Abstract
EMBO J 31 12, 2770–2783 (2012); published online May 01 2012
Caspases are widely known as initiators and executioners of cell death. Full activation of caspases leading to cleavage of many cellular substrates was long considered to be a point-of-no-return in the apoptosis pathway. However, it also has been known that activated caspases do not always have the ability to kill, but instead initiate non-apoptotic processes such as cell differentiation or activation of innate immune responses. In this issue of The EMBO Journal, Meinander et al (2012) explore the contribution of polyubiquitination of Dredd, a known initiator caspase, to the activation of innate immunity. The authors show that infection with gram-negative bacteria leads to DIAP2-dependent ubiquitylation of Dredd which in turn is required for processing of Relish (Rel) and expression of antimicrobial peptide (AMP) genes that are indispensable for fighting the infection.
To fight infection, Drosophila relies on its innate immune system that rapidly responds to pathogens by activating danger signalling pathways that lead to the expression of AMP genes. Production of AMPs is regulated by two signalling pathways: the Toll and immune deficiency (IMD) pathways (Hetru and Hoffmann, 2009). The Toll pathway is triggered by gram-positive bacteria or fungi and results in activation of the NF-κB transcription factors DIF and Dorsal. The IMD pathway is activated by DAP-type peptidoglycan (PNG), a typical cell wall component of gram-negative bacteria, and leads to nuclear translocation of the NF-κB transcription factor Rel. Depending on the NF-κB-binding motif present in the respective promotor, target genes respond to the Toll-DIF/Dorsal cascade or the IMD-Rel cascade (Lemaitre et al, 1995). Homologous signal transduction pathways are also found in mammals: Toll signalling resembles the myeloid differentiation factor 88 (MyD88)-dependent pathway used by most mammalian Toll-like receptors (TLRs) while IMD signalling strongly resembles the mammalian TNF receptor 1 (TNF-R1) pathway. Downstream of the receptor PGRP-LC in the IMD pathway, the initiator caspase Dredd and the inhibitor of apoptosis protein 2 (DIAP2) have been implicated in the Drosophila immune response (Gesellchen et al, 2005; Kleino et al, 2005).
IAPs are known to prevent caspases from induction of apoptosis by binding to them via their baculovirus IAP repeat (BIR) domain(s) or by ubiquitylation via their RING domains (Takahashi et al, 1998; Ditzel et al, 2008). Drosophila DIAP1 and DIAP2, as well as most mammalian IAPs, carry a RING domain that confers E3-ubiquitin ligase activity. DIAP2 was shown to be recruited to cleaved Imd and to promote cytoplasmic cleavage of Rel (Paquette et al, 2010). In contrast to embryonic lethal diap1 mutant flies, diap2 null flies are viable and show no defects in developmental or stress-induced apoptosis (Wang et al, 1999; Huh et al, 2007). However, these flies cannot cope with gram-negative bacterial infections, indicating that DIAP2 is required for IMD signalling.
Ubiquitylation of signalling adaptors has been implicated in several steps in NF-κB signalling. In both Drosophila IMD and mammalian TNF signalling, ligand binding induces recruitment of adaptor proteins such as Imd or TRADD. For subsequent signal transduction, post-translational modifications including phosphorylation and ubiquitylation are crucial. While phosphorylation is mediated by kinase–substrate complexes, ubiquitylation requires the sequential transfer of ubiquitin by three enzymes: a ubiquitin activating E1, a ubiquitin-conjugating E2, and a E3-ubiquitin ligase that specifically recognizes the target protein. Ubiquitin contains several internal lysine residues and can be linked to itself by ubiquitination. This creates chains that are structurally distinct and that serve as binding platforms or recognition sites for specific proteins (Grabbe et al, 2011). The best characterized linkage types are lysine 48 (K48) and K63. K48-linked polyubiquitin is usually considered as degradation signal, while K68-linked ubiquitin chains were shown to serve as recruitment platforms for signalling factors (Chen, 2005). In the IMD as well as the mammalian TNF-R1 pathway, IKKγ (dKenny/Nemo) is known to be modified by K63-linked ubiquitin, and K63-linkages present in the receptor-associated signalling complexes have been shown to be required for recruitment of other protein complexes such as the TAK–TAB complex. In Drosophila, DIAP2 seems to be the main transducer of IMD signalling-related ubiquitin modifications. In this issue of the EMBO Journal, Meinander et al, added another layer of complexity to the IMD pathway by showing that DIAP2-mediated K63-linked polyubiqitination of the initiator caspase Dredd is required for its endoproteolytic modification of the NF-κB factor Rel.
The authors explain how DIAP2 confers substrate specificity to the caspase-8 orthologue Dredd. They provide a refined model of the IMD pathway in which Dredd is rapidly ubiquitylated by DIAP2 in a signal-dependent manner, resulting in efficient cleavage of Rel/NF-κB and subsequent transcription of AMP genes (see Figure 1). Data from in vitro as well as in vivo approaches support their model. Using deletion constructs and pull-down assays, the authors demonstrated that DIAP2 directly interacts with the death-effector-domain 1 (DED1) of Dredd. It is interesting to note that DIAP2 is the first IAP found to interact with a DED-containing initiator caspase. Instead of inhibiting caspase activity, K63-linked ubiquitylation of Dredd by DIAP2 is required for proteolytic processing of Rel/NF-κB. RING mutants of DIAP2 abrogated the signal-dependent ubiquitylation of Dredd and could not rescue the lethality of diap2 mutant flies following infection with gram-negative bacteria. Employing a loss-of-function allele of Dredd that carries a missense mutation (G120R) in the sequence encoding the DED1, that authors showed that a failure to stimulate ubiquitylation at lysine 120 upon IMD pathway stimulation resulted in abrogation of Rel/NF-κB cleavage and consequently impaired expression of AMP genes.
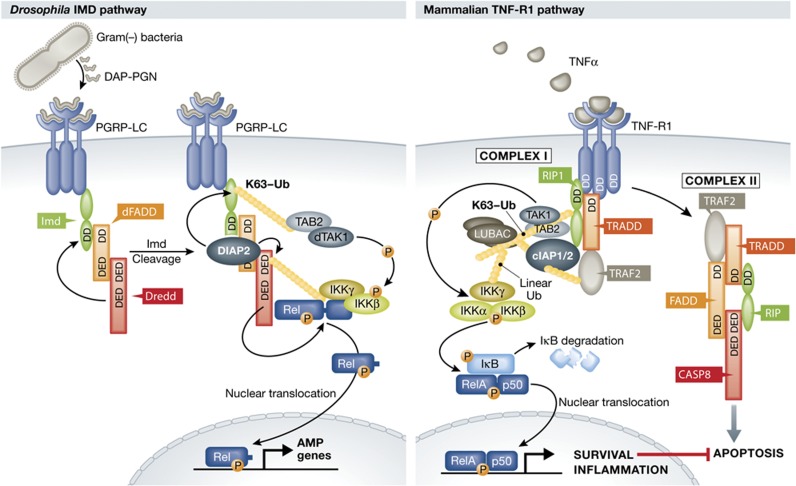
Figure 1.
Model of the Drosophila IMD and mammalian TNF signalling pathways. Ligand binding causes clustering of PGRP receptors (Drosophila) or TNF-R1 receptors (mammals) followed by recruitment of adaptor proteins such as Imd and dFADD or RIP1 and TRADD. In IMD signalling Dredd binds to dFADD and is autocatalytically activated to cleave IMD, generating a newly exposed N-terminal residue that in turn binds the E3-ligase DIAP2. DIAP2 adds K63-linked ubiquitin chains to IMD and Dredd, which are necessary for downstream signalling, ultimately leading to cleavage of Relish and induction of AMP genes. Mammalian TNF-R1 signalling involves assembly of two molecularly and spatially distinct complexes: complex I and II. The initial membrane-bound complex I consists of TRADD, RIP1 and TRAF2 and rapidly transduces inflammatory signals via NF-κB, similar to the Drosophila IMD pathway. In a second step, TRADD, RIP1 and TRAF2 dissociate from the TNF-R1 and, together with FADD and caspase-8, form complex II. In the absence of NF-κB activity from complex I, complex II can initiate caspase-8 activation and cell death.
The authors suggest that the ubiquitin chains placed on Dredd by DIAP2 form part of a new docking site for proteins with Ubiquitin-binding domains (UBD) such as Kenny (IKKy) or Tab2. As IKK is known to fulfil a structural role in Rel/NF-κB cleavage, it is likely to bring Rel/NF-κB in close proximity allowing for Dredd-mediated proteolysis. Impaired ubiquitylation of Dredd might abrogate recruitment of IKK:Rel/NF-κB complexes and therefore block Rel/NF-κB cleavage.
This elegant work shows that DIAP2-mediated ubiquitin modifications play an essential role in the IMD pathway. Instead of inhibiting the catalytic activity of caspases, as described for many IAPs, DIAP2 exert its effect on Dredd by promoting the endoproteolysis of Rel. It will be interesting to see in the future whether this concept also holds true for the mammalian TNF-R1 signalling pathway and how ubiquitination of caspases change their substrate specificity and signalling properties in general.
Acknowledgments
We thank Henning Walczak and members of the Boutros laboratory for helpful comments on the manuscript. Research in the laboratory of MB was supported by the ERASysBio+ program and an ERC Advanced Grant.
Footnotes
The authors declare that they have no conflict of interest.
References
- Chen ZJ (2005) Ubiquitin signalling in the NF-kappaB pathway. Nat Cell Biol 7: 758–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditzel M, Broemer M, Tenev T, Bolduc C, Lee TV, Rigbolt KT, Elliott R, Zvelebil M, Blagoev B, Bergmann A, Meier P (2008) Inactivation of effector caspases through nondegradative polyubiquitylation. Mol Cell 32: 540–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesellchen V, Kuttenkeuler D, Steckel M, Pelte N, Boutros M (2005) An RNA interference screen identifies Inhibitor of Apoptosis Protein 2 as a regulator of innate immune signalling in Drosophila. EMBO Rep 6: 979–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabbe C, Husnjak K, Dikic I (2011) The spatial and temporal organization of ubiquitin networks. Nat Rev Mol Cell Biol 12: 295–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetru C, Hoffmann JA (2009) NF-kappaB in the immune response of Drosophila. Cold Spring Harb Perspect Biol 1: a000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh JR, Foe I, Muro I, Chen CH, Seol JH, Yoo SJ, Guo M, Park JM, Hay BA (2007) The Drosophila inhibitor of apoptosis (IAP) DIAP2 is dispensable for cell survival, required for the innate immune response to gram-negative bacterial infection, and can be negatively regulated by the reaper/hid/grim family of IAP-binding apoptosis inducers. J Biol Chem 282: 2056–2068 [DOI] [PubMed] [Google Scholar]
- Kleino A, Valanne S, Ulvila J, Kallio J, Myllymäki H, Enwald H, Stöven S, Poidevin M, Ueda R, Hultmark D, Lemaitre B, Rämet M (2005) Inhibitor of apoptosis 2 and TAK1-binding protein are components of the Drosophila Imd pathway. EMBO J 24: 3423–3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre B, Meister M, Govind S, Georgel P, Steward R, Reichhart JM, Hoffmann JA (1995) Functional analysis and regulation of nuclear import of dorsal during the immune response in Drosophila. EMBO J 14: 536–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinander A, Runchel C, Tenev T, Chen L, Kim C-H, Ribeiro PS, Broemer M, Leulier F, Zvelebil M, Silverman N, Meier P (2012) Ubiquitylation of the initiator caspase DREDD is required for innate immune signalling. EMBO J 31: 2770–2783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette N, Broemer M, Aggarwal K, Chen L, Husson M, Erturk-Hasdemir D, Reichhart JM, Meier P, Silverman N (2010) Caspase-mediated cleavage, IAP binding, and ubiquitination: linking three mechanisms crucial for Drosophila NF-kappaB signaling. Mol Cell 37: 172–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi R, Deveraux Q, Tamm I, Welsh K, Assa-Munt N, Salvesen GS, Reed JC (1998) A single BIR domain of XIAP sufficient for inhibiting caspases. J Biol Chem 273: 7787–7790 [DOI] [PubMed] [Google Scholar]
- Wang SL, Hawkins CJ, Yoo SJ, Muller HA, Hay BA (1999) The Drosophila caspase inhibitor DIAP1 is essential for cell survival and is negatively regulated by HID. Cell 98: 453–463 [DOI] [PubMed] [Google Scholar]