Abstract
EMBO J 31 12, 2755–2769 (2012); published online April 27 2012
Stem cells, both adult and germline, are the key cells underpinning animal evolution. Yet, surprisingly little is known about the evolution of their shared key feature: pluripotency. Now using genome-wide expression profiling of pluripotent planarian adult stem cells (pASCs), Önal et al (2012) present evidence for deep molecular conservation of pluripotency. They characterise the expression profile of pASCs and identify conserved expression profiles and functions for genes required for mammalian pluripotency. Their analyses suggest that molecular pluripotency mechanisms may be conserved, and tantalisingly that pluripotency in germ stem cells (GSCs) and somatic stem cells (SSCs) may have had shared common evolutionary origins.
Pluripotency is the base ingredient for metazoan biology: the controlled production of specialised but functionally integrated cell types from an undifferentiated stem cell must have arisen early in multicellular evolution. In mammals, there is a clear transcription factor (TF)-driven programme for maintaining the pluripotency of embryonic stem cells (ESCs), such that forced induction of this programme can reprogram somatic cells to be pluripotent (Takahashi and Yamanaka, 2006).
Perhaps one obstacle to tackling the question of conservation with respect to adult stem cells has been that both, Caenorhabditis elegans and Drosophila melanogaster, lack SSCs. Instead, they set aside GSCs at the start of development and forgo pluri- and multi-potent SSCs (Ewen-Campen et al, 2010). Enter planarians, champions of tissue regeneration, a potentially rich source of SSCs to work with. pASCs have large nuclei, little cytoplasm and open chromatin, as you might expect of bonafide stem cells (Aboobaker, 2011). In addition, encircling the nuclei of pASCs are RNA-rich granules called chromatoid bodies (CBs). These structures have much in common with RNA-rich nuage or germ plasm found in GSCs. Recently, any debate over their potency was settled as individual pASCs were shown to rescue lethally irradiated animals (Wagner et al, 2011). So we could just profile pASCs and see what they express. Easy right? Wrong! Ironically over the years nobody has managed to culture pASCs despite their indefinite replicative capacity in vivo. This means that researchers have had to be creative to sample the pASC expression profile.
One obvious way to do this is to compare the profiles of planarians with the proliferative compartment removed by irradiation to an intact planarians with pASCs (Figure 1). This method has been successful in identifying genes expressed in pASCs (Rossi et al, 2007; Blythe et al, 2010). However, whole organism irradiation causes many expression changes unrelated to pASCs (Solana et al, 2012). In isolation it can’t be used to provide a clear pASC expression profile.
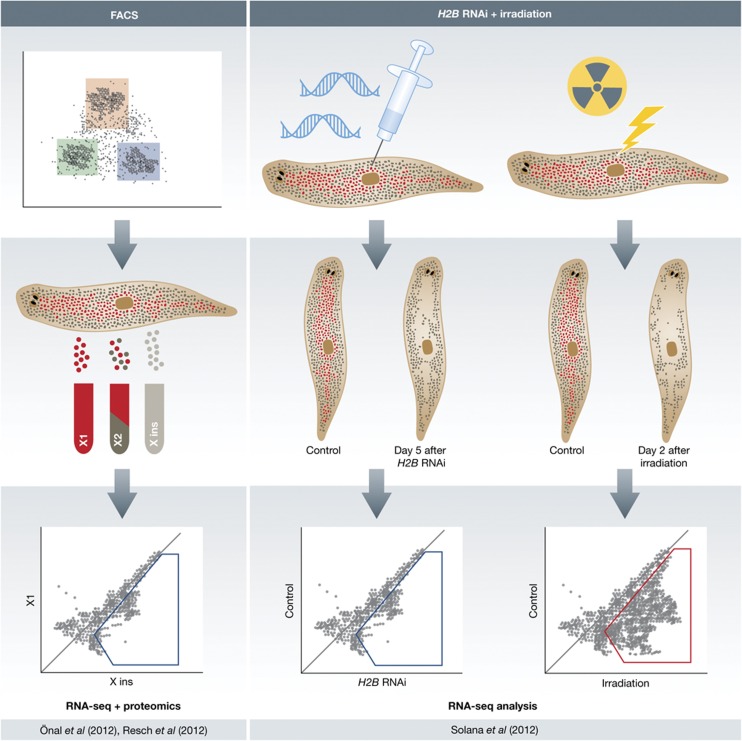
Figure 1.
Önal et al (2012) sorted planarian cells into irradiation sensitive proliferative cells (X1, red box), cells with higher nuclear to cytoplasm ratio (X2, green box) and irradiation insensitive cells (Xins, blue box). Proteomic and RNAseq analysis allowed them to identify pASC expression profile. Solana et al combined RNAi of Histone-2B to ablate stem cells and irradiation followed by RNAseq.
Önal et al (2012) tackled this by using fluorescence-assisted cell sorting (FACs) of planarian cells (Hayashi et al, 2006). This approach sorts cells by using fluorescent dyes that allow pASCs to be separated by their phenotypic characteristics. Sorting gives rise to three populations: A population of proliferating cells (X1s), cells with high nuclear to cytoplasm ratio (X2s), and a third irradiation insensitive differentiated cell population (Xins). Once sorted, cells were then used for both RNAseq and proteomic characterisation (Figure 1). An alternate approach used recently by Solana et al involved specific ablation of pASCs using RNAi of the Histone-2B gene. The RNAseq data from this genetic ablation was then cross-referenced with irradiation data to reduce noise in both data sets (Figure 1). In a third recent study profiling from FACs sorted cells was also cross-referenced with irradiation data (Resch et al, 2012).
Critically, Önal et al (2012) went on to perform a series of detailed informatic and then functional analyses to assess whether pluripotency mechanisms are conserved. First, they found that complexes related to epigenetic control known to be crucial to ESC pluripotency were enriched in pASCs, and demonstrated that members of these complexes were essential for pASC pluripotency. Moreover, they looked specifically at homologues of genes known to promote and suppress ESC pluripotency and found that they were enriched and excluded from pASCs, respectively. Together, these data provide strong evidence that the regulation of pluripotency is deeply conserved at the network level. But what about the known key TFs involved in reprogramming in mammals, Oct-4, Nanog, and Sox-2? Önal et al (2012) were able to identify many genes related to both Oct-4 (POU domain TFs) and Sox-2 (HMG-box domain TFs) but no clear candidate NANOG orthologue. Furthermore, they were able to suggest clear candidates for potential functional orthologues of Oct-4 and Sox-2 that are expressed in pASCs. Together, these results suggest that there is indeed a great deal of conservation between pASCs and ESCs, and that novel insights from planarians will be directly applicable to mammals.
From an evolutionary perspective, Önal et al (2012) make one other very exciting observation. They describe enrichment in pASCs for the expression of genes involved in post-transcriptional regulation, and many of these are implicated in germline pluripotency. A recent hypothesis proposes that an alternative germline pluripotency programme is broadly at work in animal GSCs and some SSCs (Juliano et al, 2010). Önal et al (2012) findings suggest that the TF-driven somatic and post-transcriptionally driven germline pluripotency programs may co-exist in pASCs, and thus may have existed together in the original animal pluripotent stem cell.
Footnotes
The authors declare that they have no conflict of interest.
References
- Aboobaker AA (2011) Planarian stem cells: a simple paradigm for regeneration. Trends Cell Biol 21: 304–311 [DOI] [PubMed] [Google Scholar]
- Blythe MJ, Kao D, Malla S, Rowsell J, Wilson R, Evans D, Jowett J, Hall A, Lemay V, Lam S, Aboobaker AA (2010) A dual platform approach to transcript discovery for the planarian Schmidtea mediterranea to establish RNAseq for stem cell and regeneration biology. PLoS One 5: e15617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewen-Campen B, Schwager EE, Extavour CG (2010) The molecular machinery of germ line specification. Mol Reprod Dev 77: 3–18 [DOI] [PubMed] [Google Scholar]
- Hayashi T, Asami M, Higuchi S, Shibata N, Agata K (2006) Isolation of planarian X-ray-sensitive stem cells by fluorescence-activated cell sorting. Dev Growth Differ 48: 371–380 [DOI] [PubMed] [Google Scholar]
- Juliano CE, Swartz SZ, Wessel GM (2010) A conserved germline multipotency program. Development 137: 4113–4126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Önal P, Grün D, Adamidi C, Rybak A, Solana J, Mastobuoni G, Wang Y, Rahn H-P, Chen W, Kempa S, Ziebold U, Rajewsky N (2012) Gene expression of pluripotency determinants is conserved between mammalian and planarian stem cells. EMBO J 31: 2755–2769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resch AM, Palakodeti D, Lu YC, Horowitz M, Graveley BR (2012) Transcriptome analysis reveals strain-specific and conserved stemness genes in Schmidtea mediterranea. PLoS One 7: e34447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi L, Salvetti A, Marincola FM, Lena A, Deri P, Mannini L, Batistoni R, Wang E, Gremigni V (2007) Deciphering the molecular machinery of stem cells: a look at the neoblast gene expression profile. Genome Biol 8: R62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solana J, Kao D, Mihaylova Y, Jaber-Hijazi F, Malla S, Wilson R, Aboobaker A (2012) Defining the molecular profile of planarian pluripotent stem cells using a combinatorial RNA-seq, RNAi and irradiation approach. Genome Biol 13: R19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–676 [DOI] [PubMed] [Google Scholar]
- Wagner DE, Wang IE, Reddien PW (2011) Clonogenic neoblasts are pluripotent adult stem cells that underlie planarian regeneration. Science 332: 811–816 [DOI] [PMC free article] [PubMed] [Google Scholar]