Abstract
Aberrant regulation of the Wnt signalling pathway has emerged as a prevalent theme in cancer biology. This chapter summarizes the research that provides a proof of concept for inhibiting Wnt signalling in cancer, the potential means by which this could be achieved, and some recent advances towards this goal. A brief discussion of molecular diagnostics and possible safety concerns is also provided.
Keywords: cancer, drugs, Wnt
Introduction
For the better part of the twentieth century, the progress of cancer therapy in the clinic appeared to follow a path independent of our advances in the understanding of cancer at the molecular level. Progress in the clinic, represented by the hallmark work of Frei and Freibach in mid 1960s, consisted of carpet-bombing cancers with cocktails of drugs dosed to the highest tolerable level. Mechanistically, these drugs attack cancer cells at a very fundamental level, and the combinations to some extent accounted for the heterogeneity apparent in the disease. In the early 1970s, the seminal work of Varmus, Bishop, Weinberg and others made it apparent that cancer was indeed a genetic disease (Weinberg and Bishop, 1996). Oncogenesis was ultimately associated with specific defects in endogenous human genes. Acquisition of activating mutations in proto-oncogenes could transform cells, as did inactivating mutations in tumour suppressor genes. The discovery of numerous cancer-causing genes quickly followed, yet the veritable translation of cancer genetics into the clinic would await the FDA approval of Gleevac (Imatinib) for CML in 2001.
The remarkable success of Gleevac energized the field of rational drug design by proving that drugging the driver actually worked. Drugging of the EGFR receptor in lung cancer, BRAF in melanoma, Smoothened in medulloblastoma, and so on, has further fortified efforts in designer drug therapy for cancer. Among the litany of cancer-causing genes, the adenomatous polyposis coli (APC) tumour suppressor identified in 1991 provided a vector to a therapeutic target in the vast majority colorectal cancers (Groden et al, 1991; Kinzler et al, 1991). That discovery, and that of the mechanism by which it promotes cancer, fuelled the delineation of a cancer signalling pathway consisting of secreted ligands, cell surface receptors, scaffolds, kinases, ubiquitin ligases and hydrolases, and activators and repressors of gene transcription (http://www.stanford.edu/group/nusselab/cgi-bin/wnt/) (Figure 1). Recent successes in rational drug design, together with our growing knowledge of oncogenic Wnt signalling, and its prevalence in human cancer, provides clamant motivation for drugging this pathway.
Figure 1.
Therapeutic intervention in Wnt signalling. Components of Wnt signalling at the plasma membrane (PM), in the cytoplasm and nucleus are represented with the locus of intervention for some of the inhibitors (red text) described in this chapter.
The association of genetic defects with Wnt signalling and cancer
The genesis of mammary tumours arising in mice infected with the murine mammary tumour virus was ultimately traced to the activation of the murine int-1 gene (Nusse and Varmus, 1982). The int-1 gene bore resemblance to the fly wingless gene, a secreted component of a signalling network that included zeste white, a glycogen synthase kinase homologue, and armadillo, the Drosophila version of mammalian β-catenin. The relevance of Wnt signalling to cancer was fortified by the discovery that the human tumour suppressor APC protein was associated with β-catenin (Su et al, 1993; Rubinfeld et al, 1997). That β-catenin was downregulated by APC and upregulated by Wnt-1 implicated β-catenin as a potential driver of human cancer (Hinck et al, 1994; Munemitsu et al, 1995). This was confirmed by the identification of mutations in the gene coding for β-catenin that rendered the protein refractory to regulation by APC (Morin et al, 1997; Rubinfeld et al, 1997). Finally, the means by which β-catenin promoted tumourigensis was revealed by the discovery of transcription factors that associated with it to activate growth-promoting genes (Behrens et al, 1996; Molenaar et al, 1996).
Germline mutations in APC are the cause of familial adenomatous polyposis, a heritable intestinal cancer syndrome. In addition, somatic mutations in APC are detected in the vast majority of all sporadic colorectal cancers. (Clements et al, 2003). Loss of function in both alleles is required for tumourigensis and that loss is structurally linked to the protein’s ability to regulate β-catenin protein stability (Polakis, 2007). Specifically, truncating mutations in APC remove all binding sites for Axin, a scaffold that also binds β-catenin and recruits the kinases GSK3 and CKI essential for marking β-catenin for destruction facilitated by the E3 ubiquitin ligase β-TRCP (Figure 1). Axins I and II are also tumour suppressors found mutated in both sporadic and familial cancers (Lammi et al, 2004; Salahshor and Woodgett, 2005; Marvin et al, 2011). Axins bind directly to both APC and β-catenin and are essential for the downregulation of β-catenin.
Although the GSK3 genes, which are required for the regulation of β-catenin, are putative tumour suppressors, mutations in the alleles coding for GSK3α and β have not been associated with human cancers. However, GSK3β activity may be altered by an in-frame splice deletion affecting the kinase domain identified by the Jamieson lab in chronic mylogenous leukaemia (Abrahamsson et al, 2009). The core components of the so-called β-catenin destruction complex, comprised of GSK3, Axin, APC and β-TrCP, has been expanded recently by the addition of WTX, a tumour suppressor associated with the paediatric renal cancer Wilm’s tumour (Major et al, 2007). Aberrant splicing has also been described for the Wnt coreceptor LRP5 in parathyroid and breast cancers. Here, missplicing deletes the region of LRP5 that interacts with the secreted Wnt signalling repressor DDK1 (Bjorklund et al, 2009).
While it is apparent that deregulation of Wnt signalling is a driver in the majority of colorectal cancers, as well as many other human cancers, finding an executable point of therapeutic intervention has been challenging. The following sections summarize the rationale for drugging the pathway, including potential nodes for intervention, the progress that has been made and what will be required to develop a drug.
Will Wnt inhbitors work?
Although the current success rate in targeted therapies is favourable, past performance does not guarantee future success. Trepidation is particularly warranted when considering targets that are not directly activated by mutation, as exemplified by the Wnt signalling pathway. The limits of scientific intuition, as they translate to therapy, are becoming apparent. For example, pathway logic would lead one to surmise that inhibiting the immediate downstream effector of an undruggable oncogenic protein would be therapeutically beneficial. However, inhibition of wild-type (wt) raf kinase, lying immediately downstream of oncogenic ras, fails to provide benefit and could even exacerbate progression of these tumours (Poulikakos and Rosen, 2011). Indeed, an unfortunate side effect of raf inhibitors in the clinic is their promotion of keratoacanthoma and squamous cell carcinoma, apparently due to the paradoxical activation of MAP kinase signalling in wt raf keratinocytes (Chapman et al, 2011). Even when the oncogene product itself is drugged, success might not follow. The spectacular clinical efficacy of the raf inhibitor in V600E raf melanomas has not been reproduced in V600E raf colon cancers, suggesting that the overall genetic context also modifies the effectiveness of drugging the oncogenic protein.
Drug discovery is a labour-intensive process. Drugging a single target can require the establishment of robust in vitro and cell-based assays, a high-throughput screening effort involving millions of chemical compounds and the full-time effort of dozens of medicinal chemists, as well as structural biologists, pharmacologists and toxicologists. Prior to embarking on such an endeavour, one must achieve a certain level of confidence that inhibiting the intended target will indeed negatively impact tumourigenesis. However imperfect, preclinical experiments that provide a proof of concept in model systems are essential to advance a molecule to clinical evaluation.
Inhibition of signalling in cancers driven by Wnt pathway mutations
A rich history of experiments designed to disrupt Wnt signalling strongly suggest that therapeutic intervention could be successful. Among the earliest of these attempts was the expression of wt APC in an APC mutant colorectal cancer cell line. Increased apoptosis was noted following induced expression of wt APC in cultured HT29 cells (Morin et al, 1996). Expression of Axin I, an additional tumour suppressor in the Wnt pathway, also promoted apoptosis in cancer cell lines containing mutations in either β-catenin, APC or Axin I (Satoh et al, 2000). Although these experiments show that re-introduction of wt tumour suppressors can reinstate growth control, they fall short of modelling a more practical therapy in which a positive acting element is interfered with. This can be simulated with dominant-negative mutants, like the N-terminally deleted TCF-4, which binds Wnt target genes nonproductively as a result of its inability to associate with β-catenin. Indeed, ectopic expression of the dominant-negative TCF4 in colorectal cancer cells turned off target genes resulting in their growth arrest (Tetsu and McCormick, 1999; van de Wetering et al, 2002).
The two tumour suppressors, Axin and APC, negatively regulate β-catenin, and β-catenin itself is oncogenically activated by gain-of-function mutations. These genetic findings alone point to β-catenin as a prime target for therapeutic intervention. Accordingly, a variety of proof-of-principle experiments aimed at interfering with β-catenin have provided encouraging results. In an early example of this, Gottardi et al expressed a fragment of cadherin that binds soluble β-catenin preventing its association with TCF transcription factors, thereby shutting off gene activation (Gottardi et al, 2001). This resulted in growth inhibition of SW480 colorectal cancer cells. Suppression of β-catenin by antisense oligonucleotides was also found to inhibit tumourgenicity of colon cancer cells in vitro and in vivo (Green et al, 2001; Roh et al, 2001). Disruption of the β-catenin gene by somatic cell gene targeting revealed an essential requirement for β-catenin in clonal growth of the APC mutant and β-catenin mutant colorectal cancer lines, DLD-1 and SW48, respectively (Kim et al, 2002).
More recently, the use of RNA-interfering technologies have been employed to validate β-catenin as a target in cancer therapy. Examples of this include siRNA knockdown of β-catenin transcripts, which reduced Wnt-dependent gene activation and diminished the growth of colon cancer cells in vitro and in vivo (Verma et al, 2003). Inducible shRNA was used to knockdown β-catenin in colorectal cancer cells harbouring mutations in β-catenin itself (Mologni et al, 2010). The cell lines employed, HCT116 and Ls174t, also contained K-ras mutations, and significant effects on cell growth and survival were not observed unless both the ras and β-catenin oncogenes were repressed. Under these conditions, highly effective inhibition of tumour growth in vivo was observed, whereas repression of either oncogene alone was considerably less effective.
One of the most compelling proof of principal studies was recently provided by the McLaughlin lab, which engineered the inducible knockdown of β-catenin into APC mutant colorectal cancer cells (Scholer-Dahirel et al, 2011). The growth of tumour xenografts from these cells was strongly inhibited upon shRNA knockdown of β-catenin, which was accompanied by induction of p21 and loss of Ki67 staining. Interestingly, markers of intestinal cell differentiation were also induced in the tumours upon knockdown of β-catenin. An important finding here was that the tumours quickly resumed their growth when expression of the shRNA was ceased. This suggests that any therapeutic inhibitor of β-catenin will likely require continuous administration.
In validating the Wnt signalling pathway one needs to keep in mind the dual role of β-catenin in signalling and cell adhesion. It is conceivable that the effects observed upon disruption of β-catenin expression or function could relate to its role in facilitating cadherin in the formation of cell–cell contacts. To address this caveat, Cong et al (2003) created a fusion protein that artificially recruited the E3 ubiquitin ligase β-TrCP to β-catenin. Expression of this construct downregulated the soluble, signalling pool of β-catenin, without impacting the cadherin-bound, adhesive fraction. Colon cells expressing the fusion protein lost their tumourigenic potential, including their ability to form tumours in mice.
Inhibition of Wnt signalling in cancers lacking pathway mutations
Mutations in the Wnt pathway should serve as a guide in determining where prospective inhibitors would be expected to work. In addition to colorectal cancers, where mutations are prevalent, hepatocellular cancers commonly harbour mutations in Wnt pathway components. Accordingly, RNAi-mediated knockdown of β-catenin decreased the viability, proliferation and growth in soft agar of the HepG2 human hepatoma cell line, which expresses mutant β-catenin (Zeng et al, 2007). Surprisingly, similar inhibitory effects were observed with a second cell line, Hep3b, which has wt β-catenin. Interfering with Wnt signalling was also effective in a haematopoetic model of cancer that lacked mutations in the Wnt pathway (Ashihara et al, 2009). This study showed that knockdown of β-catenin in RPMI8226 multiple myeloma cells reduced their capacity to form tumours in irradiated nude mice. Thus, interference with Wnt signalling in cancers lacking pathway mutations can be effective and suggests that aberrant activation of Wnt receptors should also be entertained as an oncogenic mechanism.
Constitutive receptor stimulation could account for hyperactive Wnt signalling in the absence of pathway mutations. This scenario invokes a source of Wnt ligand, which could arise from either the host cells or the cancer cells, themselves. In examining a panel of breast and ovarian cancer cell lines, Bafico et al (2004) noted the presence of a signalling pool of uncomplexed β-catenin, a hallmark of Wnt signalling, in cells that were otherwise wt for the Wnt pathway. Ectopic expression of the secreted Wnt ligand inhibitors, secreted frizzled-related protein (sFRP1) or dickkopf (DKK1), reduced the pool of soluble β-catenin and inhibited expression of a Wnt reporter gene. The authors ascribed the activity to autocrine Wnt signalling and subsequently reported this in non-small-cell lung cancer (NSCLC) cell lines as well (Akiri et al, 2009).
Autocrine Wnt signalling in breast cancer cell lines is consistent with reports on human mammary cancers, where Wnt pathway mutations are absent, yet hyperactive signalling is apparent. Breast cancers fitting this description appear biased towards those classified as basal-like or triple negative (Lin et al, 2000; Chung et al, 2004; Howe and Brown, 2004; Khramtsov et al, 2010; Geyer et al, 2011). Accordingly, Yang et al (2011) recently reported a proof-of-principle experiment involving the knockdown of FZD7, whose expression is characteristic of the basal-like breast cancer (Sorlie et al, 2003). Knockdown of FZD7 in cell line models of triple negative breast cancer reduced expression of Wnt target genes, inhibited tumourigenesis in vitro and greatly retarded the capacity of the MDA-MD-231 cell line to form tumours in mice. That certain breast cancers are driven by Wnt ligands is also supported by the work from the Hynes lab in which sFRP1, which binds to and effectively competes with FZD receptors for Wnt ligands, was ectopically expressed in the MDA-MB-231 cell line (Matsuda et al, 2009). Expression of sFRP1 diminished the capacity of these cells to form tumours in the mammary fat pads of mice and impaired their ability to metastasize to the lungs.
An association between Wnt signalling and metastasis was also discovered by the Massague lab, this time in lung cancers (Nguyen et al, 2009). A Wnt target gene signature was evident in metastatic lesions derived from human lung cancer models grown in mice. Functional relevance was assessed by the expression of dominant-negative TCF4 or TCF1 in the metastasis-prone derivatives of the PC9 and H2030 non-small lung cancer lines. The dominant-negative transcription factors significantly retarded metastatic spread to the bone and brain, but did not impede the growth of the primary tumour.
The genetic manipulations involving knockdown of transcripts and ectopic expression of dominant-negative genes or secreted inhibitors are pertinent lessons, yet they remain one step away from a pharmacological proof of concept. The systemic administration of an inhibitor, albeit in a model system, more directly tests the pharmacologic feasibility of a therapeutic approach. Accordingly, DeAlmeida et al (2007) tested the administration of a Wnt inhibitory fusion protein consisting of the Fc region of IgG fused to the extracellular domain of the FZD8 (FZD8CRD) Wnt receptor. The molecule appeared pharmacologically sound based on its dramatic inhibitory effect in the MMTV-Wnt-1 model of mammary cancer. The FZD8CRD also significantly inhibited the formation of tumour xenografts by two nonengineered cancer cell lines, the N-TERA2 human testicular cancer and the PA1 human ovarian cancer cell line. The PA1 was among those cells identified by Bafico et al (2004) as having autocrine Wnt signalling. Additional approaches, involving antibodies to the FZD and LRP5/6 Wnt receptors, would likely be effective and perhaps offer greater specificity relative to FZD8CRD.
Inhibition of Wnt signalling in cancer stem cells
Additional rationale for drugging Wnt comes from the purported role of Wnt signalling in the establishment and maintenance of so-called cancer stem cells (Reya and Clevers, 2005; Malanchi and Huelsken, 2009). There is ample evidence for a cellular hierarchy in some cancers, especially those of haematopoetic origin, and recent studies support a role for Wnt in maintaining their pluripotency. Excessive Wnt signalling was apparent in granulocytic-macrophage progenitors isolated from CML patients, and their capacity for proliferation and self-renewal was attenuated by the ectopic expression of the Wnt pathway inhibitor Axin (Jamieson et al, 2004). In a separate study, introduction of BCR-ABL into marrow cells from conditional β-catenin−/− knockout mice significantly reduced the onset of CML-like disease relative to that observed with wt marrow cells (Zhao et al, 2007). Nevertheless, recipients of the transformed β-catenin−/− cells went on to develop disease resembling acute lymphoblastic leukaemia. In yet another animal model of leukaemia, initiated by MLL, the mixed lineage leukaemia fusion protein, disruption of the β-catenin gene prevented pre-leukaemic stem cells from inducing leukaemia in grafted mice (Yeung et al, 2010). In this same study, shRNA knockdown of β-catenin in leukaemia stem cells severely delayed the onset of leukaemia in recipient hosts. The requirement for Wnt signalling was also tested in a model of AML, in which haematopoietic stem cells were transformed by Hoxa9 and Meis1a. The absence of β-catenin severely impaired the development of AML in recipient mice (Wang et al, 2010).
Recent evidence also points to a role for Wnt signalling in cutaneous cancer stem cells. The Huelsken lab found many similarities between normal skin stem cells and cancer stem cells isolated from murine epidermal tumours induced by carcinogens (Malanchi et al, 2008). The cancer stem cells appeared enriched for Wnt signaling, and conditional knockdown of β-catenin effectively eliminated this population of cells resulting in complete regression of the epidermal tumours. This demonstrates that a mouse epidermal tumour is indeed impacted by inhibition of Wnt signalling, and the authors also provided some evidence that Wnt signalling is exacerbated in human squamous cell carcinomas.
How can we inhibit Wnt signalling?
The biological rationale for drugging Wnt signalling in cancers that possess pathway-activating mutations is certainly compelling. Nevertheless, the tractability of developing a rational drug is gated by certain criteria. First of all, we must identify a positive acting target in the pathway that is amenable to drug inhibition. Historically, small-molecule inhibitors have enjoyed success in targeting ion channels, G-protein-coupled receptors, proteases and, more recently, kinases. Although β-catenin is the most salient positive acting element in the Wnt cancer pathway, it bears no relation to these legacy targets, and direct inhibition would likely involve blocking its interaction with the TCF/LEF transcription factors. Pharmacological disruption of protein–protein interactions with small-molecule inhibitors is not without precedent, but it is rare, difficult and highly dependent upon the specifics of the structural interface one wishes to disrupt. This of course relies upon the detailed structural information, which exists in abundance for β-catenin, including complexes with several of its partners (reviewed in Xu and Kimelman, 2007).
Targeting β-catenin protein–protein interactions
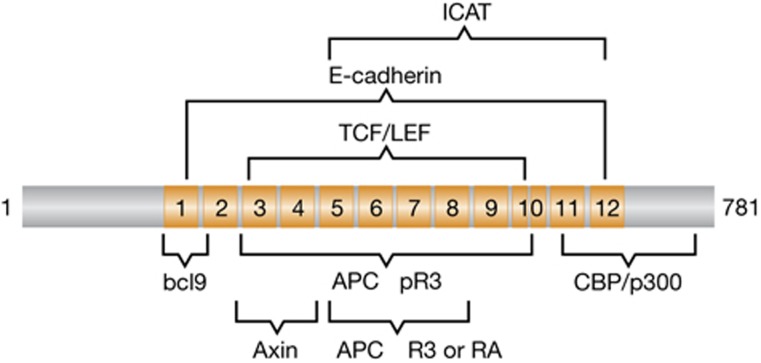
From a drug perspective, the β-catenin–TCF structural interface appears daunting. Approximately 70% of the β-catenin amino-acid sequence constitutes a core superhelical region consisting of 12 armadillo repeats of 42 amino acids each. Each repeat is comprised of three helices, and together these repeats form a large positively charged groove (Huber et al, 1997). About 51 N-terminal residues of the TCF sequence occupy this groove, forming three modules of contact defined by a β-hairpin, an extended region and an α-helix (Graham et al, 2002). Although not easily recognized by sequence, cadherin, APC and ICAT, another inhibitor of β-catenin, share with TCF a chemically conserved motif within its extended region, such that the four proteins bind competitively to β-catenin (Eklof Spink et al, 2001; Huber and Weis, 2001; Graham et al, 2002; Xing et al, 2003). All four binding partners form salt bridges with lysines 312 and 435 in the armadillo repeats. Moreover, Axin and TCF overlap in their binding to β-catenin armadillo repeats 3–4, both using a single α-helix for this contact. Thus, any compound intended to disrupt the TCF–β-catenin interaction must spare the disruption of these overlapping binding partners, three of which have been classified as human tumour suppressors. Disruption of β-catenin–cadherin binding in normal intestinal epithelium has been tested in vivo by ectopic expression of an interfering fragment of cadherin. These mice developed intestinal inflammation that led to neoplasia (Hermiston and Gordon, 1995).
The extensive interaction of β-catenin with TCF, along with the overlapping binding sites with other partners, presents some serious challenges for a small-molecule inhibitor approach (Figure 2). Nevertheless, mutational analysis suggests that the appropriate compound could selectively inhibit TCF binding only. For example, the substitution H460A in β-catenin selectively hinders binding to the TCF homologue LEF1 without impairing binding to either APC or Axin (von Kries et al, 2000). β-catenin binds other important partners through contacts more discrete and perhaps more druggable than those used for TCF binding. For instance, in the nucleus β-catenin recruits various chromatin-modifying enzymes to promote gene transcription at TCF-binding sites (Willert and Jones, 2006; Mosimann et al, 2009). Among these enzymes, the p300 acetyltransferase interacts with β-catenin C-terminal sequence, including the armadillo repeat-12 (Hecht et al, 2000; Miyagishi et al, 2000; Daniels and Weis, 2002). The isolated helical domain of the β-catenin inhibitor ICAT effectively disrupts the p300/β-catenin complex, suggesting that this transcription-relevant portion of catenin could be targeted without impacting the binding of the tumour suppressors APC or Axin (Daniels and Weis, 2002).
Figure 2.
Overlapping regions of interaction for β-catenin-binding proteins. The 12 armadillo repeats in β-catenin are depicted in orange and the generalized areas of interaction for various proteins that bind to them are shown. APC R3 is the third 20-amino-acid repeat unit in the APC protein and pR3 is the phosphorylated form. RA is the first 15-amino-acid repeat unit in APC.
The binding of bcl9 to the first armadillo repeat of β-catenin also involves a far more limited interface than that defined for the TCF interaction (Sampietro et al, 2006). A single helix comprised of a couple dozen residues in bcl9 make contacts between the second and third helix of the first armadillo domain. Half of the bcl9 helix forms hydrogen bond salt bridges with β-catenin while the remaining half binds largely through hydrophobic interactions. Unfortunately, a deep pocket, typically amenable to drug binding, is not apparent in this structure. It also remains unclear whether the disruption of this interaction would be beneficial in cancer therapy. While bcl9, and its associate pygopus, appear critical for nuclear armadillo signalling in Drosophila, it plays a more limited role in mice (Jessen et al, 2008). Importantly, intestinal adenocarcinomas, driven by aberrant Wnt signalling, occur in mice devoid of intestinal bcl9, and its homologue bcl9l, at a frequency equal to that in wt mice (Deka et al, 2010). Based on gene signature analysis, the authors speculated that bcl9 null tumours possessed fewer stem cell-like characteristics and therefore could represent a less aggressive cancer than the wt tumours. Although upstream of β-catenin, the dishevelled (DVL) PDZ domain, which interacts with FZD, is potentially amenable to small-molecule disruption. This was demonstrated with Dvl PDZ-binding peptide ligands that when internalized inhibited Wnt signalling (Zhang et al, 2009). Small molecules that target this interface have also been reported (Fujii et al, 2007; Grandy et al, 2009).
Targeting kinases in the Wnt pathway
On a wish list of oncology targets, a positively acting kinase with an oncogenic mutation would rank well above any protein–protein interaction. Unfortunately, none exist in the Wnt cancer pathway. In its absence, a druggable enzyme acting downstream of β-catenin might suffice. Accordingly, Firestein et al (2008) identified the CDK8 kinase as important for both colon cancer cell viability and oncogenic Wnt signalling. Moreover, the cdk8 gene undergoes copy number gain in some colon cancers. Although this work defines CDK8 as important for β-catenin-dependent transcription, CDK8 has a broader role as a part of the Mediator complex, which is required for activator-dependent transcription by Pol II (Conaway and Conaway, 2011). Considered in this context, CDK8 is clearly not dedicated to Wnt signalling and is also required for the expression of many non-Wnt targets, including activation of the p21 gene by the p53 tumour suppressor (Donner et al, 2007). Moreoever, CDK8 has roles outside of its participation in Mediator. Nevertheless, based on its role in Wnt signalling, the amplification of its gene in colon cancer, and the ability of kinase-active, but not kinase-dead, CDK8 to transform immortalized cells, it remains an attractive target.
Casein kinase II could also be considered as a target downstream of β-catenin. Although several studies had defined a positive role for CKII in Wnt signalling, the Jones lab localized this kinase to Wnt target genes where it enhanced transactivation by enforcing the interaction of β-catenin with LEF1 (Wang and Jones, 2006). Again, inhibition of this target would likely elicit physiological effects well beyond those associated with Wnt signalling alone. The casein kinase I family has also been implicated in Wnt signalling, however, in both negative and positive directions. The phosphorylation of the LRP5/6 Wnt coreceptors by CKIγ supports receptor activation, whereas phosphorylation of β-catenin by CK1α primes it for proteolytic destruction (Liu et al, 2002; Davidson et al, 2005). Unexpectedly, the Lee lab found a small-molecule allosteric activator of CKIα in an in vitro screen designed to read out β-catenin turnover (Thorne et al, 2010). The compound, pyrvinium, potently inhibits Wnt signalling even in cells mutant for the APC tumour suppressor. It remains unclear whether systemic activation of CKI represents a tractable approach to Wnt inhibition in vivo; however, this study underscores the potential of a highly unconventional mechanism, that of kinase activation, as a novel approach in cancer therapy.
Unconventional approaches
The challenge of targeting Wnt signalling with small molecules could provide some incentive for exploring unconventional approaches. Among these, lytic viruses engineered to replicate selectively in the background of enhanced β-catenin signalling have been developed. Replicating adenoviruses, in which the expression of essential viral antigens are placed under control of the TFC4 promotor, have demonstrated selective replication in cells with enhanced Wnt signalling (Brunori et al, 2001). Permutations on this approach include insertion of transgenes into the viral genome coding for proteins that render the infected cell susceptible to a prodrug or radionuclide (Lukashev et al, 2005; Peerlinck et al, 2009). Directly eliminating β-catenin by mRNA interference is also conceptually appealing. Silencing of β-catenin by transfection or local administration of small interfering RNA retards the growth of preclinical tumour models (Zeng et al, 2007; Ashihara et al, 2009; Yeung et al, 2010). However, systemic delivery of siRNA to tumours remains elusive. Interference with Wnt signalling could also be approached orthogonally by stimulating pathways that inhibit the Wnt pathway. For example, the orphan nuclear receptor RORα binds to and represses β-catenin-dependent gene activation (Lee et al, 2010).
Finding drugs
In vitro and cell-based assays have been exploited to identify chemical matter that could be developed into therapeutics capable of inhibiting Wnt signalling. Accordingly, the Shivdasani lab designed an in vitro assay that scored for the disruption of the β-catenin/TCF interaction and screened 7000 natural compounds for activity (Lepourcelet et al, 2004). They identified two fungal derivatives, termed PKF115-854 and CGP049090, both of which disrupted the Tcf/β-catenin complex, inhibited colon cancer cell proliferation and interfered with β-catenin-mediated axis duplication in Xenopus embryos. These compounds have not advanced to clinical testing but have shown recent promise in preclinical models of hepatocellular and haematologic cancers (Minke et al, 2009; Gandhirajan et al, 2010; Wei et al, 2010). Using a cell-based transcriptional reporter assay, the Kahn lab identified a Wnt signalling inhibitor termed ICG-001 that was efficacious in colon tumour xenografts and in the ApcMin mouse model of intestinal tumourigenesis (Eguchi et al, 2005). The target of this compound was identified as CBP, an acetyl transferase that binds β-catenin to facilitate gene activation. An analogue of ICG-001 was recently advanced to phase I clinical testing (Takahashi-Yanaga and Kahn, 2010).
Although cell-based screens yield hits, it is not always possible to identify the target of the active compound. However, secondary assays can discriminate at which level in the signalling pathway a candidate may be acting. After Chen et al (2009) identified inhibitors in a cell-based screen driven by ectopic expression of Wnt3a in mouse L-cells, they retested the compounds on L-cells activated by the addition of exogenous Wnt3a ligand. Interestingly, compounds that failed this latter test, termed IWPs, turned out to inhibit porcupine, an acyl transferase required for modification and release of Wnt ligands from the producing cell. A second class of compounds, the IWRs, did not block receptor activation by Wnt ligands, but appeared to stabilize Axin, thereby enhancing the destruction complex and the degradation of β-catenin.
The Cong lab also observed the stabilization of Axin by a small-molecule Wnt inhibitor. In this study, they discovered the small-molecule inhibitor XAV939 in a cell-based high-throughput Wnt reporter screen (Huang et al, 2009). Using a protein affinity capture technique, they identified the Tankyrases TNKS1 and 2 as the targets of XAV939. The TNKs associate with Axin and modify it by poly-ADP ribosylation, which leads to its degradation via subsequent ubiquitination. The paper also shows that IWR-1, previously reported by the Chen et al, is again an inhibitor of tankyrase activity. The ability of these compounds to inhibit hyperactive Wnt signalling resident to APC mutated colorectal cancer cells is quite exciting and highlights a new avenue for therapy. The next step will require pharmacologically sound derivatives to test this target for in vivo anti-tumour activity. More recently, two additional small-molecule Wnt inhibitors, termed JW67 and JW74, were identified in a cell-based reporter screen and, again, stabilize the Axins and promote β-catenin turnover (Waaler et al, 2011). The targets of these compounds have not been determined, although their structures appear unrelated to XAV939 or IWR-1. In a modified cell-based screen, Gonsalves et al (2011) knocked down the Wnt inhibitor Axin with RNAi to establish the Wnt signal. This ensured that any hits obtained would operate downstream of β-catenin. Accordingly, some of the compounds inhibited Wnt signalling initiated by the expression of a stabilized mutant form of β-catenin and also appeared to disrupt the β-catenin/TCF interaction.
Where do we use Wnt inhibitors?
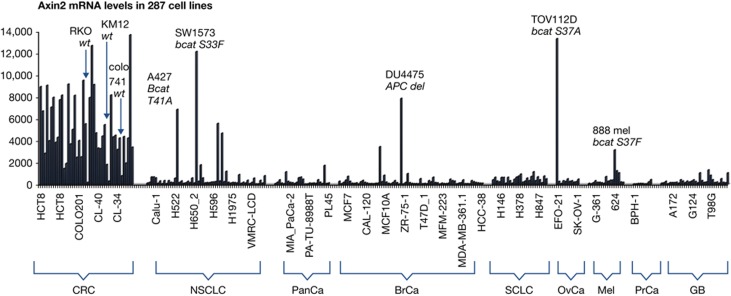
In the age of personalized medicine, a specific molecular diagnostic is needed to determine who might best benefit from a rational drug. This is particularly pertinent when the ‘wrong’ patient might not only lack a response but possibly progress more rapidly on the drug. Obviously, a drug with the capacity to inhibit β-catenin activated gene transcription in cells harbouring mutations in APC would have immediate appeal in the colorectal cancer clinic. Here, the majority of cancers are APC mutant, while a handful of others are mutant for Axin or β-catenin (Polakis, 2007). Outside of colorectal cancer, though, the prevalence of Wnt pathway mutations, albeit substantial, are less common. DNA sequencing of biopsy specimens would be needed to select qualified patients. Gene signatures, which to some extent can specify the activation of a signalling pathway, could also be helpful. Remarkably, the presence of a single mRNA transcript, encoded by the Axin II gene, correlates well with mutations in the Wnt signalling pathway when surveying human cancer cell lines (Figure 3). Thus, therapies that function downstream of hyperactive β-catenin, in cancers driven by Wnt signalling mutations, could readily find a patient population based on gene sequencing and/or transcript analysis.
Figure 3.
Relative levels of Axin II mRNA in cancer cell lines. Levels of AxinII mRNA in 287 cancer cells of various origins were measured by microarray analysis on the Affymetrix U133P platform (unpublished data). Each vertical bar (signal intensity/average difference) represents a measurement from an individual cell line from the indicated class, although specific identification is provided for only a portion of the cell lines. Axin II levels are particularly high in cell lines derived from colorectal cancers (CRC) relative to those from NSCLC, pancreatic cancer (PanCa), breast cancer (BrCa), small-cell lung cancer (SCLC), ovarian cancer (OvCa), melanoma (Mel), prostate cancer (PrCa) and glioblastoma (GB). The cell line and mutation is noted for some cell lines not in the colorectal category that exhibit high-level Axin II mRNA expression. Three colorectal cell lines, KM12, colo741 and RKO, known to be wt for Wnt signalling components, express low levels of Axin II mRNA.
Cancers with active Wnt signalling, yet lacking pathway mutations, would be significantly more difficult to identify. Immunohistochemical staining for nuclear or cytoplasmic β-catenin can be indicative of Wnt signalling, but scoring is subjective and varies with the materials and techniques employed. Even in colorectal cancers that harbour APC mutations, the staining of nuclear β-catenin can appear heterogenous or restricted to cells at the invasive front of the tumour (Brabletz et al, 2001). Nevertheless, there have been several reports in which Wnt signalling is considered active based on aberrant subcellular localization of β-catenin. In particular, a significant fraction of breast cancers exhibit nuclear or cytoplasmic staining of β-catenin (Lin et al, 2000; Chung et al, 2004). More recently, aberrant β-catenin staining in breast cancers has been associated with so-called triple negative and basal-like subtypes of breast cancer (Khramtsov et al, 2010; Geyer et al, 2011). It is noteworthy that the murine MMTV-Wnt-1 transgenic model of mammary cancer also exhibits hallmarks of the human basal-like breast cancers (Herschkowitz et al, 2007).
As noted above, there is increasing evidence that Wnt signalling may be associated with the maintenance or survival of tumour re-initiating cells or cancer stem cells (Reya and Clevers, 2005; Malanchi and Huelsken, 2009). In this scenario, the impact of inhibiting Wnt signalling on tumour burden might only be realized following a prolonged period of cellular attrition. Alternatively, Wnt inhibition could be combined or sequenced with standard of care chemotherapy to eliminate the remaining presumptive tumour re-initiating cells. Such an approach would require an element of faith, as it would be difficult to determine whether these remaining cells exhibit any evidence of Wnt signalling. The implication for Wnt signalling in metastasis has also been demonstrated preclinically (Nguyen et al, 2009), but objective clinical evaluation of drugs intended to interfere only with metastatic spread could be quite challenging.
Safety concerns
Naturally, sustained systemic inhibition of Wnt signalling would likely be accompanied by toxic side effects, particularly when considering its role in stem cell biology. Some of these side effects, such as damage to the intestinal mucosa, reduced bone density, alopecia and other pathologies associated with regenerative tissues, might be anticipated from our current understanding of Wnt signalling (Chen et al, 2009). Indeed, interference with Wnt signalling by genetic manipulation or systemic exposure to high levels of the Wnt inhibitor DKK1 has catastrophic effects on the cellular architecture in the intestine (Pinto et al, 2003; Kuhnert et al, 2004). However, the complete genetic ablation of TCF4, or uncontrolled in vivo expression of DKK1, is hardly pharmacologic. As with all drugs, proper dosing and scheduling of Wnt inhibitors will be required to minimize any side effects. It is encouraging that the acute elimination of the lgr5+ presumptive stem cells in the intestine did not disrupt cellular homeostasis and was followed by recovery and renewal of this Wnt-regulated stem cell population (Tian et al, 2011). The emerging model of intestinal homeostasis posits that the Wnt-regulated stem cell population is not fixed or finite, but regenerative, and can be re-populated by a quiescent stem cell refractory to Wnt perturbations (Tian et al, 2011; Yan et al, 2011). The exacerbation of Wnt signaling in tumours relative to normal tissue, along with the potential for normal tissue to recover, could provide a margin of safety for Wnt inhibitors in the clinic.
Conclusion
It is clear that aberrant Wnt signaling contributes to human cancer. When taken together, myriad proof of concept studies strongly suggest that interfering with this signal would lead to clinical benefit. Although some promising therapeutic leads have been advanced, they either lack specificity or act at a distance from the true driver of pathway activation. Safety concerns are also apparent, but this complication comes with all drugs and the potential benefit justifies the risk. Moreover, there exists a clear path forward to identify the appropriate patient population for a specific inhibitor.
Footnotes
The author declares that he has no conflict of interest.
References
- Abrahamsson AE, Geron I, Gotlib J, Dao KH, Barroga CF, Newton IG, Giles FJ, Durocher J, Creusot RS, Karimi M, Jones C, Zehnder JL, Keating A, Negrin RS, Weissman IL, Jamieson CH (2009) Glycogen synthase kinase 3beta missplicing contributes to leukemia stem cell generation. Proc Natl Acad Sci USA 106: 3925–3929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiri G, Cherian MM, Vijayakumar S, Liu G, Bafico A, Aaronson SA (2009) Wnt pathway aberrations including autocrine Wnt activation occur at high frequency in human non-small-cell lung carcinoma. Oncogene 28: 2163–2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashihara E, Kawata E, Nakagawa Y, Shimazaski C, Kuroda J, Taniguchi K, Uchiyama H, Tanaka R, Yokota A, Takeuchi M, Kamitsuji Y, Inaba T, Taniwaki M, Kimura S, Maekawa T (2009) Beta-catenin small interfering RNA successfully suppressed progression of multiple myeloma in a mouse model. Clin Cancer Res 15: 2731–2738 [DOI] [PubMed] [Google Scholar]
- Bafico A, Liu G, Goldin L, Harris V, Aaronson SA (2004) An autocrine mechanism for constitutive Wnt pathway activation in human cancer cells. Cancer Cell 6: 497–506 [DOI] [PubMed] [Google Scholar]
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W (1996) Functional interaction of beta-catenin with the transcription factor LEF-1. Nature 382: 638–642 [DOI] [PubMed] [Google Scholar]
- Bjorklund P, Svedlund J, Olsson AK, Akerstrom G, Westin G (2009) The internally truncated LRP5 receptor presents a therapeutic target in breast cancer. PLoS One 4: e4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabletz T, Jung A, Reu S, Porzner M, Hlubek F, Kunz-Schughart LA, Knuechel R, Kirchner T (2001) Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc Natl Acad Sci USA 98: 10356–10361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunori M, Malerba M, Kashiwazaki H, Iggo R (2001) Replicating adenoviruses that target tumors with constitutive activation of the Wnt signaling pathway. J Virol 75: 2857–2865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, Hogg D, Lorigan P, Lebbe C, Jouary T, Schadendorf D, Ribas A, O’Day SJ, Sosman JA, Kirkwood JM, Eggermont AM et al. (2011) Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 364: 2507–2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan CW, Wei S, Hao W, Kilgore J, Williams NS, Roth MG, Amatruda JF, Chen C, Lum L (2009) Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol 5: 100–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung GG, Zerkowski MP, Ocal IT, Dolled-Filhart M, Kang JY, Psyrri A, Camp RL, Rimm DL (2004) Beta-cSatenin and p53 analyses of a breast carcinoma tissue microarray. Cancer 100: 2084–2092 [DOI] [PubMed] [Google Scholar]
- Clements WM, Lowy AM, Groden J (2003) Adenomatous polyposis coli/beta-catenin interaction and downstream targets: altered gene expression in gastrointestinal tumors. Clin Colorectal Cancer 3: 113–120 [DOI] [PubMed] [Google Scholar]
- Conaway RC, Conaway JW (2011) Function and regulation of the mediator complex. Curr Opin Genet Dev 21: 225–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong F, Zhang J, Pao W, Zhou P, Varmus H (2003) A protein knockdown strategy to study the function of beta-catenin in tumorigenesis. BMC Mol Biol 4: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels DL, Weis WI (2002) ICAT inhibits beta-catenin binding to Tcf/Lef-family transcription factors and the general coactivator p300 using independent structural modules. Mol Cell 10: 573–584 [DOI] [PubMed] [Google Scholar]
- Davidson G, Wu W, Shen J, Bilic J, Fenger U, Stannek P, Glinka A, Niehrs C (2005) Casein kinase 1 gamma couples Wnt receptor activation to cytoplasmic signal transduction. Nature 438: 867–872 [DOI] [PubMed] [Google Scholar]
- DeAlmeida VI, Miao L, Ernst JA, Koeppen H, Polakis P, Rubinfeld B (2007) The soluble Wnt receptor Frizzled8CRD-hFc inhibits the growth of teratocarcinomas in vivo. Cancer Res 67: 5371–5379 [DOI] [PubMed] [Google Scholar]
- Deka J, Wiedemann N, Anderle P, Murphy-Seiler F, Bultinck J, Eyckerman S, Stehle JC, Andre S, Vilain N, Zilian O, Robine S, Delorenzi M, Basler K, Aguet M (2010) Bcl9/Bcl9l are critical for Wnt-mediated regulation of stem cell traits in colon epithelium and adenocarcinomas. Cancer Res 70: 6619–6628 [DOI] [PubMed] [Google Scholar]
- Donner AJ, Szostek S, Hoover JM, Espinosa JM (2007) CDK8 is a stimulus-specific positive coregulator of p53 target genes. Mol Cell 27: 121–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi M, Nguyen C, Lee SC, Kahn M (2005) ICG-001, a novel small molecule regulator of TCF/beta-catenin transcription. Med Chem 1: 467–472 [DOI] [PubMed] [Google Scholar]
- Eklof Spink K, Fridman SG, Weis WI (2001) Molecular mechanisms of beta-catenin recognition by adenomatous polyposis coli revealed by the structure of an APC-beta-catenin complex. EMBO J 20: 6203–6212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein R, Bass AJ, Kim SY, Dunn IF, Silver SJ, Guney I, Freed E, Ligon AH, Vena N, Ogino S, Chheda MG, Tamayo P, Finn S, Shrestha Y, Boehm JS, Jain S, Bojarski E, Mermel C, Barretina J, Chan JA et al. (2008) CDK8 is a colorectal cancer oncogene that regulates beta-catenin activity. Nature 455: 547–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii N, You L, Xu Z, Uematsu K, Shan J, He B, Mikami I, Edmondson LR, Neale G, Zheng J, Guy RK, Jablons DM (2007) An antagonist of dishevelled protein-protein interaction suppresses beta-catenin-dependent tumor cell growth. Cancer Res 67: 573–579 [DOI] [PubMed] [Google Scholar]
- Gandhirajan RK, Staib PA, Minke K, Gehrke I, Plickert G, Schlosser A, Schmitt EK, Hallek M, Kreuzer KA (2010) Small molecule inhibitors of Wnt/beta-catenin/lef-1 signaling induces apoptosis in chronic lymphocytic leukemia cells in vitro and in vivo. Neoplasia 12: 326–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer FC, Lacroix-Triki M, Savage K, Arnedos M, Lambros MB, MacKay A, Natrajan R, Reis-Filho JS (2011) Beta-catenin pathway activation in breast cancer is associated with triple-negative phenotype but not with CTNNB1 mutation. Mod Pathol 24: 209–231 [DOI] [PubMed] [Google Scholar]
- Gonsalves FC, Klein K, Carson BB, Katz S, Ekas LA, Evans S, Nagourney R, Cardozo T, Brown AM, DasGupta R (2011) An RNAi-based chemical genetic screen identifies three small-molecule inhibitors of the Wnt/wingless signaling pathway. Proc Natl Acad Sci USA 108: 5954–5963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottardi CJ, Wong E, Gumbiner BM (2001) E-cadherin suppresses cellular transformation by inhibiting beta-catenin signaling in an adhesion-independent manner. J Cell Biol 153: 1049–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham TA, Clements WK, Kimelman D, Xu W (2002) The crystal structure of the beta-catenin/ICAT complex reveals the inhibitory mechanism of ICAT. Mol Cell 10: 563–571 [DOI] [PubMed] [Google Scholar]
- Grandy D, Shan J, Zhang X, Rao S, Akunuru S, Li H, Zhang Y, Alpatov I, Zhang XA, Lang RA, Shi DL, Zheng JJ (2009) Discovery and characterization of a small molecule inhibitor of the PDZ domain of dishevelled. J Biol Chem 284: 16256–16263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DW, Roh H, Pippin JA, Drebin JA (2001) Beta-catenin antisense treatment decreases beta-catenin expression and tumor growth rate in colon carcinoma xenografts. J Surg Res 101: 16–20 [DOI] [PubMed] [Google Scholar]
- Groden J, Thliveris A, Samowitz W, Carlson M, Gelbert L, Albertsen H, Joslyn G, Stevens J, Spirio L, Robertson M, Sargeant L, Krapcho K, Wolff E, Burt R, Hughes JP, Warrington J, McPherson J, Wasmuth J, Le Paslier D, Abderrahim H et al. (1991) Identification and characterization of the familial adenomatous polyposis coli gene. Cell 66: 589–600 [DOI] [PubMed] [Google Scholar]
- Hecht A, Vleminckx K, Stemmler MP, van Roy F, Kemler R (2000) The p300/CBP acetyltransferases function as transcriptional coactivators of beta-catenin in vertebrates. EMBO J 19: 1839–1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermiston ML, Gordon JI (1995) Inflammatory bowel disease and adenomas in mice expressing a dominant negative N-cadherin. Science 270: 1203–1207 [DOI] [PubMed] [Google Scholar]
- Herschkowitz JI, Simin K, Weigman VJ, Mikaelian I, Usary J, Hu Z, Rasmussen KE, Jones LP, Assefnia S, Chandrasekharan S, Backlund MG, Yin Y, Khramtsov AI, Bastein R, Quackenbush J, Glazer RI, Brown PH, Green JE, Kopelovich L, Furth PA et al. (2007) Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biol 8: R76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinck L, Nelson WJ, Papkoff J (1994) Wnt-1 modulates cell-cell adhesion in mammalian cells by stabilizing beta-catenin binding to the cell adhesion protein cadherin. J Cell Biol 124: 729–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe LR, Brown AM (2004) Wnt signaling and breast cancer. Cancer Biol Ther 3: 36–41 [DOI] [PubMed] [Google Scholar]
- Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner S, Hild M, Shi X, Wilson CJ, Mickanin C, Myer V, Fazal A, Tomlinson R, Serluca F, Shao W, Cheng H et al. (2009) Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature 461: 614–620 [DOI] [PubMed] [Google Scholar]
- Huber AH, Nelson WJ, Weis WI (1997) Three-dimensional structure of the armadillo repeat region of beta-catenin. Cell 90: 871–882 [DOI] [PubMed] [Google Scholar]
- Huber AH, Weis WI (2001) The structure of the beta-catenin/E-cadherin complex and the molecular basis of diverse ligand recognition by beta-catenin. Cell 105: 391–402 [DOI] [PubMed] [Google Scholar]
- Jamieson CH, Ailles LE, Dylla SJ, Muijtjens M, Jones C, Zehnder JL, Gotlib J, Li K, Manz MG, Keating A, Sawyers CL, Weissman IL (2004) Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med 351: 657–667 [DOI] [PubMed] [Google Scholar]
- Jessen S, Gu B, Dai X (2008) Pygopus and the Wnt signaling pathway: a diverse set of connections. Bioessays 30: 448–456 [DOI] [PubMed] [Google Scholar]
- Khramtsov AI, Khramtsova GF, Tretiakova M, Huo D, Olopade OI, Goss KH (2010) Wnt/beta-catenin pathway activation is enriched in basal-like breast cancers and predicts poor outcome. Am J Pathol 176: 2911–2920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Crooks H, Foxworth A, Waldman T (2002) Proof-of-principle: oncogenic beta-catenin is a valid molecular target for the development of pharmacological inhibitors. Mol Cancer Ther 1: 1355–1359 [PubMed] [Google Scholar]
- Kinzler KW, Nilbert MC, Su L-K, Vogelstein B, Bryan TM, Levy DB, Smith KJ, Preisinger AC, Hedge P, McKechnie D, Finniear R, Matrkam A, Groffen J, Boguski MS, Altschul SF, Hrrii A, Ando H, Miyoshi Y, Miki Y, Nishisho I et al. (1991) Identification of FAP locus genes from chromosome 5q21. Science 253: 661–665 [DOI] [PubMed] [Google Scholar]
- Kuhnert F, Davis CR, Wang HT, Chu P, Lee M, Yuan J, Nusse R, Kuo CJ (2004) Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc Natl Acad Sci USA 101: 266–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammi L, Arte S, Somer M, Jarvinen H, Lahermo P, Thesleff I, Pirinen S, Nieminen P (2004) Mutations in AXIN2 cause familial tooth agenesis and predispose to colorectal cancer. Am J Hum Genet 74: 1043–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, Kim IS, Kim H, Lee JS, Kim K, Yim HY, Jeong J, Kim JH, Kim JY, Lee H, Seo SB, Kim H, Rosenfeld MG, Kim KI, Baek SH (2010) RORalpha attenuates Wnt/beta-catenin signaling by PKCalpha-dependent phosphorylation in colon cancer. Mol Cell 37: 183–195 [DOI] [PubMed] [Google Scholar]
- Lepourcelet M, Chen YN, France DS, Wang H, Crews P, Petersen F, Bruseo C, Wood AW, Shivdasani RA (2004) Small-molecule antagonists of the oncogenic Tcf/beta-catenin protein complex. Cancer Cell 5: 91–102 [DOI] [PubMed] [Google Scholar]
- Lin SY, Xia W, Wang JC, Kwong KY, Spohn B, Wen Y, Pestell RG, Hung MC (2000) Beta-catenin, a novel prognostic marker for breast cancer: its roles in cyclin D1 expression and cancer progression. Proc Natl Acad Sci USA 97: 4262–4266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X (2002) Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108: 837–847 [DOI] [PubMed] [Google Scholar]
- Lukashev AN, Fuerer C, Chen MJ, Searle P, Iggo R (2005) Late expression of nitroreductase in an oncolytic adenovirus sensitizes colon cancer cells to the prodrug CB1954. Hum Gene Ther 16: 1473–1483 [DOI] [PubMed] [Google Scholar]
- Major MB, Camp ND, Berndt JD, Yi X, Goldenberg SJ, Hubbert C, Biechele TL, Gingras AC, Zheng N, Maccoss MJ, Angers S, Moon RT (2007) Wilms tumor suppressor WTX negatively regulates WNT/beta-catenin signaling. Science 316: 1043–1046 [DOI] [PubMed] [Google Scholar]
- Malanchi I, Huelsken J (2009) Cancer stem cells: never Wnt away from the niche. Curr Opin Oncol 21: 41–46 [DOI] [PubMed] [Google Scholar]
- Malanchi I, Peinado H, Kassen D, Hussenet T, Metzger D, Chambon P, Huber M, Hohl D, Cano A, Birchmeier W, Huelsken J (2008) Cutaneous cancer stem cell maintenance is dependent on beta-catenin signalling. Nature 452: 650–653 [DOI] [PubMed] [Google Scholar]
- Marvin ML, Mazzoni SM, Herron CM, Edwards S, Gruber SB, Petty EM (2011) AXIN2-associated autosomal dominant ectodermal dysplasia and neoplastic syndrome. Am J Med Genet A 155A: 898–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda Y, Schlange T, Oakeley EJ, Boulay A, Hynes NE (2009) WNT signaling enhances breast cancer cell motility and blockade of the WNT pathway by sFRP1 suppresses MDA-MB-231 xenograft growth. Breast Cancer Res 11: R32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minke KS, Staib P, Puetter A, Gehrke I, Gandhirajan RK, Schlosser A, Schmitt EK, Hallek M, Kreuzer KA (2009) Small molecule inhibitors of WNT signaling effectively induce apoptosis in acute myeloid leukemia cells. Eur J Haematol 82: 165–175 [DOI] [PubMed] [Google Scholar]
- Miyagishi M, Fujii R, Hatta M, Yoshida E, Araya N, Nagafuchi A, Ishihara S, Nakajima T, Fukamizu A (2000) Regulation of Lef-mediated transcription and p53-dependent pathway by associating beta-catenin with CBP/p300. J Biol Chem 275: 35170–35175 [DOI] [PubMed] [Google Scholar]
- Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destree O, Clevers H (1996) XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell 86: 391–399 [DOI] [PubMed] [Google Scholar]
- Mologni L, Dekhil H, Ceccon M, Purgante S, Lan C, Cleris L, Magistroni V, Formelli F, Gambacorti-Passerini CB (2010) Colorectal tumors are effectively eradicated by combined inhibition of {beta}-catenin, KRAS, and the oncogenic transcription factor ITF2. Cancer Res 70: 7253–7263 [DOI] [PubMed] [Google Scholar]
- Morin PJ, Vogelstein B, Kinzler KW (1996) Apoptosis and APC in colorectal tumorigenesis. Proc Natl Acad Sci USA 93: 7950–7954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW (1997) Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science 275: 1787–1790 [DOI] [PubMed] [Google Scholar]
- Mosimann C, Hausmann G, Basler K (2009) Beta-catenin hits chromatin: regulation of Wnt target gene activation. Nat Rev Mol Cell Biol 10: 276–286 [DOI] [PubMed] [Google Scholar]
- Munemitsu S, Albert I, Souza B, Rubinfeld B, Polakis P (1995) Regulation of intracellular beta-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc Natl Acad Sci USA 92: 3046–3050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen DX, Chiang AC, Zhang XH, Kim JY, Kris MG, Ladanyi M, Gerald WL, Massague J (2009) WNT/TCF signaling through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis. Cell 138: 51–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R, Varmus HE (1982) Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell 31: 99–109 [DOI] [PubMed] [Google Scholar]
- Peerlinck I, Merron A, Baril P, Conchon S, Martin-Duque P, Hindorf C, Burnet J, Quintanilla M, Hingorani M, Iggo R, Lemoine NR, Harrington K, Vassaux G (2009) Targeted radionuclide therapy using a Wnt-targeted replicating adenovirus encoding the Na/I symporter. Clin Cancer Res 15: 6595–6601 [DOI] [PubMed] [Google Scholar]
- Pinto D, Gregorieff A, Begthel H, Clevers H (2003) Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev 17: 1709–1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakis P (2007) The many ways of Wnt in cancer. Curr Opin Genet Dev 17: 45–51 [DOI] [PubMed] [Google Scholar]
- Poulikakos PI, Rosen N (2011) Mutant BRAF melanomas--dependence and resistance. Cancer Cell 19: 11–15 [DOI] [PubMed] [Google Scholar]
- Reya T, Clevers H (2005) Wnt signalling in stem cells and cancer. Nature 434: 843–850 [DOI] [PubMed] [Google Scholar]
- Roh H, Green DW, Boswell CB, Pippin JA, Drebin JA (2001) Suppression of beta-catenin inhibits the neoplastic growth of APC-mutant colon cancer cells. Cancer Res 61: 6563–6568 [PubMed] [Google Scholar]
- Rubinfeld B, Robbins P, El-Gamil M, Albert I, Porfiri E, Polakis P (1997) Stabilization of beta-catenin by genetic defects in melanoma cell lines. Science 275: 1790–1792 [DOI] [PubMed] [Google Scholar]
- Salahshor S, Woodgett JR (2005) The links between axin and carcinogenesis. J Clin Pathol 58: 225–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampietro J, Dahlberg CL, Cho US, Hinds TR, Kimelman D, Xu W (2006) Crystal structure of a beta-catenin/BCL9/Tcf4 complex. Mol Cell 24: 293–300 [DOI] [PubMed] [Google Scholar]
- Satoh S, Daigo Y, Furukawa Y, Kato T, Miwa N, Nishiwaki T, Kawasoe T, Ishiguro H, Fujita M, Tokino T, Sasaki Y, Imaoka S, Murata M, Shimano T, Yamaoka Y, Nakamura Y (2000) AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nat Genet 24: 245–250 [DOI] [PubMed] [Google Scholar]
- Scholer-Dahirel A, Schlabach MR, Loo A, Bagdasarian L, Meyer R, Guo R, Woolfenden S, Yu KK, Markovits J, Killary K, Sonkin D, Yao YM, Warmuth M, Sellers WR, Schlegel R, Stegmeier F, Mosher RE, McLaughlin ME (2011) Maintenance of adenomatous polyposis coli (APC)-mutant colorectal cancer is dependent on Wnt/beta-catenin signaling. Proc Natl Acad Sci USA 108: 17135–17140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, Demeter J, Perou CM, Lonning PE, Brown PO, Borresen-Dale AL, Botstein D (2003) Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA 100: 8418–8423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su LK, Vogelstein B, Kinzler KW (1993) Association of the APC tumor suppressor protein with catenins. Science 262: 1734–1737 [DOI] [PubMed] [Google Scholar]
- Takahashi-Yanaga F, Kahn M (2010) Targeting Wnt signaling: can we safely eradicate cancer stem cells? Clin Cancer Res 16: 3153–3162 [DOI] [PubMed] [Google Scholar]
- Tetsu O, McCormick F (1999) Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398: 422–426 [DOI] [PubMed] [Google Scholar]
- Thorne CA, Hanson AJ, Schneider J, Tahinci E, Orton D, Cselenyi CS, Jernigan KK, Meyers KC, Hang BI, Waterson AG, Kim K, Melancon B, Ghidu VP, Sulikowski GA, LaFleur B, Salic A, Lee LA, Miller DM III, Lee E (2010) Small-molecule inhibition of Wnt signaling through activation of casein kinase 1alpha. Nat Chem Biol 6: 829–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, de Sauvage FJ (2011) A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature 478: 255–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A, van der Horn K, Batlle E, Coudreuse D, Haramis AP, Tjon-Pon-Fong M, Moerer P, van den Born M, Soete G, Pals S, Eilers M, Medema R, Clevers H (2002) The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 111: 241–250 [DOI] [PubMed] [Google Scholar]
- Verma UN, Surabhi RM, Schmaltieg A, Becerra C, Gaynor RB (2003) Small interfering RNAs directed against beta-catenin inhibit the in vitro and in vivo growth of colon cancer cells. Clin Cancer Res 9: 1291–1300 [PubMed] [Google Scholar]
- von Kries JP, Winbeck G, Asbrand C, Schwarz-Romond T, Sochnikova N, Dell'Oro A, Behrens J, Birchmeier W (2000) Hot spots in beta-catenin for interactions with LEF-1, conductin and APC. Nat Struct Biol 7: 800–807 [DOI] [PubMed] [Google Scholar]
- Waaler J, Machon O, von Kries JP, Wilson SR, Lundenes E, Wedlich D, Gradl D, Paulsen JE, Machonova O, Dembinski JL, Dinh H, Krauss S (2011) Novel synthetic antagonists of canonical Wnt signaling inhibit colorectal cancer cell growth. Cancer Res 71: 197–205 [DOI] [PubMed] [Google Scholar]
- Wang S, Jones KA (2006) CK2 controls the recruitment of Wnt regulators to target genes in vivo. Curr Biol 16: 2239–2244 [DOI] [PubMed] [Google Scholar]
- Wang Y, Krivtsov AV, Sinha AU, North TE, Goessling W, Feng Z, Zon LI, Armstrong SA (2010) The Wnt/beta-catenin pathway is required for the development of leukemia stem cells in AML. Science 327: 1650–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Chua MS, Grepper S, So S (2010) Small molecule antagonists of Tcf4/beta-catenin complex inhibit the growth of HCC cells in vitro and in vivo. Int J Cancer 126: 2426–2436 [DOI] [PubMed] [Google Scholar]
- Weinberg RA, Bishop JM (1996) Molecular Oncology New York: Scientific American [Google Scholar]
- Willert K, Jones KA (2006) Wnt signaling: is the party in the nucleus? Genes Dev 20: 1394–1404 [DOI] [PubMed] [Google Scholar]
- Xing Y, Clements WK, Kimelman D, Xu W (2003) Crystal structure of a beta-catenin/axin complex suggests a mechanism for the beta-catenin destruction complex. Genes Dev 17: 2753–2764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Kimelman D (2007) Mechanistic insights from structural studies of beta-catenin and its binding partners. J Cell Sci 120: 3337–3344 [DOI] [PubMed] [Google Scholar]
- Yan KS, Chia LA, Li X, Ootani A, Su J, Lee JY, Su N, Luo Y, Heilshorn SC, Amieva MR, Sangiorgi E, Capecchi MR, Kuo CJ (2011) The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc Natl Acad Sci USA 109: 466–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Wu X, Wang Y, Zhang K, Wu J, Yuan YC, Deng X, Chen L, Kim CC, Lau S, Somlo G, Yen Y (2011) FZD7 has a critical role in cell proliferation in triple negative breast cancer. Oncogene 30: 4437–4446 [DOI] [PubMed] [Google Scholar]
- Yeung J, Esposito MT, Gandillet A, Zeisig BB, Griessinger E, Bonnet D, So CW (2010) beta-Catenin mediates the establishment and drug resistance of MLL leukemic stem cells. Cancer Cell 18: 606–618 [DOI] [PubMed] [Google Scholar]
- Zeng G, Apte U, Cieply B, Singh S, Monga SP (2007) siRNA-mediated beta-catenin knockdown in human hepatoma cells results in decreased growth and survival. Neoplasia 9: 951–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Appleton BA, Wiesmann C, Lau T, Costa M, Hannoush RN, Sidhu SS (2009) Inhibition of Wnt signalling by Dishevelled PDZ peptides. Nat Chem Biol 5: 217–219 [DOI] [PubMed] [Google Scholar]
- Zhao C, Blum J, Chen A, Kwon HY, Jung SH, Cook JM, Lagoo A, Reya T (2007) Loss of beta-catenin impairs the renewal of normal and CML stem cells in vivo. Cancer Cell 12: 528–541 [DOI] [PMC free article] [PubMed] [Google Scholar]