Abstract
Objectives. Histological examination of pathological tendon generally does not reveal signs of inflammation. However, the inflammatory cytokine IL-6 has been shown to be expressed in ruptured rotator cuff tendon. The aim of this study was to investigate the expression of IL-6 family members in painful posterior tibialis tendon (PTT) and in painful and ruptured Achilles tendon (AT) compared with normal tendon.
Methods. AT samples were obtained from cadavers (normal) or from patients undergoing surgical procedures to treat chronic painful tendinopathy or ruptured tendon. PTT samples were obtained from patients undergoing surgery for other reasons (normal) and from patients with PTT dysfunction (painful). Total RNA was extracted and mRNA expression was analysed by quantitative real-time PCR.
Results. Collagen type I α-chain I (COL1A1) expression was increased in both painful PTT and AT compared with normal. Ciliary neurotrophic factor levels were increased in painful PTT only. In the painful AT, cyclooxygenase-2 (COX2) and IL-6 expression increased compared with normal. In the ruptured AT, levels of VEGF A, COX2, oncostatin-M, leukaemia inhibitory factor and IL-6 expression were higher compared with both normal and painful AT. IL-6R expression decreased in both painful and ruptured AT compared with normal.
Conclusion. Painful AT and PTT show different expression patterns, indicating a substantial difference between those two tendinopathies. Inflammatory markers are up-regulated in painful and particularly in ruptured AT, pointing towards a role of inflammation not only in rupture healing, but also in Achilles tendinopathy.
Keywords: gene expression, tendinopathy, tendon rupture, OSM, LIF, CNTF, IL-6, Achilles tendon, posterior tibialis tendon
Introduction
Tendinopathy, characterized by chronic tendon pain, swelling and impaired performance, is a common syndrome in both recreational and elite athletes as well as in the general population. Although its aetiology remains poorly understood, tendinopathy is considered to be a primarily degenerative condition, as histological examination of pathological tendon generally does not detect inflammatory cells in or around the lesion [1]. However, inflammation may still play a role in the early initiation of the disease, as some studies indicate that acute overloading may induce an inflammatory response in tendon tissue.
Fatigue loading of the rat patellar tendon in vivo caused structural damage, accompanied by increased mRNA expression of the inflammatory cytokine IL-1β [2]. Furthermore, cyclic stretching of cultured human tendon fibroblasts increased the production of the inflammatory mediators prostaglandin E2 (PGE2), cyclooxygenase-1 and cyclooxygenase-2 (COX2) [3], while COX2 expression was up-regulated in the ruptured rat Achilles tendon (AT) when allowed free mobilization post-surgery [4]. IL-6, a cytokine with a central role in inflammation and tissue injury has also been shown to be secreted at an increased level upon cyclical stretching of human tendon fibroblasts [5]. In vivo, an intense running bout led to increased IL-6 concentrations in the peritendinous tissue around the AT in humans [6].
Further studies have suggested that inflammatory cytokines and mediators may play a role during the progression of tendinopathic conditions, as they have also been detected in painful and ruptured tendon [1, 7]. COX2, also known as PG-endoperoxide synthase 2, leads to increased synthesis of pro-inflammatory PGs such as PGE2. Comparing tissue sections of tendinopathic and healthy human patellar tendon, immunohistochemical staining has revealed that the percentage of COX2 immunopositive cells was more than twice as high in the tendinopathic samples [1]. This also coincided with an increased secretion of PGE2 by cultured fibroblasts from tendinopathic tissue [1]. IL-6 mRNA has been shown to be highly expressed in ruptured human rotator cuff tendon, with immunohistochemical analysis indicating that the positive staining was mainly in proliferative vessels and to a lesser extent in fibroblasts [7]. Unfortunately, these samples were not compared with healthy controls. Furthermore, it is unknown if and how other members of the IL-6 family are expressed in response to tendinopathic changes or tendon rupture. The family of IL-6-type cytokines includes IL-6, IL-11, oncostatin M (OSM), leukaemia inhibitory factor (LIF), ciliary neurotrophic factor (CNTF) and cardiotrophin-1, which all signal through the common receptor subunit gp130 [8].
The aim of this study was to investigate the gene expression of IL-6 and other members of the IL-6 family in painful and ruptured human tendon compared with healthy controls. Expressions of COX2 and other genes known to be up-regulated in tendinopathy [collagen type 1 α-chain 1 (COL1A1) and VEGF A] were also investigated. We analysed samples from normal, painful and ruptured AT as well as normal and painful posterior tibialis tendon (PTT) to test whether similar patterns of gene expression are observed in similar pathologies of different tendon types.
Materials and methods
Tendon specimens
Morphologically normal AT specimens from the mid-region 5–6 cm from the insertion were obtained post-mortem within 9–54 h from nine males [age 45.3 (16.4) years] with no known history of tendon problems. Painful AT samples were obtained from the lesion site from 20 patients [13 males and 7 females; age 47.3 (7.2) years] undergoing surgical procedures to treat chronic painful Achilles tendinopathy of at least 6 months duration. Ruptured AT samples were obtained from the site of rupture from 18 patients [14 males and 4 females; age 46.3 (12.3) years] undergoing surgical repair of the damaged AT. Morphologically normal PTT specimens were obtained from 10 patients [6 males and 4 females; age 47.5 (11.7) years] undergoing unrelated surgery (e.g. osteosarcoma). Painful PTT samples were obtained from the lesion site from 20 patients [6 males and 14 females; age 53.9 (11.9) years] clinically classified to have Stage II dysfunction (correctable without fixed deformity) and undergoing surgical repair by tendon transfer of the neighbouring flexor digitorum longus tendon to restore functional support of the foot arch. Both normal and painful PTT tissues were harvested from the region that wraps around the medial malleolus. The study had local ethics committee approval (Cambridge Research Ethics Committee and Joint Royal National Orthopaedic Hospital/Institute of Orthopaedics and Musculoskeletal Science (RNOH/IOMS) Research Ethics Committee), and appropriate informed consent was obtained from the patients or their relatives.
Gene expression analysis
Total RNA was isolated from frozen tissue samples by a modified Tri-Spin protocol as described previously [9]. The concentration of RNA was estimated using a NanoDrop spectrophotometer and the absorbance ratio A260/A280 was 1.82 (0.15) for the AT and 1.60 (0.07) for the PTT specimens [mean (s.e.)]. cDNA was prepared using SuperScript II (Invitrogen, UK) and primed using random hexamers. The mRNA levels of eight genes of interest were quantified using quantitative real-time PCR: COL1A1, VEGF, COX2, OSM, LIF, CNTF, IL-6 and IL-6 receptor (IL-6R). 18S ribosomal RNA was used to check the quality of the RNA. Topo-1 and eukaryotic initiation factor 4A-II (EIF4A2) were used as housekeeping genes. Specific primers were obtained from Applied Biosystems (Paisley, UK) and PrimerDesign (Southampton, UK) or designed using Primer Express 1.0 software (Applied Biosystems, Warrington, UK) and the Roche Universal ProbeLibrary Assay Design Centre (for primer and probe sequences see supplementary data available at Rheumatology Online). The relative quantitation of genes was performed using the 7500 Real-Time PCR System (Applied Biosystems, Paisley, UK). Conditions for the PCR were 2 min at 50°C, 10 min at 95°C and then 45 cycles, each consisting of 15 s at 95°C and 1 min at 60°C. The cycle number (Ct) at which amplification entered the exponential phase was determined. Gene expression data are presented as 2−ΔCt = 2−[Ct gene of interest − ((Ct EIF4A2 + Ct topo-1)/2)], normalizing the expression of the genes of interest to the mean Ct of topo-1 and EIF4A2.
Statistical analyses
As we did not detect any gender differences using a two-way analysis of variance (ANOVA), results for males and females are presented together. To detect differences in gene expression between groups, a Mann–Whitney U-test was performed on the 2−ΔCt values using SPSS 16.0 for Windows. For all statistical tests, significance was established at P ≤ 0.05.
Results
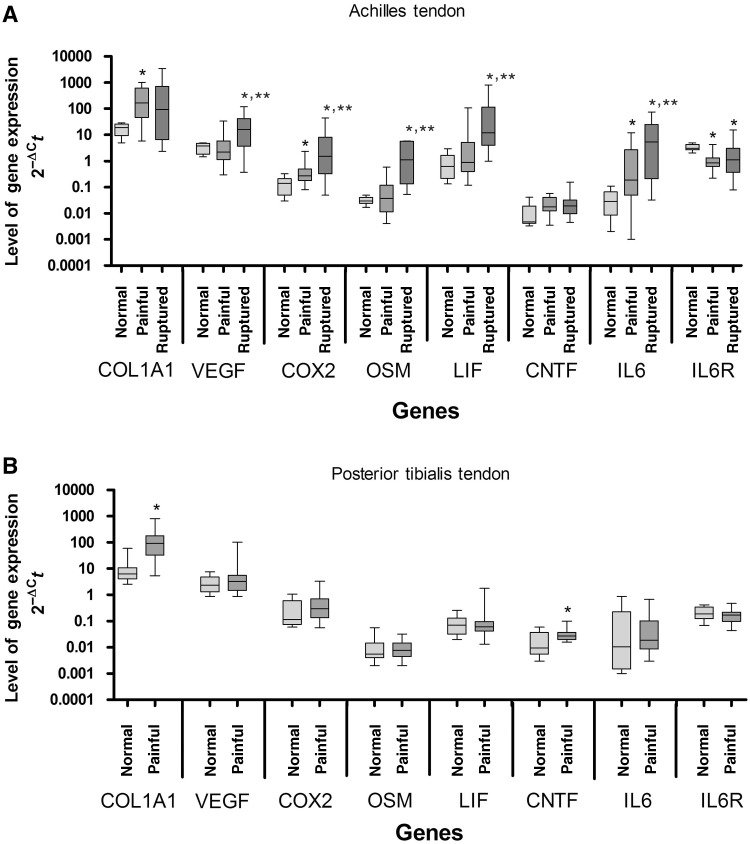
COL1A1 and CNTF expression were significantly elevated 14- and 2.9-fold, respectively, in painful PTT compared with normal PTT (Fig. 1B). In painful AT, COL1A1 expression significantly increased 9-fold compared with controls. Although the median CNTF level was 3.9-fold higher in painful than in normal AT, this difference was not significant. COX2 and IL-6 expression significantly increased 2-fold and 6.3-fold, respectively, in painful compared with normal AT, while IL-6R expression decreased 3.6-fold. In the ruptured AT, VEGF, COX2, OSM, LIF and IL-6 expression were all significantly higher compared with both normal AT (4.1-, 11-, 39-, 20- and 180-fold, respectively) and painful AT (7.2-, 5.6-, 31-, 14- and 28-fold, respectively). IL-6R expression in the ruptured AT was 2.7-fold less compared with normal (Fig. 1A).
Fig. 1.
Expression of mRNA in (A) normal, painful and ruptured AT and (B) normal and painful PTT. Values are normalized to the mean of EIF4A2 and topo-1. Data are presented as box-and-whisker plots. The median is represented by the line within the box, while the box extends from the 25th to the 75th percentile. The whiskers show the highest and lowest values. *Significantly different from control specimens; **significant difference between painful and ruptured specimens (P ≤ 0.05; Mann–Whitney U-test).
Discussion
The expression of some IL-6 family members was found to be up-regulated in pathological tendon. However, contrary to our expectation, painful AT and PTT showed different expression patterns, indicating a substantial difference between those two tendinopathies. The up-regulation of inflammatory markers in ruptured AT might point towards a role of inflammation in rupture healing. However, inflammatory markers were also up-regulated in painful AT, suggesting a potential role of inflammation in Achilles tendinopathy also. In contrast, the increase in expression of IL-6 and COX2 found in the painful AT does not occur in the painful PTT. Such a finding indicates that the inflammatory response may not simply be a cell response to degeneration or inflammation, but could also be influenced by differences in gross mechanical loading of the AT and PTT. The mechanical properties of the AT have been shown to be altered by tendinopathy, leading to lower tendon stiffness and higher in vivo strains during isometric plantar flexion compared with healthy AT [10]. Previous studies have demonstrated that the concentration of IL-6 and COX2 rises with increased mechanical strain [3, 6]. Hence the higher strains perceived by cells in tendinopathic AT may be the cause of the up-regulation of IL-6 and COX2 seen in the painful AT in our study. In contrast, the PTT appears to lengthen permanently with tendinopathy, resulting in a loss of support of the foot arch (A. Robinson, consultant orthopaedic surgeon, Addenbrooke's Hospital, Cambridge, personal observation). This elongation likely reduces the mechanical strain on the cells during loading and may explain the lack of inflammatory response in this tendon. The down-regulation of IL-6R in painful and ruptured AT could be a response to high levels of IL-6.
The expression of the IL-6 family member's OSM and LIF was increased in ruptured compared with normal and painful AT. It is unknown whether these changes preceded the rupture or were a result of the rupture and following healing process. Both OSM and LIF have been shown to stimulate release and suppress synthesis of proteoglycans in articular cartilage [11, 12].
Similarly CNTF, which was up-regulated in painful PTT, has also been shown to inhibit proteoglycan synthesis, although without affecting proteoglycan release [12]. One might assume that OSM, LIF and CNTF might function in a similar way in tendon, thereby decreasing proteoglycan content. However, this seems surprising, since GAG content and mRNA expression of the proteoglycans aggrecan and biglycan has been shown to increase in AT tendinopathy [13] while the expression of decorin either increased [14] or decreased [13] in AT rupture.
Collagen metabolism has also been shown to be influenced by IL-6 and OSM. Collagen degradation is stimulated in tendon tissue treated with OSM in combination with IL-1a [15] and in cartilage tissue treated with the combination of IL-1a and either IL-6 (in the presence of its soluble receptor) or OSM [16]. However, IL-6 can also stimulate collagen synthesis, as IL-6 injections led to an increased concentration of a procollagen marker in the peritendinous space around the human AT [17]. An increased COL1A1 expression is a common response to tendinopathy [9, 18], and one we confirm in this study, showing an increased expression in both painful AT and PTT. Such a response seems to indicate an attempt of the tendon to restore normal tissue composition and function.
An increase in expression of the angiogenic factor VEGF has also been described as a common sign of pathological tendon, as it is critical for neovascularization that usually does not occur in healthy adult tendon [19–21]. However, VEGF expression is highly variable in tissue samples of painful tendinopathy [19, 21], suggesting that expression might depend on the stage of the disease. A study on patellar tendinopathy showed that the subset of patients demonstrating VEGF expression suffered from tendinopathy for a considerably shorter time period than the patients with no detectable VEGF [21]. This indicates that VEGF might only be up-regulated early in the onset of the disease or the healing process, and might explain why we found VEGF to be up-regulated in ruptured AT but not in painful AT or PTT.
Limitations of the study and potential future directions
The extent to which the altered gene expression of IL-6 family members in painful and ruptured tendon is translated into protein remains to be established. Immunohistochemistry could determine where in the tissue those proteins are expressed, focusing particularly on vascular expression, as blood vessels have been shown to be a major source of IL-6 expression in ruptured tendon [7] and neovascularization has been linked to tendinopathy. Also, the response of the tissue could be affected by gender or age, e.g. mRNA expression levels tend to decrease with age (see supplementary data available at Rheumatology Online).
Conclusion
We have shown that IL-6 family members are expressed in tendon and that they are up-regulated in tendon pathology. However, as IL-6 has been attributed both pro- and anti-inflammatory function [22], expression of IL-6 and COX2 in pathological tendon does not necessarily indicate a state of inflammation. The role and function of the IL-6 family members in tendons needs to be further clarified. It remains to be established whether the difference in gene expression between pathological AT and PTT is due to differences in loading, tendon structure or composition or reflects a difference in the type, progression or healing of tendinopathy. Given those differences, it is possible that the success of different treatment options may depend on the anatomical site of the tendinopathy.

Supplementary data
Supplementary data are available at Rheumatology Online.
Acknowledgements
The authors would like to thank Helen Birch, Andrew (Fred) Robinson and Tomas Movin for the provision of tendon specimens.
Funding: This work was supported by Arthritis Research UK (grant number 18424). G.P.R. is an Arthritis Research UK Senior Research Fellow (grant number 17826).
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1.Fu SC, Wang W, Pau HM, et al. Increased expression of transforming growth factor-beta1 in patellar tendinosis. Clin Orthop Relat Res. 2002;400:174–83. doi: 10.1097/00003086-200207000-00022. [DOI] [PubMed] [Google Scholar]
- 2.Sun HB, Li Y, Fung DT, et al. Coordinate regulation of IL-1beta and MMP-13 in rat tendons following subrupture fatigue damage. Clin Orthop Relat Res. 2008;466:1555–61. doi: 10.1007/s11999-008-0278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang JH, Jia F, Yang G, et al. Cyclic mechanical stretching of human tendon fibroblasts increases the production of prostaglandin E2 and levels of cyclooxygenase expression: a novel in vitro model study. Connect Tissue Res. 2003;44:128–33. doi: 10.1080/03008200390223909. [DOI] [PubMed] [Google Scholar]
- 4.Bring D, Reno C, Renstrom P, et al. Prolonged immobilization compromises up-regulation of repair genes after tendon rupture in a rat model. Scand J Med Sci Sports. 2010;20:411–7. doi: 10.1111/j.1600-0838.2009.00954.x. [DOI] [PubMed] [Google Scholar]
- 5.Skutek M, van Griensven M, Zeichen J, et al. Cyclic mechanical stretching enhances secretion of interleukin 6 in human tendon fibroblasts. Knee Surg Sports Traumatol Arthrosc. 2001;9:322–6. doi: 10.1007/s001670100217. [DOI] [PubMed] [Google Scholar]
- 6.Langberg H, Olesen JL, Gemmer C, et al. Substantial elevation of interleukin-6 concentration in peritendinous tissue, in contrast to muscle, following prolonged exercise in humans. J Physiol. 2002;542:985–90. doi: 10.1113/jphysiol.2002.019141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakama K, Gotoh M, Yamada T, et al. Interleukin-6-induced activation of signal transducer and activator of transcription-3 in ruptured rotator cuff tendon. J Int Med Res. 2006;34:624–31. doi: 10.1177/147323000603400607. [DOI] [PubMed] [Google Scholar]
- 8.Heinrich PC, Behrmann I, Muller-Newen G, et al. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J. 1998;334(Pt 2):297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ireland D, Harrall R, Curry V, et al. Multiple changes in gene expression in chronic human Achilles tendinopathy. Matrix Biol. 2001;20:159–69. doi: 10.1016/s0945-053x(01)00128-7. [DOI] [PubMed] [Google Scholar]
- 10.Arya S, Kulig K. Tendinopathy alters mechanical and material properties of the Achilles tendon. J Appl Physiol. 2010;108:670–5. doi: 10.1152/japplphysiol.00259.2009. [DOI] [PubMed] [Google Scholar]
- 11.Carroll GJ, Bell MC. Leukaemia inhibitory factor stimulates proteoglycan resorption in porcine articular cartilage. Rheumatol Int. 1993;13:5–8. doi: 10.1007/BF00290327. [DOI] [PubMed] [Google Scholar]
- 12.Hui W, Bell M, Carroll G. Oncostatin M (OSM) stimulates resorption and inhibits synthesis of proteoglycan in porcine articular cartilage explants. Cytokine. 1996;8:495–500. doi: 10.1006/cyto.1996.0067. [DOI] [PubMed] [Google Scholar]
- 13.Corps AN, Robinson AH, Movin T, et al. Increased expression of aggrecan and biglycan mRNA in Achilles tendinopathy. Rheumatology. 2006;45:291–4. doi: 10.1093/rheumatology/kei152. [DOI] [PubMed] [Google Scholar]
- 14.Karousou E, Ronga M, Vigetti D, et al. Collagens, proteoglycans, MMP-2, MMP-9 and TIMPs in human Achilles tendon rupture. Clin Orthop Relat Res. 2008;466:1577–82. doi: 10.1007/s11999-008-0255-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cawston TE, Curry VA, Summers CA, et al. The role of oncostatin M in animal and human connective tissue collagen turnover and its localization within the rheumatoid joint. Arthritis Rheum. 1998;41:1760–71. doi: 10.1002/1529-0131(199810)41:10<1760::AID-ART8>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 16.Rowan AD, Koshy PJ, Shingleton WD, et al. Synergistic effects of glycoprotein 130 binding cytokines in combination with interleukin-1 on cartilage collagen breakdown. Arthritis Rheum. 2001;44:1620–32. doi: 10.1002/1529-0131(200107)44:7<1620::AID-ART285>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 17.Andersen MB, Pingel J, Kjaer M, et al. Interleukin-6: a growth factor stimulating collagen synthesis in human tendon. J Appl Physiol. 2011;110:1549–54. doi: 10.1152/japplphysiol.00037.2010. [DOI] [PubMed] [Google Scholar]
- 18.Corps AN, Robinson AH, Movin T, et al. Versican splice variant messenger RNA expression in normal human Achilles tendon and tendinopathies. Rheumatology. 2004;43:969–72. doi: 10.1093/rheumatology/keh222. [DOI] [PubMed] [Google Scholar]
- 19.Alfredson H, Lorentzon M, Backman S, et al. cDNA-arrays and real-time quantitative PCR techniques in the investigation of chronic Achilles tendinosis. J Orthop Res. 2003;21:970–5. doi: 10.1016/S0736-0266(03)00107-4. [DOI] [PubMed] [Google Scholar]
- 20.Pufe T, Petersen W, Tillmann B, et al. The angiogenic peptide vascular endothelial growth factor is expressed in foetal and ruptured tendons. Virchows Arch. 2001;439:579–85. doi: 10.1007/s004280100422. [DOI] [PubMed] [Google Scholar]
- 21.Scott A, Lian O, Bahr R, et al. VEGF expression in patellar tendinopathy: a preliminary study. Clin Orthop Relat Res. 2008;466:1598–604. doi: 10.1007/s11999-008-0272-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fisman EZ, Tenenbaum A. The ubiquitous interleukin-6: a time for reappraisal. Cardiovasc Diabetol. 2010;9:62–8. doi: 10.1186/1475-2840-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.