Abstract
After its discovery as oncogen and morphogen, studies on Wnt focused initially on its role in animal development. With the finding that the colorectal tumour suppressor gene APC is a negative regulator of the Wnt pathway in (colorectal) cancer, attention gradually shifted to the study of the role of Wnt signalling in the adult. The first indication that adult Wnt signalling controls stem cells came from a Tcf4 knockout experiment: mutant mice failed to build crypt stem cell compartments. This observation was followed by similar findings in multiple other tissues. Recent studies have indicated that Wnt agonists of the R-spondin family provide potent growth stimuli for crypts in vivo and in vitro. Independently, Lgr5 was found as an exquisite marker for these crypt stem cells. The story has come full circle with the finding that the stem cell marker Lgr5 constitutes the receptor for R-spondins and occurs in complex with Frizzled/Lrp.
Keywords: intestine, Lgr5, stem cells, Wnt
Wnt in intestinal crypt homeostasis and colon cancer
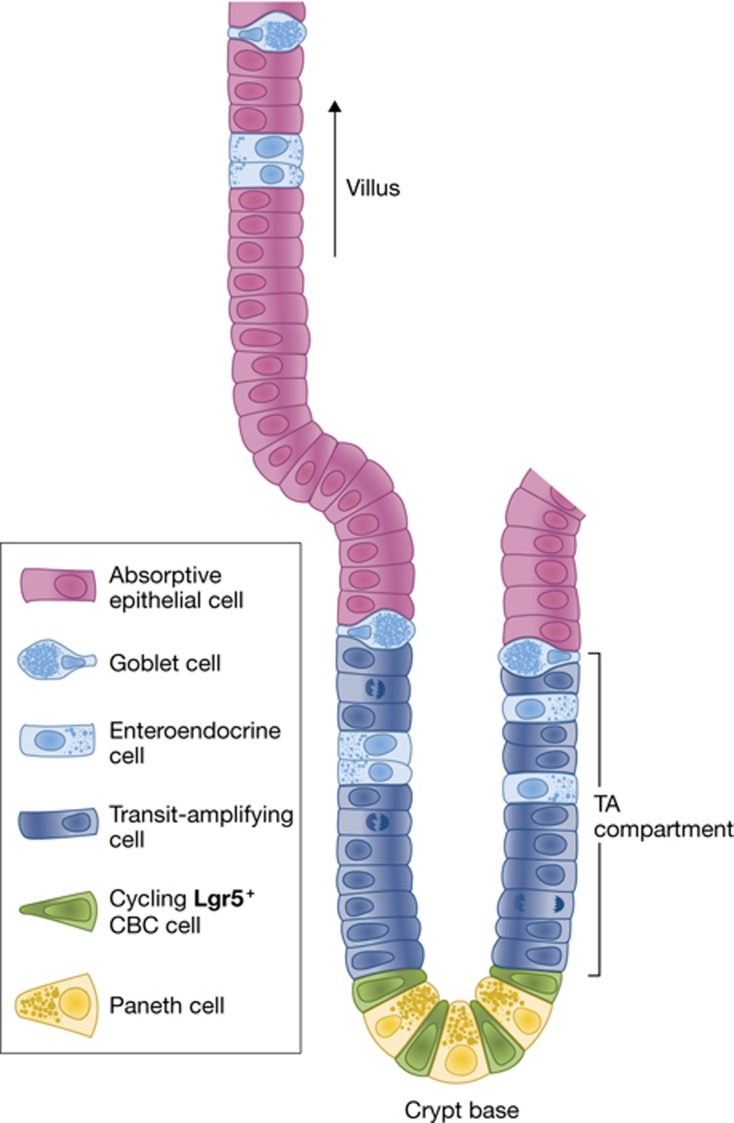
The inner surface epithelium of the intestine undergoes constant homeostatic renewal as it endures continuous mechanical and chemical stress. With its self-renewal cycle of 4–5 days, it is the fastest proliferating tissue in adult animals (Cheng and Leblond, 1974a). The epithelium of the small intestine is folded into large numbers of villi that protrude into the lumen, thus maximizing the surface to allow nutrient uptake (Figure 1A, B). The crypts of Lieberkühn, where active proliferation takes place, are invaginations that are located at the base of the villi into the underlying connective tissue (Figure 1C). The colon is similarly folded into crypts, but has a flat surface epithelium rather than carrying villi. Stem cells reside at crypt bottoms in both parts of the bowel; transit-amplifying (TA) cells make up the remainder of the crypts (Figure 1D). TA cells rapidly divide 4 to 5 times before differentiation upon crossing the crypt–villus junction (Marshman et al, 2002). This organization allows proliferating stem cells to literally push their progeny up the crypt–villus axis in a conveyor-belt-like fashion. When differentiated cells ultimately reach the tip of the villus they undergo apoptosis and are lost into the lumen. The proliferation and the acquisition of particular cell fates is coordinated by a small number of highly evolutionarily conserved signalling pathways, including the Wnt/β-catenin and the Notch signalling pathways.
Figure 1.
Structure of the intestinal epithelium. (A) The inner surface epithelium of the small intestine is folded into large numbers of villi. (B, C) In-between the villi are the crypts of lieberkühn. (D) Electron microscopy image of an intestinal crypt with slender crypt base columnar (CBC) cells residing in-between granulated Paneth cells (P). Reproduced with the kind permission of Prof Dr Wim A Buurman (Maastricht University). (E) Schematic representation of a single small intestinal crypt for reference.
The molecular workings of the Wnt pathway are discussed elsewhere in this issue (Nusse and Varmus, 2012). The key event that is activated by Wnt signals is the stabilization of β-catenin and the subsequent formation of nuclear β-catenin/Tcf complexes that can drive expression of Wnt target genes. Mutations in cancer mimick these events (Nusse and Varmus, 1982). Loss of function of the tumour suppressors APC or Axin2 lead to accumulation of nuclear β-catenin and induces the formation of intestinal adenomas (Rubinfeld et al, 1993; Su et al, 1993; Korinek et al, 1997; Liu et al, 2000). Oncogenic point mutations in β-catenin that prevent its degradation have similar outcomes (Morin et al, 1997; Polakis, 1999). Tcf4/Tcf7l2, the fourth Tcf family member to be cloned (Korinek et al, 1998b), mediates Wnt signalling in normal and malignant intestinal cells (Korinek et al, 1997). Deletion of the Tcf4 transcription factor blocks the development of the intestinal crypts (Korinek et al, 1998a), while abrogation in adult animals by expression of the Wnt inhibitor Dickkopf-1 (DKK1; Pinto et al, 2003; Kuhnert et al, 2004) or by deletion of the genes encoding β-catenin (Fevr et al, 2007) or Tcf4 (van Es et al, 2012) stops crypt proliferation completely.
Self-renewal and cancer of the gut thus appear to represent two sides of the same coin. When micro-arraying first became available, we exploited this paradigm by determining the Wnt-driven genetic programme that is activated inappropriately in APC-mutant human colon cancer cells (van de Wetering et al, 2002). This programme consists of a core of about 80 genes (Van der Flier et al, 2007). Histological expression studies for each of the 80 Wnt target genes revealed that they were invariably expressed in crypts. However, while most Tcf4 target genes were expressed by the TA cells, a handful were specifically expressed by postmitotic Paneth cells at crypt bottoms (Van der Flier et al, 2007). One of the Tcf4 target genes, Lgr5/Gpr49, was expressed in a unique fashion: it appeared to be specifically active in small, cycling cells that are interspersed between the Paneth cells. These so-called ‘crypt base columnar’ (CBC) cells were identified originally by Leblond and colleagues by electron microscopy more than 35 years ago (Cheng and Leblond, 1974b), when they were postulated to represent the intestinal stem cells. Since then, these cells were all but forgotten.
Lineage tracing of Lgr5 stem cells in the intestine
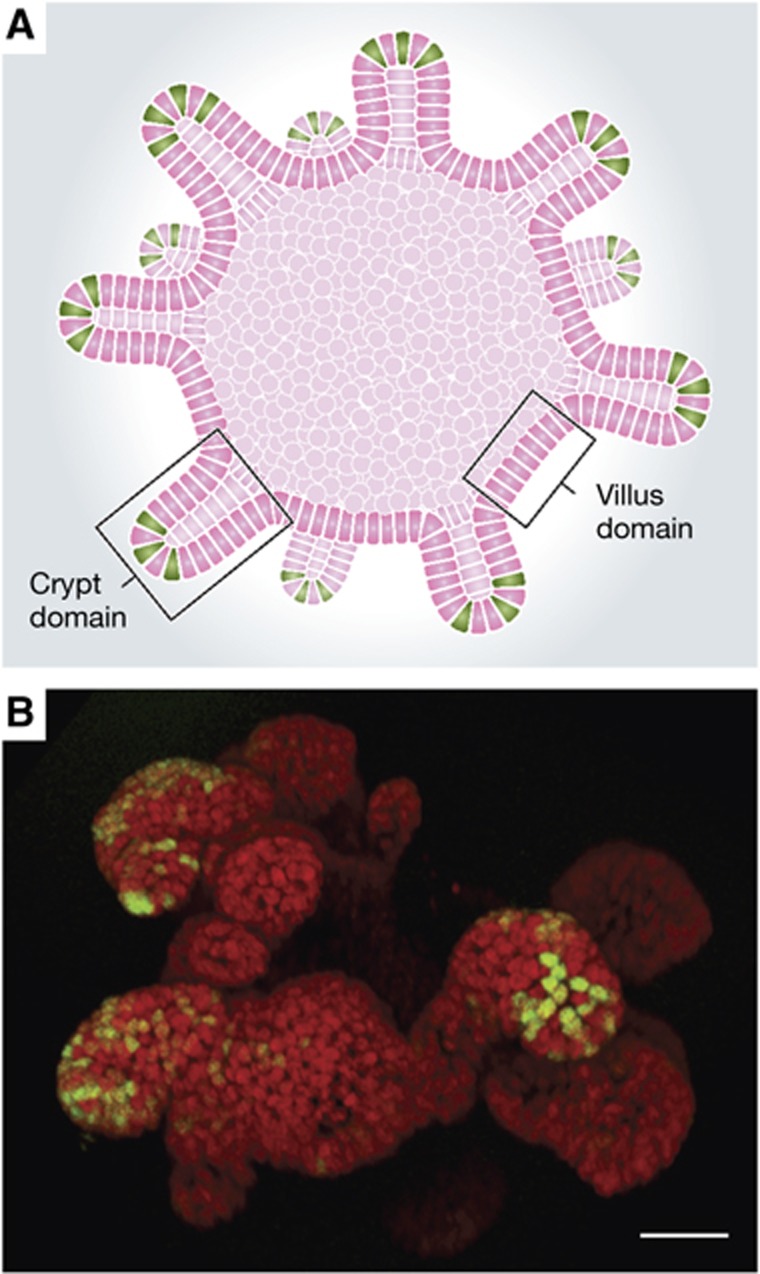
To further document the highly specific expression pattern of Lgr5, a knock-in genetic model was generated by insertion of a LacZ reading frame into the Lgr5 locus. This allele allowed the visualization of rare scattered Lgr5+ cells a.o. in the eye, brain, hair follicle, mammary gland, reproductive organs, stomach and intestinal tract (Barker et al, 2007). A second Lgr5 allele was then engineered to drive expression of GFP and CreERT2 in the CBC cells. While the GFP was useful in verifying the expression pattern of Lgr5, lineage tracing using the inducible CreERT2 protein provided crucial evidence for CBC stemness. The Lgr5 knock-in allele was crossed into a reporter mouse that carries a LacZ expression cassette inserted into the ubiquitously expressed Rosa26 locus (Soriano, 1999). This would allow ubiquitous LacZ expression, if not for the presence of a transcriptional roadblock inserted in front of the LacZ reading frame. This roadblock is flanked by loxp sites, thus allowing removal by the Cre enzyme. Once the roadblock is removed, the LacZ enzyme is expressed in a given CBC cell, but also in all progeny that this cell might generate afterwards. One day after Cre induction, only single Lgr5+ cells at the very bottom of the crypt expressed Rosa26-LacZ. Analysis at different time points after induction revealed rapid multiplication of the CBCs and their progeny. Within 5 days, marked ‘ribbons’ spanning the entire crypt–villus axis were formed (Barker et al, 2007). Morphological and marker expression studies revealed that all differentiated cell types in the intestinal epithelium originated from Lgr5+ CBC cells, that is, enterocytes, paneth cells, goblet cells, enteroendocrine cells, tuft cells (Gerbe et al, 2011) and M-cells (Figure 2). Lineage tracing persisted over the lifetime of the animal, thus demonstrating that Lgr5+ stem cells are long-lived and multipotent.
Figure 2.
Stem cell compartment of the small intestine and its cell types. Lgr5+ CBC stem cells reside in the bottom of the crypt and push their progeny up the villus.
Classically, stem cells are most often defined as being multipotent, long-lived, slow cycling/quiescent and asymmetrically dividing. While Lgr5+ cells are long-lived and multipotent, they divide once every 24 h (Barker et al, 2007; Schepers et al, 2011). Additionally, Schepers et al (2011) observed that stem cells do not segregate their DNA asymmetrically (Escobar et al, 2011). In order to achieve homeostasis, how is the balance between stem cell self-renewal and differentiation regulated?
The intestinal niche
Paneth cells, producers of a variety of bactericidal products (Wilson et al, 1999; Ayabe et al, 2000; Salzman et al, 2010), are intermingled with the Lgr5 stem cells (Figure 1D). Sato and colleagues investigated if these cells represent the niche cells for the Lgr5 stem cells (Sato et al, 2011b). Several lines of evidence indicate that Paneth cells serve as the stem cell niche. Ablation of Paneth cells by mutation of Gfi1 (Shroyer et al, 2005), transgenic expression of diphtheria toxin A under the Paneth-cell-specific cryptdin 2 promoter (Garabedian et al, 1997) or by conditional deletion of Sox9 (Bastide et al, 2007; Mori-Akiyama et al, 2007) induced a severe reduction in stem cell numbers and proliferation. Low numbers of residual Paneth cells were observed in all three approaches. In vitro culture of Lgr5 stem cells confirmed their dependence on adjacent Paneth cells (Sato et al, 2011b).
Which niche signals are provided by Paneth cells? Microarraying revealed expression of Wnt3, Egf and Tgfα, and of the Notch ligands Dll1 and Dll4. Genetic removal of Wnt3 has no effect in vivo, but abruptly blocks the Lgr5 stem cells in in vitro organoid culture. Thus, extraepithelial sources of Wnt contribute to the maintenance of Lgr5 stem cells. Given that Notch receptors and Delta ligands are membrane-bound, it would appear that only the neighbouring Paneth cells can maintain active Notch signalling in Lgr5 stem cells. Inhibition of Notch signalling activates expression of Math1/ATOH1 in progenitor cells, which drives the cells into the secretory lineage and inhibits proliferation (van Es et al, 2005; Riccio et al, 2008). This effect is also seen in Lgr5 stem cells (van Es et al, 2010; Pellegrinet et al, 2011). In a recent study, Kim et al (2012) used conditional deletion of the Notch-repressed target gene Math1/ATOH1 to completely eliminate Paneth cells. Unexpectedly, they observed that stem cells normally proliferated in these Paneth-less mice, yet exclusively generated cells of the enterocyte lineage (Kim et al, 2012). We propose that—by removing the pivotal differentiation factor Math1—Kim et al removed their dependence on Notch signals, which in effect renders Math1-mutant Lgr5 stem cells independent of the neighbouring Paneth cells. Similarly, mice that have a constitutively activated Kras allele lack Paneth cells, but retain stem cells. The Kras activation also activates expression of the transcriptional repressor Hes1, which in turn prohibits Math1 expression and Paneth cell differentiation (Feng et al, 2011). So, while Paneth cells do not supply all the stimuli needed for stem cells to survive, they are certainly crucial in providing many niche signals, permitting stemness at the bottom of the crypt.
The niche as a permissive zone for stemness allows for a simple model of homeostatic self-renewal in the crypt. Symmetrically dividing stem cells continuously fill the limited niche space, while stem cells that are ‘evicted’ populate the TA compartment to ultimately give rise to the individual lineages of the intestinal epithelium. Snippert et al (2010b) used multicolour lineage tracing to demonstrate this principle in the crypts of the small intestine. Examination of the dynamics of this process led to the notion that intestinal stem cells divide symmetrically such that every stem cell division gives rise to two equipotent daughters. These daughters can remain as stem cells or can become TA cells, depending on their location relative to the Paneth cell niche. This model of ‘neutral competition’ (that is, stem cells compete, but have equal chances to win) implies that longevity is an attribute of the stem cell population, but not of individual stem cells. The stem cell population size results directly from the size of the niche. Thus, the exact control of Paneth cell numbers at crypt bottoms is the central driver of homeostatic self-renewal.
Intestinal organoids
The generation of Lgr5-GFP mice has allowed purification of Lgr5 stem cells from the small intestine by fluorescence activated cell sorting (FACS). Isolated cells can be grown in a 3D culture system using Matrigel and Egf, Noggin and R spondin 1 (Rspo1) (Sato et al, 2009; Figure 3). R-spondins were known enhancers of low-dose Wnt signals (Kazanskaya et al, 2004). In vivo, Rspo1 strongly stimulates crypt proliferation (Kim et al, 2005). Cultured cells multiply indefinitely and form spheres with a central lumen and protruding buds, collectively called ‘miniguts’ or ‘organoids’. The central lumen of these organoids corresponds to the intestinal lumen with the surrounding epithelial cells tightly packed, properly polarized and fully differentiated. Differentiated cells ultimately apoptose and are shed into the lumen of the organoid. The protruding buds constitute crypt-like domains with Lgr5+ stem cells at the bottom interspersed with Lysozyme-positive, granulated Paneth cells. Rapidly dividing TA cells fill the remainder of these crypt-like structures. Complete organoids with a gut-like architecture and containing all epithelial cell types can be grown from a single Lgr5 stem cell. Establishment of culture conditions for colonic organoids allowed Yui et al (2012) to expand a single isolated colon Lgr5-GFP+ stem cell (additionally labelled with RFP) into millions of offspring, allowing engraftment into superficially damaged recipient colons of immunocompromised (rag2−/−) mice. Control animals lost weight due to the dextran sulphate sodium-induced colitis. Transplanted mice, however, displayed reduced weight loss. Closer examination showed successful engraftment of RFP-positive cultured cells. Grafted cells contributed to the epithelium, restored barrier function and harboured all differentiated cell types of the colon epithelium. Follow-up studies showed that—even at 25 weeks after transplantation—the grafts still contributed to the epithelium and showed no signs of adenomatous or dysplastic change.
Figure 3.
Cultured Lgr5+ stem cells form organoids. (A) Schematic representation of an organoid with the lumen corresponding to the intestinal lumen. (B) Organoid expressing GFP under the Lgr5 promoter.
This technology of ex vivo stem cell expansion may be clinically applied for regenerative medicine. Jung et al (2011) were able to enrich colonic stem cells from human biopsies using Ephrin type-B receptor 2 as a marker for stem cells. These cells could be cultured into spheroid structures for up to 3 months under modified culture conditions. Human colonic cultures require nicotinamide, prostaglandin E2 and Wnt3a in addition to the murine small intestinal culture conditions. The spheroid cultures largely consist of undifferentiated cells, however, and removal of Wnt3a and PGE2 as well as blocking the Notch pathway with a γ-secretase inhibitor is necessary to achieve differentiation. Further optimization of human colon culture conditions involved the addition of gastrin, an Alk4/5/7 inhibitor (A83-01) and a p38 inhibitor (SB202190) to the cultures in order to obtain budding organoids similar to those of murine origin (Sato et al, 2011a). Differentiation, however, still requires removal of nicotinamide, the inhibitors and Wnt as well as blocking Notch signalling.
Crypt base columnar stem cells are the cells of origin of intestinal cancer
The potential of the intestinal stem cells to generate large amounts of epithelium from a single cell begs the question if these cells lose their tight control on division rate, and transform into tumour cells. The role of the Wnt pathway and its inhibitor APC in intestinal cancer has been well documented (Korinek et al, 1997; Morin et al, 1997). Barker et al (2009) used the Lgr5 knock-in allele to delete the APC tumour suppressor in stem cells and observed rapid transformation. The transformed cells remained at the bottom of the crypts and gave rise to microadenomas with high levels of nuclear β-catenin. Within 3 to 5 weeks, these developed into macroscopic adenomas throughout the small intestine and colon. Deletion of APC in non-stem cells using a cytochrome p450-driven Cre enzyme induced with a limiting dose of β-naphtoflavone provided an essential control. Villus cells that were transformed by this regimen continued to travel upwards and died off within 4 to 5 days. Crypt cells that were hit by β-naphtoflavone also transformed, but persisted longer and were able to form microadenomas as well. These microadenoms rarely developed into macroscopic adenomas, however, and β-naphtoflavone-induced animals were followed for more than 9 months without significant progression. Similar results were reported using another CBC-specific Cre driver, Cd133-CreERT2 (Zhu et al, 2009).
Wnt signalling in stomach stem cells
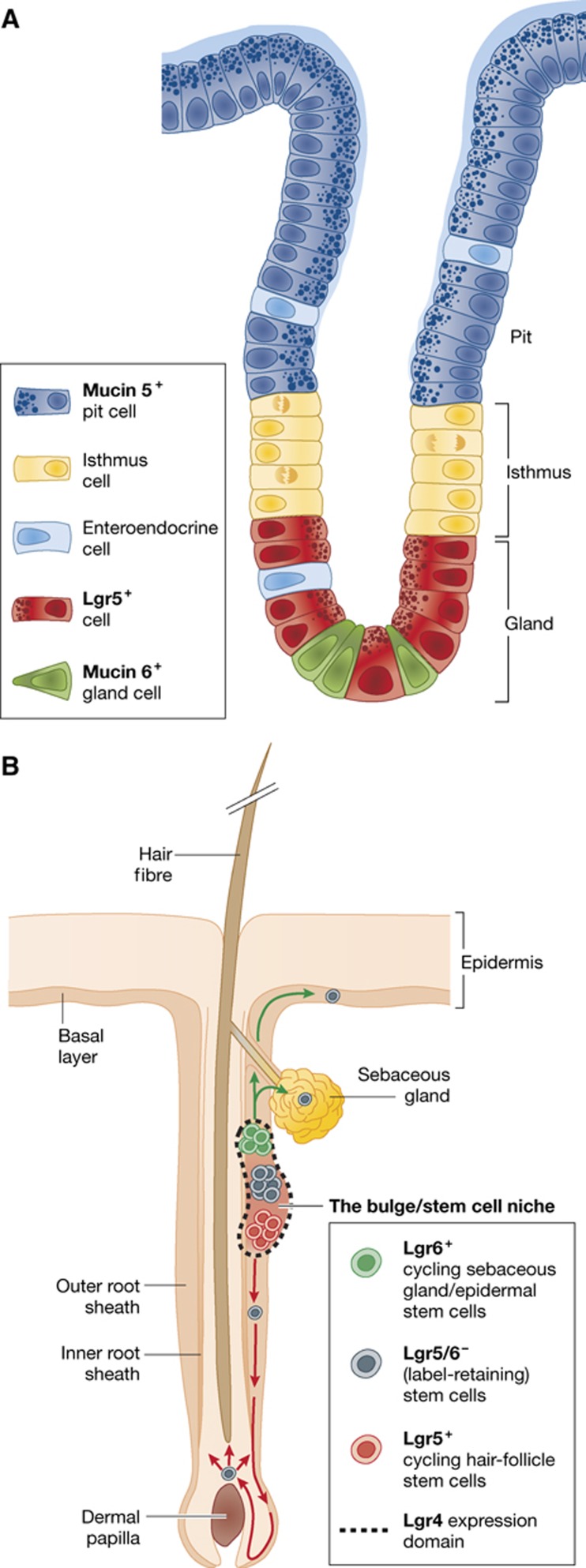
More rostrally in the gastrointestinal tract, Lgr5 marks stem cells in the pyloric region of the stomach. These cells reside at the bottoms of the epithelial glands. The gland, the regenerative unit of this tissue, is divided into three subcompartments: pit, isthmus and gland from top to bottom (Figure 4a). Lgr5 stomach stem cells residing at the gland bottom push their progeny upwards and into differentiation. Lineage tracing revealed that all differentiated stomach epithelial cell types originate from these Lgr5+ cells (Barker et al, 2010). As said, Lgr5-expressing cells only occur in the pylorus, the most distal segment of the stomach, at the junction with the duodenum. During the first weeks after birth, the stomach undergoes a process of morphogenetic rearrangement, maturing into a fully functional organ. When lineage tracing was initiated in neonates, cells from all parts of the stomach epithelium were found to originate from Lgr5-expressing cells. Over the first 4 postnatal weeks, Lgr5 expression gradually disappeared in the corpus of the stomach. Much like the situation in the intestine, Apc deletion (induced using Lgr5-CreERT2) resulted in the formation of Wnt pathway-driven stomach adenomas. Of note, the Lgr5+ cells from the pylorus can be isolated and cultured to form ever-expanding gastric organoids in vitro (Barker et al, 2010). In addition to the small intestinal culture conditions, these cells require Wnt3a and FGF10 to grow and form spheroid cultures with protruding buds. For the differentiated lineages to appear, Wnt3a must be removed from the culture conditions. So far, no human gastric organoid cultures have been reported.
Figure 4.
Stomach and hair follicle. (A) The gastric glands of the stomach are subdivided in the pit, isthmus and gland. Lgr5+ cells reside at the bottom and regenerate the epithelium. (B) Multiple stem cell populations are responsible for homeostasis of the adult skin.
Wnt signalling in the hair follicle
The skin is the largest organ in the mammalian body, allowing interaction with and providing protection from the surrounding world. The interfollicular epidermis and sebaceous gland constantly self-renew, while the hair follicles undergo cyclic changes of growth, involution and resting phases. Mouse hair follicle stem cells are thought to reside in the hair follicle bulge (Cotsarelis et al, 1990) and are characterized by expression of the CD34 cell-surface marker (Trempus et al, 2003), expression of cytokeratin 15 (Morris et al, 2004) and retention of either DNA or histone-labels over long periods (Cotsarelis et al, 1990; Tumbar et al, 2004). Keratinocytes with stem cell properties have also been isolated from other areas of the hair follicle (Ito et al, 2004; Tumbar et al, 2004; Figure 4B).
Wnt signalling provides one of the mitogenic stimuli that are necessary for hair growth (Gat et al, 1998; Niemann et al, 2003; Nguyen et al, 2009) and wound healing (Okuse et al, 2005; Cheon et al, 2006; Fathke et al, 2006). β-Catenin stabilizing mutations cause pilomatricomas and trichofolliculomas (Chan et al, 1999). Keratin 14 (K14) promoter-driven transgenic expression of stabilized B-catenin induces de novo hair growth and the formation of extra skin (Gat et al, 1998). Transient β-catenin stabilization in adult animals induced lesions, but only continuous β-catenin accumulation was sufficient to maintain hair follicle tumours (Celso et al, 2006). Conversely, removal of β-catenin in K14-expressing cells rescued chemically induced papillomas (Malanchi et al, 2008) and injection of the soluble Wnt inhibitor DKK1 into the hypodermis of mice prematurely induced the involution phase (catagen) and shortened hair follicles (Kwack et al, 2012). In tumours of the sebaceous gland, inactivating mutations of the Tcf family member LEF1 were found (Takeda et al, 2006), and indeed transgenic expression of an N-terminally truncated form of Lef1 (ΔNLef1)—which is no longer able to transactivate with β-catenin, but still occupies DNA—induced sebaceous tumours (Niemann et al, 2002; Niemann et al, 2007). The counter-intuitive tumour activating effect of ΔNLef1 is explained by the lack of Wnt-driven ARF activation and the resulting absence of the p53 tumour suppressor protein, rendering cells more sensitive to transforming mutations. Complete removal of two other members of the Tcf/Lef family, Tcf3 and Tcf4, in K14-expressing cells resulted in reduced thickness of the skin and reduced hair growth (Nguyen et al, 2009).
Given that Wnt signalling is implied in hair follicle stem cell biology, it came as no surprise that active stem cells in the bulge express Lgr5. Indeed, at different stages of the hair cycle, Lgr5 expression was observed in several distinct locations in the hair follicle. During the growth phase (anagen), Jaks et al (2008) detect Lgr5 expression in cells of the outer root sheath. They also observed expression in cells, which link the mesenchymal component of the hair follicle (the dermal papilla) with the bulge area when hair follicles are in the destructive (catagen) phase and cells of the bulge region during the resting phase (telogen). These bulge cells initiate rapid cycling at the initiation of the hair growth phase. By lineage tracing, Lgr5+ cells generated all lineages of the hair follicle. FACS profiling of these Lgr5+ cells showed that 80% also expresses the previously identified stem cell marker CD34, yet they only constitute part of the total CD34+ population. Sorted Lgr5+ cells could be transplanted to form a complete new hair follicle in recipient nude mice, where they outperformed the mixed CD34+ population.
Lgr6 expression in the skin
Lgr6 is a close relative of Lgr5 and—like Lgr5—is expressed in rare cells in multiple organs. Snippert et al (2010a) identified a population of Lgr6-expressing cells in the hair follicle (Figure 4b). Using Lgr6-LacZ and Lgr6-GFP-IRES-CreERT2 alleles, Lgr6 expression was detected in the isthmus part of the hair follicle. Expression profiling after FACS revealed this population of cells to be distinct from the Lgr5-marked one. Lineage tracing initiated in animals indicated that Lgr6 progeny contributes to the sebaceous gland, interfollicular epidermis as well as the hair, particularly when lineage tracing was started at embryonic stage 17.5. Like Lgr5+ cells, transplanted Lgr6+ cells could generate entire, new hair follicles. When lineage tracing was initiated prior to wound formation, Lgr6 cells were able to contribute to the newly generated epidermis over long periods of time, as well as to newly formed hair follicles.
Common traits of Lgr-marked stem cells
The tissues where Lgr5 and -6 have thus far been identified as markers of constitutively active stem cells share key characteristics. They are all rapidly proliferating epithelia, in which the niche allows unidirectional displacement of daughter cells. They are known to be dependent on Wnt signals (of note, Lgr5 is a Wnt target gene). And tumours arising from the pertinent tissues are often driven by mutationally activated Wnt signals. However, Lgr5 expression is not restricted to the stem cells in the stomach, small intestine, colon and hair follicle, but also marks cells a.o. in the mammary gland, brain and—upon damage—in the pancreas and liver (Barker et al, 2007). The role of these cells is the subject of ongoing research. The highly specific expression of the Lgr family members in stem cells allows for identification and characterization of these cells, but also begs the question if they play an important function in stem cells. What are these leucine-rich receptors and what do they do?
Structure of the Lgr4, Lgr5 and Lgr6 receptors
In 1998, Hsueh and colleagues cloned two molecules that were related to the hormone receptors for thyroid-stimulating hormone (TSH), follicle-stimulating hormone (FSH) and leutinizing hormone (LH) (Hsu et al, 1998). These hormone receptors belong to the large, G-protein–coupled, 7-transmembrane (7TM) family of proteins. They are unique in that they have a large N-terminal extracellular (ecto-) domain that contains a series of leucine-rich repeats. In the LH, FSH and TSH receptor molecules, the ectodomain is crucial for binding of the glycoprotein hormones. The two novel G-protein–coupled receptors were termed leucine-rich repeat containing, G-protein–coupled receptors 4 and 5 (Lgr4 and Lgr5). In 2000, the same investigators identified a third member of this subfamily, Lgr6 (Hsu et al, 2000). These three receptors are of ancient evolutionary origin, as homologous proteins are found in invertebrates including sea anemone (Nothacker and Grimmelikhuijzen, 1993), mollusk (Herpin et al, 2004; Tensen et al, 1994), the nematode Caenorhabditis elegans (Kudo et al, 2000) and Drosophila melanogaster (Hauser et al, 1997; Nishi et al, 2000). The ectodomains of Lgr4, Lgr5 and Lgr6 consist of a central array of multiple leucine-rich repeats (18 in Lgr4 and Lgr5, and 13 in Lgr6) that are flanked by N- and C-terminal cysteine-rich sequences. In comparison, only nine leucine-rich repeats are found in the glycoprotein hormone receptors. The leucine-rich repeats of Lgr4, Lgr5 and Lgr6 each consist of 24 amino acids and show similarity to repeats found in functionally unrelated proteins such as slit, decorin, and Toll (Barker and Clevers, 2010). The junctions between the ectodomain and the first transmembrane region, as well as the rhodopsin-like 7TM domains, are highly conserved between Lgr4, Lgr5 and Lgr6. In the glycoprotein hormone receptors, ligand-induced recognition and activation steps are performed by separate domains of the proteins. Binding of the cognate hormones involves the leucine-rich N-terminal ectodomain, which induces a conformational change in the receptor that allows the ectodomain to activate the rhodopsin-like 7TM region of the receptor.
Function of Lgrs
Lgr4 mutation has pleiotropic effects
A gene-trap screen identified a mouse in which the Lgr4 gene was disrupted by insertion of a galactosidase LacZ fusion protein and placental alkaline phosphatase. This allowed Van Schoore et al (2005) to accurately evaluate the expression pattern of Lgr4. A broad expression pattern was noted, with particularly strong activity in cartilage, heart, hair follicles, kidneys, reproductive tracts and the nervous system cells. Thus, Lgr4 is expressed broadly within proliferative compartments, but definitely is not restricted to rare stem cells within such compartments. Using a different model with β-galactosidase inserted in the Lgr4 locus, Mazerbourg et al (2004) observed that only 40% of Lgr4-null mice were born. Most of these died within the first 2 days after birth, displaying a pleiotropic phenotype (Mazerbourg et al, 2004). All Lgr4-null embryos displayed intrauterine growth retardation. After crossing this allele into a CD1 background, Mendive et al (2006) reported that homozygous mice survived to adulthood. Lgr4-knockout male mice were infertile, tube elongation did not occur in the male reproductive tract. The investigators concluded that these reproductive tract defects resulted from a loss of Lgr4 regulation of c-AMP-dependent oestrogen receptor activity (Li et al, 2010). Song et al (2008) described the severe effects on the anterior segment structure of the eye, which included microphthalmia and cataracts (Weng et al, 2008). Lgr4 mutant embryos of this strain displayed abnormal definitive erythropoiesis and delayed osteoblast differentiation and mineralization (Luo et al, 2009).
An Lgr4 hypomorph was generated by Hoshii et al (2007). The homozygous mutant mice expressed 10% of normal levels of Lgr4 messenger RNA and 60% survived to adulthood. The male mice were also infertile, displaying morphological abnormalities similar to those of the Lgr4-knockout mice. Hypomorphic embryos developed a normal gall bladder bud, but it did not undergo elongation beyond midgestation. These mice did not develop a gall bladder or cystic duct, although there were no discernable effects on liver or pancreas development (Yamashita et al, 2009).
Kato et al (2006) generated mice with a conditional knockout allele that facilitated inducible deletion of the exon encoding the 7TM domain of Lgr4. Embryonic deletion of this exon using mice expressing Cre in all tissues resulted in embryonic/perinatal lethality. Neonatal Lgr4-null mice displayed renal hypoplasia. Jin et al (2008) (Kato et al, 2007) observed that mutant pups were born with opened eyes, an abnormality that might result from a defect in motility of keratinocytes in the skin. They confirmed this eye-open phenotype by conditional deletion of Lgr4 in the skin. These Lgr4 KO mice survived to adulthood, with sparse head hair and focal alopecia behind their ears. Lgr4fl/fl mice showed similar abnormalities in hair follicles (Mohri et al, 2008).
Taken together, Lgr4 appears to be involved in the development of a wide variety of embryonic tissues. However, very little was known about its function in adult animals or the pathway by which Lgr4 was able to influence this wide variety of developmental processes.
Lgr5 and -6
Not much was known about mammalian Lgr5 before 2007. Lgr5/Gpr49 is a Wnt target gene as well as a cancer gene; it was on the original list of Wnt/Tcf4 targets active in colorectal cancers (van de Wetering et al, 2002) and is overexpressed in tumours of the ovary and liver, likely because of mutational activation of the Wnt pathway in these tumours (Yamamoto et al, 2003; McClanahan et al, 2006; Zucman-Rossi et al, 2007). Additionally, Lgr5 expression was observed in basal cell carcinomas (Tanese et al, 2008) and in healthy cyclic endometrium (Krusche et al, 2007). The phenotype of a mouse mutant for Lgr5 was published by Morita et al (2004). This strain harbours a lacZ reporter gene just N-terminal to the region of Lgr5 that encodes the first transmembrane domain, essentially creating a null allele (Morita et al, 2004). Homozygous disruption of Lgr5 resulted in neonatal lethality, characterized by ingestion of air at birth, in gastrointestinal tract dilation and the absence of milk from the stomach. Macroscopic and histological examination revealed fusion of the tongue to the floor of the oral cavity in newborns, a condition called ankyloglossia. Lgr5 was found to be expressed in the epithelium of the tongue and in the mandible of wild-type embryos. The observed phenotype indicated that Lgr5 is an essential gene, yet the lethal neonatal phenotype precluded the study of the role of Lgr5 in adult tissues. Garcia et al (2009) observed that the same Lgr5-null strain also had accelerated maturation of Paneth cells in the developing intestine. They proposed a role for Lgr5 in negatively regulating Wnt signalling during neonatal development of the intestine.
In a study from de Lau et al (2011) compound mutant mice were described conditionally deleting either Lgr4, 5 or both Lgrs in the intestinal epithelium. Deletion of Lgr5 in adult animals revealed no phenotype, while deletion of Lgr4 reduced the proliferation in intestinal crypts and led to crypts being disconnected from the epithelium, leaving the long-lived paneth cells in isolated nests. The combined removal of Lgr4 and 5 aggravated this phenotype, severely disrupting crypts and halting villus repopulation, which eventually led to the death of the animal. Finally, homozygous mutant Lgr6 mice have no apparent phenotype (Snippert et al, 2010a).
Until recently, Lgr4/5/6 were designated orphan receptors in the absence of a known ligand. In 2011, three groups reported in separate studies that R-spondins are the ligands for the stem-cell-specific Lgrs (below).
R-spondins
R(oof plate-specific) spondin 1 was initially observed to be expressed in the neural tube at the boundary between the roof plate and the neuroepithelium 10 and 12 dpc (Kamata et al, 2004). Later, three more R-spondins were identified (Chen et al, 2002; Kazanskaya et al, 2004; Kim et al, 2006). Homologues of the R-spondins are found in all vertebrates, but not in invertebrates such as D. melanogaster or C. elegans (de Lau et al, 2012). These excreted proteins are characterized by two Furin-like cysteine-rich repeats near the N terminus of the mature protein, a Thrombospondin type I repeat in the centre of the protein, and a positively charged C-terminal region. The Furin-like repeats are also found in growth factor receptors like the EGF, insulin, hepatocite growth factor and neurotrophic factor receptors. The thrombospondin domain is in turn implicated in glycosaminoglycan/proteoglycan binding. Indeed, Ohkawara et al (2011) recently observed Rspo3 binding to the transmembrane proteoglycan Syndecan 4 in Xenopus embryos, activating non-canonical Wnt signalling through endocytosis of Syndecan 4.
R-spondins are able to synergize with the Wnt pathway, enhancing Wnt signalling only in the presence of canonical Wnt ligands (Kazanskaya et al, 2004; Kim et al, 2008; Li et al, 2009). As such, their effect can be blocked by the extracellular Wnt receptor inhibitor DKK1 (Kazanskaya et al, 2004). The ability to potentiate Wnt signalling suggested that R-spondins may be pivotal during many of the Wnt-dependent stages of development.
R-spondin 1
In human the sex of an embryo depends on the presence or absence of a Y chromosome. Genetic males (XY) form testes that in turn produce steroid hormones that drive phenotypic sex determination. However, if the testes do not form properly and these hormones are lacking, default female development occurs. This is called XY sex conversion. The opposite, XX sex conversion, is rare and usually caused by translocation of the Y-chromosomal SRY gene (that is responsible for male development) to an X chromosome (Koopman et al, 1991). In some cases, XX sex conversion can have another cause: Dax1 Foxl2 and Wnt4 have been implicated in (the lack of) female gonad development (Brennan and Capel, 2004; Ross and Capel, 2005; Yao, 2005; Wilhelm and Koopman, 2006). Through studying two Italian consanguineous families Parma et al (2006) identified disruption of the Rspo1 gene in sex-converted individuals. Careful expression analysis then revealed Rspo1 to be specifically activated in the female gonads starting at E12.5 in mouse embryos. In situ hybridization further revealed Rspo1 expression in the developing kidney, dermis and later in the adult dermal papilla of the hair in addition to the previously observed Rspo1 expression in the roof plate and various parts of the developing brain. Analysis of Rspo1−/− mice confirmed the sex reversal phenotype (Tomizuka et al, 2008), which is also observed in Wnt4−/− animals (Vainio et al, 1999). In conclusion, activation of Wnt signalling through Wnt4/Rspo1 appears to be necessary for proper ovary development.
In a separate study Kim et al (2005) used a knock-in approach that allows B-cell restricted transgene expression under control of an immunoglobulin-κ promoter to ectopically express Rspo1 in mice. They observed a massive enlargement of the gastrointestinal tract, in particular of the small intestine and colon. Rspo1 induced hyperproliferation in these compartments, leading to a doubling in diameter and weight of the small intestine and a 25% increase in length of the colon of these animals. Hyperproliferating cells were observed in enlarged crypts that also exhibited increased nuclear β-catenin levels.
R-spondin 2
During limb development, two key structures, the apical ectodermal ridge (AER) and the zone of polarizing activity, control proximal–distal outgrowth and anterior–posterior patterning, respectively. Bell et al (2008) described the Footless mutant that expresses a hypomorphic variant of the Rspo2 gene. These animals immediately die after birth and display severe malformation of the forelimbs and stunted growth of the hindlimbs. Rspo2 expression occurs in the AER of wild-type animals. Indeed, several of the Wnt-driven markers for the AER were not expressed in the absence of Rspo2 (Aoki et al, 2007; Nam et al, 2007). The researchers also used a TOPGAL reporter mouse line to evaluate the Wnt activation in Rspo2-affected tissues and observed a severe reduction in Wnt activation in the AER.
In addition to the limb phenotype, Bell et al (2008) reported malformed cartilage of the trachea and hypomorphic lungs in their Footless mutants. In order to investigate the link with the Wnt pathway, these animals were crossed with Lrp6−/− mice reducing Wnt signalling to a minimum. Removal of Lrp6 increased the penetrance of the limb phenotypes and aggravated the trachea and lung phenotypes (Bell et al, 2008).
In a genetic study surveying >180 dog breeds, Cadieu et al (2009) identified Rspo2 as one of the three genes that together determine the structure of a dog’s coat. Single-nucleotide polymorphism mapping revealed Fgf5 and Krt71 as the other two of the triad that defines coat length, curliness and ‘furnishings’ (the growth pattern marked by a moustache and eyebrows). An insertion in the 3′UTR of the Rspo2 gene, in particular dog breeds, induced a threefold increased expression that in turn induced characteristic extra hair growth on the muzzle of these animals.
R-spondin 3
There are three critical events in the development of the mouse placenta. First, chorioallantoic fusion takes place on embryonic day E8.5. Second, allantoic mesoderm and underlying fetal blood vessels fuse with chorionic trophoblast cells forming simple branches around E9.0. Third, further chorioallantoic branching results in a functional labyrinth with highly branched villi, which enable effective exchange of gases, nutrients and waste products between mother and fetus. R-spondin 3 is expressed in early embryogenesis in a complex pattern, including the brain, neural tube, tail, heart, somites and limbs. Aoki et al (2007) deleted Rspo3 and observed that chorioallantoic fusion occurs normally, but there was no penetration of the fetal blood vessels into the chorion or chorioallantoic branching, phenocopying Fzd5−/− mice (Ishikawa et al, 2001). Kazanskaya et al (2008) also observed that removal of R-spondin 3 by morpholino in Xenopus or genetic deletion in mouse hampered angiogenesis in the placenta. They further identified lack of Vegf induction in the placenta as the cause for the lack of angiogenesis, and showed that Vegf expression was controlled by R-spondin 3/Wnt in the placenta.
R-spondin 4
Lack of toe and fingernails, Anonychia, is considered to be a mild disorder. It is usually observed as a feature of autosomal-dominant inherited syndromes like Coffis-Siris syndrome or Nail-Patella syndrome. In rare cases it is an isolated disorder that follows an autosomal-recessive inheritance pattern. The Rspo4 gene was found to be mutated in studies of anonychial individuals of European as well as Asian descent (Blaydon et al, 2006; Bruchle et al, 2008; Ishii et al, 2008). Together, these studies identified 10 mutations: 4 in the first and 4 in the second furin domain of Rspo4, as well as 2 splice site mutations. Li et al (2009) mutated the corresponding cysteine residues in the furin domains of R-spondin 2 and tested them in vitro. The mutations affected R-spondin secretion, most likely due to the inability to form necessary cysteine bridges.
R-spondins are the ligands for Lgr4, 5 and 6
After expression profiling of intestinal crypts in the Lgr4/Lgr5 compound knockout model, de Lau et al (2011) noticed that the gene programme that is lost upon deletion of the Lgrs largely coincides with the gene programme that is activated upon Wnt overactivation by APC deletion (Sansom et al, 2007) . This apparent regulation of Wnt target genes by Lgrs prompted further investigation of a possible Wnt interaction. Co-immunoprecipitations of the surface Wnt pathway components Frizzeld5/7 and Lrp5/6 with Lgr4 and 5 provided further evidence for a functional link. Precipitation of tagged Rspo1 after incubation with Lgr-expressing cells revealed Lgr4, 5 and 6 to be the only transmembrane interactors in HEK293 or Ls174 cells. Lgr family members 1, 3, 7, 8 were also tested, but were found not to bind R-spondins. Similarly, Carmon et al (2011) observed that Fc-tagged Rspo1 bound to the membrane of cells expressing Lgr4 or 5, and found that Lgr’s and R-spondins were internalized together. Depletion of Lgrs abrogated the synergistic effects of R-spondins on Wnt signalling. Conversely, organoid lethality upon the loss of Lgr4 and 5 was rescued by activating the Wnt pathway downstream of Lgrs using the Gsk3β inhibitor CHIR99021. Thus, all four R-spondins bind to Lgr4, Lgr5 and Lgr6 and enhance Wnt signalling.
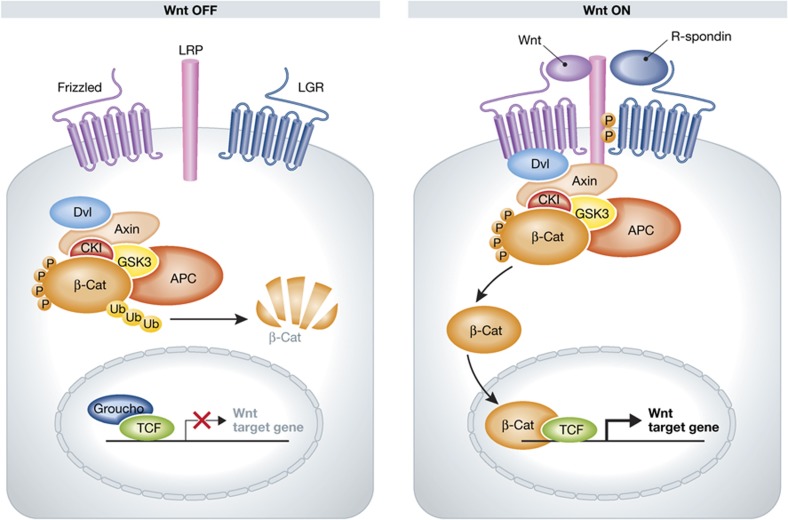
What intracellular processes mediate this signal? G-proteins were the first candidates to be extensively tested monitoring the stimulation/inhibition of cAMP production, Ca2+ mobilization and β-arrestin. No activation was observed using any combination of R-spondins and Lgrs (Carmon et al, 2011). The interaction with Lrp allows for a model in which R-spondin/Lgr directly stimulates the Wnt pathway without further separate intracellular signalling. Indeed, Carmon et al (2011) observed loss of R-spondin-induced Lrp6 phosphorylation upon removal of Lgr5. However, overexpression of Lgr5 in this system did not lead to an increase in Lrp6 phosphorylation. Instead, it limited maximal phosphorylation and the duration of the stimulation; that is, β-catenin stabilization. Glinka et al (2011) showed that the internalization of Rspo3 with Lgr4 is clathrin-dependent and imply Lgr4 and 5 in Wnt/PCP signalling during Xenopus embryogenesis. In conclusion, R-spondins synergise with Wnt signalling through the Lgr family members 4, 5 and 6. The mechanisms involved in this activation are unclear, but Lgrs are found to physically interact with Lrp5/6 and Frizzelds, placing them at the heart of the receptor complex for Wnt ligands (Figure 5).
Figure 5.
Lgrs in the Wnt pathway. Protein interaction data show that the Lgrs are in direct contact with Lrp5/6 and Frizzelds, placing them in the heart of the receptor complex for Wnt ligands.
Future directions
The Lgr receptor family has proven to be a useful tool in identifying a multitude of adult stem cell populations throughout epithelium of the mammalian body. The specific expression of Lgr5 and 6 by adult stem cells reinforces the notion that Wnt signalling and adult stem cell biology are closely linked. Moreover, it appears now possible to exploit this Lgr5/Wnt axis ex vivo by Rspo-based 3D culture technologies for multiple adult tissue types. These organoid cultures allow detailed studies of adult stem cells in their tissue of origin, but in isolation from confounding phenomena such as adjacent tissues, the immune system or the microbiome. Wnt/Rspo-based organoid culture also holds promise in the realm of regenerative medicine. In particular, the fact that single stem cells can be extensively expanded outside the body allows gene therapy-type approaches.
Acknowledgments
We thank Inge de Vries for helping with preparation of the figures.
Footnotes
The authors declare that they have no conflict of interest.
References
- Aoki M, Mieda M, Ikeda T, Hamada Y, Nakamura H, Okamoto H (2007) R-spondin3 is required for mouse placental development. Dev Biol 301: 218–226 [DOI] [PubMed] [Google Scholar]
- Ayabe T, Satchell DP, Wilson CL, Parks WC, Selsted ME, Ouellette AJ (2000) Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol 1: 113–118 [DOI] [PubMed] [Google Scholar]
- Barker N, Clevers H (2010) Leucine-rich repeat-containing G-protein-coupled receptors as markers of adult stem cells. Gastroenterology 138: 1681–1696 [DOI] [PubMed] [Google Scholar]
- Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H, van den Born M, Danenberg E, van den Brink S, Korving J, Abo A, Peters PJ, Wright N, Poulsom R, Clevers H (2010) Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 6: 25–36 [DOI] [PubMed] [Google Scholar]
- Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H (2009) Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 457: 608–611 [DOI] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H (2007) Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007 [DOI] [PubMed] [Google Scholar]
- Bastide P, Darido C, Pannequin J, Kist R, Robine S, Marty-Double C, Bibeau F, Scherer G, Joubert D, Hollande F, Blache P, Jay P (2007) Sox9 regulates cell proliferation and is required for Paneth cell differentiation in the intestinal epithelium. J Cell Biol 178: 635–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell SM, Schreiner CM, Wert SE, Mucenski ML, Scott WJ, Whitsett JA (2008) R-spondin 2 is required for normal laryngeal-tracheal, lung and limb morphogenesis. Development 135: 1049–1058 [DOI] [PubMed] [Google Scholar]
- Blaydon DC, Ishii Y, O'Toole EA, Unsworth HC, Teh MT, Ruschendorf F, Sinclair C, Hopsu-Havu VK, Tidman N, Moss C, Watson R, de Berker D, Wajid M, Christiano AM, Kelsell DP (2006) The gene encoding R-spondin 4 (RSPO4), a secreted protein implicated in Wnt signaling, is mutated in inherited anonychia. Nat Genet 38: 1245–1247 [DOI] [PubMed] [Google Scholar]
- Brennan J, Capel B (2004) One tissue, two fates: molecular genetic events that underlie testis versus ovary development. Nat Rev Genet 5: 509–521 [DOI] [PubMed] [Google Scholar]
- Bruchle NO, Frank J, Frank V, Senderek J, Akar A, Koc E, Rigopoulos D, van Steensel M, Zerres K, Bergmann C (2008) RSPO4 is the major gene in autosomal-recessive anonychia and mutations cluster in the furin-like cysteine-rich domains of the Wnt signaling ligand R-spondin 4. J Invest Dermatol 128: 791–796 [DOI] [PubMed] [Google Scholar]
- Cadieu E, Neff MW, Quignon P, Walsh K, Chase K, Parker HG, Vonholdt BM, Rhue A, Boyko A, Byers A, Wong A, Mosher DS, Elkahloun AG, Spady TC, Andre C, Lark KG, Cargill M, Bustamante CD, Wayne RK, Ostrander EA (2009) Coat variation in the domestic dog is governed by variants in three genes. Science 326: 150–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmon KS, Gong X, Lin Q, Thomas A, Liu Q (2011) R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta-catenin signaling. Proc Natl Acad Sci USA 108: 11452–11457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celso B, Tepas J, Langland-Orban B, Pracht E, Papa L, Lottenberg L, Flint L (2006) A systematic review and meta-analysis comparing outcome of severely injured patients treated in trauma centers following the establishment of trauma systems. J Trauma 60: 371–378discussion 378 [DOI] [PubMed] [Google Scholar]
- Chan EF, Gat U, McNiff JM, Fuchs E (1999) A common human skin tumour is caused by activating mutations in beta-catenin. Nat Genet 21: 410–413 [DOI] [PubMed] [Google Scholar]
- Chen JZ, Wang S, Tang R, Yang QS, Zhao E, Chao Y, Ying K, Xie Y, Mao YM (2002) Cloning and identification of a cDNA that encodes a novel human protein with thrombospondin type I repeat domain, hPWTSR. Mol Biol Rep 29: 287–292 [DOI] [PubMed] [Google Scholar]
- Cheng H, Leblond CP (1974a) Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. I. Columnar cell. Am J Anat 141: 461–479 [DOI] [PubMed] [Google Scholar]
- Cheng H, Leblond CP (1974b) Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am J Anat 141: 537–561 [DOI] [PubMed] [Google Scholar]
- Cheon SS, Wei Q, Gurung A, Youn A, Bright T, Poon R, Whetstone H, Guha A, Alman BA (2006) Beta-catenin regulates wound size and mediates the effect of TGF-beta in cutaneous healing. FASEB J 20: 692–701 [DOI] [PubMed] [Google Scholar]
- Cotsarelis G, Sun TT, Lavker RM (1990) Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 61: 1329–1337 [DOI] [PubMed] [Google Scholar]
- de Lau W, Barker N, Low TY, Koo BK, Li VS, Teunissen H, Kujala P, Haegebarth A, Peters PJ, van de Wetering M, Stange DE, van Es JE, Guardavaccaro D, Schasfoort RB, Mohri Y, Nishimori K, Mohammed S, Heck AJ, Clevers H (2011) Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature 476: 293–297 [DOI] [PubMed] [Google Scholar]
- de Lau W, Snel B, Clevers HC (2012) The R-spondin protein family. Genome Biol 13: 242–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar M, Nicolas P, Sangar F, Laurent-Chabalier S, Clair P, Joubert D, Jay P, Legraverend C (2011) Intestinal epithelial stem cells do not protect their genome by asymmetric chromosome segregation. Nat Commun 2: 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathke C, Wilson L, Shah K, Kim B, Hocking A, Moon R, Isik F (2006) Wnt signaling induces epithelial differentiation during cutaneous wound healing. BMC Cell Biol 7: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Bommer GT, Zhao J, Green M, Sands E, Zhai Y, Brown K, Burberry A, Cho KR, Fearon ER (2011) Mutant KRAS promotes hyperplasia and alters differentiation in the colon epithelium but does not expand the presumptive stem cell pool. Gastroenterology 141: 1003-1013e1001–e1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fevr T, Robine S, Louvard D, Huelsken J (2007) Wnt/beta-catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Mol Cell Biol 27: 7551–7559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garabedian EM, Roberts LJ, McNevin MS, Gordon JI (1997) Examining the role of Paneth cells in the small intestine by lineage ablation in transgenic mice. J Biol Chem 272: 23729–23740 [DOI] [PubMed] [Google Scholar]
- Garcia MI, Ghiani M, Lefort A, Libert F, Strollo S, Vassart G (2009) LGR5 deficiency deregulates Wnt signaling and leads to precocious Paneth cell differentiation in the fetal intestine. Dev Biol 331: 58–67 [DOI] [PubMed] [Google Scholar]
- Gat U, DasGupta R, Degenstein L, Fuchs E (1998) De novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell 95: 605–614 [DOI] [PubMed] [Google Scholar]
- Gerbe F, van Es JH, Makrini L, Brulin B, Mellitzer G, Robine S, Romagnolo B, Shroyer NF, Bourgaux JF, Pignodel C, Clevers H, Jay P (2011) Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. J Cell Biol 192: 767–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinka A, Dolde C, Kirsch N, Huang YL, Kazanskaya O, Ingelfinger D, Boutros M, Cruciat CM, Niehrs C (2011) LGR4 and LGR5 are R-spondin receptors mediating Wnt/beta-catenin and Wnt/PCP signalling. EMBO Rep 12: 1055–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser F, Nothacker HP, Grimmelikhuijzen CJ (1997) Molecular cloning, genomic organization, and developmental regulation of a novel receptor from Drosophila melanogaster structurally related to members of the thyroid-stimulating hormone, follicle-stimulating hormone, luteinizing hormone/choriogonadotropin receptor family from mammals. J Biol Chem 272: 1002–1010 [DOI] [PubMed] [Google Scholar]
- Herpin A, Badariotti F, Rodet F, Favrel P (2004) Molecular characterization of a new leucine-rich repeat-containing G protein-coupled receptor from a bivalve mollusc: evolutionary implications. Biochim Biophys Acta 1680: 137–144 [DOI] [PubMed] [Google Scholar]
- Hoshii T, Takeo T, Nakagata N, Takeya M, Araki K, Yamamura K (2007) LGR4 regulates the postnatal development and integrity of male reproductive tracts in mice. Biol Reprod 76: 303–313 [DOI] [PubMed] [Google Scholar]
- Hsu SY, Kudo M, Chen T, Nakabayashi K, Bhalla A, van der Spek PJ, van Duin M, Hsueh AJ (2000) The three subfamilies of leucine-rich repeat-containing G protein-coupled receptors (LGR): identification of LGR6 and LGR7 and the signaling mechanism for LGR7. Mol Endocrinol 14: 1257–1271 [DOI] [PubMed] [Google Scholar]
- Hsu SY, Liang SG, Hsueh AJ (1998) Characterization of two LGR genes homologous to gonadotropin and thyrotropin receptors with extracellular leucine-rich repeats and a G protein-coupled, seven-transmembrane region. Mol Endocrinol 12: 1830–1845 [DOI] [PubMed] [Google Scholar]
- Ishii Y, Wajid M, Bazzi H, Fantauzzo KA, Barber AG, Blaydon DC, Nam JS, Yoon JK, Kelsell DP, Christiano AM (2008) Mutations in R-spondin 4 (RSPO4) underlie inherited anonychia. J Invest Dermatol 128: 867–870 [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Tamai Y, Zorn AM, Yoshida H, Seldin MF, Nishikawa S, Taketo MM (2001) Mouse Wnt receptor gene Fzd5 is essential for yolk sac and placental angiogenesis. Development 128: 25–33 [DOI] [PubMed] [Google Scholar]
- Ito M, Kizawa K, Hamada K, Cotsarelis G (2004) Hair follicle stem cells in the lower bulge form the secondary germ, a biochemically distinct but functionally equivalent progenitor cell population, at the termination of catagen. Differentiation 72: 548–557 [DOI] [PubMed] [Google Scholar]
- Jaks V, Barker N, Kasper M, van Es JH, Snippert HJ, Clevers H, Toftgard R (2008) Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet 40: 1291–1299 [DOI] [PubMed] [Google Scholar]
- Jin C, Yin F, Lin M, Li H, Wang Z, Weng J, Liu M, Da Dong X, Qu J, Tu L (2008) GPR48 regulates epithelial cell proliferation and migration by activating EGFR during eyelid development. Invest Ophthalmol Vis Sci 49: 4245–4253 [DOI] [PubMed] [Google Scholar]
- Jung P, Sato T, Merlos-Suarez A, Barriga FM, Iglesias M, Rossell D, Auer H, Gallardo M, Blasco MA, Sancho E, Clevers H, Batlle E (2011) Isolation and in vitro expansion of human colonic stem cells. Nat Med 17: 1225–1227 [DOI] [PubMed] [Google Scholar]
- Kamata T, Katsube K, Michikawa M, Yamada M, Takada S, Mizusawa H (2004) R-spondin, a novel gene with thrombospondin type 1 domain, was expressed in the dorsal neural tube and affected in Wnts mutants. Biochim Biophys Acta 1676: 51–62 [DOI] [PubMed] [Google Scholar]
- Kato S, Matsubara M, Matsuo T, Mohri Y, Kazama I, Hatano R, Umezawa A, Nishimori K (2006) Leucine-rich repeat-containing G protein-coupled receptor-4 (LGR4, Gpr48) is essential for renal development in mice. Nephron Exp Nephrol 104: e63–e75 [DOI] [PubMed] [Google Scholar]
- Kato S, Mohri Y, Matsuo T, Ogawa E, Umezawa A, Okuyama R, Nishimori K (2007) Eye-open at birth phenotype with reduced keratinocyte motility in LGR4 null mice. FEBS Lett 581: 4685–4690 [DOI] [PubMed] [Google Scholar]
- Kazanskaya O, Glinka A, del Barco Barrantes I, Stannek P, Niehrs C, Wu W (2004) R-Spondin2 is a secreted activator of Wnt/beta-catenin signaling and is required for Xenopus myogenesis. Dev Cell 7: 525–534 [DOI] [PubMed] [Google Scholar]
- Kazanskaya O, Ohkawara B, Heroult M, Wu W, Maltry N, Augustin HG, Niehrs C (2008) The Wnt signaling regulator R-spondin 3 promotes angioblast and vascular development. Development 135: 3655–3664 [DOI] [PubMed] [Google Scholar]
- Kim KA, Kakitani M, Zhao J, Oshima T, Tang T, Binnerts M, Liu Y, Boyle B, Park E, Emtage P, Funk WD, Tomizuka K (2005) Mitogenic influence of human R-spondin1 on the intestinal epithelium. Science 309: 1256–1259 [DOI] [PubMed] [Google Scholar]
- Kim KA, Wagle M, Tran K, Zhan X, Dixon MA, Liu S, Gros D, Korver W, Yonkovich S, Tomasevic N, Binnerts M, Abo A (2008) R-Spondin family members regulate the Wnt pathway by a common mechanism. Mol Biol Cell 19: 2588–2596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KA, Zhao J, Andarmani S, Kakitani M, Oshima T, Binnerts ME, Abo A, Tomizuka K, Funk WD (2006) R-Spondin proteins: a novel link to beta-catenin activation. Cell Cycle 5: 23–26 [DOI] [PubMed] [Google Scholar]
- Kim TH, Escudero S, Shivdasani RA (2012) Intact function of Lgr5 receptor-expressing intestinal stem cells in the absence of Paneth cells. Proc Natl Acad Sci USA 109: 3932–3937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R (1991) Male development of chromosomally female mice transgenic for Sry. Nature 351: 117–121 [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H (1998a) Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet 19: 379–383 [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H (1997) Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science 275: 1784–1787 [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Willert K, Molenaar M, Roose J, Wagenaar G, Markman M, Lamers W, Destree O, Clevers H (1998b) Two members of the Tcf family implicated in Wnt/beta-catenin signaling during embryogenesis in the mouse. Mol Cell Biol 18: 1248–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krusche CA, Kroll T, Beier HM, Classen-Linke I (2007) Expression of leucine-rich repeat-containing G-protein-coupled receptors in the human cyclic endometrium. Fertil Steril 87: 1428–1437 [DOI] [PubMed] [Google Scholar]
- Kudo M, Chen T, Nakabayashi K, Hsu SY, Hsueh AJ (2000) The nematode leucine-rich repeat-containing, G protein-coupled receptor (LGR) protein homologous to vertebrate gonadotropin and thyrotropin receptors is constitutively active in mammalian cells. Mol Endocrinol 14: 272–284 [DOI] [PubMed] [Google Scholar]
- Kuhnert F, Davis CR, Wang HT, Chu P, Lee M, Yuan J, Nusse R, Kuo CJ (2004) Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc Natl Acad Sci USA 101: 266–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwack MH, Kim MK, Kim JC, Sung YK (2012) Dickkopf 1 promotes regression of hair follicles. J Invest Dermatol 132: 1554–1560 [DOI] [PubMed] [Google Scholar]
- Li SJ, Yen TY, Endo Y, Klauzinska M, Baljinnyam B, Macher B, Callahan R, Rubin JS (2009) Loss-of-function point mutations and two-furin domain derivatives provide insights about R-spondin2 structure and function. Cell Signal 21: 916–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XY, Lu Y, Sun HY, Wang JQ, Yang J, Zhang HJ, Fan NG, Xu J, Jiang JJ, Liu RY, Li DL, Liu MY, Ning G (2010) G protein-coupled receptor 48 upregulates estrogen receptor alpha expression via cAMP/PKA signaling in the male reproductive tract. Development 137: 151–157 [DOI] [PubMed] [Google Scholar]
- Liu W, Dong X, Mai M, Seelan RS, Taniguchi K, Krishnadath KK, Halling KC, Cunningham JM, Boardman LA, Qian C, Christensen E, Schmidt SS, Roche PC, Smith DI, Thibodeau SN (2000) Mutations in AXIN2 cause colorectal cancer with defective mismatch repair by activating beta-catenin/TCF signalling. Nat Genet 26: 146–147 [DOI] [PubMed] [Google Scholar]
- Luo J, Zhou W, Zhou X, Li D, Weng J, Yi Z, Cho SG, Li C, Yi T, Wu X, Li XY, de Crombrugghe B, Hook M, Liu M (2009) Regulation of bone formation and remodeling by G-protein-coupled receptor 48. Development 136: 2747–2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malanchi I, Peinado H, Kassen D, Hussenet T, Metzger D, Chambon P, Huber M, Hohl D, Cano A, Birchmeier W, Huelsken J (2008) Cutaneous cancer stem cell maintenance is dependent on beta-catenin signalling. Nature 452: 650–653 [DOI] [PubMed] [Google Scholar]
- Marshman E, Booth C, Potten CS (2002) The intestinal epithelial stem cell. Bioessays 24: 91–98 [DOI] [PubMed] [Google Scholar]
- Mazerbourg S, Bouley DM, Sudo S, Klein CA, Zhang JV, Kawamura K, Goodrich LV, Rayburn H, Tessier-Lavigne M, Hsueh AJ (2004) Leucine-rich repeat-containing, G protein-coupled receptor 4 null mice exhibit intrauterine growth retardation associated with embryonic and perinatal lethality. Mol Endocrinol 18: 2241–2254 [DOI] [PubMed] [Google Scholar]
- McClanahan T, Koseoglu S, Smith K, Grein J, Gustafson E, Black S, Kirschmeier P, Samatar AA (2006) Identification of overexpression of orphan G protein-coupled receptor GPR49 in human colon and ovarian primary tumors. Cancer Biol Ther 5: 419–426 [DOI] [PubMed] [Google Scholar]
- Mendive F, Laurent P, Van Schoore G, Skarnes W, Pochet R, Vassart G (2006) Defective postnatal development of the male reproductive tract in LGR4 knockout mice. Dev Biol 290: 421–434 [DOI] [PubMed] [Google Scholar]
- Mohri Y, Kato S, Umezawa A, Okuyama R, Nishimori K (2008) Impaired hair placode formation with reduced expression of hair follicle-related genes in mice lacking Lgr4. Dev Dyn 237: 2235–2242 [DOI] [PubMed] [Google Scholar]
- Mori-Akiyama Y, van den Born M, van Es JH, Hamilton SR, Adams HP, Zhang J, Clevers H, de Crombrugghe B (2007) SOX9 is required for the differentiation of paneth cells in the intestinal epithelium. Gastroenterology 133: 539–546 [DOI] [PubMed] [Google Scholar]
- Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW (1997) Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science 275: 1787–1790 [DOI] [PubMed] [Google Scholar]
- Morita H, Mazerbourg S, Bouley DM, Luo CW, Kawamura K, Kuwabara Y, Baribault H, Tian H, Hsueh AJ (2004) Neonatal lethality of LGR5 null mice is associated with ankyloglossia and gastrointestinal distension. Mol Cell Biol 24: 9736–9743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G (2004) Capturing and profiling adult hair follicle stem cells. Nat Biotechnol 22: 411–417 [DOI] [PubMed] [Google Scholar]
- Nam JS, Turcotte TJ, Yoon JK (2007) Dynamic expression of R-spondin family genes in mouse development. Gene Expr Patterns 7: 306–312 [DOI] [PubMed] [Google Scholar]
- Nguyen H, Merrill BJ, Polak L, Nikolova M, Rendl M, Shaver TM, Pasolli HA, Fuchs E (2009) Tcf3 and Tcf4 are essential for long-term homeostasis of skin epithelia. Nat Genet 41: 1068–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemann C, Owens DM, Hulsken J, Birchmeier W, Watt FM (2002) Expression of DeltaNLef1 in mouse epidermis results in differentiation of hair follicles into squamous epidermal cysts and formation of skin tumours. Development 129: 95–109 [DOI] [PubMed] [Google Scholar]
- Niemann C, Owens DM, Schettina P, Watt FM (2007) Dual role of inactivating Lef1 mutations in epidermis: tumor promotion and specification of tumor type. Cancer Res 67: 2916–2921 [DOI] [PubMed] [Google Scholar]
- Niemann C, Unden AB, Lyle S, Zouboulis Ch C, Toftgard R, Watt FM (2003) Indian hedgehog and beta-catenin signaling: role in the sebaceous lineage of normal and neoplastic mammalian epidermis. Proc Natl Acad Sci USA 100(Suppl 1): 11873–11880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi S, Hsu SY, Zell K, Hsueh AJ (2000) Characterization of two fly LGR (leucine-rich repeat-containing, G protein-coupled receptor) proteins homologous to vertebrate glycoprotein hormone receptors: constitutive activation of wild-type fly LGR1 but not LGR2 in transfected mammalian cells. Endocrinology 141: 4081–4090 [DOI] [PubMed] [Google Scholar]
- Nothacker HP, Grimmelikhuijzen CJ (1993) Molecular cloning of a novel, putative G protein-coupled receptor from sea anemones structurally related to members of the FSH, TSH, LH/CG receptor family from mammals. Biochem Biophys Res Commun 197: 1062–1069 [DOI] [PubMed] [Google Scholar]
- Nusse R, Varmus HE (1982) Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell 31: 99–109 [DOI] [PubMed] [Google Scholar]
- Nusse R, Varmus H (2012) Three decades of Wnts: a personal perspective on how a scientific field developed. EMBO J 31: 2670–2684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawara B, Glinka A, Niehrs C (2011) Rspo3 binds syndecan 4 and induces Wnt/PCP signaling via clathrin-mediated endocytosis to promote morphogenesis. Dev Cell 20: 303–314 [DOI] [PubMed] [Google Scholar]
- Okuse T, Chiba T, Katsuumi I, Imai K (2005) Differential expression and localization of WNTs in an animal model of skin wound healing. Wound Repair Regen 13: 491–497 [DOI] [PubMed] [Google Scholar]
- Parma P, Radi O, Vidal V, Chaboissier MC, Dellambra E, Valentini S, Guerra L, Schedl A, Camerino G (2006) R-spondin1 is essential in sex determination, skin differentiation and malignancy. Nat Genet 38: 1304–1309 [DOI] [PubMed] [Google Scholar]
- Pellegrinet L, Rodilla V, Liu Z, Chen S, Koch U, Espinosa L, Kaestner KH, Kopan R, Lewis J, Radtke F (2011) Dll1- and dll4-mediated notch signaling are required for homeostasis of intestinal stem cells. Gastroenterology 140: 1230-1240e1231–e1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto D, Gregorieff A, Begthel H, Clevers H (2003) Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev 17: 1709–1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakis P (1999) The oncogenic activation of beta-catenin. Curr Opin Genet Dev 9: 15–21 [DOI] [PubMed] [Google Scholar]
- Riccio O, van Gijn ME, Bezdek AC, Pellegrinet L, van Es JH, Zimber-Strobl U, Strobl LJ, Honjo T, Clevers H, Radtke F (2008) Loss of intestinal crypt progenitor cells owing to inactivation of both Notch1 and Notch2 is accompanied by derepression of CDK inhibitors p27Kip1 and p57Kip2. EMBO Rep 9: 377–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AJ, Capel B (2005) Signaling at the crossroads of gonad development. Trends Endocrinol Metab 16: 19–25 [DOI] [PubMed] [Google Scholar]
- Rubinfeld B, Souza B, Albert I, Muller O, Chamberlain SH, Masiarz FR, Munemitsu S, Polakis P (1993) Association of the APC gene product with beta-catenin. Science 262: 1731–1734 [DOI] [PubMed] [Google Scholar]
- Salzman NH, Hung K, Haribhai D, Chu H, Karlsson-Sjoberg J, Amir E, Teggatz P, Barman M, Hayward M, Eastwood D, Stoel M, Zhou Y, Sodergren E, Weinstock GM, Bevins CL, Williams CB, Bos NA (2010) Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol 11: 76–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansom OJ, Meniel VS, Muncan V, Phesse TJ, Wilkins JA, Reed KR, Vass JK, Athineos D, Clevers H, Clarke AR (2007) Myc deletion rescues Apc deficiency in the small intestine. Nature 446: 676–679 [DOI] [PubMed] [Google Scholar]
- Sato T, Stange DE, Ferrante M, Vries RG, Van Es JH, Van den Brink S, Van Houdt WJ, Pronk A, Van Gorp J, Siersema PD, Clevers H (2011a) Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology 141: 1762–1772 [DOI] [PubMed] [Google Scholar]
- Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H (2011b) Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469: 415–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H (2009) Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459: 262–265 [DOI] [PubMed] [Google Scholar]
- Schepers AG, Vries R, van den Born M, van de Wetering M, Clevers H (2011) Lgr5 intestinal stem cells have high telomerase activity and randomly segregate their chromosomes. EMBO J 30: 1104–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shroyer NF, Wallis D, Venken KJ, Bellen HJ, Zoghbi HY (2005) Gfi1 functions downstream of Math1 to control intestinal secretory cell subtype allocation and differentiation. Genes Dev 19: 2412–2417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snippert HJ, Haegebarth A, Kasper M, Jaks V, van Es JH, Barker N, van de Wetering M, van den Born M, Begthel H, Vries RG, Stange DE, Toftgard R, Clevers H (2010a) Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science 327: 1385–1389 [DOI] [PubMed] [Google Scholar]
- Snippert HJ, van der Flier LG, Sato T, van Es JH, van den Born M, Kroon-Veenboer C, Barker N, Klein AM, van Rheenen J, Simons BD, Clevers H (2010b) Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 143: 134–144 [DOI] [PubMed] [Google Scholar]
- Song H, Luo J, Luo W, Weng J, Wang Z, Li B, Li D, Liu M (2008) Inactivation of G-protein-coupled receptor 48 (Gpr48/Lgr4) impairs definitive erythropoiesis at midgestation through down-regulation of the ATF4 signaling pathway. J Biol Chem 283: 36687–36697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P (1999) Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 21: 70–71 [DOI] [PubMed] [Google Scholar]
- Su LK, Vogelstein B, Kinzler KW (1993) Association of the APC tumor suppressor protein with catenins. Science 262: 1734–1737 [DOI] [PubMed] [Google Scholar]
- Takeda H, Lyle S, Lazar AJ, Zouboulis CC, Smyth I, Watt FM (2006) Human sebaceous tumors harbor inactivating mutations in LEF1. Nat Med 12: 395–397 [DOI] [PubMed] [Google Scholar]
- Tanese K, Fukuma M, Yamada T, Mori T, Yoshikawa T, Watanabe W, Ishiko A, Amagai M, Nishikawa T, Sakamoto M (2008) G-protein-coupled receptor GPR49 is up-regulated in basal cell carcinoma and promotes cell proliferation and tumor formation. Am J Pathol 173: 835–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tensen CP, Van Kesteren ER, Planta RJ, Cox KJ, Burke JF, van Heerikhuizen H, Vreugdenhil E (1994) A G protein-coupled receptor with low density lipoprotein-binding motifs suggests a role for lipoproteins in G-linked signal transduction. Proc Natl Acad Sci USA 91: 4816–4820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizuka K, Horikoshi K, Kitada R, Sugawara Y, Iba Y, Kojima A, Yoshitome A, Yamawaki K, Amagai M, Inoue A, Oshima T, Kakitani M (2008) R-spondin1 plays an essential role in ovarian development through positively regulating Wnt-4 signaling. Hum Mol Genet 17: 1278–1291 [DOI] [PubMed] [Google Scholar]
- Trempus CS, Morris RJ, Bortner CD, Cotsarelis G, Faircloth RS, Reece JM, Tennant RW (2003) Enrichment for living murine keratinocytes from the hair follicle bulge with the cell surface marker CD34. J Invest Dermatol 120: 501–511 [DOI] [PubMed] [Google Scholar]
- Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E (2004) Defining the epithelial stem cell niche in skin. Science 303: 359–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainio S, Heikkila M, Kispert A, Chin N, McMahon AP (1999) Female development in mammals is regulated by Wnt-4 signalling. Nature 397: 405–409 [DOI] [PubMed] [Google Scholar]
- van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A, van der Horn K, Batlle E, Coudreuse D, Haramis AP, Tjon-Pon-Fong M, Moerer P, van den Born M, Soete G, Pals S, Eilers M, Medema R, Clevers H (2002) The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 111: 241–250 [DOI] [PubMed] [Google Scholar]
- Van der Flier LG, Sabates-Bellver J, Oving I, Haegebarth A, De Palo M, Anti M, Van Gijn ME, Suijkerbuijk S, Van de Wetering M, Marra G, Clevers H (2007) The intestinal Wnt/TCF signature. Gastroenterology 132: 628–632 [DOI] [PubMed] [Google Scholar]
- van Es JH, de Geest N, van de Born M, Clevers H, Hassan BA (2010) Intestinal stem cells lacking the Math1 tumour suppressor are refractory to Notch inhibitors. Nat Commun 1: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Es JH, Haegebarth A, Kujala P, Itzkovitz S, Koo BK, Boj SF, Korving J, van den Born M, van Oudenaarden A, Robine S, Clevers H (2012) A critical role for the wnt effector tcf4 in adult intestinal homeostatic self-renewal. Mol Cell Biol 32: 1918–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, Cozijnsen M, Robine S, Winton DJ, Radtke F, Clevers H (2005) Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature 435: 959–963 [DOI] [PubMed] [Google Scholar]
- Van Schoore G, Mendive F, Pochet R, Vassart G (2005) Expression pattern of the orphan receptor LGR4/GPR48 gene in the mouse. Histochem Cell Biol 124: 35–50 [DOI] [PubMed] [Google Scholar]
- Weng J, Luo J, Cheng X, Jin C, Zhou X, Qu J, Tu L, Ai D, Li D, Wang J, Martin JF, Amendt BA, Liu M (2008) Deletion of G protein-coupled receptor 48 leads to ocular anterior segment dysgenesis (ASD) through down-regulation of Pitx2. Proc Natl Acad Sci USA 105: 6081–6086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm D, Koopman P (2006) The makings of maleness: towards an integrated view of male sexual development. Nat Rev Genet 7: 620–631 [DOI] [PubMed] [Google Scholar]
- Wilson CL, Ouellette AJ, Satchell DP, Ayabe T, Lopez-Boado YS, Stratman JL, Hultgren SJ, Matrisian LM, Parks WC (1999) Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defense. Science 286: 113–117 [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Sakamoto M, Fujii G, Tsuiji H, Kenetaka K, Asaka M, Hirohashi S (2003) Overexpression of orphan G-protein-coupled receptor, Gpr49, in human hepatocellular carcinomas with beta-catenin mutations. Hepatology 37: 528–533 [DOI] [PubMed] [Google Scholar]
- Yamashita R, Takegawa Y, Sakumoto M, Nakahara M, Kawazu H, Hoshii T, Araki K, Yokouchi Y, Yamamura K (2009) Defective development of the gall bladder and cystic duct in Lgr4- hypomorphic mice. Dev Dyn 238: 993–1000 [DOI] [PubMed] [Google Scholar]
- Yao HH (2005) The pathway to femaleness: current knowledge on embryonic development of the ovary. Mol Cell Endocrinol 230: 87–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yui S, Nakamura T, Sato T, Nemoto Y, Mizutani T, Zheng X, Ichinose S, Nagaishi T, Okamoto R, Tsuchiya K, Clevers H, Watanabe M (2012) Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5(+) stem cell. Nat Med 18: 618–623 [DOI] [PubMed] [Google Scholar]
- Zhu L, Gibson P, Currle DS, Tong Y, Richardson RJ, Bayazitov IT, Poppleton H, Zakharenko S, Ellison DW, Gilbertson RJ (2009) Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature 457: 603–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucman-Rossi J, Benhamouche S, Godard C, Boyault S, Grimber G, Balabaud C, Cunha AS, Bioulac-Sage P, Perret C (2007) Differential effects of inactivated Axin1 and activated beta-catenin mutations in human hepatocellular carcinomas. Oncogene 26: 774–780 [DOI] [PubMed] [Google Scholar]