Abstract
Wnt proteins play important roles in wiring neural circuits. Wnts regulate many aspects of neural circuit generation through their receptors and distinct signalling pathways. In this review, we discuss recent findings on the functions of Wnts in various aspects of neural circuit formation, including neuronal polarity, axon guidance, synapse formation, and synaptic plasticity in vertebrate and invertebrate nervous systems.
Keywords: neurobiology, neuronal circuits, synapse formation, Wnts
Introduction
Wnt proteins are key regulators of a variety of developmental processes, including embryonic patterning, cell specification, and cell polarity (van Amerongen and Nusse, 2009; Budnik and Salinas, 2011). Recent studies have started to reveal that Wnt signalling plays important roles in various aspects of synaptic development and plasticity. Wnt proteins are involved in regulating axon guidance, dendritic morphogenesis, and synapse formation and thus contribute to the formation of neural connectivity (Salinas, 2005; Speese and Budnik, 2007).
Many in vitro and in vivo experiments have demonstrated the function of Wnts in guidance and polarity of neuronal axons. The in vivo role of Wnts in establishing neuronal polarity has been studied mainly in C. elegans by investigating anterior (A) and posterior (P) neuronal polarity (Hilliard and Bargmann, 2006; Prasad and Clark, 2006). Wnts modulate axon pathfinding, and can generate both attractive and repulsive responses through diverse receptors and signalling pathways; most likely, Ryk/Derailed receptor mediates repulsive responses, whereas Fz/LIN-17-type receptors mediate attractive responses. Interestingly, Wnts play roles in synaptogenesis by both promoting and inhibiting synapse formation. These synapse-promoting and synapse-inhibiting effects of Wnts are related to the activation of canonical and non-canonical Wnt signalling pathways, respectively (Davis et al, 2008). Furthermore, recent work has provided evidence that Wnt signalling is essential for synaptic plasticity and neurotransmission. It has been reported that neuronal activity regulates trafficking of Wnt signalling molecule to and out of synapses, and this contributes to activity-mediated synapse remodelling (Sahores et al, 2010). In hippocampal slices, Wnt signalling affects long-term potentiation (LTP; Chen et al, 2006).
In this review, we discuss recent findings on the functions of Wnts in the various aspects of neural circuit formation, including neuronal polarity, axon guidance, synapse formation, and synaptic plasticity in the nervous system.
Wnts in neuronal polarity
The most salient morphological features of neuronal cells are the highly polarized neurites which include both axonal and dendritic processes. The proper compartmentalization of membrane and cytosolic contents of axons and dendrites is critical for the development and function of neurons. While axodendritic polarity can be achieved in dissociated neuron culture (Dotti et al, 1988), suggestive of its cell-intrinsic origin, it is likely that extracellular cues function to regulate the development of axons and dendrites in vivo. The in vitro and in vivo aspects of Wnt functions in neuronal polarity have been studied mainly in cultured hippocampal neurons and C. elegans, respectively. In C. elegans, Wnts regulate both anterior (A) and posterior (P) neuronal polarity. Based on the specific combination of Wnts and Wnt receptors, neuronal axons can be guided towards or away from the Wnt source.
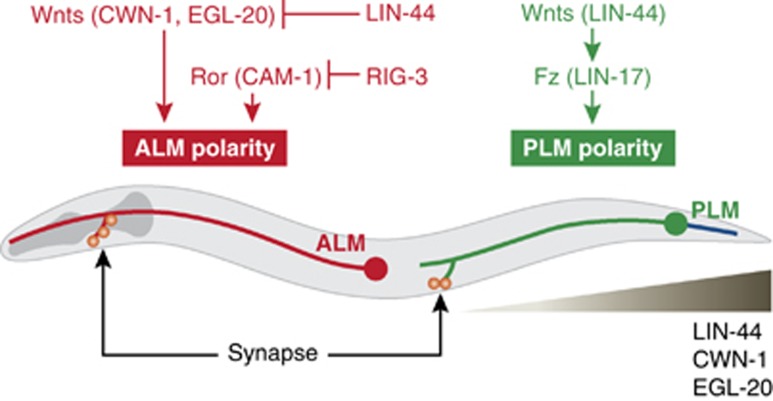
In C. elegans, the posterior lateral microtubule (PLM) and anterior lateral microtubule (ALM) mechanosensory neurons exhibit distinct A-P axon-dendrite polarity (Figure 1). The cell bodies of PLM neurons are located in the posterior lumbar ganglion. Two processes extend from these cell bodies: a long anterior process that extends towards the centre of the animal, and a short posterior process that extends towards the tail. As indicated by their name, ALM neurons have cell bodies located in the anterior half of the animal. ALM neurons extend a single long process towards the head, and this long process has a short ventral branch.
Figure 1.
Wnts regulate neuronal polarity. In C. elegans, ALM neuronal polarity is shaped by the Wnts CWN-1 and EGL-20 and the Ror receptor CAM-1, whereas PLM neuronal polarity is shaped by the Wnt LIN-44 and the Fz receptor LIN-17. Note that the Wnt (LIN-44, CWN-1, EGL-20) gradients high in posterior and low in anterior.
A-P axon polarity is shaped by Wnt/LIN-44 through its interaction with Frizzled/LIN-17 receptors (Hilliard and Bargmann, 2006; Prasad and Clark, 2006; Figure 1). In lin-44 and lin-17 mutants, the polarity of PLM neurons is reversed, with the long process containing presynaptic components posterior instead of anterior to its cell body. LIN-17 is asymmetrically localized to the posterior process of PLM neurons, and its localization is LIN-44 dependent. In a similar way, the polarity of ALM neurons is reversed in cwn-1 egl-20 Wnt double mutants, suggesting that neuronal polarity in different body regions is regulated by different Wnt signals (Figure 1). The effects of the two Wnts, CWN-1 and EGL-20, on ALM polarity are antagonized by the action of another Wnt–LIN-44 (Prasad and Clark, 2006; Fleming et al, 2010). Recently, a cell surface Ig superfamily protein (IgSF), RIG-3, has been reported to regulate the A-P polarity of ALM neurons (Babu et al, 2011). The effect of RIG-3 on ALM polarity is mediated by changes in Wnt signalling. Inactivating RIG-3 reduces the severity of ALM polarity reversal defects in cwn-1; egl-20 double mutants. This effect of RIG-3 on ALM polarity is mediated by inhibition of CAM-1, an Ror-type receptor tyrosine kinase that binds Wnt ligands. Together, these results suggest that RIG-3 is a regulator of Wnt signalling.
The retromer, a multiprotein complex involved in intracellular trafficking, such as transcytosis and endosome-to-Golgi trafficking, functions in the establishment of the Wnt gradient formed by EGL-20 (Coudreuse et al, 2006; Prasad and Clark, 2006). Deletion mutations in retromer subunits also cause defects in ALM and PLM axon polarity and other Wnt-related signalling (Prasad and Clark, 2006). How is Wnt secretion regulated by membrane trafficking? DPY-23, the C. elegans mu subunit of the clathrin adaptor AP-2, mediates the endocytosis of membrane proteins. dpy-23 mutants display a neuronal polarity defect similar to those found in Wnt mutants, suggesting that DPY-23 might be required in Wnt signalling (Prasad and Clark, 2006; Pan et al, 2008). MIG-14, the homologue of the Wnt secretion factor Wntless, acts to control Wnt function. In dpy-23 mutants, MIG-14 accumulates at or near the plasma membrane, whereas in retromer mutants, MIG-14 accumulates in intracellular compartments. These results suggest that intracellular trafficking of MIG-14 by AP-2 and the retromer plays an important role in Wnt secretion. Together, these data provide a link between membrane trafficking and secretion of Wnts.
Studies in cultured hippocampal neurons have demonstrated that the Par3-Par6-atypical protein kinase C (aPKC) polarity complex is involved in axonal polarity determination (Shi et al, 2003; Nishimura et al, 2004). Importantly, in cultured hippocampal neurons, aPKC is directly regulated by the Wnt signalling molecule, Dvl (Zhang et al, 2007; Figure 1). Downregulation of Dvl disturbs axon specification, whereas overexpression of Dvl results in the formation of multiple axons. Interestingly, association of Dvl with aPKC can stabilize and activate aPKC. Wnt5a, a non-canonical Wnt, activates aPKC and promotes axon differentiation. The Wnt5a effect on axon differentiation is attenuated by downregulation of Dvl or inhibition of aPKC. These results together suggest that interaction between Dvl and aPKC can promote axon differentiation mediated by the PAR3–PAR6–aPKC complex. However, in Drosophila, deletion of the Par3–Par6–aPKC complex does not result in detectable defects in axon-dendrite polarity (Rolls and Doe, 2004). Therefore, Wnt’s role in neurite polarity in mammalian systems needs to be further explored by in vivo experiments.
Wnts in axon guidance
Long-distance navigation by axons during development is critical for the correct wiring of circuits; both diffuse and membrane-attached cues play important roles in this process (Tessier-Lavigne and Goodman, 1996). Axon guidance along the A-P axis is controlled mainly by Wnts and their receptors (Killeen and Sybingco, 2008). A large body of evidence suggests that Wnts modulate axon pathfinding and target selection. Furthermore, through diverse receptors and signalling pathways, Wnts can generate both attractive and repulsive responses.
In nervous systems with bilateral symmetry, many neurons project axons across the midline to the opposite side through commissures. In Drosophila, ectopic expression of DWnt5 leads to specific disruption of the commissural axon tracts of the central nervous system (Fradkin et al, 1995), likely indicating a role for DWnt5 in axonal growth in the midline. In a subsequent study, Yoshikawa et al (2003) showed that DWnt5 acts as a repulsive signal through Derailed, an atypical receptor-tyrosine kinase that keeps the axons out of the posterior commissure. In vertebrate systems, Wnt5a gradients surround the corpus callosum and guide callosal axons after crossing the midline (postcrossing) by Wnt5a-induced repulsion via Ryk (receptor related to tyrosine kinase), the vertebrate homologue of Drosophila Derailed (Hutchins et al, 2011, 2012). Ryk receptor knockdown by siRNA reduces outgrowth rates of postcrossing but not precrossing axons and causes axon misrouting. Interestingly, axon guidance defects with Ryk knockdown result from reduced calcium activity (Hutchins et al, 2011, 2012). In the corpus callosum, inhibition of calcium/calmodulin-dependent kinase II (CaMKII) causes severe guidance errors that result from reduced Wnt-mediated repulsion.
In rodents, Wnt1 and Wnt5a are expressed in the dorsal spinal cord as a gradient in the A-P axis. This Wnt gradient attracts ascending somatosensory axons that project from the spinal cord to the brain and repel descending corticospinal tract (CST) axons in the opposite direction that grow from the brain to the spinal cord (Dickson, 2005; Liu et al, 2005). The repulsive activity of Wnt5a is mediated by Ryk (Keeble et al, 2006), which is expressed in CST axons. Intrathecal injection of anti-Ryk antibodies blocks the posterior growth of CST axons. In Ryk-deficient mice, cortical axons project aberrantly across the major forebrain commissure, the corpus callosum (Keeble et al, 2006). Therefore, Wnt5a acts as a chemorepulsive ligand for Ryk, driving callosal axons towards the contralateral hemisphere after crossing the midline. In addition to the chemorepulsive functions described in the above studies (Yoshikawa et al, 2003; Liu et al, 2005; Keeble et al, 2006), Ryk is also reported to be required for attractive responses to Wnt3 in dorsal root ganglion (DRG) cell axons (Lu et al, 2004). In Ryk-deficient mice, DRG axons exhibit defects in Wnt3-mediated attraction. Together, these data indicate that Wnt-Ryk-calcium is a conserved signalling pathway essential for the proper guidance of axons.
A number of experiments also suggest that Wnt can attract axonal growth cones. Commissural neurons in the vertebrate dorsal spinal cord project axons ventrally towards the floor plate, where they cross the midline and turn anteriorly towards the brain. Wnt4, which is expressed in a decreasing A to P gradient, attracts postcrossing commissural axons (Lyuksyutova et al, 2003). In mice lacking the Wnt receptor Frizzled-3, commissural axons exhibit A-P guidance defects after midline crossing. Thus, Wnt-Frizzled signalling directs spinal cord commissural axons to turn anteriorly after midline crossing through an attractive mechanism. aPKC is required for this Wnt4-mediated attraction of commissural axons and proper A-P pathfinding. In addition, PI3K could act as a switch to activate Wnt responsiveness during midline crossing (Wolf et al, 2008). Specific blockade of aPKC signalling causes commissural axons to turn randomly along the A-P axis. Overexpression of p110γ, the catalytic subunit of PI3Kγ, causes precocious anterior turning of commissural axons before midline crossing. Outside of the spinal cord, monoaminergic neurons, such as serotonergic (5-HT) and dopaminergic (mdDA) neurons in the brainstem, project axons along the A-P axis. In Frizzled-3 mutant mice, monoaminergic axons exhibit A-P guidance defects, and many cell bodies of 5-HT and mdDA neurons are oriented abnormally along the direction of their aberrant axon projections (Fenstermaker et al, 2010). These data suggest that Wnt signalling may be a global A-P guidance mechanism, underlying axonal and cellular organization.
In C. elegans, Wnts act directly on the mechanosensory neuron PLM via the Frizzled/LIN-17 pathway, and this pathway is required for axon branching and anteriorly directed axon growth (Prasad and Clark, 2006). In Drosophila, the axons of retinal photoreceptor cells extend to the first optic ganglion, the lamina, and DWnt4 acts as the ventral cue for the lamina (Sato et al, 2006). In DWnt4 mutants, ventral retinal axons misproject to the dorsal lamina. Drosophila Frizzled-2 (DFz2) and Dvl mutants also cause DWnt4 mutant-like defects. In zebrafish, Wnt11r organizes the central muscle zone by binding to the unplugged/muscle-specific kinase (MuSK) ectodomain (Jing et al, 2009). Inducible unplugged/MuSK transgene showed that organization of the central muscle zone is essential for motor growth cone guidance. Blockade of Dishevelled signalling in muscle fibres causes a growth cone guidance defect. Thus, it is supposed that Wnt activates unplugged/MuSK signalling in muscle fibres to guide growth cone.
In summary, Wnt signalling plays conserved roles in guiding axons to their destinations. Because of the distribution of Wnt-secreting cells, diffuse gradients of Wnt molecules are established. Axons interpret these gradients with receptors on their growth cones. The majority of the experimental data support the notion that the Ryk/Derailed receptor mediates repulsive responses, while Fz/LIN-17-type receptors mediate attractive responses. Therefore, the logic of axonal migration patterns is determined both by the nature of the Wnt gradient and by the receptors expressed by neurons.
Wnts in synaptogenesis
A large body of evidence suggests that Wnts also play important roles in the development and function of synapses. This question has been tested in both vertebrate and invertebrate models. Interestingly, Wnts have been found to promote synapse formation in some cases and to inhibit synapse formation in other cases, suggesting that the actions of Wnts in synapse formation and maintenance are complex.
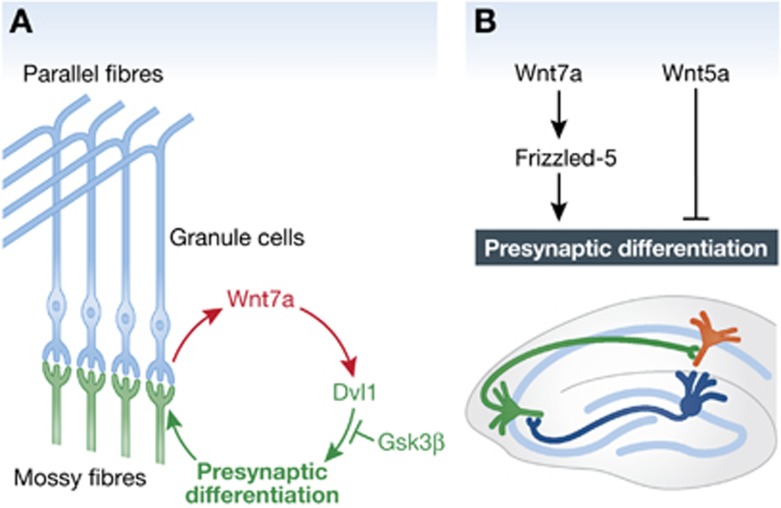
Wnts in cerebellar granule cell neurons have been reported to be involved in the maturation of neuronal connections (Lucas and Salinas, 1997; Hall et al, 2000). In the cerebellum, mossy fibres undergo extensive remodelling as they contact several granule cells and form complex, multisynaptic glomerular rosettes. Axon and growth cone remodelling in mossy fibres is blocked by the Wnt antagonist Sfrp-1 (secreted Frizzled-related protein-1; Bovolenta et al, 2008) and accelerated by Wnt7a, which is expressed by granule cells (Hall et al, 2000). Wnt7a also induces synapsin I clustering at remodelled areas of mossy fibres. In Wnt7a knockout mice, the morphological maturation of glomerular rosettes and the clustering of synapsin I are delayed (Hall et al, 2000), suggesting that Wnt7a functions as a synaptogenic factor to promote the formation of complex presynaptic terminals.
A number of signalling molecules in Wnt pathways have also been implicated in synapse development. Similarly to the effect of Wnt7a, inhibition of Gsk3β, a serine/threonine kinase, leads to axonal remodelling and clustering of synapsin I in developing neurons (Hall et al, 2000, 2002). Loss of function of Dvl1 mimics and enhances the Wnt7a phenotype in the cerebellum (Ahmad-Annuar et al, 2006), indicating a requirement for Dvl1 function. Electrophysiological recordings at mossy fibre-granule cell synapses in Wnt7a Dvl1 double-mutant mice revealed a significant decrease in the frequency but not the amplitude of miniature excitatory postsynaptic currents (mEPSCs), suggesting a defect in presynaptic neurotransmitter release (Ahmad-Annuar et al, 2006). Deficiency in Wnts affects the localization of synaptic proteins but does not affect their expression levels (Ahmad-Annuar et al, 2006). Taken together, these findings demonstrate that Wnt7a acts as a retrograde signal to regulate presynaptic assembly through Dvl- and Gsk3β-mediated signalling pathways (Figure 2).
Figure 2.
Wnts are involved in synaptogenesis by promoting synapse formation. (A) In the cerebellum, Wnt7a acts as a retrograde signal to regulate presynaptic assembly through Dvl- and Gsk3β-mediated signalling pathways. (B) In hippocampal neurons, Fz5 is required for Wnt7a-mediated synapse formation. The activation of Wnt7a increases presynaptic inputs, whereas the activation of Wnt5a decreases the number of presynaptic terminals.
Similarly, in rat hippocampal neurons, Wnt7a was found to stimulate clustering of presynaptic proteins and induce recycling and exocytosis of synaptic vesicles using FM dyes (Cerpa et al, 2008). Electrophysiological analysis on adult rat hippocampal slices showed that Wnt7a, but not Wnt5a, decreases paired pulse facilitation and increases mEPSC frequency, resulting in an increase in neurotransmitter release in CA3-CA1 synapses (Cerpa et al, 2008). These results indicate that Wnt signalling modulates the presynaptic function of rat hippocampal neurons. Frizzled-5 (Fz5) is expressed during the peak of synapse formation and localizes to synaptic sites in the hippocampus (Sahores et al, 2010). Expression of Fz5 increases the number of presynaptic sites, whereas knockdown of Fz5 blocks the ability of Wnt7a to promote synaptogenesis. In hippocampal neurons, therefore, Fz5 is required for Wnt7a-mediated synapse formation (Figure 2).
Interestingly, the synapse-promoting and synapse-inhibiting effects of Wnt proteins are related to the activation of canonical and non-canonical Wnt signalling pathways, respectively (Davis et al, 2008). In hippocampal cultures, activation of the canonical Wnt7a pathway increases presynaptic inputs, whereas activation of non-canonical Wnt5a signalling pathway decreases the number of presynaptic terminals (Figure 2). In addition to having a presynaptic function (Davis et al, 2008), several studies indicate that Wnt5a also has postsynaptic function (Farias et al, 2009; Cuitino et al, 2010). Wnt5a induces clustering of PSD95 and promotes the recruitment of a diffuse pool of PSD95 to the membrane pool to form new PSD95 clusters in dendritic spines (Farias et al, 2009). Moreover, Wnt5a acting as a non-canonical ligand functions through a JNK-dependent signalling pathway to regulate PSD95 distribution. Wnt5a increases the amplitude of glutamatergic synaptic transmission without affecting paired pulse facilitation, indicating a postsynaptic mechanism. Furthermore, Wnt5a also appears to regulate postsynaptic specializations in inhibitory synapses in the hippocampus (Cuitino et al, 2010). Wnt5a induces GABA(A)-Rs insertion and clustering in the membrane, increases the amplitude of GABA currents, and increases the recycling of GABA(A)-Rs. Interestingly, all of these effects on GABA(A)-Rs are mediated by CaMKII. Therefore, Wnt-5a, through CaMKII activation, induces the recycling of functional GABA(A)-Rs on mature hippocampal neurons.
Most studies on Wnt7a functions have indicated that Wnt7a is involved in the formation of presynapses. Recently, however, Wnt7a has also been reported to support postsynaptic function (Ciani et al, 2011). Wnt7a and postsynaptic expression of Dvl1 increase both mEPSC frequency and amplitude. Wnt7a also induces increased dendritic spine density and maturity. In Wnt7a-Dvl1-deficient mice, mossy fibre-CA3 synaptic transmission and spine morphogenesis are defective. Wnt7a rapidly activates CaMKII in spines, and inhibition of CaMKII abolishes the effects of Wnt7a on spine growth and excitatory synaptic strength. Together, these results indicate that Wnt7a signalling is critical for the regulation of spine growth and synaptic strength through the local activation of CaMKII in the spines. Interestingly, Wnt7a has been shown to preferentially stimulate excitatory but not inhibitory synapse formation and function (Ciani et al, 2011).
Studies of neuromuscular junctions (NMJs) have provided ample insights into the roles of Wnts in synapse development. In Drosophila, the Wnt Wingless (Wg) pathway plays an essential role during synapse development. At Drosophila glutamatergic NMJs, Wg is secreted by motor neurons and binds to postsynaptic seven-pass transmembrane DFz2 receptors. Loss of Wg leads to compromised synaptic growth and structural defects in presynaptic and postsynaptic specializations (Packard et al, 2002). Interestingly, the cytoplasmic terminus of DFz2 (DFz2-C) is cleaved, and the DFz2-C fragment is translocated into the nucleus (Mathew et al, 2005; Ataman et al, 2006). Translocation of DFz2-C into the nucleus, but not its cleavage and transport, depends on Wg signalling. Disruption of this receptor pathway interferes with the formation of new synaptic boutons and leads to aberrant synaptic structures (Ataman et al, 2006). Taken together, therefore, Wg signalling is required for coordinated development of the neuromuscular synapse on both presynaptic and postsynaptic sides. In zebrafish, Wnt11r and Wnt4a induce the translocation of MuSK on muscle membranes to the recycling endosome, a transition which is crucial for the accumulation of acetylcholine receptors (AChRs) at future synaptic sites (Gordon et al, 2012). Knockdown of several Wnt components disrupts the translocation of MuSK to the endosome and AChR localization. These results suggest a role for Wnt in synapse formation through induction of MuSK endocytosis, which initiates a signalling pathway to synapse assembly.
In vertebrate muscle cells, Dvl1 has been shown to play important roles in Agrin/MuSK-induced AChR clustering (Luo et al, 2002; Strochlic et al, 2005). Dvl interacts with the MuSK, a receptor for Agrin, a secreted molecule important for vertebrate NMJ formation. Disruption of the MuSK–Dvl interaction inhibits the clustering of postsynaptic AChRs in cultured myocytes. Consistent with this finding, dominant-negative Dvl1 expression in postsynaptic muscle cells reduces the amplitude of spontaneous synaptic currents at the NMJ (Luo et al, 2002). Agrin activates the p21 kinase PAK1, and this process requires Dvl. Furthermore, inhibition of PAK1 activity leads to attenuation of the clustering of AChRs. Adenomatous polyposis coli (APC), another Wnt signalling molecule, has also been shown to be involved in AChR clustering (Wang et al, 2003). APC colocalizes with AChRs at the mature vertebrate NMJ, and disruption of APC–AChR interaction causes reduced AChR clustering induced by Agrin. In cultured myotubes, Wnt3 increases the number and size of AChR clusters induced by Agrin (Henriquez et al, 2008). Wnt3 drives the rapid formation of unstable AChR microclusters through Rac1 activation. Such unstable microclusters become large clusters only in the presence of Agrin. Therefore, Wnt3 signalling is necessary for the formation of AChR clusters (Henriquez et al, 2008; Korkut and Budnik, 2009; Henriquez and Salinas, 2012). Taken together, cross-talk between Wnt and Agrin signalling through Rac1 activation controls postsynaptic assembly at the vertebrate NMJ.
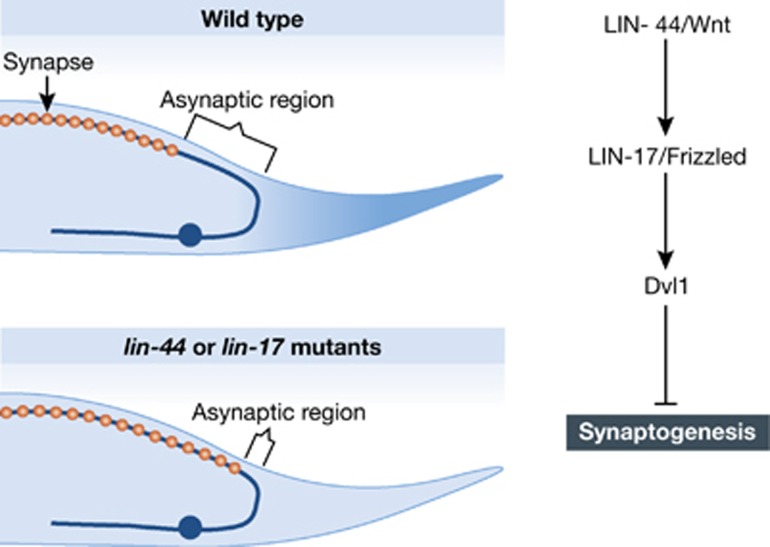
While Wnts positively regulate synapse formation as described above, Wnts also inhibit synapse formation in a number of systems (Davis and Ghosh, 2007; Inaki et al, 2007; Klassen and Shen, 2007; Salinas and Zou, 2008; Korkut and Budnik, 2009; Shen and Scheiffele, 2010). Wnt4 has been shown to play an important role in determining target specificity by preventing synapse formation (Inaki et al, 2007). Of two adjacent muscles M12 and M13 in Drosophila, Wnt4 is enriched in M13 but not in M12. In the absence of Wnt4, or its receptors Frizzled-2 and Derailed-2 or Dishevelled, motor neurons normally targeted to M12 form weak synapses on M12 and ectopic synapses on M13. Ectopic expression of Wnt4 in M12 inhibits synapse formation by MN12s. These data suggest that Wnt4, via Frizzled-2, Derailed-2, and Dishevelled, generates target specificity by preventing synapse formation on a non-target muscle. In C. elegans, Wnt/LIN-44 (Herman et al, 1995), through its receptor Frizzled/LIN-17 (Sawa et al, 1996), inhibits synapse formation in DA9 axons (Klassen and Shen, 2007). The DA9 motor neuron synapses onto the postsynaptic target dorsal muscle in a specific subdomain of the entire axon; the posterior axonal segment of DA9 is devoid of presynaptic terminals. The development of this asynaptic region is determined by a putative Wnt gradient formed by two Wnts, LIN-44 and EGL-20, which are locally secreted and inhibit synapse formation in the region. Wnt/LIN-44 localizes the Wnt receptor Frizzled/LIN-17 to the asynaptic region of DA9 axons. In lin-44 and lin-17 mutants, however, synapses form ectopically in the asynaptic region. Conversely, overexpression of LIN-44 in cells adjacent to DA9 is sufficient to localize LIN-17 within DA9 axons. Therefore, a local Wnt gradient negatively regulates DA9 synapse formation through its anti-synaptogenic activity (Figure 3).
Figure 3.
Wnts are involved in synaptogenesis through inhibition of synapse formation. A local Wnt LIN-44 gradient negatively regulates DA9 synapse formation through its anti-synaptogenic activity.
Wnts in activity-dependent synaptic plasticity
Neuronal activity plays a key role in several aspects of neuronal circuit generation by modulating synapse structure and function. Recent studies have suggested that Wnt signalling is involved in synaptic plasticity (Chen et al, 2006; Wayman et al, 2006; Beaumont et al, 2007; Ataman et al, 2008; Cerpa et al, 2008; Gogolla et al, 2009; Avila et al, 2010; Sahores et al, 2010; Varela-Nallar et al, 2010; Jensen et al, 2012; Salinas, 2012). In hippocampal slices, blockade of Wnt signalling impairs LTP, whereas activation of Wnt signalling facilitates LTP (Chen et al, 2006). These findings suggest that Wnt signalling plays a critical role in regulating synaptic plasticity. In cultured hippocampal neurons and slices, neuronal activity coupled to an NMDA receptor-mediated and Ca2+-dependent signalling pathway enhances the expression of Wnt2, and expression of Wnt2 promotes dendritic arborization (Wayman et al, 2006), indicating that Wnt2 contributes to dynamic remodelling of dendritic structure in response to neuronal activity. In Drosophila, glutamatergic NMJs undergo rapid changes in synaptic structure and function in response to patterned stimulation (Ataman et al, 2008). Wnt1/Wg is released from synaptic boutons by evoked activity, and secreted Wg stimulates both a presynaptic pathway involving GSK-3β/Shaggy and a postsynaptic DFz2 nuclear import pathway. This bidirectional Wg signalling operates downstream of synaptic activity to induce modifications in synaptic structure and function.
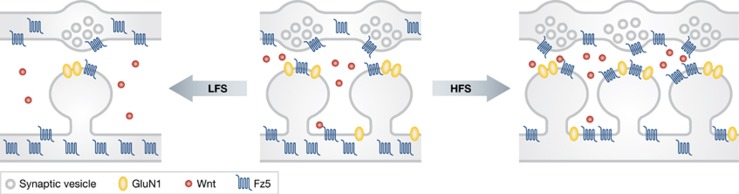
In the vertebrate system, high-frequency stimulation (HFS) is a classical stimulus for the induction of LTP, which is accompanied by synapse formation. HFS increases the surface and synaptic localization of Fz5, and this effect is blocked by the Wnt antagonist Sfrp and the soluble extracellular domain (CRD) of Fz5 (Fz5CRD), which binds to Wnt7a (Sahores et al, 2010). These results indicate that endogenous Wnts mediate the effect of HFS on Fz5 mobilization to the surface (Figure 4). Interestingly, Fz5CRD abolishes HFS-induced synapse formation. In contrast, low-frequency stimulation (LFS), which decreases the number of synapses, decreases surface Fz5 levels and the percentage of synapses containing Fz5. Taken together, these findings support the idea that neuronal activity regulates Fz5 trafficking to and out of synapses, contributing to activity-mediated synapse remodelling (Figure 4). Similarly to the involvement of Wnt in Fz5 mobilization to the synapse, a recent study in C. elegans showed that a Wnt signalling regulates the translocation of an AChR ACR-16/α7 to the synapse, thus modifying synaptic strength (Jensen et al, 2012). Wnt-signalling mutants exhibit defective translocation of ACR-16/α7 to the synapse and resultant reduction of synaptic currents. Furthermore, optogenetic stimulation of nerve cells reveals that neurons possess plastic synapses, and this synaptic plasticity is mediated by ACR-16/α7 translocation through Wnt−LIN-17/CAM-1 heteromeric receptors signalling (Jensen et al, 2012). Enriched environment (EE) increases the level of Wnt7a/b in postsynaptic CA3 pyramidal neurons and the complexity and number of large mossy fibre terminals in the CA3 region (Gogolla et al, 2009). Local application of Sfrp-1 to block Wnt signalling suppresses the effects of EE-induced remodelling. Therefore, behavioural experience regulates synapse remodelling and thus network through Wnt signalling in the hippocampus.
Figure 4.
Wnts play a role in activity-dependent synapse remodelling. Neuronal activity regulates Fz5 trafficking to and out of synapses, contributing to activity-mediated synapse remodelling. High-frequency stimulation (HFS) increases surface and synaptic localization of Fz5, whereas low-frequency stimulation (LFS) decreases surface and synaptic localization of Fz5.
Perspectives
Wnt proteins are known to play important roles in patterning neural circuits. Although many studies have uncovered roles for Wnts in neural circuit formation, detailed cellular and molecular mechanisms still need to be investigated. There are many different Wnts and Wnt receptors: 19 Wnts and 10 Fzs in vertebrate and 5 Wnts (CWN-1, CWN-2, EGL-20, LIN-44, and MOM-2; Shackleford et al, 1993; Herman et al, 1995; Thorpe et al, 1997; Whangbo and Kenyon, 1999), 4 Fzs (CFZ-2, LIN-17, MIG-1, and MOM-5; Sawa et al, 1996; Rocheleau et al, 1997; Zinovyeva and Forrester, 2005; Pan et al, 2006), and a Ryk (LIN-18; Inoue et al, 2004) in C. elegans. Thus, different combinations of Wnts and their receptors could be responsible for the different aspects of processes for neural circuit formation. In addition to the canonical pathway, in which Wnts signal through Fzs and the low-density lipoprotein receptor-related proteins 5/6 (LRP5/6), Wnts also signal through at least two other receptors, Ryk/Derailed, a receptor tyrosine kinase-like protein, and the receptor tyrosine kinase, Ror2, in non-canonical pathways (Wu et al, 2004; Cadigan and Liu, 2006; Mikels and Nusse, 2006). Interestingly, a recent study reports that a heteromeric CAM-1/LIN-17 receptor complex is involved in Wnt-mediated signalling (Jensen et al, 2012), supporting the diversity of Wnt pathway. Therefore, with ongoing increases in the number of known non-canonical Wnt pathways, it is highly possible that new pathways will be discovered as researchers investigate the detailed molecular mechanisms underlying Wnt functions in neural circuit formation.
Wnt function in synaptic plasticity suggests that this family of molecules might be involved in synapse formation and elimination during synapse remodelling (Figure 4). However, the detailed intracellular molecular mechanisms by which Wnts affect synaptic plasticity are still unknown. Recently, Cyclin Y was shown to function in synapse remodelling in C. elegans (Park et al, 2011). LRP5/6 is a transmembrane coreceptor for the canonical Wnt pathway. Since Cyclin Y is associated with the plasma membrane (Jiang et al, 2009) and is required for in vivo phosphorylation of LRP6 (Davidson et al, 2009), it is conceivable that Cyclin Y might contribute to Wnt signalling near the membrane by recruiting its cognate kinase, cyclin-dependent kinase 14 (CDK14), which phosphorylates LRP6. Taken together, these studies provide a possible mechanism linking synapse remodelling and Wnt signalling. Future studies on the function of Wnts during the formation, maintenance, and remodelling of neural circuits will provide important insights into the field of Wnts in neuronal circuitry.
The multitude of roles played by Wnts in synapse development and function predicts that abnormal activity of Wnts might be responsible for some aspects of neurological diseases. Indeed, altered Wnt signalling is linked to neurological disorders, such as Alzheimer's disease, Williams syndrome, schizophrenia, and mood disorders (Zhao et al, 2005; De Ferrari et al, 2007; Lovestone et al, 2007; Singh et al, 2010; Budnik and Salinas, 2011). Thus, discovering the molecular mechanisms by which Wnts regulate diverse aspects of structural and functional neural circuit formation will provide a basis for developing therapeutic targets for Wnt-related neurological disorders.
Acknowledgments
The work in M Park laboratory was supported by the World Class Institute (WCI) Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology of Korea (MEST) (NRF Grant Number: WCI 2009-003). The work in K Shen laboratory was supported by the Howard Hughes Medical Institute.
Footnotes
The authors declare that they have no conflict of interest.
References
- Ahmad-Annuar A, Ciani L, Simeonidis I, Herreros J, Fredj NB, Rosso SB, Hall A, Brickley S, Salinas PC (2006) Signaling across the synapse: a role for Wnt and Dishevelled in presynaptic assembly and neurotransmitter release. J Cell Biol 174: 127–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ataman B, Ashley J, Gorczyca D, Gorczyca M, Mathew D, Wichmann C, Sigrist SJ, Budnik V (2006) Nuclear trafficking of Drosophila Frizzled-2 during synapse development requires the PDZ protein dGRIP. Proc Natl Acad Sci USA 103: 7841–7846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ataman B, Ashley J, Gorczyca M, Ramachandran P, Fouquet W, Sigrist SJ, Budnik V (2008) Rapid activity-dependent modifications in synaptic structure and function require bidirectional Wnt signaling. Neuron 57: 705–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila ME, Sepulveda FJ, Burgos CF, Moraga-Cid G, Parodi J, Moon RT, Aguayo LG, Opazo C, De Ferrari GV (2010) Canonical Wnt3a modulates intracellular calcium and enhances excitatory neurotransmission in hippocampal neurons. J Biol Chem 285: 18939–18947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu K, Hu Z, Chien SC, Garriga G, Kaplan JM (2011) The immunoglobulin super family protein RIG-3 prevents synaptic potentiation and regulates Wnt signaling. Neuron 71: 103–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont V, Thompson SA, Choudhry F, Nuthall H, Glantschnig H, Lipfert L, David GR, Swain CJ, McAllister G, Munoz-Sanjuan I (2007) Evidence for an enhancement of excitatory transmission in adult CNS by Wnt signaling pathway modulation. Mol Cell Neurosci 35: 513–524 [DOI] [PubMed] [Google Scholar]
- Bovolenta P, Esteve P, Ruiz JM, Cisneros E, Lopez-Rios J (2008) Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J Cell Sci 121: 737–746 [DOI] [PubMed] [Google Scholar]
- Budnik V, Salinas PC (2011) Wnt signaling during synaptic development and plasticity. Curr Opin Neurobiol 21: 151–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadigan KM, Liu YI (2006) Wnt signaling: complexity at the surface. J Cell Sci 119: 395–402 [DOI] [PubMed] [Google Scholar]
- Cerpa W, Godoy JA, Alfaro I, Farias GG, Metcalfe MJ, Fuentealba R, Bonansco C, Inestrosa NC (2008) Wnt-7a modulates the synaptic vesicle cycle and synaptic transmission in hippocampal neurons. J Biol Chem 283: 5918–5927 [DOI] [PubMed] [Google Scholar]
- Chen J, Park CS, Tang SJ (2006) Activity-dependent synaptic Wnt release regulates hippocampal long term potentiation. J Biol Chem 281: 11910–11916 [DOI] [PubMed] [Google Scholar]
- Ciani L, Boyle KA, Dickins E, Sahores M, Anane D, Lopes DM, Gibb AJ, Salinas PC (2011) Wnt7a signaling promotes dendritic spine growth and synaptic strength through Ca(2)/Calmodulin-dependent protein kinase II. Proc Natl Acad Sci USA 108: 10732–10737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coudreuse DY, Roel G, Betist MC, Destree O, Korswagen HC (2006) Wnt gradient formation requires retromer function in Wnt-producing cells. Science 312: 921–924 [DOI] [PubMed] [Google Scholar]
- Cuitino L, Godoy JA, Farias GG, Couve A, Bonansco C, Fuenzalida M, Inestrosa NC (2010) Wnt-5a modulates recycling of functional GABAA receptors on hippocampal neurons. J Neurosci 30: 8411–8420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson G, Shen J, Huang YL, Su Y, Karaulanov E, Bartscherer K, Hassler C, Stannek P, Boutros M, Niehrs C (2009) Cell cycle control of wnt receptor activation. Dev Cell 17: 788–799 [DOI] [PubMed] [Google Scholar]
- Davis E, Ghosh A (2007) Should I stay or should I go: Wnt signals at the synapse. Cell 130: 593–596 [DOI] [PubMed] [Google Scholar]
- Davis EK, Zou Y, Ghosh A (2008) Wnts acting through canonical and noncanonical signaling pathways exert opposite effects on hippocampal synapse formation. Neural Dev 3: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ferrari GV, Papassotiropoulos A, Biechele T, Wavrant De-Vrieze F, Avila ME, Major MB, Myers A, Saez K, Henriquez JP, Zhao A, Wollmer MA, Nitsch RM, Hock C, Morris CM, Hardy J, Moon RT (2007) Common genetic variation within the low-density lipoprotein receptor-related protein 6 and late-onset Alzheimer’s disease. Proc Natl Acad Sci USA 104: 9434–9439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson BJ (2005) Wnts send axons up and down the spinal cord. Nat Neurosci 8: 1130–1132 [DOI] [PubMed] [Google Scholar]
- Dotti CG, Sullivan CA, Banker GA (1988) The establishment of polarity by hippocampal neurons in culture. J Neurosci 8: 1454–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias GG, Alfaro IE, Cerpa W, Grabowski CP, Godoy JA, Bonansco C, Inestrosa NC (2009) Wnt-5a/JNK signaling promotes the clustering of PSD-95 in hippocampal neurons. J Biol Chem 284: 15857–15866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenstermaker AG, Prasad AA, Bechara A, Adolfs Y, Tissir F, Goffinet A, Zou Y, Pasterkamp RJ (2010) Wnt/planar cell polarity signaling controls the anterior-posterior organization of monoaminergic axons in the brainstem. J Neurosci 30: 16053–16064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming T, Chien SC, Vanderzalm PJ, Dell M, Gavin MK, Forrester WC, Garriga G (2010) The role of C. elegans Ena/VASP homolog UNC-34 in neuronal polarity and motility. Dev Biol 344: 94–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fradkin LG, Noordermeer JN, Nusse R (1995) The Drosophila Wnt protein DWnt-3 is a secreted glycoprotein localized on the axon tracts of the embryonic CNS. Dev Biol 168: 202–213 [DOI] [PubMed] [Google Scholar]
- Gogolla N, Galimberti I, Deguchi Y, Caroni P (2009) Wnt signaling mediates experience-related regulation of synapse numbers and mossy fiber connectivities in the adult hippocampus. Neuron 62: 510–525 [DOI] [PubMed] [Google Scholar]
- Gordon LR, Gribble KD, Syrett CM, Granato M (2012) Initiation of synapse formation by Wnt-induced MuSK endocytosis. Development 139: 1023–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AC, Brennan A, Goold RG, Cleverley K, Lucas FR, Gordon-Weeks PR, Salinas PC (2002) Valproate regulates GSK-3-mediated axonal remodeling and synapsin I clustering in developing neurons. Mol Cell Neurosci 20: 257–270 [DOI] [PubMed] [Google Scholar]
- Hall AC, Lucas FR, Salinas PC (2000) Axonal remodeling and synaptic differentiation in the cerebellum is regulated by WNT-7a signaling. Cell 100: 525–535 [DOI] [PubMed] [Google Scholar]
- Henriquez JP, Salinas PC (2012) Dual roles for Wnt signalling during the formation of the vertebrate neuromuscular junction. Acta Physiol 204: 128–136 [DOI] [PubMed] [Google Scholar]
- Henriquez JP, Webb A, Bence M, Bildsoe H, Sahores M, Hughes SM, Salinas PC (2008) Wnt signaling promotes AChR aggregation at the neuromuscular synapse in collaboration with agrin. Proc Natl Acad Sci USA 105: 18812–18817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman MA, Vassilieva LL, Horvitz HR, Shaw JE, Herman RK (1995) The C. elegans gene lin-44, which controls the polarity of certain asymmetric cell divisions, encodes a Wnt protein and acts cell nonautonomously. Cell 83: 101–110 [DOI] [PubMed] [Google Scholar]
- Hilliard MA, Bargmann CI (2006) Wnt signals and frizzled activity orient anterior-posterior axon outgrowth in C. elegans. Dev Cell 10: 379–390 [DOI] [PubMed] [Google Scholar]
- Hutchins BI, Li L, Kalil K (2011) Wnt/calcium signaling mediates axon growth and guidance in the developing corpus callosum. Dev Neurobiol 71: 269–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchins BI, Li L, Kalil K (2012) Wnt-induced calcium signaling mediates axon growth and guidance in the developing corpus callosum. Sci Signal 5: pt1. [DOI] [PubMed] [Google Scholar]
- Inaki M, Yoshikawa S, Thomas JB, Aburatani H, Nose A (2007) Wnt4 is a local repulsive cue that determines synaptic target specificity. Curr Biol 17: 1574–1579 [DOI] [PubMed] [Google Scholar]
- Inoue T, Oz HS, Wiland D, Gharib S, Deshpande R, Hill RJ, Katz WS, Sternberg PW (2004) C. elegans LIN-18 is a Ryk ortholog and functions in parallel to LIN-17/Frizzled in Wnt signaling. Cell 118: 795–806 [DOI] [PubMed] [Google Scholar]
- Jensen M, Hoerndli FJ, Brockie PJ, Wang R, Johnson E, Maxfield D, Francis MM, Madsen DM, Maricq AV (2012) Wnt signaling regulates acetylcholine receptor translocation and synaptic plasticity in the adult nervous system. Cell 149: 173–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Gao Y, Yang T, Zhu X, Chen J (2009) Cyclin Y, a novel membrane-associated cyclin, interacts with PFTK1. FEBS Lett 583: 2171–2178 [DOI] [PubMed] [Google Scholar]
- Jing L, Lefebvre JL, Gordon LR, Granato M (2009) Wnt signals organize synaptic prepattern and axon guidance through the zebrafish unplugged/MuSK receptor. Neuron 61: 721–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeble TR, Halford MM, Seaman C, Kee N, Macheda M, Anderson RB, Stacker SA, Cooper HM (2006) The Wnt receptor Ryk is required for Wnt5a-mediated axon guidance on the contralateral side of the corpus callosum. J Neurosci 26: 5840–5848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killeen MT, Sybingco SS (2008) Netrin, Slit and Wnt receptors allow axons to choose the axis of migration. Dev Biol 323: 143–151 [DOI] [PubMed] [Google Scholar]
- Klassen MP, Shen K (2007) Wnt signaling positions neuromuscular connectivity by inhibiting synapse formation in C. elegans. Cell 130: 704–716 [DOI] [PubMed] [Google Scholar]
- Korkut C, Budnik V (2009) WNTs tune up the neuromuscular junction. Nat Rev Neurosci 10: 627–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Shi J, Lu CC, Wang ZB, Lyuksyutova AI, Song XJ, Zou Y (2005) Ryk-mediated Wnt repulsion regulates posterior-directed growth of corticospinal tract. Nat Neurosci 8: 1151–1159 [DOI] [PubMed] [Google Scholar]
- Lovestone S, Killick R, Di Forti M, Murray R (2007) Schizophrenia as a GSK-3 dysregulation disorder. Trends Neurosci 30: 142–149 [DOI] [PubMed] [Google Scholar]
- Lu W, Yamamoto V, Ortega B, Baltimore D (2004) Mammalian Ryk is a Wnt coreceptor required for stimulation of neurite outgrowth. Cell 119: 97–108 [DOI] [PubMed] [Google Scholar]
- Lucas FR, Salinas PC (1997) WNT-7a induces axonal remodeling and increases synapsin I levels in cerebellar neurons. Dev Biol 192: 31–44 [DOI] [PubMed] [Google Scholar]
- Luo ZG, Wang Q, Zhou JZ, Wang J, Luo Z, Liu M, He X, Wynshaw-Boris A, Xiong WC, Lu B, Mei L (2002) Regulation of AChR clustering by Dishevelled interacting with MuSK and PAK1. Neuron 35: 489–505 [DOI] [PubMed] [Google Scholar]
- Lyuksyutova AI, Lu CC, Milanesio N, King LA, Guo N, Wang Y, Nathans J, Tessier-Lavigne M, Zou Y (2003) Anterior-posterior guidance of commissural axons by Wnt-frizzled signaling. Science 302: 1984–1988 [DOI] [PubMed] [Google Scholar]
- Mathew D, Ataman B, Chen J, Zhang Y, Cumberledge S, Budnik V (2005) Wingless signaling at synapses is through cleavage and nuclear import of receptor DFrizzled2. Science 310: 1344–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikels AJ, Nusse R (2006) Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol 4: e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T, Kato K, Yamaguchi T, Fukata Y, Ohno S, Kaibuchi K (2004) Role of the PAR-3-KIF3 complex in the establishment of neuronal polarity. Nat Cell Biol 6: 328–334 [DOI] [PubMed] [Google Scholar]
- Packard M, Koo ES, Gorczyca M, Sharpe J, Cumberledge S, Budnik V (2002) The Drosophila Wnt, wingless, provides an essential signal for pre- and postsynaptic differentiation. Cell 111: 319–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan CL, Baum PD, Gu M, Jorgensen EM, Clark SG, Garriga G (2008) C. elegans AP-2 and retromer control Wnt signaling by regulating mig-14/Wntless. Dev Cell 14: 132–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan CL, Howell JE, Clark SG, Hilliard M, Cordes S, Bargmann CI, Garriga G (2006) Multiple Wnts and frizzled receptors regulate anteriorly directed cell and growth cone migrations in Caenorhabditis elegans. Dev Cell 10: 367–377 [DOI] [PubMed] [Google Scholar]
- Park M, Watanabe S, Poon VY, Ou CY, Jorgensen EM, Shen K (2011) CYY-1/cyclin Y and CDK-5 differentially regulate synapse elimination and formation for rewiring neural circuits. Neuron 70: 742–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad BC, Clark SG (2006) Wnt signaling establishes anteroposterior neuronal polarity and requires retromer in C. elegans. Development 133: 1757–1766 [DOI] [PubMed] [Google Scholar]
- Rocheleau CE, Downs WD, Lin R, Wittmann C, Bei Y, Cha YH, Ali M, Priess JR, Mello CC (1997) Wnt signaling and an APC-related gene specify endoderm in early C. elegans embryos. Cell 90: 707–716 [DOI] [PubMed] [Google Scholar]
- Rolls MM, Doe CQ (2004) Baz, Par-6 and aPKC are not required for axon or dendrite specification in Drosophila. Nat Neurosci 7: 1293–1295 [DOI] [PubMed] [Google Scholar]
- Sahores M, Gibb A, Salinas PC (2010) Frizzled-5, a receptor for the synaptic organizer Wnt7a, regulates activity-mediated synaptogenesis. Development 137: 2215–2225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas PC (2005) Retrograde signalling at the synapse: a role for Wnt proteins. Biochem Soc Trans 33: 1295–1298 [DOI] [PubMed] [Google Scholar]
- Salinas PC (2012) Wnt signaling in the vertebrate central nervous system: from axon guidance to synaptic function. Cold Spring Harb Perspect Biol 4: pii: a008003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas PC, Zou Y (2008) Wnt signaling in neural circuit assembly. Annu Rev Neurosci 31: 339–358 [DOI] [PubMed] [Google Scholar]
- Sato M, Umetsu D, Murakami S, Yasugi T, Tabata T (2006) DWnt4 regulates the dorsoventral specificity of retinal projections in the Drosophila melanogaster visual system. Nat Neurosci 9: 67–75 [DOI] [PubMed] [Google Scholar]
- Sawa H, Lobel L, Horvitz HR (1996) The Caenorhabditis elegans gene lin-17, which is required for certain asymmetric cell divisions, encodes a putative seven-transmembrane protein similar to the Drosophila frizzled protein. Genes Dev 10: 2189–2197 [DOI] [PubMed] [Google Scholar]
- Shackleford GM, Shivakumar S, Shiue L, Mason J, Kenyon C, Varmus HE (1993) Two wnt genes in Caenorhabditis elegans. Oncogene 8: 1857–1864 [PubMed] [Google Scholar]
- Shen K, Scheiffele P (2010) Genetics and cell biology of building specific synaptic connectivity. Annu Rev Neurosci 33: 473–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi SH, Jan LY, Jan YN (2003) Hippocampal neuronal polarity specified by spatially localized mPar3/mPar6 and PI 3-kinase activity. Cell 112: 63–75 [DOI] [PubMed] [Google Scholar]
- Singh KK, Ge X, Mao Y, Drane L, Meletis K, Samuels BA, Tsai LH (2010) Dixdc1 is a critical regulator of DISC1 and embryonic cortical development. Neuron 67: 33–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speese SD, Budnik V (2007) Wnts: up-and-coming at the synapse. Trends Neurosci 30: 268–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strochlic L, Cartaud A, Cartaud J (2005) The synaptic muscle-specific kinase (MuSK) complex: new partners, new functions. Bioessays 27: 1129–1135 [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Goodman CS (1996) The molecular biology of axon guidance. Science 274: 1123–1133 [DOI] [PubMed] [Google Scholar]
- Thorpe CJ, Schlesinger A, Carter JC, Bowerman B (1997) Wnt signaling polarizes an early C. elegans blastomere to distinguish endoderm from mesoderm. Cell 90: 695–705 [DOI] [PubMed] [Google Scholar]
- van Amerongen R, Nusse R (2009) Towards an integrated view of Wnt signaling in development. Development 136: 3205–3214 [DOI] [PubMed] [Google Scholar]
- Varela-Nallar L, Alfaro IE, Serrano FG, Parodi J, Inestrosa NC (2010) Wingless-type family member 5A (Wnt-5a) stimulates synaptic differentiation and function of glutamatergic synapses. Proc Natl Acad Sci USA 107: 21164–21169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Jing Z, Zhang L, Zhou G, Braun J, Yao Y, Wang ZZ (2003) Regulation of acetylcholine receptor clustering by the tumor suppressor APC. Nat Neurosci 6: 1017–1018 [DOI] [PubMed] [Google Scholar]
- Wayman GA, Impey S, Marks D, Saneyoshi T, Grant WF, Derkach V, Soderling TR (2006) Activity-dependent dendritic arborization mediated by CaM-kinase I activation and enhanced CREB-dependent transcription of Wnt-2. Neuron 50: 897–909 [DOI] [PubMed] [Google Scholar]
- Whangbo J, Kenyon C (1999) A Wnt signaling system that specifies two patterns of cell migration in C. elegans. Mol Cell 4: 851–858 [DOI] [PubMed] [Google Scholar]
- Wolf AM, Lyuksyutova AI, Fenstermaker AG, Shafer B, Lo CG, Zou Y (2008) Phosphatidylinositol-3-kinase-atypical protein kinase C signaling is required for Wnt attraction and anterior-posterior axon guidance. J Neurosci 28: 3456–3467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Klein TJ, Mlodzik M (2004) Subcellular localization of frizzled receptors, mediated by their cytoplasmic tails, regulates signaling pathway specificity. PLoS Biol 2: E158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa S, McKinnon RD, Kokel M, Thomas JB (2003) Wnt-mediated axon guidance via the Drosophila Derailed receptor. Nature 422: 583–588 [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhu J, Yang GY, Wang QJ, Qian L, Chen YM, Chen F, Tao Y, Hu HS, Wang T, Luo ZG (2007) Dishevelled promotes axon differentiation by regulating atypical protein kinase C. Nat Cell Biol 9: 743–754 [DOI] [PubMed] [Google Scholar]
- Zhao C, Aviles C, Abel RA, Almli CR, McQuillen P, Pleasure SJ (2005) Hippocampal and visuospatial learning defects in mice with a deletion of frizzled 9, a gene in the Williams syndrome deletion interval. Development 132: 2917–2927 [DOI] [PubMed] [Google Scholar]
- Zinovyeva AY, Forrester WC (2005) The C. elegans Frizzled CFZ-2 is required for cell migration and interacts with multiple Wnt signaling pathways. Dev Biol 285: 447–461 [DOI] [PubMed] [Google Scholar]