddD44 mutants similarly failed to induce Relish-mediated expression of attacin D and diptericin. AMP induction in DreddD44 mutants was as impaired as in DreddB118 animals that harbour a stop codon in the DREDD prodomain, which is consistent with an earlier report (Leulier et al, 2000). Given that DREDDD44 fails to be ubiquitylated in a signal-dependent manner, but otherwise behaves like WT DREDD with regards to its protein stability, catalytic potential and its ability to bind to DIAP2 and dFADD, these data suggest that signal-dependent ubiquitylation of DREDD is essential for Relish-mediated induction of AMP genes.
Figure 5.
The G120R mutation impairs signal-dependent cleavage of Relish and Imd, and abrogates activation of AMP gene expression. (A) Quantitative RT–PCR analysis of attacin D and diptericin induction in the indicated fly strains. rp49 was used as the experimental expression standard. Shown are the relative expression ratios of attacin D/rp49 and diptericin/rp49 5 h post-infection. Values are normalized to non-pricked CantonS. Np stands for non-pricked. Error bars indicate SEM from three independent experimental repeats using at least 20 flies per repeat. (B) DreddD44 mutant flies die upon infection with Ecc15. Adult flies of the indicated genotypes were subjected to Ecc15-mediated septic injury and their survival rates were monitored at the indicated time points. Error bars indicate SEM from three independent experimental repeats using at least 20 flies per repeat. (C) Immunoblot analysis assessing ubiquitylation of IMD, which requires DREDD-mediated cleavage of IMD (Paquette et al, 2010). The indicated fly strains were exposed to septic injury and fly lysates were used to immunoprecipitate IMD. The presence and modification of IMD was subsequently analysed by immunoblotting with the indicated antibodies. DD1 and w1118 fly strains are WT strains that served as controls. (D) IMD-mediated activation of JNK in the indicated fly strains was measured by evaluating expression of puckered, a bona fide downstream target of the JNK signal transduction cascade. Values were normalized to mock injections. The asterisks indicate a P-value of P<0.01, calculated on raw data before normalization. Ns designates non-significant differences. (E) Immunoblot analysis assessing cleavage of Relish in the indicated fly strains. While Relish cleavage occurred in a signal-dependent manner in yw flies, Relish failed to be cleaved in DreddB118 and DreddD44 following septic injury with Ecc15. In contrast, Relish was partially cleaved in Tak11 mutant flies. Equal loading was assessed with α-actin antibodies. An asterisk indicates a cross-reactive band.
To investigate the consequence of DREDD ubiquitylation in protecting flies against bacterial infection, we determined the survival profile of DreddD44 mutant flies that were infected with Ecc15. Consistent with the notion that DreddD44 mutants fail to induce AMP genes, DreddD44 flies were highly susceptible to injection with the Gram-negative bacteria Ecc15 (Figure 5B) (Leulier et al, 2000). Likewise, DreddB118, DreddL23, Diap27c, RelishE20 and Tak11 mutant animals also died following infection by Ecc15. DreddD44 and Diap27c mutant animals did not impair AMP production in response to infection with Micrococcus luteus, a Gram-positive bacteria known to activate the Toll pathway (data not shown). Taken together, these data indicate that loss of the Ub-E3 ligase for DREDD, or loss of signal-dependent ubiquitylation of DREDD, as seen in DreddD44 flies, dramatically sensitizes animals to infection by Gram-negative bacteria.
To gain some mechanistic insights into why DreddD44 mutants fail to induce AMP production, we performed a time course of immune stimulation and examined whether endogenous IMD and Relish were processed in DreddD44 mutant animals in a signal-dependent manner. We previously have shown that IMD is ubiquitylated following DREDD-mediated cleavage (Paquette et al, 2010). Therefore, we used IMD ubiquitylation as a proxy to investigate DREDD activation. Intriguingly, we found that IMD failed to become ubiquitylated in DreddD44 mutant flies (Figure 5C), which indicates that cleavage of IMD is impaired in these animals. Moreover, JNK activation (as measured by expression of puckered, see below) in response to immune stimulation is blocked in DreddD44 mutant flies (Figure 5D). This is consistent with the observation that IMD ubiquitylation, which is required for activation of dTAK1, a JNK upstream kinase, is lost in DreddD44 mutant animals. Consistent with the notion that ubiquitylation of DREDD is required for DREDD activation in response to septic injury with Ecc15, we found that DreddD44 mutant flies also failed to cleave Relish (Figure 5E). While cleaved Relish was detected in extracts from WT flies within 45 min of infection with Ecc15, DreddD44 mutant animals completely failed to induce Relish cleavage following immune stimulation. Likewise, Relish cleavage was similarly impaired in DreddB118 mutants. In contrast, Tak11 mutants partially cleaved Relish as previously described (Delaney et al, 2006). This indicates that impaired ubiquitylation of DREDD, through point mutation of DREDD (G120R), prevents DREDD-mediated cleavage of IMD and Relish. The observation that inefficient ubiquitylation of DREDD prevents IMD and Relish cleavage indicates that signal-dependent ubiquitylation of DREDD is a key step in innate immune-mediated activation of NF-κB.
Discussion
Ub-dependent signalling is critically important for the ability of the Drosophila fat body to produce AMP genes in response to Gram-negative bacterial infection (Zhou et al, 2005; Paquette et al, 2010). Previous studies have implicated ubiquitylation of IMD in innate immune signalling (Paquette et al, 2010). We now show that one of the key steps in innate immune-mediated activation of NF-κB appears to be ubiquitylation of DREDD. Several lines of evidence support the notion that DIAP2-mediated ubiquitylation of DREDD is critically important for activation of AMP genes. First, the E3 ligase DIAP2, which is indispensable for innate immune-mediated activation of NF-κB and protection from Gram-negative bacterial infection, physically interacts with DREDD and targets it for non-degradative polyubiquitylation. DIAP2-mediated ubiquitylation of DREDD, thereby, relies on the presence of a functional RING finger of DIAP2, which strongly suggests that DIAP2 is directly ubiquitylating DREDD. Second, ubiquitylation of DREDD occurs in a signal-dependent manner. Third, failure to stimulate DREDD ubiquitylation, as seen in DreddD44, is associated with a severe loss-of-function phenotype abrogating IMD cleavage and ubiquitylation as well as Relish maturation. DREDD activation may follow a similar principle as the one of caspase-8 in mammals, where the Ub receptor p62 promotes aggregation and activation of CUL3-modified caspase-8 (Jin et al, 2009).
While innate immune signalling induces prominent and transient ubiquitylation of WT DREDD, treatment with DAP-PGN failed to promote ubiquitylation of DREDDD44. The G120R mutation maps to DED1 of DREDD, and although it impairs DIAP2-mediated ubiquitylation, this mutation does not affect the ability of DREDD to bind to itself, DIAP2, and dFADD. It also does neither destabilize DREDDD44 nor affect its inherent catalytic centre or substrate entry. Although DREDDD44 behaves like WT DREDD in most aspects, the G120R mutation abrogates its ability to be ubiquitylated in a signal-dependent manner. Substitution of G to R at position 120 generates DREDD with a positively charged side chain protruding from DED1. Most likely, the protruding positively charged side chain prevents DIAP2-mediated ubiquitylation allosterically, possibly by re-positioning DREDD in respect to DIAP2’s E2, which moves the Ub-acceptor lysines (K) of DREDD out of reach for the E2, making them no longer available for Ub transfer. Recent structural studies indicate that Ub-acceptor K residues must be positioned within 50 angstrom (Å) of the active site cysteine of the E2 for Ub transfer (Duda et al, 2008). Ultimately, crystal structure analysis of DIAP2/E2 bound to WT and mutant DREDD will be required to validate this model.
Intriguingly, expression of attacin D and diptericin is blocked to the same degree in dreddD44 and DreddB118 animals, which lack DREDD expression due to a premature stop codon at position 127 (Leulier et al, 2000). The strong phenotype associated with the G120R mutation together with the observation that this mutation selectively affects signal-dependent ubiquitylation strongly suggests that ubiquitylation of DREDD is essential for DREDD’s function in immunity. Consistently, we find that flies harbouring the G120R mutation fail to cleave IMD and Relish and induce expression of AMP genes in response to septic injury with Gram-negative bacteria. DreddD44 mutant flies also exhibit significantly lower basal expression of attacin D and diptericin under non-challenged conditions (data not shown), indicating that production of AMPs in response to commensal microbiota is also affected in these animals. Together, these data are consistent with a model whereby ubiquitylation of DREDD is required for signal-dependent activation of DREDD. This subsequently leads to processing of IMD, which exposes an IBM that associates with DIAP2 allowing DIAP2-mediated ubiquitylation of IMD. In addition, Ub-dependent activation of Relish is also required for the induction of AMPs.
The observation that impaired DREDD ubiquitylation correlates with loss of IMD and Relish cleavage may suggest that ubiquitylation of DREDD is required for DREDD activation under physiological conditions. This view is supported by a recent study indicating that the mammalian Ub receptor p62 promotes activation of ubiquitylated caspase-8 (Jin et al, 2009). While Ub-mediated clustering of DREDD might further support DREDD dimerization and activation, it is also possible that the Ub chains on DREDD and IMD might serve as docking sites for other proteins with UBD. In this respect, it is important to note that several downstream components, such as TAB2 and Kenny, carry putative UBDs. The Ub chains on DREDD and IMD may thereby bind and recruit the dTAK1/TAB2 and IKK (IRD5(IKKβ)/Kenny(IKKγ)) kinase complexes via the Ub receptors TAB2 and Kenny, respectively. Importantly, previous data indicate that IKK is required for signal-dependent processing of Relish (Silverman et al, 2000; Stoven et al, 2003; Erturk-Hasdemir et al, 2009). While IKK is necessary for DREDD-mediated cleavage of Relish, dTAK1 is surprisingly dispensable. This is evident because knockdown or genetic ablation of dTAK1 does not alter signal-dependent cleavage of Relish (Delaney et al, 2006; Erturk-Hasdemir et al, 2009). In contrast, loss of IKK completely blocks Relish cleavage in response to Gram-negative bacterial infection (Erturk-Hasdemir et al, 2009). Intriguingly, the kinase activity of IKK is not required for DREDD-mediated cleavage of Relish. Relish cleavage is rescued in ird51 mutant flies reconstituted with either WT or catalytically inactive mutant IRD5 (IRD5K50A). This demonstrates that IKK not only functions as the kinase phosphorylating Relish but also fulfils a structural role that facilitates Relish cleavage. The observation that the kinase activity of IKK is dispensable for Relish cleavage is also supported by the observation that IKK-mediated phosphorylation of serines 528 and 529 of Relish is not required for its cleavage (Erturk-Hasdemir et al, 2009). While phosphorylation of Relish is dispensable for Relish cleavage, it is clearly required for the transcriptional activity of Relish. Taken together, these data also indicate that mere activation of DREDD is not sufficient for DREDD-mediated processing of Relish. In addition to DREDD activation, IKK protein, but not IKK-mediated phosphorylation of Relish, is also required. At present it remains unclear how IKK protein facilitates DREDD-mediated processing of Relish. However, given that IKK directly phosphorylates Relish (Silverman et al, 2000), IKK must be able to bind to Relish. In this respect, it is interesting to note that phosphorylation of human p105 by IKKβ requires direct binding of IKKβ to a motif in the PEST region of p105 (Heissmeyer et al, 2001; Salmeron et al, 2001). Hence, a similar motif might exist in Relish that enables its recruitment to IKK. Consistently, the C-terminal 107 residues of Relish are required for its cleavage (Stoven et al, 2003), suggesting that the IKK interaction motif is located within this stretch.
On the basis of the new findings, we propose a refined model for IMD signalling (Figure 6). As previously established, ligand binding induces recruitment of IMD, Dredd and dFADD to PGRP-LCx (step 1). Recruitment of DIAP2 targets DREDD for K63-linked ubiquitylation (step 2), which allows Ub-mediated aggregation and activation of DIAP2-modified DREDD (step 3). Active DREDD subsequently cleaves IMD (step 4), which exposes an IBM at the neo-amino-terminus of cleaved IMD. The IBM of IMD binds to the BIR2/3 of DIAP2. This provides DIAP2 with an additional contact point within the signalling complex, reinforcing complex stability and allowing DIAP2-mediated ubiquitylation of IMD, and quite possibly other components of the signalling complex (step 5). The Ub chains on IMD and DREDD may serve as scaffolds for the recruitment of dTAK1 and IKK (step 6). Since IKK can bind to Relish, as evidenced by its ability to phosphorylate Relish, IKK might also bring Relish into close proximity of ubiquitylated and active DREDD, allowing DREDD-mediated proteolysis of Relish. The proximity to the signalling complex will also allow phospho-mediated activation of Relish (step 7). Subsequently, modified Relish translocates to the nucleus (step 8) where it drives expression of AMP genes (step 9).
Figure 6.
Model depicting Ub-dependent activation of NF-κB during IMD signalling. (1) Ligand binding induces recruitment of IMD, DREDD and dFADD to PGRP-LCx. (2) Recruitment of DIAP2 targets DREDD for K63-linked ubiquitylation. (3) This allows Ub-mediated aggregation, activation and processing of DIAP2-modified DREDD. (4) Active DREDD subsequently cleaves IMD, which exposes an IBM at the neo-amino-terminus of cleaved IMD. The IBM of IMD subsequently binds to the BIR2/3 of DIAP2. (5) This provides DIAP2 with an additional contact point within the signalling complex, reinforcing complex stability and permitting DIAP2-mediated ubiquitylation of IMD, and quite possibly other components of the signalling complex. (6) The Ub chains on IMD and DREDD may serve as scaffolds for the recruitment of dTAK1 and IKK. Since IKK can bind to Relish, as evidenced by its ability to phosphorylate Relish, IKK might also bring Relish into close proximity of ubiquitylated and active DREDD, allowing DREDD-mediated proteolysis of Relish. (7) The proximity to the signalling complex also allows phospho-mediated activation of Relish. (8) Subsequently, modified Relish translocates to the nucleus where (9) it drives expression of AMP target genes.
The direct involvement of ubiquitylation in caspase-mediated endoproteolysis of substrates represents a novel mechanism for Ub-dependent activation of NF-κB. Interestingly, a similar mechanism might also exist in mammals. For example, loss of caspase-8 function in human patients reportedly is connected with defective activation of lymphocytes (Chun et al, 2002), a process that is known to require NF-κB. Moreover, mammalian p62 can promote aggregation, activation and processing of ubiquitylated caspase-8 in a stimulus-dependent manner (Jin et al, 2009). As DREDD is the orthologue of mammalian caspase-8, it will be interesting to examine whether ubiquitylation of caspase-8 might convert caspase-8 from a pro-death molecule into an NF-κB-activating signalling component.
Materials and methods
Constructs
DIAP2 constructs were cloned into pAc5, pMT or pMT-IZ without tag or with C-terminal GST, HA or V5 tags. HA or V5 C-terminal tagged DREDD constructs were cloned into pMT. dFADD was cloned into pMT vector with C-terminal HA or Myc-tag. IMD was cloned into pMT or pAc5 and expressed as full-length protein (for Figure 1G) or alternatively as Ub-fusion protein to generate IMD exposing an N-terminal IBM (used in Figure 2I), corresponding to the DREDD-cleaved form of IMD (aa 31–273) (Paquette et al, 2010). Relish and Ub were cloned into pAc5 with N-terminal FLAG, HA or HIS-tag, respectively. Point mutants were generated by site-directed mutagenesis (Stratagene) according to the manufacturer’s instructions. All constructs were cloned by PCR and verified by DNA sequencing.
Fly stocks
The following fly strains were used: CantonS, w1118, yw, DD1 (wild-type) (Jung et al, 2001), Diap27c(Leulier et al, 2006), DreddD44, DreddB118, DreddL23 (Leulier et al, 2000), Tak11 (Vidal et al, 2001), RelishE20 (Hedengren et al, 1999), c564-GAL4 (fat-body-specific driver), and Da-GAL4. The UAS-Diap2 constructs encoding WT or mutant DIAP2 were generated by cloning the Diap2 open reading frame in EcoRI/XhoI-digested pUAST or FlyC31 pUASTattB vector. w1118 flies or the landing platform fly strain 24749 were used to generate UAS-Diap2 transgenic flies. Drosophila stocks and crosses were maintained at 25°C, unless stated otherwise.
Bacterial strains, infection experiments and survival analysis
In all, 2- to 4-day-old adult flies were used for infection experiments. Microbial septic injuries were performed by pricking adult flies in the lateral part of the thorax with a thin needle previously dipped into a concentrated (optical density of 200) culture of Erwinia carotovora carotovora 15 (Ecc15) or Escherichia coli. M. luteus was used as Gram-positive bacteria. Following septic injury, flies were incubated at 29°C and examined at different time points to monitor survival. The infected flies were transferred to fresh vials daily. For RT–qPCR analysis, adult flies were injected using the Nanoject II (Drummond Scientific). In all, 13.8 nl of cultured Ecc15 bacterial solution (optical density of 300) was injected and flies were incubated at 25°C. The experiments were performed in triplicates using 20–40 flies for each genotype.
Quantitative RT–PCR
For quantitative analysis of attacin D, diptericin, puckered and rp49 mRNA expression, RNA was extracted from whole animals using the RNeasy Mini kit (QIAGEN). cDNAs were synthesized using Quantitect Reverse transcription kit (QIAGEN) and quantitative PCR was performed using MesaBlue qPCR mastermix Plus and SYBR green (Eurogentech). SYBR green analysis was performed on a Lightcycler (Roche Diagnostics). The amount of mRNA detected was normalized to control rp49 mRNA values. We used normalized data to quantify the relative levels of a given mRNA according to cycling threshold analysis.
Tissue culture and treatments
Drosophila S2 cells were cultured as described previously (Tenev et al, 2005) and transfected with Effectene (QIAGEN) according to the manufacturer’s instructions. Peptidoglycan (DAP-PGN) isolated from E. coli was used at a concentration of 1 μg/ml for the indicated time points. Cycloheximide (Sigma) was used at a concentration of 50 μg/ml.
Immunoprecipitation and immunoblot analysis
Immunoprecipitation assays were performed as described previously (Tenev et al, 2005). In brief, cells were lysed in a buffer containing 50 mM Tris, pH 7.5, 150 mM NaCl, 1% Triton X-100, 10% glycerol and 1 mM EDTA, and extracts were spun at 14 000 r.p.m. for 10 min at 4°C. HA-, or V5-tagged proteins were purified using antibody-IgG-coupled agarose beads (GE Healthcare, Sigma Aldrich, respectively). After 2 h incubation at 4°C, beads were washed three times in 10 mM Tris, pH 7.5, 150 mM NaCl, 0.1% Triton X-100 and 5% glycerol. The following antibodies were used for immunoblotting: α-V5 (Serotec), α-HA (Roche), α-DIAP2 (Leulier et al, 2006), α-Myc (Sigma), α-Relish (in house), α-IMD (in house), α-FLAG (Sigma), α-K63-Ub, α-K48-Ub (Millipore), α-Ub (Cell Signaling), α-GST (Amersham) and α-Actin (Santa Cruz). Signals were visualized by chemiluminescence (GE Healthcare) or Odyssey Technology (LI-COR Biosciences). For total cell lysates, cells were lysed directly in Laemmli sample buffer before immunoblot analysis.
Ubiquitylation assays
For immunoprecipitation of ubiquitylated proteins, S2 cells were harvested in 200 μl of boiling 1% SDS in PBS, and the resulting lysate was heated at 100°C for 5 min. Lysates were diluted with 1 ml of 0.5% BSA, 1% Triton X-100 in PBS. DNA was sheared by sonication, and the material centrifuged at 14 000g for 10 min. HA-tagged ubiquitylated proteins were purified from the supernatant using α-HA agarose beads. Purified proteins were washed four times with 0.5% BSA, 1% Triton X-100 in PBS and the eluate analysed by immunoblotting. For Figure 1G, HA-tagged DREDD and IMD were purified under denaturing conditions and the presence of the respective Ub chains were detected using Ub-linkage specific antibodies.
Supplementary Material
Acknowledgments
We thank Bruno Lemaitre, Eric Baehrecke and Dan Hultmark for reagents, and Rebecca Wilson for technical assistance. We also thank members of the Meier laboratory for discussions. AM acknowledges the Academy of Finland and the Magnus Ehrnrooth Foundation for financial support, and NS was supported by NIH Grants AI60025 and AI082663 as well as the Burroughs Welcome Fund. We acknowledge NHS funding to the NIHR Biomedical Research Centre.
Author contributions: AM and CR contributed equally to the design and execution of most of the experiments, data analysis and writing of the manuscript. TT planned and performed binding and competition assays, while LC and CHK performed ubiquitylation assays in cells and in vivo. PSR, FL and MB made specific contributions to the initial phase of the project. MZ performed 3D modelling and sequence analysis. NS and PM contributed to the writing and data analysis of this manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Bischof J, Maeda RK, Hediger M, Karch F, Basler K (2007) An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc Natl Acad Sci USA 104: 3312–3317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Rodriguez A, Erskine R, Thach T, Abrams JM (1998) Dredd, a novel effector of the apoptosis activators reaper, grim, and hid in Drosophila. Dev Biol 201: 202–216 [DOI] [PubMed] [Google Scholar]
- Choe KM, Lee H, Anderson KV (2005) Drosophila peptidoglycan recognition protein LC (PGRP-LC) acts as a signal-transducing innate immune receptor. Proc Natl Acad Sci USA 102: 1122–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe KM, Werner T, Stoven S, Hultmark D, Anderson KV (2002) Requirement for a peptidoglycan recognition protein (PGRP) in Relish activation and antibacterial immune responses in Drosophila. Science 296: 359–362 [DOI] [PubMed] [Google Scholar]
- Chun HJ, Zheng L, Ahmad M, Wang J, Speirs CK, Siegel RM, Dale JK, Puck J, Davis J, Hall CG, Skoda-Smith S, Atkinson TP, Straus SE, Lenardo MJ (2002) Pleiotropic defects in lymphocyte activation caused by caspase-8 mutations lead to human immunodeficiency. Nature 419: 395–399 [DOI] [PubMed] [Google Scholar]
- Delaney JR, Stoven S, Uvell H, Anderson KV, Engstrom Y, Mlodzik M (2006) Cooperative control of Drosophila immune responses by the JNK and NF-kappaB signaling pathways. EMBO J 25: 3068–3077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikic I, Wakatsuki S, Walters KJ (2009) Ubiquitin-binding domains – from structures to functions. Nat Rev Mol Cell Biol 10: 659–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditzel M, Broemer M, Tenev T, Bolduc C, Lee TV, Rigbolt KT, Elliott R, Zvelebil M, Blagoev B, Bergmann A, Meier P (2008) Inactivation of effector caspases through nondegradative polyubiquitylation. Mol Cell 32: 540–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duda DM, Borg LA, Scott DC, Hunt HW, Hammel M, Schulman BA (2008) Structural insights into NEDD8 activation of cullin-RING ligases: conformational control of conjugation. Cell 134: 995–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckelman BP, Drag M, Snipas SJ, Salvesen GS (2008) The mechanism of peptide-binding specificity of IAP BIR domains. Cell Death Differ 15: 920–928 [DOI] [PubMed] [Google Scholar]
- Elrod-Erickson M, Mishra S, Schneider D (2000) Interactions between the cellular and humoral immune responses in Drosophila. Curr Biol 10: 781–784 [DOI] [PubMed] [Google Scholar]
- Erturk-Hasdemir D, Broemer M, Leulier F, Lane WS, Paquette N, Hwang D, Kim CH, Stoven S, Meier P, Silverman N (2009) Two roles for the Drosophila IKK complex in the activation of Relish and the induction of antimicrobial peptide genes. Proc Natl Acad Sci USA 106: 9779–9784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrandon D, Imler JL, Hetru C, Hoffmann JA (2007) The Drosophila systemic immune response: sensing and signalling during bacterial and fungal infections. Nat Rev Immunol 7: 862–874 [DOI] [PubMed] [Google Scholar]
- Georgel P, Naitza S, Kappler C, Ferrandon D, Zachary D, Swimmer C, Kopczynski C, Duyk G, Reichhart JM, Hoffmann JA (2001) Drosophila immune deficiency (IMD) is a death domain protein that activates antibacterial defense and can promote apoptosis. Dev Cell 1: 503–514 [DOI] [PubMed] [Google Scholar]
- Gesellchen V, Kuttenkeuler D, Steckel M, Pelte N, Boutros M (2005) An RNA interference screen identifies Inhibitor of Apoptosis Protein 2 as a regulator of innate immune signalling in Drosophila. EMBO Rep 6: 979–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuking P, Narasimamurthy R, Lemaitre B, Basler K, Leulier F (2009) A non-redundant role for Drosophila Mkk4 and hemipterous/Mkk7 in TAK1-mediated activation of JNK. PLoS ONE 4: e7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottar M, Gobert V, Michel T, Belvin M, Duyk G, Hoffmann JA, Ferrandon D, Royet J (2002) The Drosophila immune response against Gram-negative bacteria is mediated by a peptidoglycan recognition protein. Nature 416: 640–644 [DOI] [PubMed] [Google Scholar]
- Grabbe C, Dikic I (2009) Functional roles of ubiquitin-like domain (ULD) and ubiquitin-binding domain (UBD) containing proteins. Chem Rev 109: 1481–1494 [DOI] [PubMed] [Google Scholar]
- Grivennikov SI, Greten FR, Karin M (2010) Immunity, inflammation, and cancer. Cell 140: 883–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyrd-Hansen M, Meier P (2010) IAPs: from caspase inhibitors to modulators of NF-kappaB, inflammation and cancer. Nat Rev Cancer 10: 561–574 [DOI] [PubMed] [Google Scholar]
- Hedengren M, Asling B, Dushay MS, Ando I, Ekengren S, Wihlborg M, Hultmark D (1999) Relish, a central factor in the control of humoral but not cellular immunity in Drosophila. Mol Cell 4: 827–837 [DOI] [PubMed] [Google Scholar]
- Heissmeyer V, Krappmann D, Hatada EN, Scheidereit C (2001) Shared pathways of IkappaB kinase-induced SCF(betaTrCP)-mediated ubiquitination and degradation for the NF-kappaB precursor p105 and IkappaBalpha. Mol Cell Biol 21: 1024–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeller D, Dikic I (2009) Targeting the ubiquitin system in cancer therapy. Nature 458: 438–444 [DOI] [PubMed] [Google Scholar]
- Hu S, Yang X (2000) dFADD, a novel death domain-containing adapter protein for the Drosophila caspase DREDD. J Biol Chem 275: 30761–30764 [DOI] [PubMed] [Google Scholar]
- Huh JR, Foe I, Muro I, Chen CH, Seol JH, Yoo SJ, Guo M, Park JM, Hay BA (2007) The Drosophila inhibitor of apoptosis (IAP) DIAP2 is dispensable for cell survival, required for the innate immune response to gram-negative bacterial infection, and can be negatively regulated by the reaper/hid/grim family of IAP-binding apoptosis inducers. J Biol Chem 282: 2056–2068 [DOI] [PubMed] [Google Scholar]
- Jin Z, Li Y, Pitti R, Lawrence D, Pham VC, Lill JR, Ashkenazi A (2009) Cullin3-based polyubiquitination and p62-dependent aggregation of caspase-8 mediate extrinsic apoptosis signaling. Cell 137: 721–735 [DOI] [PubMed] [Google Scholar]
- Jung AC, Criqui MC, Rutschmann S, Hoffmann JA, Ferrandon D (2001) Microfluorometer assay to measure the expression of beta-galactosidase and green fluorescent protein reporter genes in single Drosophila flies. Biotechniques 30: 594-598,600–591 [DOI] [PubMed] [Google Scholar]
- Kanayama A, Seth RB, Sun L, Ea CK, Hong M, Shaito A, Chiu YH, Deng L, Chen ZJ (2004) TAB2 and TAB3 activate the NF-kappaB pathway through binding to polyubiquitin chains. Mol Cell 15: 535–548 [DOI] [PubMed] [Google Scholar]
- Kaneko T, Goldman WE, Mellroth P, Steiner H, Fukase K, Kusumoto S, Harley W, Fox A, Golenbock D, Silverman N (2004) Monomeric and polymeric gram-negative peptidoglycan but not purified LPS stimulate the Drosophila IMD pathway. Immunity 20: 637–649 [DOI] [PubMed] [Google Scholar]
- Kaneko T, Yano T, Aggarwal K, Lim JH, Ueda K, Oshima Y, Peach C, Erturk-Hasdemir D, Goldman WE, Oh BH, Kurata S, Silverman N (2006) PGRP-LC and PGRP-LE have essential yet distinct functions in the drosophila immune response to monomeric DAP-type peptidoglycan. Nat Immunol 7: 715–723 [DOI] [PubMed] [Google Scholar]
- Karin M, Greten FR (2005) NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol 5: 749–759 [DOI] [PubMed] [Google Scholar]
- Kleino A, Valanne S, Ulvila J, Kallio J, Myllymaki H, Enwald H, Stoven S, Poidevin M, Ueda R, Hultmark D, Lemaitre B, Ramet M (2005) Inhibitor of apoptosis 2 and TAK1-binding protein are components of the Drosophila Imd pathway. EMBO J 24: 3423–3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komander D (2009) The emerging complexity of protein ubiquitination. Biochem Soc Trans 37: 937–953 [DOI] [PubMed] [Google Scholar]
- Lemaitre B, Hoffmann J (2007) The host defense of Drosophila melanogaster. Annu Rev Immunol 25: 697–743 [DOI] [PubMed] [Google Scholar]
- Lemaitre B, Kromer-Metzger E, Michaut L, Nicolas E, Meister M, Georgel P, Reichhart JM, Hoffmann JA (1995) A recessive mutation, immune deficiency (imd), defines two distinct control pathways in the Drosophila host defense. Proc Natl Acad Sci USA 92: 9465–9469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA (1996) The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86: 973–983 [DOI] [PubMed] [Google Scholar]
- Lemaitre B, Reichhart JM, Hoffmann JA (1997) Drosophila host defense: differential induction of antimicrobial peptide genes after infection by various classes of microorganisms. Proc Natl Acad Sci USA 94: 14614–14619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leulier F, Lhocine N, Lemaitre B, Meier P (2006) The Drosophila inhibitor of apoptosis protein DIAP2 functions in innate immunity and is essential to resist gram-negative bacterial infection. Mol Cell Biol 26: 7821–7831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leulier F, Parquet C, Pili-Floury S, Ryu JH, Caroff M, Lee WJ, Mengin-Lecreulx D, Lemaitre B (2003) The Drosophila immune system detects bacteria through specific peptidoglycan recognition. Nat Immunol 4: 478–484 [DOI] [PubMed] [Google Scholar]
- Leulier F, Rodriguez A, Khush RS, Abrams JM, Lemaitre B (2000) The Drosophila caspase Dredd is required to resist gram-negative bacterial infection. EMBO Rep 1: 353–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leulier F, Vidal S, Saigo K, Ueda R, Lemaitre B (2002) Inducible expression of double-stranded RNA reveals a role for dFADD in the regulation of the antibacterial response in Drosophila adults. Curr Biol 12: 996–1000 [DOI] [PubMed] [Google Scholar]
- Lu Y, Wu LP, Anderson KV (2001) The antibacterial arm of the drosophila innate immune response requires an IkappaB kinase. Genes Dev 15: 104–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfruelli P, Reichhart JM, Steward R, Hoffmann JA, Lemaitre B (1999) A mosaic analysis in Drosophila fat body cells of the control of antimicrobial peptide genes by the Rel proteins Dorsal and DIF. EMBO J 18: 3380–3391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Khanuja BS, Ip YT (1999) Toll receptor-mediated Drosophila immune response requires Dif, an NF-kappaB factor. Genes Dev 13: 792–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naitza S, Rosse C, Kappler C, Georgel P, Belvin M, Gubb D, Camonis J, Hoffmann JA, Reichhart JM (2002) The Drosophila immune defense against gram-negative infection requires the death protein dFADD. Immunity 17: 575–581 [DOI] [PubMed] [Google Scholar]
- Nathan C, Ding A (2010) Nonresolving inflammation. Cell 140: 871–882 [DOI] [PubMed] [Google Scholar]
- Paquette N, Broemer M, Aggarwal K, Chen L, Husson M, Erturk-Hasdemir D, Reichhart JM, Meier P, Silverman N (2010) Caspase-mediated cleavage, IAP binding, and ubiquitination: linking three mechanisms crucial for Drosophila NF-kappaB signaling. Mol Cell 37: 172–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramet M, Manfruelli P, Pearson A, Mathey-Prevot B, Ezekowitz RA (2002) Functional genomic analysis of phagocytosis and identification of a Drosophila receptor for E. coli. Nature 416: 644–648 [DOI] [PubMed] [Google Scholar]
- Ribeiro PS, Kuranaga E, Tenev T, Leulier F, Miura M, Meier P (2007) DIAP2 functions as a mechanism-based regulator of drICE that contributes to the caspase activity threshold in living cells. J Cell Biol 179: 1467–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutschmann S, Jung AC, Hetru C, Reichhart JM, Hoffmann JA, Ferrandon D (2000a) The Rel protein DIF mediates the antifungal but not the antibacterial host defense in Drosophila. Immunity 12: 569–580 [DOI] [PubMed] [Google Scholar]
- Rutschmann S, Jung AC, Zhou R, Silverman N, Hoffmann JA, Ferrandon D (2000b) Role of Drosophila IKK gamma in a toll-independent antibacterial immune response. Nat Immunol 1: 342–347 [DOI] [PubMed] [Google Scholar]
- Rutschmann S, Kilinc A, Ferrandon D (2002) Cutting edge: the toll pathway is required for resistance to gram-positive bacterial infections in Drosophila. J Immunol 168: 1542–1546 [DOI] [PubMed] [Google Scholar]
- Salmeron A, Janzen J, Soneji Y, Bump N, Kamens J, Allen H, Ley SC (2001) Direct phosphorylation of NF-kappaB1 p105 by the IkappaB kinase complex on serine 927 is essential for signal-induced p105 proteolysis. J Biol Chem 276: 22215–22222 [DOI] [PubMed] [Google Scholar]
- Silverman N, Zhou R, Erlich RL, Hunter M, Bernstein E, Schneider D, Maniatis T (2003) Immune activation of NF-kappaB and JNK requires Drosophila TAK1. J Biol Chem 278: 48928–48934 [DOI] [PubMed] [Google Scholar]
- Silverman N, Zhou R, Stoven S, Pandey N, Hultmark D, Maniatis T (2000) A Drosophila IkappaB kinase complex required for Relish cleavage and antibacterial immunity. Genes Dev 14: 2461–2471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaug B, Jiang X, Chen ZJ (2009) The role of ubiquitin in NF-kappaB regulatory pathways. Annu Rev Biochem 78: 769–796 [DOI] [PubMed] [Google Scholar]
- Stoven S, Ando I, Kadalayil L, Engstrom Y, Hultmark D (2000) Activation of the Drosophila NF-kappaB factor Relish by rapid endoproteolytic cleavage. EMBO Rep 1: 347–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoven S, Silverman N, Junell A, Hedengren-Olcott M, Erturk D, Engstrom Y, Maniatis T, Hultmark D (2003) Caspase-mediated processing of the Drosophila NF-kappaB factor Relish. Proc Natl Acad Sci USA 100: 5991–5996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanji T, Ip YT (2005) Regulators of the Toll and Imd pathways in the Drosophila innate immune response. Trends Immunol 26: 193–198 [DOI] [PubMed] [Google Scholar]
- Tenev T, Zachariou A, Wilson R, Ditzel M, Meier P (2005) IAPs are functionally non-equivalent and regulate effector caspases through distinct mechanisms. Nat Cell Biol 7: 70–77 [DOI] [PubMed] [Google Scholar]
- Thevenon D, Engel E, Avet-Rochex A, Gottar M, Bergeret E, Tricoire H, Benaud C, Baudier J, Taillebourg E, Fauvarque MO (2009) The Drosophila ubiquitin-specific protease dUSP36/Scny targets IMD to prevent constitutive immune signaling. Cell Host Microbe 6: 309–320 [DOI] [PubMed] [Google Scholar]
- Tsichritzis T, Gaentzsch PC, Kosmidis S, Brown AE, Skoulakis EM, Ligoxygakis P, Mosialos G (2007) A Drosophila ortholog of the human cylindromatosis tumor suppressor gene regulates triglyceride content and antibacterial defense. Development 134: 2605–2614 [DOI] [PubMed] [Google Scholar]
- Tzou P, Ohresser S, Ferrandon D, Capovilla M, Reichhart JM, Lemaitre B, Hoffmann JA, Imler JL (2000) Tissue-specific inducible expression of antimicrobial peptide genes in Drosophila surface epithelia. Immunity 13: 737–748 [DOI] [PubMed] [Google Scholar]
- Varshavsky A (2000) Ubiquitin fusion technique and its descendants. Methods Enzymol 327: 578–593 [DOI] [PubMed] [Google Scholar]
- Vidal S, Khush RS, Leulier F, Tzou P, Nakamura M, Lemaitre B (2001) Mutations in the Drosophila dTAK1 gene reveal a conserved function for MAPKKKs in the control of rel/NF-kappaB-dependent innate immune responses. Genes Dev 15: 1900–1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JK, Wang L, Zheng L, Wan F, Ahmed M, Lenardo MJ, Wu H (2005) Crystal structure of MC159 reveals molecular mechanism of DISC assembly and FLIP inhibition. Mol Cell 20: 939–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Silverman N, Hong M, Liao DS, Chung Y, Chen ZJ, Maniatis T (2005) The role of ubiquitination in Drosophila innate immunity. J Biol Chem 280: 34048–34055 [DOI] [PubMed] [Google Scholar]
- Zhuang ZH, Sun L, Kong L, Hu JH, Yu MC, Reinach P, Zang JW, Ge BX (2006) Drosophila TAB2 is required for the immune activation of JNK and NF-kappaB. Cell Signal 18: 964–970 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.