Abstract
Objectives
Paracetamol is the most commonly administered medicine to children. A recent study highlighted the risk of overdose of paracetamol using British National Formulary for Children (BNFC) age-based dosing guidelines. This current study assesses the safety of changes to the UK paracetamol product dosing system proposed by the Medicines and Healthcare products Regulatory Authority (MHRA) which include a larger number of narrower age bands and a single dose per age band.
Design
Theoretical comparison of the proposed MHRA dosing system with the product dosing instructions of a commonly prescribed form of paracetamol in the UK.
Setting
United Kingdom
Participants
Proposed MHRA dosing recommendations and current product dosing instructions were compared using a previously validated model.
Main outcome measures
For both dosing recommendations, single and cumulative daily doses of paracetamol for boys and girls at the 9th, 50th and 91st centiles for weight were calculated for 3 month, 1 year, 6 year and 12 year age groups.
Results
With the current product dosing instructions, underweight children are at risk of receiving approximately two times the recommended single and cumulative daily dose of paracetamol, particularly at age 1 year and 6 years. This risk is negligible when the same model is applied to the proposed MHRA dosing system, whereby underweight, average weight and overweight children at all ages receive doses marginally above or within the recommended dose range or limit.
Conclusion
The proposed MHRA dosing recommendations for paracetamol use in children are effective at reducing the risk of paracetamol overdose in children of all ages, when compared with current product dosing instructions.
Introduction
Paracetamol (acetaminophen) is one of the most common medicines administered to children worldwide. In the UK, paediatric formulations of paracetamol are sold over-the- counter without prescription and contain labels with age-based dosing instructions. Age-based dosing has been found to be imprecise and can result in inaccurate dosing due to marked variations in weight of children of the same age.1 The risks of both overdosing and underdosing have recently been demonstrated in an analysis of the age-based paracetamol dosing guidelines in the British National Formulary for Children (BNFC) 2010–2011.2
In order to improve the effectiveness and safety of paracetamol labelling, the Medicines and Healthcare products Regulatory Agency (MHRA) proposes to introduce new dosing instructions on all children's paracetamol products, to take effect by the end of 2011.3 The changes include moving to seven narrower age bands with a defined single dose per age band, differing from current labels which contain only three age bands and have a dose range within each band. The aim of this research was to assess the safety of the proposed changes using a previously validated model,2 in order to estimate the dosage received by children of varying weights and ages. Comparison has been made with current dosing instructions detailed on the label of a commonly used brand of children's paracetamol (Boots Pain Relief 3 Months Plus Paracetamol 120 mg/5 ml Suspension).
Methods
The proposed MHRA dosing instructions3 were reviewed for children aged 3 months to 12 years and compared with an example of current paracetamol product dosing information. Dosing instructions from the Boots Pain Relief 3 Months Plus Paracetamol 120 mg/5 ml and Boots Pain Relief 6 years Plus Paracetamol 250 mg/5 ml Suspensions were used as the comparators due to the reputation of the brand as UK's leading pharmacy-led health retailer.4 Methods were similar to those used in our previous analysis of BNFC dosing guidelines:2 Paediatric growth charts were used to estimate the average weight of boys and girls respectively in the 9th, 50th and 91st weight centiles at the ages of 3 months, 1 year, 6 years and 12 years (the 1 month age group was omitted as paracetamol labels do not recommend paracetamol administration to children under the age of 3 months without consultation with a doctor). UK-World Health Organization (WHO) growth charts were used for children aged 0 to 4 years and UK90 charts for children older than 4 years as recommended by the Royal Society of Paediatrics and Child Health.5
Based on our previously validated model,2 calculations were performed to determine the range of potential doses (mg/kg) when using either the proposed MHRA or Boots product dosing system for either a 120 mg/5 ml or 240 mg/5 ml formulation of paracetamol. A high-strength dose of 240 mg/5 ml was used instead of 250 mg/5 ml to reflect the proposed MHRA guidelines which specify high-strength paracetamol formulations as 240–250 mg/5 ml. The upper doses were calculated by dividing the highest dose of paracetamol specified at each age, by the weight of a child in the 9th centile for weight. Average doses were calculated by dividing the average (middle) dose of paracetamol specified at each age, by the weight of a child in the 50th centile for weight. The lower doses were calculated by dividing the lowest dose of paracetamol specified at each age, by the weight of a child in the 91st centile for weight. Assuming that a child was to be given ongoing doses of paracetamol over a 24 hour period, the cumulative daily dose was also calculated. For all ages, a maximum of 4 doses in 24 hours is recommended in both the current and proposed dosing systems, and so the cumulative daily dose was calculated by multiplying the single dose by 4.
For a single dose, 10–15 mg/kg was used as the recommended therapeutic dose range, based on standard textbook recommendations.6 A recommended maximum daily therapeutic limit of 60 mg/kg/24 hrs was selected based on a summary of World Health Organization policy regarding paracetamol.7
Results
The proposed MHRA dosing instructions contain 4 age bands for infant suspensions (120 mg/5 ml) with a single dose within each band and 3 age bands for 6 year plus suspensions (240 mg/5 ml or 250 mg/5 ml) with a single dose within each band (Table 1). The Boots Pain Relief 3 Months Plus Paracetamol 120 mg/5 ml Suspension dosing instructions contain 3 age bands with a range of doses within each band (Table 1). The Boots brand also has a higher-strength formulation available (Boots Pain Relief 6 years Plus Paracetamol Suspension 250 mg/5 ml) which contains 2 age bands with a range of doses within each age (Table 1). Both dosing systems suggest dosing to a maximum of 4 times in 24 hours.
Table 1.
Boots paracetamol dosing instructions and proposed MHRA paracetamol dosing recommendations
| Infant paracetamol suspension (120 mg/5 ml) | |||||
|---|---|---|---|---|---|
| Boots Pain Relief 3 months Plus Dosing Instructions | Proposed MHRA Dosing Instructions | ||||
| Age | Dose | How often (in 24 hours) | Age | Dose | How often (in 24 hours) |
| 3–11 months | 2.5–5 ml | 4 times | 3–6 months | 2.5 ml | 4 times |
| 1–5 years | 5–10 ml | 4 times | 6–24 months | 5 ml | 4 times |
| 6–12 years | 10–20 ml | 4 times | 2–4 years | 7.5 ml | 4 times |
| 4–6 years | 10 ml | 4 times | |||
| Paracetamol six plus suspension (240/250 mg/5 ml) | |||||
| Boots Pain Relief 6 years Plus Dosing Instructions | Proposed MHRA Dosing Instructions | ||||
| Age | Dose | How often (in 24 hours) | Age | Dose | How often (in 24 hours) |
| 6–11 years | 2.5–5 ml | 4 times | 6–8 years | 5 ml | 4 times |
| 12 years + | 5–10 ml | 4 times | 8–10 years | 7.5 ml | 4 times |
| 10–12 years | 10 ml | 4 times | |||
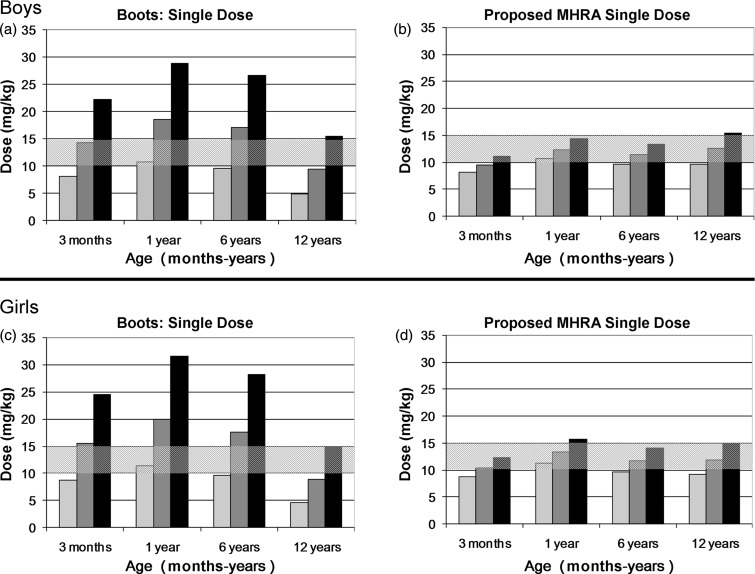
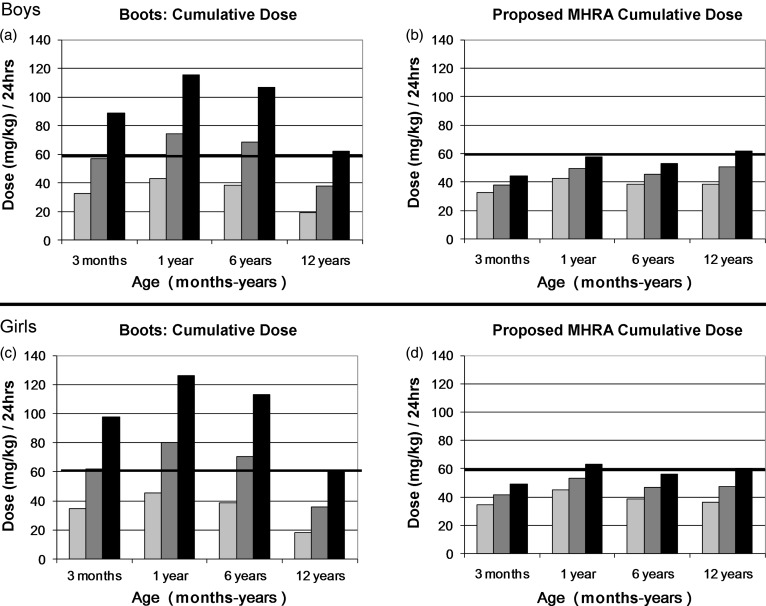
When the proposed MHRA dosing instructions were assessed, the upper single dose of paracetamol marginally exceeded the recommended therapeutic range (10–15 mg/kg) for boys at the 9th centile for weight at age 12 years and girls at the 9th centile for weight at age 1 year, with doses between 15.5 mg/kg and 15.8 mg/kg (3%–5% greater than maximum recommended dose) (Figures 1b and 1d). All other age and weight combinations were within safe single dose limits (Figures 1b and 1d). Similarly, the upper cumulative daily dose marginally exceeded the recommended maximum daily therapeutic limit of 60 mg/kg/24 hrs for boys in the 9th centile for weight at age 12 years and girls at the 9th centile for weight at age 1 year, with doses of between 61.9 mg/kg/24 hr and 63.2 mg/kg/24 hr (3%–5% greater than maximum recommended cumulative dose) (Figure 2b and 2d). All other age and weight combinations were within safe cumulative daily dose limits (Figure 2b and 2d).
Figure 1.
a) and b) Comparison of Boots dosing instructions and proposed MHRA dosing recommendations for single paracetamol doses (mg/kg) for low (9th centile), average (50th centile) and high (91st centile) weights for boys aged 3 months to 12 years.
 Lowest dose in age range/91 st centile weight
Lowest dose in age range/91 st centile weight
 Average dose in age range / 50th centile weight
Average dose in age range / 50th centile weight
 Highest dose in age range 9th centile weight
Highest dose in age range 9th centile weight
 Hashed area represents recommended dose range
Hashed area represents recommended dose range
c) and d) Comparison of Boots dosing instructions and proposed MHRA dosing recommendations for single paracetamol doses (mg/kg) for low (9th centile), average (50th centile) and high (91st centile) weights for girls aged 3 months to 12 years.
 Lowest dose in age range/91st centile weight
Lowest dose in age range/91st centile weight
 Average dose in age range / 50th centile weight
Average dose in age range / 50th centile weight
 Highest dose in age range/9th centile weight
Highest dose in age range/9th centile weight
 Hashed area represents recommended dose range
Hashed area represents recommended dose range
Figure 2.
a) and b) Comparison of Boots dosing instructions and proposed MHRA dosing recommendations for cumulative daily paracetamol doses (mg/kg/24hrs) for low (9th centile), average (50th centile) and high (91st centile) weights for boys aged 3 months to 12 years.
 (Lowest dose in age range/91st centile weight) × maximum number of doses daily
(Lowest dose in age range/91st centile weight) × maximum number of doses daily
 (Average dose in age range/50th centile weight) × maximum number of doses daily
(Average dose in age range/50th centile weight) × maximum number of doses daily
 (Highest dose in age range/9th centile weight) × maximum number of doses daily
(Highest dose in age range/9th centile weight) × maximum number of doses daily
 Bold line denotes maximum cumulative daily limit
Bold line denotes maximum cumulative daily limit
c) and d) Comparison of Boots dosing instructions and proposed MHRA dosing recommendations for cumulative daily paracetamol doses (mg/kg/24hrs) for low (9th centile), average (50th centile) and high (91st centile) weights for girls aged 3 months to 12 years.
 (Lowest dose in age range/91st centile weight) × maximum number of doses daily
(Lowest dose in age range/91st centile weight) × maximum number of doses daily
 (Average dose in age range/50th centile weight) × maximum number of doses daily
(Average dose in age range/50th centile weight) × maximum number of doses daily
 (Highest dose in age range/9th centile weight) × maximum number of doses daily
(Highest dose in age range/9th centile weight) × maximum number of doses daily
 Bold line denotes maximum cumulative daily limit
Bold line denotes maximum cumulative daily limit
When the Boots dosing instructions were assessed, the upper single dose of paracetamol exceeded the recommended therapeutic range (10–15 mg/kg) for boys at the 9th centile for weight at all age groups and girls at the 9th centile for weight at age 3 months, 1 year and 6 years with doses between 15.5 mg/kg and 31.6 mg/kg (3%– 111% greater than maximum recommended dose) (Figures 1a and 1c). For boys at the 50th centile for weight at age 1 year and 6 years, and for girls at the 50th centile for weight at age 3 months, 1 year or 6 years, the average single dose of paracetamol exceeded the recommended therapeutic range with doses of between 15.5 mg/kg and 20 mg/kg (3%–33% greater than maximum recommended dose) (Figures 1a and 1c). For boys and girls at the 91st centile for weight in all age groups, the lower dose limit of a single dose of paracetamol was within or below the recommended therapeutic range, with doses of between 4.6 mg/kg and 11.3 mg/kg (Figures 1a and 1c).
Similarly, the upper cumulative daily dose exceeded the recommended maximum daily therapeutic limit of 60 mg/kg/24 hrs for boys and girls at the 9th centile for weight at all age groups (excluding girls in the 12 year age group) with doses of between 61.9 mg/kg/24 hrs and 126.3 mg/kg/24 hrs (3%–111% greater than maximum recommended cumulative dose) (Figure 2A and 2C). Boys at the 50th centile for weight at age 1 year and 3 years, and girls at the 50th centile for weight at age 3 months, 1 year and 6 years had an average cumulative daily dose above the recommended maximum daily therapeutic limit of 60 mg/kg/24 hrs, with doses of between 62.1 mg/kg/24 hr and 80 mg/kg/24 hr (4%–33% greater than maximum recommended cumulative dose) (Figure 2a and 2c). Children at the 91st centile for weight in all age groups had cumulative daily doses below the recommended maximum daily therapeutic limit (Figure 2a and 2c).
Discussion
This study confirmed that the proposed MHRA changes to paracetamol product dosing recommendations, which include a larger number of narrower age bands and a single dose per age band, are effective at reducing the risk of overdose in underweight and average weight children at all ages, when compared with current product dosing instructions.
Based on current Boots paracetamol product dosing instructions, underweight children are at risk of receiving over twice the recommended single and cumulative daily dose of paracetamol and average weight children are at risk of receiving up to 133% of recommended single and cumulative daily dose of paracetamol, with children aged 1 year and 6 years at particular risk. This risk is negligible when the same dosing model is applied to the proposed MHRA dosing recommendations, where the highest dose calculated for any weight or age was only 8% above the recommended dose limit.
Age-based dosing has come under scrutiny due to the potential risk of misdosing children who are underweight or overweight for their age.1 On the 18th May 2011, the United States Food and Drug Administration (FDA) federal advisory panel unanimously recommended that paracetamol dosing for children be primarily based on weight instead of age due to safety concerns regarding age-based guidelines and the risk of overdose when recommended doses are exceeded.8 The FDA will now move to enforce weight-based labels on all paracetamol packaging. As our analysis shows, current age-based paracetamol dosing instructions on UK children's paracetamol products do put underweight children at risk of overdose. However, our analysis also demonstrated that the risks associated with age-based dosing can be minimized by specifying narrower age bands that contain only one recommended dose instead of a range of doses. This has important public health significance, as age-based dosing instructions are clear and easy for parents to follow, whereas weight-based guidelines are more complex, requiring parents to perform a dosage calculation based on an accurate weight measurement. Although formal health literacy statistics are not available for the UK, on average over 50% of OECD populations have poor levels of health literacy,9 and therefore many parents in the UK may lack the necessary health literacy skills to comprehend complex dosing instructions.10 The proposed MHRA changes provide an effective solution to this problem, by applying dosing instructions which are both easy to follow and safe for children of varying weights and ages. Another positive initiative to be implemented under the proposed changes is the supply of a dosing device such as a plastic spoon, cup or measuring syringe with all children's medicines in order to minimize dosing errors. However, whilst in theory this is clearly good practice, often in busy families these dosing devices are mislaid, and it is therefore important for dosing instructions to be as clear and easy to follow as possible.
Despite the improvement in safety of the new MHRA guidelines, one important issue to consider is the overlap of ages which occurs at each age band. For example, according to the proposed guidelines, a child aged 6 months would fit into both the 3–6 month age band and the 6–24 month age band, meaning that they could receive a dose of either 60 mg or 120 mg depending on which age band was chosen. This was not fully evaluated in our calculations due to the pre-specification of the same 4 age groups used in our previous analysis. Only one of the age groups selected for analysis (6 years) was on the cusp of two age bands, and in this particular instance the age bands recommended the same dose (4–6 years: 10 ml of 120 mg/5 ml/6–8 years: 5 ml of 240 mg/5 ml or 250 mg/5 ml). We would recommend changing the age bands so that no overlap occurs, as is the case in the current Boots brand dosing guidelines; for example 3–5 months for the first band and 6–24 months in the second band.
Another issue to consider is the variation in strengths of current paracetamol formulations. For simplicity, the 120 mg/5 ml and 240 mg/ml formulation was used in our calculations for all ages. However, for ages 6 years and above a 250 mg/5 ml suspension is also available which, if used in our model, would have resulted in slightly higher doses for older children, although these differences would result in only minor changes to overall dose calculations and would be unlikely to change the overall recommendations of the study.
The issues regarding safe prescribing of medicines for children is not limited to paracetamol. There is a paucity of pharmacokinetic data on which many paediatric medicine dosing and age recommendations are based, commonly leading to unlicensed and ‘off label’ prescribing.11,12 It would be beneficial for similar theoretical analyses to be performed on other commonly used paediatric medicines, as well as further pharmacokinetic and/or pharmacodynamic studies undertaken, not only in regards to oral administration but rectal and intravenous dosing as well.
Conclusion
The proposed MHRA changes to dosing recommendations for paracetamol use in children are effective at reducing the risk of overdose of paracetamol, particularly in underweight and average weight children. The changes, including a larger number of narrower age bands and the inclusion of a single dose per age band, ensure that children of all weights and ages receive safe single and cumulative doses of paracetamol when instructions are followed correctly. This demonstrates the safety of well designed age-based paediatric dosing guidelines, without the need to change to weight-based guidelines which may be more difficult for many parents to understand and follow.
DECLARATIONS
Competing interests
None declared
Funding
No specific funding was sought for this research project
Ethical approval
Ethical approval was not required as the study was a modelling study, which did not involve any research of individuals
Guarantor
Sally Eyers
Contributorship
SE developed the concept for the study. SE, KP and RB were involved with study design and data analysis. SE and RB were involved with the drafting of the manuscript. SE, KP, JF and RB were involved with manuscript revision. JF was involved with figure design and data-checking
Acknowledgments
None
Reviewer
Peter Helms
References
- 1.Donald C, Duncan R, Blair L, Thakore S, Clark M Paediatric analgesia in the emergency department, are we getting it right? Eur J Emerg Med 2007;14:157–9 [DOI] [PubMed] [Google Scholar]
- 2.Eyers S, Fingleton J, Eastwood A, Perrin K, Beasley R British National Formulary for Children: the risk of inappropriate paracetamol prescribing. Archives of Disease in Childhood 2011; doi:10.1136/archdoschild- 2011-300464 [DOI] [PubMed]
- 3.Press release: More exact paracetamol dosing for children to be introduced June 6th 2011. Available at: http://www.mhra.gov.uk/NewsCentre/Pressreleases/CON120251 (last accessed 29 August 2011)
- 4.Alliance Boots. Available at: http://en.wikipedia.org/wiki/Alliance_Boots (last accessed 12 September 2011)
- 5.UK-WHO growth charts: early years 21st March 2011 Available at: http://www.rcpch.ac.uk/what-we-do/college-projects/research-projects/uk-who-growth-charts-early-years/uk-who-growth-charts (last accessed 20 May 2011)
- 6.Kliegman R, Berhman R, Jenson H, et al. (editors) Nelson Textbook of Paediatrics. Philidelphia, PA: Saunders, Elsevier; 2007 [Google Scholar]
- 7.Russell FM, Shann F, Curtis N, Mulholland K Evidence on the use of paracetamol in febrile children. Bull World Health Organ 2003;81:367–72 [PMC free article] [PubMed] [Google Scholar]
- 8.Correct Acetaminophen Dose Depends on Children's Weight. Medscape News, 2011. Available at: http://www.medscape.com/viewarticle/743004 (last accessed 10 October 2011)
- 9.Health Literacy Statistics. 2010. Available at: http://www.healthliteracy.org.nz/about-health-literacy/health-literacy-statistics/ (last accessed 10 October 2011)
- 10.The Skills for Life survey: A national needs and impact survey of literacy, numeracy and ICT skills. 2003. Available at: https://www.education.gov.uk/publications/standard/publicationDetail/Page1/RR490 (last accessed 8 September 2011)
- 11.Marcovitch H Safer prescribing for children. BMJ 2005;331:646–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McPhillips H, Stille C, Smith D, et al. Methodological Challenges in Describing Medication Dosing Errors in Children. Advances in Patient Safety 2005;2:213–23