Abstract
Vitronectin is present in large concentrations in serum and the extracellular matrix. Although vitronectin is known to modulate neutrophil adhesion and chemotaxis, and to contribute to neutrophil-associated proinflammatory processes, a role in apoptosis has not been demonstrated. In the present studies, we found that neutrophils demonstrated more rapid progression to spontaneous or TNF-related apoptosis-inducing ligand–induced apoptosis when incubated under vitronectin-free conditions than when vitronectin was present. The ability of native vitronectin to delay neutrophil apoptosis was not recapitulated by the vitronectin somatomedin B domain. In contrast, inclusion of the cyclo[Arg-Gly-Asp-D-Phe-Val] peptide in cultures containing vitronectin resulted in enhanced neutrophil apoptosis, showing that the vitronectin RGD motif (Arg-Gly-Asp motif) was responsible for the antiapoptotic effects of vitronectin. Addition of antibodies to β1, β3, or β5, but not to β2 or β4 integrins, reversed the ability of vitronectin to diminish neutrophil apoptosis. The ability of vitronectin to enhance neutrophil viability was dependent on activation of phosphatidylinositol 3-kinase and extracellular signal–regulated kinase 1/2 kinases, but not on the p38 kinase. Increased numbers of apoptotic neutrophils were present in the lungs of LPS-treated transgenic vitronectin-deficient mice, as compared with control mice. These results demonstrate a novel antiapoptotic function for vitronectin.
Keywords: vitronectin, inflammation, neutrophils, apoptosis, lung injury
Clinical Relevance
These studies demonstrate a novel role for vitronectin diminishing neutrophil apoptosis. Our experiments, showing that exposure to vitronectin extends the lifespan of activated neutrophils, provide new insights into the mechanisms underlying acute lung injury. These studies also suggest that therapeutic approaches that inhibit interaction between vitronectin and activated neutrophils may be beneficial in diminishing the severity of acute lung injury and other inflammatory processes in which neutrophils play a major role.
Although neutrophils play a central role in host defense against invading microbes, organ injury can result if neutrophils activated to produce proinflammatory mediators remain in tissues for prolonged periods. The timely progression of neutrophils to apoptotic cell death and subsequent clearance is essential for the successful resolution of neutrophil-associated inflammatory processes (1). Under normal conditions, neutrophils are short lived, but during inflammatory responses, such as acute lung injury, they demonstrate prolonged viability. The importance of diminished neutrophil apoptosis in promoting tissue injury during inflammatory processes has been demonstrated by studies in which pharmacologically induced increase in apoptosis resulted in diminished severity of organ dysfunction, such as lung injury induced by LPS or hemorrhage (2–4).
Vitronectin is a glycoprotein present in large concentrations in serum, extracellular matrix, and platelets, and participates in the regulation of coagulation, fibrinolysis, and complement activation (5, 6). Tissue levels of vitronectin markedly increase in pathophysiologic settings associated with acute inflammation, such as severe sepsis, and appear to contribute to organ injury in such conditions (7, 8). Vitronectin promotes neutrophil adhesion and migration through interaction with integrins, including αvβ3, and other ligands, such as the urokinase plasminogen activator (uPA) receptor (uPAR) (9, 10). The first 43 amino acids of N-terminal–end vitronectin consist of the somatomedin B (SMB) domain followed by a short RGD motif (Arg-Gly-Asp) that is responsible for binding to integrins (45–47 aa). Integrins play an essential role in many cellular processes, including cell survival, proliferation, migration, and matrix attachment. The vitronectin SMB domain has been shown to bind plasminogen activator inhibitor (PAI) -1, uPAR, collagen, and thrombin–antithrombin complexes. Previous studies demonstrated that vitronectin and its binding partners, PAI-1 and the uPA, can diminish the phagocytosis of apoptotic cells by macrophages, a process referred to as efferocytosis (11–13). However, a role for vitronectin in modulating neutrophil apoptosis has not been described.
In the present studies, we found that vitronectin has potent effects in diminishing spontaneous and extrinsically induced neutrophil apoptosis under in vitro and in vivo conditions. These results demonstrate a novel antiapoptotic function for vitronectin and provide a potential mechanism for the ability of vitronectin to enhance neutrophil-associated proinflammatory responses.
Materials and Methods
Mice
Vitronectin-deficient mice (B6.129S2(D2)-Vtntm1Dgi/J), as well as control mice (C57BL/6J), were purchased from The Jackson Laboratory (Bar Harbor, ME). Vitronectin knockout male mice were crossed to B6D2F1/J female mice, and then backcrossed to C57BL/6J for 12 generations before being interbred. Male mice (8–12 wk of age) were used for experiments. All experiments were conducted in accordance with institutional review board–approved protocols (University of Alabama at Birmingham Institutional Animal Care and Use Committee).
Reagents and Antibodies
Antibodies to total and phospho-(S473)Akt, phospho-(T202/Y204) extracellular signal–regulated kinases (Erk)1/2, and phospho-(T180/Y182)p38, as well as to induced myeloid leukemia cell differentiation protein Mcl-1 were purchased from Cell Signaling (Beverly, MA). Antibodies to β-actin were from Sigma (St. Louis, MO). Propidium iodide and antibodies to annexin V were purchased from EMD Chemicals (Gibbstown, NJ). Mouse vitronectin was purchased from Abcam (Cambridge, MA). Vitronectin SMB domain deletion mutant (vtn ΔSMB) vitronectin or vitronectin SMB domain were expressed in Drosophila S2 cells using methods described by Schar and colleagues (14), and proteins purified as described by Thompson and colleagues (15). Mouse anti–integrin-β1, -β2, β3, or -β4 antibodies and specific isotype control IgG were purchased from BD Biosciences (San Diego, CA), whereas monoclonal human anti–integrin-β5 (cross-reacting with mouse, clone KN52) was purchased from eBioscience (San Diego, CA). RGDfv [cyclo(Arg-Gly-Asp-D-Phe-Val)] and RADfv [cyclo(Arg-Ala-Asp-D-Phe-Val)] were purchased from Enzo Life Science (Plymouth Meeting, PA). Recombinant mouse TNF-related apoptosis-inducing ligand (TRAIL)/tumor necrosis factor ligand superfamily member 10 (TNFSF10) was obtained from R&D Systems (Minneapolis, MN).
Neutrophil Isolation and Culture
Bone marrow neutrophils were isolated using methods previously described (16) and as described in the supplemental Materials and Methods.
Measurement of Neutrophil Apoptosis
The percentage of early and late apoptotic cells was determined by staining with annexin V–FITC and propidium iodide, followed by flow cytometry (17) (see supplemental Materials and Methods).
Western Blot Analysis
Western blot analysis was performed as previously described (16, 18) (see supplemental Materials and Methods).
Acute Lung Injury Model
Mice were treated with LPS (1 mg/kg) in saline or saline alone, administered intratracheally, in 50 μl saline, as previously described (16, 18) (see supplemental Materials and Methods).
Measurement of Neutrophil Apoptosis In Vivo
Infiltrating leukocytes were isolated from minced lung tissue by treatment with collagenase-B (2 mg/ml; Roche) and DNase I (0.02 mg/ml; Sigma) in Iscove's modified Dulbecco's medium supplemented with 1 mM sodium pyruvate, 2 mM l-glutamine, 10 μg/ml penicillin–streptomycin, 25 μM 2-mercaptoethanol, and 0.1 mM nonessential amino acids (Life Technologies, Grand Island, NY) at 37°C for 30 minutes. To determine the percentage of apoptotic neutrophils, the cells were incubated with annexin V–FITC (Calbiochem, La Jolla, CA) and Gr1-PE (BD Bioscience) antibodies and then analyzed by flow cytometry. Caspase-9 activity was measured in CD-11b–stained cells from bronchoalveolar lavage fluids using standard fluorogenic substrates (Calbiochem).
Statistical Analyses
Statistical significance was determined by the Wilcoxon rank sum test (independent two-group Mann-Whitney U test) as well as Student's t test for comparisons between two groups. Multigroup comparisons were performed using one-way ANOVA with Tukey's post hoc test. A value of P less than 0.05 was considered significant. Analyses were performed on SPSS version 16.0 (IBM, Armonk, NY) for Windows (Microsoft Corp., Redmond, WA).
Results
Vitronectin Increases Neutrophil Viability
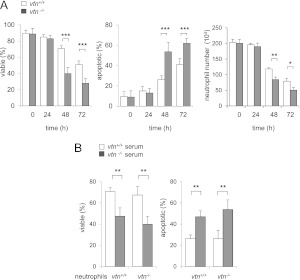
In a first set of experiments, cell viability was measured in neutrophils isolated from the bone marrow of vitronectin control (vtn+/+) or vitronectin-deficient (vtn−/−) mice that were then incubated in vitronectin-deficient or normal mouse serum. As shown in Figure 1A, neutrophils in each group (i.e., vtn−/− neutrophils/vtn−/− serum or vtn+/+ neutrophils/vtn+/+ serum) had similar profiles of viability (∼90% viable) after culture for 24 hours. However, at 48 and 72 hours in culture, a marked increase in the percentage of apoptotic cells was found among the vtn−/− neutrophils incubated in serum obtained from vtn−/− mice as compared with vtn+/+ cells. Although culture of neutrophils was initiated using the same number of cells, there was also a significant decrease in the total number of vtn−/− cells compared with wild-type vtn+/+ neutrophils, consistent with increased death of the vtn−/− neutrophils.
Figure 1.
Effects of vitronectin on neutrophil viability. (A) Neutrophils obtained from the bone marrow of vitronectin control (vtn+/+) mice were incubated with 5% serum from vtn+/+ mice, and neutrophils obtained from the bone marrow of vitronectin-deficient (vtn−/−) mice were incubated with 5% serum from vtn−/− mice. Viable and apoptotic neutrophils obtained at 0, 24, 48, or 72 hours after the initiation of culture were identified by flow cytometry using staining with annexin V and propidium iodide (PI). The percentage of viable neutrophils was determined by subtraction of PI and annexin V–positive cells from the total population of cells. The numbers of neutrophils, calculated after 0, 24, 48 or 72 hours of culture, are shown in the right panel. Means (±SD) (n = 4 independent sets of neutrophils to determine viable and apoptotic cells; n = 3 to determine number of cells) are shown. *P < 0.05 (P = 0.02), **P < 0.01 (P = 0.006), ***P < 0.001 (P = 0.0002) compared with vtn+/+ cells. (B) Percentages of viable and apoptotic neutrophils were determined among vtn+/+ or vtn−/− neutrophils after 48-hour incubation in medium containing 5% vtn+/+ or vtn−/− serum. Means (±SD) (n = 3 independent sets of neutrophils) are shown. Left panel: **P < 0.01 (0.009) comparing vtn+/+ neutrophils or **P < 0.01 (P = 0.003) comparing vtn−/− neutrophils cultured in serum deficient or containing vitronectin. Right panel: **P < 0.01 (P = 0.0097) comparing vtn+/+ neutrophils or **P < 0.01 (P = 0.002) comparing vtn−/− neutrophils cultured in serum deficient or containing vitronectin.
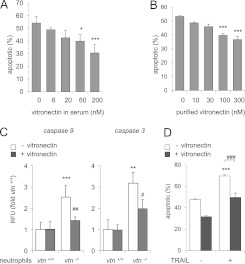
We next determined if vitronectin-dependent enhancement of neutrophil viability was mediated by cellularly produced vitronectin or vitronectin present in the serum that had been added to the cultures. To examine this question, vtn+/+ or vtn−/− bone marrow neutrophils were incubated with serum obtained from vtn−/− or vtn+/+ mice. As shown in Figure 1B, the presence of vitronectin in the culture medium, but not the vitronectin-expressing status of the neutrophils, was responsible for the preservation of neutrophil viability. In particular, both vtn−/− and vtn+/+ neutrophils were more susceptible to apoptosis when cultured in vtn−/− serum than when exposed to vtn+/+ serum. Similarly, inclusion of vitronectin-containing serum in the cell cultures increased the percentage of viable vtn+/+ and vtn−/− neutrophils. Additional experiments confirmed that vitronectin dose-dependently decreased apoptosis among vtn−/− neutrophils (Figure 2A). Similarly, the percentage of apoptotic neutrophils was diminished in a dose-dependent manner by inclusion of purified vitronectin in the cultures (Figure 2B). As shown in Figure E1 in the online supplement, exposure of mature neutrophils isolated from peritoneal exudates to vitronectin also diminished progression to apoptosis. In particular, we found increased apoptosis and diminished numbers of viable cells over time in cultures of vtn−/− as compared with wild-type (vtn+/+) peritoneal neutrophils. Consistent with these results, culture of peritoneal neutrophils (vtn−/−) with serum containing vitronectin resulted in decreased percentages of apoptotic cells (Figure E1B).
Figure 2.
Effects of vitronectin on spontaneous or TNF-related apoptosis-inducing ligand (TRAIL)–induced neutrophil apoptosis. (A) Vtn−/− neutrophils were cultured with vtn−/− serum or medium that was dose-dependently supplemented with serum from vtn+/+ mice. Means (±SD) (n = 4 independent sets of neutrophils) are shown. *P < 0.05 (P = 0.025), ***P < 0.001 (P = 0.0006). (B) Vtn−/− neutrophils were treated with purified vitronectin at the indicated concentrations for 30 minutes. Cells were then cultured in media containing 5% serum from vtn−/− mice for 48 hours and the percentages of apoptotic cells determined by flow cytometry. Means (±SD) (n = 3 independent sets of neutrophils) are shown. ***P < 0.001 (P = 0.0002) compared with untreated cells. (C) vtn+/+ or vtn−/− neutrophils were treated with purified vitronectin (0 or 100 nM) for 30 minutes, then cultured in media containing 5% serum from vtn−/− mice for 48 hours, followed by measurement of the activity of caspase 9 and caspase 3. Means (±SD) (n = 3 independent sets of neutrophils). **P < 0.01 (P = 0.002), ***P < 0.001 (P = 0.0006) compared with vtn+/+ cells, or #P < 0.05 (P = 0.03), ##P < 0.01 (P = 0.002) compared with vtn−/− untreated cells. (D) The percentage of apoptotic cells was determined after exposure of neutrophils to purified vitronectin (0 or 100 nM) for 4 hours, followed by inclusion of TRAIL (0 or 30 ng/ml) for an additional 44 hours. Means (±SD) (n = 3 independent sets of neutrophils) are shown. ***P < 0.001 (P = 0.0002) compared with untreated cells or ###P < 0.001 (P = 0.0002) compared with cells treated with TRAIL alone.
Exposure of bone marrow neutrophils to vitronectin diminished activation of caspase 9 and caspase 3 (Figure 2C). Of note, the antiapoptotic effect of vitronectin was not limited to spontaneous death among neutrophils, but also diminished extrinsic apoptotic death induced through the TRAIL/tumor necrosis factor receptor type 1–associated DEATH domain protein (TRADD) signaling pathway (Figure 2D).
The Antiapoptotic Effects of Vitronectin Are Dependent on the RGD Domain
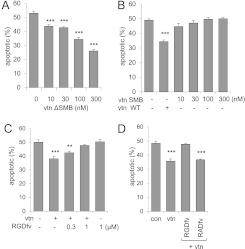
The SMB domain and RGD motifs of vitronectin have previously been shown to modulate cellular viability in nonmyeloid cell populations (19–26). To determine the importance of SMB domain in affecting neutrophil viability and apoptosis, vtn−/− bone marrow neutrophils were incubated with purified SMB domain or recombinant vitronectin that lacked the SMB domain (i.e., vtn ΔSMB). In a manner similar to wild-type vitronectin (Figure 2B), vtn ΔSMB dose-dependently diminished neutrophil apoptosis (Figure 3A). To confirm that the SMB domain was not responsible for the antiapoptotic effects of vitronectin, we cultured neutrophils with purified SMB domain and found no effects on the percentage of apoptotic neutrophils, unlike the reduction in neutrophil apoptosis induced by incubation with wild-type vitronectin (Figure 3B). Of note, the effects of recombinant vitronectin proteins were not due to potential contamination with LPS. As shown in Figures E2A and E2B, inclusion of polymyxin B in the cell cultures did not modify the effects of vitronectin ΔSMB or the vitronectin SMB domain on neutrophil apoptosis.
Figure 3.
The RGD motif is required for the antiapoptotic effect of vitronectin. vtn−/− neutrophils were cultured with (A) vitronectin lacking the somatomedin B (SMB) domain (ΔSMB domain), or (B) wild-type vitronectin (0 or 100 nM) or purified SMB domain (0–300 nM) for 30 minutes. Cells were cultured in 5% serum from vtn−/− mice for 48 hours and the percentage of apoptotic neutrophils determined. vtn−/− neutrophils were treated with (C) RGDfv [cyclo(Arg-Gly-Asp-D-Phe-Val)] (0, 0.3, or 1 μM) or (D) RGDfv (0 or 1 μM) or RADfv [cyclo(Arg-Ala-Asp-D-Phe-Val)] (0 or 1 μM) for 30 minutes before exposure to vitronectin. The percentages of apoptotic and viable cells were determined by flow cytometry after 48 hours of culture. Means (±SD) (n = 3 independent sets of neutrophils) are shown. (A) ***P < 0.001 (P = 0.0002); (B) ***P < 0.001 (P = 0.002); (C) ***P < 0.001 (P = 0.0002), **P < 0.01 (P = 0.006); and (D) ***P < 0.001 (P = 0.0002) compared with untreated cells.
We next determined whether the RGD motif is responsible for the effects of vitronectin on neutrophil viability. To examine this possibility, we used the RGD-blocking peptide, RGDfv, and compared its effects on neutrophil apoptosis with RADfv, a structurally similar analog that is unable to interact with the RGD motif (27). As shown in Figures 3C and 3D, inclusion of RGDfv in the cultures dose-dependently diminished the effects of vitronectin on neutrophil viability, but exposure to RADfv had no effect when compared with treatment of the cells with vitronectin alone. Taken together, these results show that the antiapoptotic effect of vitronectin is mediated by the RGD motif, but not the SMB domain.
Interactions between Vitronectin and Integrins Result in Diminished Neutrophil Apoptosis
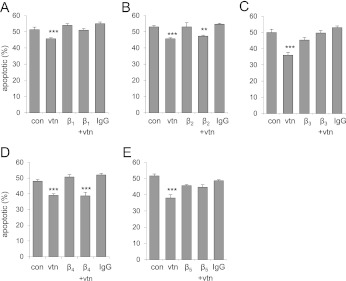
Previous studies have shown that the vitronectin RGD motif binds αvβ1, αvβ3, and αvβ5 integrins (28). Given that blockade of the vitronectin RGD domain with RGDfv diminished the antiapoptotic effects of vitronectin, suggesting that RGD-binding integrins were responsible for the properties of vitronectin on neutrophil viability, we examined the effect of specific antibodies to β1, β2, β3, β4, or β5 integrins on vitronectin-induced decrease in spontaneous neutrophil apoptosis. As shown in Figure 4A, antibodies to β1, β3, or β5, but not β2 or β4, integrins diminished vitronectin-dependent increases in neutrophil viability. These results indicate that vitronectin enhances viability of neutrophils through interaction with specific integrins, particularly β1, β3, and β5.
Figure 4.
Interactions between integrins and vitronectin modulate neutrophil survival. (A–E) vtn−/− neutrophils were cultured with antibodies (1 μg/ml) to β1, β2, β3, β4, or β5 integrins or isotype-specific IgG (1 μg/ml) for 30 minutes. The cells were then washed and incubated with purified vitronectin (0 or 100 nM) for 30 minutes, followed by culture with media containing 5% serum from vtn−/− mice for 48 hours. Means (±SD) (n = 3 independent sets of neutrophils) are shown. (A) ***P < 0.001 (P = 0.0002); (B) **P < 0.01 (P = 0.003), ***P < 0.001 (P = 0.0005); (C) ***P < 0.001 (P = 0.0002); (D) ***P < 0.001 (P = 0.0002 and 0.0003); and (E) ***P < 0.001 (P = 0.0002) compared with untreated control cells (con).
Involvement of Phosphatidylinositol 3-Kinases and Erk in Vitronectin-Induced Enhancement of Neutrophil Viability
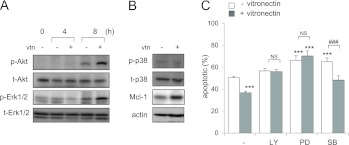
Treatment of bone marrow neutrophils with vitronectin resulted in enhanced phosphorylation of Akt and Erk1/2, but not of p38 mitogen-activated protein kinase, and increased expression of the antiapoptotic protein, Mcl-1 (Figures 5A and 5B). To establish which of these kinases might be involved in mediating the antiapoptotic effects of vitronectin, neutrophils were cultured with combinations of vitronectin and specific inhibitors of phosphatidylinositol 3-kinases (PI3-K) (LY294002), Erk1/2 (PD98059), or p38 (SB203580). Inhibition of PI3-K, Erk1/2, or p38 resulted in increased apoptosis of neutrophils, as previously reported (29). Inclusion of vitronectin in the cultures did not affect the increase in neutrophil apoptosis induced by PI3-K or Erk1/2 inhibitors, but did reduce the enhanced rate of apoptosis found after p38 inhibition (Figure 5C). These results implicate the activation of PI3-K/Akt and Erk1/2 in mediating the antiapoptotic effects of vitronectin in neutrophils.
Figure 5.
Effects of vitronectin on kinase activation. vtn−/− neutrophils were cultured with purified mouse vitronectin (0 or 100 nM) for 4 or 8 hours. (A) Representative Western blots show the levels of total and phosphorylated protein kinase B (Akt) and extracellular signal–regulated kinases (Erk)1/2 after incubation of neutrophils with or without vitronectin for 0, 4, or 8 hours, whereas (B) shows the amounts of total and phospho-p38 and levels of induced myeloid leukemia cell differentiation protein (Mcl-1) obtained from neutrophils cultured with vitronectin (0 or 100 nM) for 8 hours. Similar results were obtained from a second independent experiment. (C) The percentage of apoptotic neutrophils was determined after culture for 48 hours with LY294002 (LY; 0 or 10 μM), PD98059 (PD; 0 or 50 μM), or SB203580 (SB; 0 or 10 μM) and purified mouse vitronectin (0 or 100 nM) for 48 hours. Means (±SD) (n = 3 independent sets of neutrophils) are shown. ***P < 0.001 (from left to right: P = 0.0003, 0.0003, 0.0002, and 0.0007) compared with untreated cells; ###P < 0.001 (P = 0.0003) compared with cells treated with SB203580 alone. NS, not significant.
Vitronectin Is Associated with Prolonged Longevity of Neutrophils during LPS-Induced Acute Lung Injury
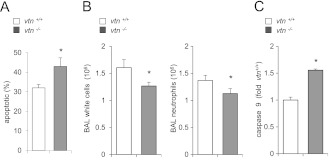
Given our results showing that vitronectin has potent antiapoptotic effects in cultured neutrophils, we next determined if vitronectin has similar effects in vivo. To examine this question, acute lung injury was induced in control (vtn+/+) and vtn−/− mice by intratracheal administration of LPS, and then the percentage of apoptotic neutrophils in the lung interstitium determined 24 hours after LPS exposure, the time of peak inflammatory response. As shown in Figure 6A, the percentage of apoptotic neutrophils present in the lungs was significantly increased in vtn−/− as compared with vtn+/+ mice. Similar to the results obtained from flow cytometry using staining with annexin V, increased activity of caspase 9 and decreased numbers of neutrophils were found in the lungs of vtn−/− as compared with vtn+/+ mice during acute lung injury (Figures 6B and 6C). These in vivo findings are similar to those showing that addition of vitronectin to neutrophils cultured with LPS resulted in diminished levels of apoptosis (Figure E3).
Figure 6.
Vitronectin deficiency is associated with enhanced neutrophil apoptosis in the lungs of mice with LPS-induced acute lung injury. (A) Number of apoptotic neutrophils (annexin V/GR1-FITC) obtained from the lungs of vtn+/+ or vtn−/− mice 24 hours after LPS (1 mg/kg, intratracheally) administration. Means (±SD) (n = 4 mice in each group). *P < 0.05 (P = 0.02) compared with vtn+/+. (B and C) Total white cell and neutrophil numbers, and caspase 9 activity in neutrophils obtained from bronchoalveolar lavage 24 hours after LPS administration to vtn+/+ or vtn−/− mice. (B) Means (±SD) (n = 4 mice in each group). *P < 0.05 (P = 0.02) compared with vtn+/+. (C) Results obtained from three mice per group. P < 0.1 compared with vtn+/+.
Discussion
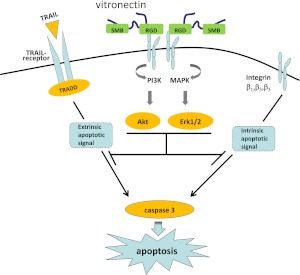
In the present experiments, we found that vitronectin has potent regulatory effects on neutrophil viability (Figure 7). In particular, exposure to vitronectin under both in vitro and in vivo conditions was associated with reduced apoptosis in neutrophils. The antiapoptotic properties of vitronectin were dependent on the RGD, but not the SMB domain, consistent with interactions between RGD-binding integrins and vitronectin being responsible for the effects on neutrophil viability. Blocking experiments demonstrated that the antiapoptotic actions of vitronectin were due to interactions with β1, β3, and β5, but not β2 or β4 integrins, confirming that the mechanism through which vitronectin enhances viability of neutrophils is by interaction with specific integrins, particularly those that include the β1, β3, or β5 subunits, consistent with well described interactions between vitronectin and these integrin subunits (30). Such results are also concordant with previously reported findings in nonmyeloid cell populations, including microvascular endothelial cells, human umbilical vein endothelial cells, and human glioma cell lines, in which antiapoptotic actions of vitronectin were mediated through interactions with β3 and β5 integrins (31, 32).
Figure 7.
Vitronectin-dependent activation of integrin signaling pathways inhibits neutrophil apoptosis. Interactions between the vitronectin RGD motif and integrins induce activation of Akt and Erk1/2, inhibiting both spontaneous and extrinsically induced apoptosis in neutrophils. MAPK, mitogen-activated protein kinase; TRADD, tumor necrosis factor receptor type 1–associated DEATH domain protein.
Engagement of integrins has been shown to diminish neutrophil apoptosis through activation of PI3-K/Akt and Erk1/2, generating survival signals, including phosphorylation of Bcl-2–associated death promoter (Bad) and Bcl-2–like protein 4 (Bax), followed by dissociation of phosphorylated Bad and Bax from Mcl-1 and stabilization of intracellular levels of Mcl-1 (29, 33). Consistent with the antiapoptotic effects of vitronectin arising from interactions with integrins, the present experiments demonstrated that culture of neutrophils with vitronectin increased activation of PI3-K/Akt and Erk1/2, as well as increased levels of Mcl-1. Moreover, pharmacologic inhibition of PI3-K or Erk1/2 abrogated the antiapoptotic effects of vitronectin.
The ability of vitronectin to diminish neutrophil apoptosis was not limited in these studies to spontaneous cell death, but also was present when apoptosis was induced through engagement of death receptors by TRAIL. The mechanism by which vitronectin diminishes the effects of TRAIL on neutrophil apoptosis may be through activation of Akt, which has been shown to inhibit TRAIL receptor–induced cell death (34, 35).
Under in vivo conditions, vitronectin, primarily through interactions with its SMB domain, is often associated with proteins, such as PAI-1 and the uPAR, that can affect apoptosis of neutrophils and other cell populations (9, 36). However, in the present studies, purified vitronectin SMB domain had no effect on neutrophil apoptosis, whereas mutant vitronectin lacking the SMB domain (vtn ∆SMB) still retained antiapoptotic activity, indicating that the effects of vitronectin on neutrophil viability did not occur through binding of secondary proteins to the SMB domain. The RGD sequence of vitronectin is located adjacent to the SMB domain (37, 38), and is included in the vtn ∆SMB mutant used in these experiments. The ability of the RGD-blocking peptide, RGDfv, to reverse the antiapoptotic effects of vitronectin strongly suggests not only that the RGD domain of vitronectin is responsible for such actions, but also that the effects of vitronectin on neutrophil viability and apoptosis are directly due to interactions between vitronectin itself and integrins, without involvement of vitronectin-binding intermediary proteins.
After LPS-induced lung injury, there was a higher percentage of apoptotic cells among the neutrophils infiltrating the lungs of transgenic mice lacking vitronectin as compared with control mice. These results are consistent with our in vitro experiments showing that vitronectin decreases spontaneous or extrinsically mediated apoptosis in cultured neutrophils, as well as in neutrophils stimulated with LPS. Of note, data analysis for Figure 6C performed with Mann-Whitney U test shows a P value of 0.1; however, a significant difference (P < 0.05) was obtained using Student's t test with the assumption that the variables are normally distributed. Increased concentrations of vitronectin are present in the alveoli and pulmonary interstitium of mice with LPS-induced acute lung injury and in patients with the acute respiratory distress syndrome (7, 8). In these settings, large numbers of neutrophils activated to produce increased amounts of proinflammatory mediators, including cytokines and reactive oxygen species, accumulate in the airways and parenchyma of the lungs (39, 40). Exposure of neutrophils that migrate into the pulmonary interstitium and alveoli to high concentrations of vitronectin may result in diminished apoptosis and prolonged presence in the lungs, thereby enhancing the severity of tissue damage. Of note, diminished neutrophil apoptosis is a well characterized finding during acute lung injury in experimental models and in patients with this condition (39, 41, 42). Furthermore, in addition to its antiapoptotic effects, vitronectin may also exacerbate the severity of acute lung injury through decreasing the uptake and clearance of apoptotic neutrophils by alveolar and tissue-based macrophages (12), thereby allowing the apoptotic neutrophils to progress to necrotic death with associated release of proinflammatory contents into the pulmonary parenchyma.
These experiments describe a novel antiapoptotic function for vitronectin that may contribute to its role in neutrophil-driven proinflammatory processes, such as acute lung injury. Because interactions between vitronectin and multiple integrins appear to be responsible for its actions in delaying neutrophil apoptosis, interventions specifically aimed at the RGD domain of vitronectin may have greater efficacy in reversing the antiapoptotic effects of vitronectin than would therapies directed at blocking individual integrins. Future work will be necessary to explore these issues.
Supplementary Material
Acknowledgments
The authors thank Celeste P. Bell for and Enid Keyser for technical support.
Footnotes
This work was supported in part by National Institutes of Health (NIH) grants HL76206 (E.A.) and GM87748 (J.W.Z.), and American Heart Association grant 10GRNT4430033 (C.B.P.), as well as NIH grant P30 AR 48,311 to the Flow Cytometry Core, Arthritis and Musculoskeletal Center, University of Alabama.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2011-0187OC on January 26, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Duffin R, Leitch AE, Fox S, Haslett C, Rossi AG. Targeting granulocyte apoptosis: mechanisms, models, and therapies. Immunol Rev 2010;236:28–40 [DOI] [PubMed] [Google Scholar]
- 2.Rossi AG, Sawatzky DA, Walker A, Ward C, Sheldrake TA, Riley NA, Caldicott A, Martinez-Losa M, Walker TR, Duffin R, et al. Cyclin-dependent kinase inhibitors enhance the resolution of inflammation by promoting inflammatory cell apoptosis. Nat Med 2006;12:1056–1064 [DOI] [PubMed] [Google Scholar]
- 3.Gilroy DW, Lawrence T, Perretti M, Rossi AG. Inflammatory resolution: new opportunities for drug discovery. Nat Rev Drug Discov 2004;3:401–416 [DOI] [PubMed] [Google Scholar]
- 4.Abraham E, Carmody A, Shenkar R, Arcaroli J. Neutrophils as early immunologic effectors in hemorrhage- or endotoxemia-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol 2000;279:L1137–L1145 [DOI] [PubMed] [Google Scholar]
- 5.Preissner KT, Jenne D. Vitronectin: a new molecular connection in haemostasis. Thromb Haemost 1991;66:189–194 [PubMed] [Google Scholar]
- 6.Tomasini BR, Mosher DF. Vitronectin. Prog Hemost Thromb 1991;10:269–305 [PubMed] [Google Scholar]
- 7.Singh B, Janardhan KS, Kanthan R. Expression of angiostatin, integrin alphavbeta3, and vitronectin in human lungs in sepsis. Exp Lung Res 2005;31:771–782 [DOI] [PubMed] [Google Scholar]
- 8.Tsuruta Y, Park Y-J, Siegal GP, Liu G, Abraham E. Involvement of vitronectin in lipopolysaccaride-induced acute lung injury. J Immunol 2007;179:7079–7086 [DOI] [PubMed] [Google Scholar]
- 9.Blasi F, Carmeliet P. uPAR: a versatile signalling orchestrator. Nat Rev Mol Cell Biol 2002;3:932–943 [DOI] [PubMed] [Google Scholar]
- 10.Reuning U, Magdolen V, Hapke S, Schmitt M. Molecular and functional interdependence of the urokinase-type plasminogen activator system with integrins. Biol Chem 2003;384:1119–1131 [DOI] [PubMed] [Google Scholar]
- 11.Savill J, Dransfield I, Hogg N, Haslett C. Vitronectin receptor–mediated phagocytosis of cells undergoing apoptosis. Nature 1990;343:170–173 [DOI] [PubMed] [Google Scholar]
- 12.Park YJ, Liu G, Lorne EF, Zhao X, Wang J, Tsuruta Y, Zmijewski J, Abraham E. PAI-1 inhibits neutrophil efferocytosis. Proc Natl Acad Sci USA 2008;105:11784–11789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Y, Friggeri A, Banerjee S, Bdeir K, Cines DB, Liu G, Abraham E. Urokinase-type plasminogen activator inhibits efferocytosis of neutrophils. Am J Respir Crit Care Med 2010;182:1516–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schar CR, Blouse GE, Minor KH, Peterson CB. A deletion mutant of vitronectin lacking the somatomedin B domain exhibits residual plasminogen activator inhibitor-1–binding activity. J Biol Chem 2008;283:10297–10309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson LC, Goswami S, Ginsberg DS, Day DE, Verhamme IM, Peterson CB. Metals affect the structure and activity of human plasminogen activator inhibitor-1. I. Modulation of stability and protease inhibition. Protein Sci 2011;20:353–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zmijewski JW, Lorne E, Zhao X, Tsuruta Y, Sha Y, Liu G, Abraham E. Antiinflammatory effects of hydrogen peroxide in neutrophil activation and acute lung injury. Am J Respir Crit Care Med 2009;179:694–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darzynkiewicz Z, Juan G, Li X, Gorczyca W, Murakami T, Traganos F. Cytometry in cell necrobiology: analysis of apoptosis and accidental cell death (necrosis). Cytometry 1997;27:1–20 [PubMed] [Google Scholar]
- 18.Zmijewski JW, Lorne E, Zhao X, Tsuruta Y, Sha Y, Liu G, Siegal GP, Abraham E. Mitochondrial respiratory complex I regulates neutrophil activation and severity of lung injury. Am J Respir Crit Care Med 2008;178:168–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gibson AD, Lamerdin JA, Zhuang P, Baburaj K, Serpersu EH, Peterson CB. Orientation of heparin-binding sites in native vitronectin: analyses of ligand binding to the primary glycosaminoglycan-binding site indicate that putative secondary sites are not functional. J Biol Chem 1999;274:6432–6442 [DOI] [PubMed] [Google Scholar]
- 20.Smith HW, Marshall CJ. Regulation of cell signalling by uPAR. Nat Rev Mol Cell Biol 2010;11:23–36 [DOI] [PubMed] [Google Scholar]
- 21.Rømer MU, Larsen L, Offenberg H, Brünner N, Lademann UA. Plasminogen activator inhibitor 1 protects fibrosarcoma cells from etoposide-induced apoptosis through activation of the PI3K/Akt cell survival pathway. Neoplasia 2008;10:1083–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossignol P, Anglès-Cano E, Lijnen HR. Plasminogen activator inhibitor-1 impairs plasminogen activation–mediated vascular smooth muscle cell apoptosis. Thromb Haemost 2006;96:665–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prager GW, Mihaly J, Brunner PM, Koshelnick Y, Hoyer-Hansen G, Binder BR. Urokinase mediates endothelial cell survival via induction of the X-linked inhibitor of apoptosis protein. Blood 2009;113:1383–1390 [DOI] [PubMed] [Google Scholar]
- 24.Zhao H, Ross FP, Teitelbaum SL. Unoccupied alpha(v)beta3 integrin regulates osteoclast apoptosis by transmitting a positive death signal. Mol Endocrinol 2005;19:771–780 [DOI] [PubMed] [Google Scholar]
- 25.Zhou X, Murphy FR, Gehdu N, Zhang J, Iredale JP, Benyon RC. Engagement of alphavbeta3 integrin regulates proliferation and apoptosis of hepatic stellate cells. J Biol Chem 2004;279:23996–24006 [DOI] [PubMed] [Google Scholar]
- 26.Xu J, Millard M, Ren X, Cox OT, Erdreich-Epstein A. c-Abl mediates endothelial apoptosis induced by inhibition of integrins alphavbeta3 and alphavbeta5 and by disruption of actin. Blood 2010;115:2709–2718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chatterjee S, Brite KH, Matsumura A. Induction of apoptosis of integrin-expressing human prostate cancer cells by cyclic Arg-Gly-Asp peptides. Clin Cancer Res 2001;7:3006–3011 [PubMed] [Google Scholar]
- 28.Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res 2010;339:269–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Filep JG, El Kebir D. Neutrophil apoptosis: a target for enhancing the resolution of inflammation. J Cell Biochem 2009;108:1039–1046 [DOI] [PubMed] [Google Scholar]
- 30.Stefansson S, Lawrence DA. The serpin PAI-1 inhibits cell migration by blocking integrin alpha V beta 3 binding to vitronectin. Nature 1996;383:441–443 [DOI] [PubMed] [Google Scholar]
- 31.Isik FF, Gibran NS, Jang YC, Sandell L, Schwartz SM. Vitronectin decreases microvascular endothelial cell apoptosis. J Cell Physiol 1998;175:149–155 [DOI] [PubMed] [Google Scholar]
- 32.Uhm JH, Dooley NP, Kyritsis AP, Rao JS, Gladson CL. Vitronectin, a glioma-derived extracellular matrix protein, protects tumor cells from apoptotic death. Clin Cancer Res 1999;5:1587–1594 [PubMed] [Google Scholar]
- 33.Saldanha-Gama RF, Moraes JA, Mariano-Oliveira A, Coelho AL, Walsh EM, Marcinkiewicz C, Barja-Fidalgo C. Alpha(9)beta(1) integrin engagement inhibits neutrophil spontaneous apoptosis: involvement of Bcl-2 family members. Biochim Biophys Acta 2010;1803:848–857 [DOI] [PubMed] [Google Scholar]
- 34.Lane D, Goncharenko-Khaider N, Rancourt C, Piche A. Ovarian cancer ascites protects from TRAIL-induced cell death through alphavbeta5 integrin–mediated focal adhesion kinase and Akt activation. Oncogene 2010;29:3519–3531 [DOI] [PubMed] [Google Scholar]
- 35.Li P, Jayarama S, Ganesh L, Mordi D, Carr R, Kanteti P, Hay N, Prabhakar BS. Akt-phosphorylated mitogen-activated kinase-activating death domain protein (MADD) inhibits TRAIL-induced apoptosis by blocking Fas-associated death domain (FADD) association with death receptor 4. J Biol Chem 2010;285:22713–22722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu J, Peng L, McMahon GA, Lawrence DA, Fay WP. Recombinant plasminogen activator inhibitor-1 inhibits intimal hyperplasia. Arterioscler Thromb Vasc Biol 2009;29:1565–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh B, Su Y-C, Riesbeck K. Vitronectin in bacterial pathogenesis: a host protein used in complement escape and cellular invasion. Mol Microbiol 2010;78:545–560 [DOI] [PubMed] [Google Scholar]
- 38.Chillakuri CR, Jones C, Mardon HJ. Heparin binding domain in vitronectin is required for oligomerization and thus enhances integrin mediated cell adhesion and spreading. FEBS Lett 2010;584:3287–3291 [DOI] [PubMed] [Google Scholar]
- 39.Abraham E. Neutrophils and acute lung injury. Crit Care Med 2003; 31(4, Suppl)S195–S199 [DOI] [PubMed] [Google Scholar]
- 40.Parsey MV, Tuder RM, Abraham E. Neutrophils are major contributors to intraparenchymal lung IL-1 beta expression after hemorrhage and endotoxemia. J Immunol 1998;160:1007–1013 [PubMed] [Google Scholar]
- 41.Matute-Bello G, Liles WC, Radella F, II, Steinberg KP, Ruzinski JT, Jonas M, Chi EY, Hudson LD, Martin TR. Neutrophil apoptosis in the acute respiratory distress syndrome. Am J Respir Crit Care Med 1997;156:1969–1977 [DOI] [PubMed] [Google Scholar]
- 42.Parsey MV, Kaneko D, Shenkar R, Abraham E. Neutrophil apoptosis in the lung after hemorrhage or endotoxemia: apoptosis and migration are independent of interleukin-1beta. Chest 1999;116(1 Suppl)67S–68S [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.