Abstract
Pulmonary endothelial functions are critical to maintain the low pressure of the pulmonary circulation and effective diffusion capacity of the lung. To investigate pulmonary endothelial cell biology in healthy or diseased lungs, we developed methods to harvest and culture pure populations of primary pulmonary arterial endothelial cells and microvascular endothelial cells from human lung explanted at time of transplantation or from donor lungs not used in transplantation. The purity and characteristics of cultured endothelial cells is ascertained by morphologic criteria using phase contrast and electron microscopy; phenotypic expression profile for endothelial specific proteins such as endothelial nitric oxide synthase, platelet/endothelial cell adhesion molecule, and von Willbrand factor; and endothelial function assays such as Dil-acetylated low-density lipoprotein uptake and tube formation. This detailed method provides researchers with the ability to establish cells for molecular, genetic, and biochemical investigation of human pulmonary vascular diseases.
Keywords: lung, endothelium, smooth muscle cells, microvascular cells, pulmonary hypertension
Pulmonary endothelium plays an important role in vascular homeostasis and in the ventilation perfusion matching that enables efficient gas exchange in the lungs (1–4). Given the important role of endothelial cells in healthy or diseased lungs, cultured pulmonary arterial endothelial cells (PAECs) and microvascular endothelial cells (MVECs) are useful for elucidation of cellular and molecular mechanisms of vascular biology. However, the lung is structurally complex, and the diversity of cell types from which it is composed greatly complicates the isolation and culture of pure PAECs and MVECs. Cell culture models thus far have used animal-derived endothelial cells from bovine, murine, or rodent sources (5, 6). A few previous studies have demonstrated that human PAECs and MVECs can be isolated for in vitro studies (7–11). However, the limited numbers of reports may reflect the difficulties associated with isolation, purity, and maintenance of these cells in culture (9). The most common human endothelial cell culture system is derived from the human umbilical vein due to the ease of retrieval of endothelial veins and simplicity of harvest. However, venous endothelium may differ substantially from arterial or capillary endothelial function.
Pulmonary arteries are of three types based on histology and diameter: elastic, muscular, and nonmuscular (12, 13). The main pulmonary artery and its branches to a diameter of approximately 500 μm have an elastic structure with interrupted elastic fibers around medial smooth muscle. Muscular arteries (70–500 μm) have circumferential smooth muscle bound by internal and external elastic lamina. From diameters 30 to 150 μm, the continuous muscle gives rise to a spiral, so arteries are partially muscular with a layer of connective tissue with embedded pericytic cells (12). Arteries less than 70 μm in diameter generally are nonmuscular arterioles and extend into the alveolar capillaries (12).
Primary PAECs derived from elastic and muscular arteries and MVECs derived from nonmuscular arterioles and extending into the alveolar capillaries may be harvested from explanted diseased lungs and from donor lungs not used for transplantation. Here, we provide information on methodologies that we have established to harvest and culture pure populations of primary PAECs and primary pulmonary MVECs for the study of human pulmonary vascular diseases. The purity and characteristics of cultured endothelial cells is ascertained by morphologic criteria using phase contrast and electron microscopy; phenotypic expression profile for endothelial specific proteins such as endothelial nitric oxide synthase (eNOS), platelet/endothelial cell adhesion molecule (PECAM-1, or CD31), and von Willbrand factor (vWF); and endothelial function assays such as Dil-acetylated low-density lipoprotein uptake and tube formation.
Materials and Methods
All explanted lungs were collected at the Cleveland Clinic, and Institutional Review Board approval was obtained. To maintain sterility and to avoid contamination of the cell cultures, all work was performed using aseptic techniques in the sterile environment of a tissue culture hood. Additionally, all glassware and plastics used for cell culture were opened in a sterile work environment. This protocol is detailed to give investigators the ability to study more reliably the role of pulmonary endothelial cells in lung vascular diseases (14–24).
Dissection of Pulmonary Artery Branches and Tissue
Pulmonary arteries to the third and fourth branches were dissected from explanted pulmonary arterial hypertension (PAH) lungs and donor lungs not used for transplant. The main artery, the first and second branches, the third branch, and the fourth branch were dissected from the explanted lungs and placed in a conical tube containing Dulbecco’s modified Eagle medium (DMEM) with a 1% penicillin/streptomycin/ fungizone solution (Invitrogen, Grand Island, NY). The tissue used for culturing Microvascular endothelial cells was obtained from peripheral lobes. The lung pleura and the outer most layer of tissue (0.5 cm) were removed with a scalpel to reduce the possible cell contamination. The remaining tissue was minced in small pieces and placed in a conical tube containing DMEM with a 1% penicillin/streptomycin/ fungizone solution (Invitrogen).
Isolation and Culturing of Pulmonary Vascular Cells: PAECs, Smooth Muscle Cells, and MVECs
Within 5 to 20 hours of surgery, human PAECs were isolated from elastic and muscular arteries, and microvascular endothelial cells were isolated from lung tissue. The culturing procedure for PAECs was derived from modifications of general endothelial culture methods originally described by Hoshi and McKeehan (25) and Thornton and colleagues (26), whereas the method for microvascular endothelial cell culture was based on Kessler and colleagues (27). The pulmonary arterial smooth muscle cells were cultured using a modified collagenase/DNase digestion protocol (28).
PAECs.
After careful removal of the excess fat and connective tissue, the main artery was cut into 2-cm pieces, and the smaller arteries are cut into 3- to 4-cm pieces. The arteries were rinsed three times with Hanks’ balanced salt solution (Invitrogen) to remove unwanted blood cells. The arteries were placed in 10 to 15 ml phosphate-buffered saline (PBS) containing 2 mg/ml type II collagenase (Worthington Biochem, Lakewood, NJ) and kept at 37°C and 5% CO2 with 90% humidity for 20 minutes. The arteries were massaged with a spatula followed by gentle shaking to detach the endothelial cells into MCDB-107 medium containing 14.8 g/l MCDB-105 (Sigma), 90 mg/l heparin (Sigma), 115 mg endothelial cell growth supplement (Sigma), 15.1 mg/l glycine, 149 mg/l potassium chloride (KCl), 10% heat-inactivated FBS (Lonza, Walkersville, MA), and 1% penicillin/streptomycin/fungizone solution (Invitrogen) with pH 7.3. After removal of the arterial segments, the endothelial cells were collected by centrifugation at 330 × g for 7 minutes at room temperature. The cell pellet was resuspended in MCDB-107 medium and seeded on fibronectin (1 mg/cm2) (CalBiochem, La Jolla, CA)-coated cell culture plates. The cells were incubated at 37°C, 5% CO2 with 90% humidity followed by media changes at 24 hours and every 3 days until confluence.
MVECs.
After removal of lung pleura and the outermost layer of tissue (0.5 cm), the tissue was cut into 2- to 4-cm sections and digested in 15 ml of PBS containing 2 mg/ml type II collagenase (Worthington Biochem, Lakewood, NJ) at 37°C and 5% CO2 with 90% humidity for 20 minutes. After the enzymatic digestion, the fragments were transferred into a sterile Petri dish containing endothelial MCDB 107 growth medium. Mechanical compression with the blunt edge of a no. 21 scalpel blade was applied to the center of each tissue fragment, squeezing in an outward direction to express clusters of MVECs. The culture medium containing expressed cells was filtered through a sterile 70-μm pore nylon cell strainer to remove tissue fragments. The filtered cells were suspended in 10 ml of MCDB-107 medium. This cell suspension, containing clusters of endothelial cells, was passed through a second nylon filter with a 20-μm pore diameter. Trapped cells, enriched for endothelial clusters, were flushed off the filter with MCDB-107 medium, collected, and centrifuged at 330 × g for 7 minutes at room temperature. The cell pellet was resuspended in MCDB-107 medium and seeded on fibronectin (1 mg/cm2)-coated cell culture plates. The cells were incubated at 37°C, 5% CO2 with 90% humidity followed by media changes at 48 hours and every 4 days until confluence.
Pulmonary arterial smooth muscle cells.
After removing the PAECs, the arteries were minced and digested overnight in 100 ml of Hanks’ balanced salt solution containing collagenase and DNase (0.1 mg/ml each), 2.5 ml Hepes buffer (Sigma), penicillin (250 units/ml), streptomycin (250 mg/ml), and fungizone (0.625 mg/ml) (Invitrogen). The liberated cells were filtered from the undigested debris with a 100-μm-pore nylon cell strainer (BD Falcon, Bedford, MA), cultured in DMEM/F-12 medium supplemented with 10% FBS (Bio-Whittaker, Walkersville, MD) and 2.5% penicillin/streptomycin/ fungizone solution antibiotics, and incubated at 37°C, 5% CO2 with 90% humidity follow by media changes at 24 hours and every 4 days until confluence. Cultured smooth muscle cells obtained by this method routinely stain positively for α-smooth muscle cell actin (Sigma/Aldrich, St. Louis, MO).
Subculture of Human PAECs and MVECs
Primary endothelial cultures that form uniform monolayers were selected for subculturing. To detach, the cells were washed with PBS followed by incubation with 1 ml of 0.05% trypsin-EDTA (Invitrogen) at 37°C. The cells were closely monitored on an inverted microscope to determine the optimal length of time of exposure to the trypsin-EDTA solution. Detached cells were transferred to a conical tube containing 5 ml of MCDB-107 and centrifuged at 330 × g for 7 minutes. A confluent 100-mm tissue culture plate was split into five plates with MCDB-107 medium.
Immunohistochemistry
PAECs and MVECs.
CD31 was identified in cells by immunohistochemistry. Cultures were grown on Lab Tek II chamber slide systems, fixed with 4% paraformaldehyde in PBS (pH 7.4), and rinsed with PBS. Mouse monoclonal anti-CD31 Ab (1:80; Dako, Carpinteria, CA) was applied to the surface of the slide. Diaminobenzidine (Dako) and hydrogen peroxide were applied to develop color. Immunostaining was performed by an automated biotin-avidin peroxidase system (Ventana-320-ES; Ventana Medical Systems, Tucson, AZ). Negative control of secondary antibody alone was performed on each section of tissue studied. All slides were counterstained with hematoxylin.
Lung tissue.
Immunohistochemical detection of eNOS and CD31 was performed as previously described (23). Briefly, histologic sections of formalin-fixed, paraffin-embedded lung tissues were dehydrated followed by antigen retrieval with Antigen Retrieval Citra Plus (Bio Genex, San Ramon, CA). The rabbit polyclonal antibody directed against eNOS (PA1–037; Affinity Bioreagents, Golden, CO) (1:30) and the mouse monoclonal anti-CD31 Ab (1:80; Dako) were used. Diaminobenzidine and hydrogen peroxide were applied to develop color. Immunostaining was performed by an automated biotin-avidin peroxidase system (Ventana-320-ES; Ventana Medical Systems). Negative control of secondary antibody alone was performed on each section of tissue studied. All slides were counterstained with hematoxylin.
Fluorescence Histochemistry for Confocal Microscopy
PAEC and MVEC.
Cultures were grown on Lab Tek II chamber slide systems fixed with 4% paraformaldehyde in PBS (pH 7.4) and rinsed with PBS. After a 15-minute incubation in PBS with 2% FBS, rabbit anti-human von Willebrand Factor VIII-related antigen (vWF) (1:200), FITC-labeled G. simplicifolia (1:1,000), and FITC-labeled H. pomatia (1:1,000) (Sigma) were applied and incubated at room temperature for 1 hour in the dark. The slides were washed with PBS, and coverslips were affixed to the slides in Vectashield mounting medium containing 4,6-diamindino-2-phenylindole (Vector Laboratories, Youngstown, OH) as a nuclear stain.
Lung tissue.
Histologic sections of formalin-fixed, paraffin-embedded lung tissues were rehydrated followed by antigen retrieval with a preheated citrate buffer (Dako). After a 15-minute incubation in PBS with 2% FBS, the FITC-labeled Griffonia simplicifolia and the FITC-labeled Helix pomatia (Sigma) in a 1:1,000 dilution, rabbit anti-human vWF in a dilution of 1:200 were applied and incubated at room temperature for 1 hour in the dark. The slides were washed three times with PBS and incubated with Alexa-633 conjugated anti-rabbit antibody (1:1,000) in PBS containing 2% FBS for 1 hour at room temp. The slides were washed with PBS, and coverslips were affixed to the slides in Vectashield mounting medium containing 4,6-diamindino-2-phenylindole (Vector Laboratories) as a nuclear stain. Confocal images were obtained using a Leica TCS-SP laser-scanning confocal microscope (Leica, Heidelberg, Germany), which was equipped with three lasers and photo detectors that permit detection of three distinct fluorochromes.
Ultrastructural Analyses by Electron Microscopy
An ultrastructural analysis of pulmonary arterial cells and lung tissue was performed as previous described (24). Briefly, cells and tissue were fixed at 4°C for 1 hour in 0.1 M sodium cacodylate buffer (pH 7.4) containing 2.5% glutaraldehyde and 4% formaldehyde. After washing three times, cells were postfixed with 1% aqueous osmium tetroxide for 1 hour at 4°C, washed in sodium cacodylate buffer followed by maleate buffer (pH 5.1), and dehydrated with ascending grades of ethanol and propylene oxide. Samples were embedded using a Eponate12 kit (Ted Pella, Inc., Redding, CA), polymerized at 70°C for 48 hours, trimmed, sectioned at 70 to 90 nm, poststained in saturated uranyl acetate and lead citrate, and examined with a transmission electron microscope (Philips CM12; Philips, Eindhoven, The Netherlands).
Western Blot Analysis
Cytosolic extracts of PAECs were prepared according to the manufacturer's protocol (Panomics Inc., Fremont, CA). Whole cell lysates were prepared as previously described (23). Protein was separated by electrophoresis on a 4 to 15% Tris-HCl precast gel (Bio-Rad Lab, Hercules, CA) and transferred onto polyvinylidene difluoride membranes (Millipore Corp., Bedford, MA). Alternatively, XT Precast Gels (Bio-Rad) and 3-(N-morpholino) propanesulfonic acid buffer were used for SDS-PAGE. Antibodies used for Western analyses included an anti-eNOS Ab (Affinity Bioreagents). Monoclonal anti-mouse Caveolin Ab (1:250; BD Transduction Laboratories) was used as a protein-loading marker.
Endothelial Cell Low-Density Lipoprotein Uptake
The ability to identify endothelial cells based on their specific uptake of acetylated low-density lipoprotein (Ac-LDL) was examined using Ac-LDL labeled with the fluorescent probe 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine. Cultures grown on Lab Tek II chamber slide systems were incubated with 15 μg/ml Dil-Ac LDL (Invitrogen) in growth medium for 20 minutes, washed with PBS (pH 7.4), and immediately observed by inverted fluorescence microscopy using a rhodamine filter.
In Vitro Tube Formation Assay
The angiogenic potential of the cultured endothelial cells was evaluated by using a matrigel endothelial cell tube formation assay (Chemicon International, Temecula, CA) (22). Tube formation was complete between 0 and 8 hours and evaluated by measuring capillary tube length, width, total length of the network, and numbers of branches in three random fields using Image-Pro Plus software (MediaCybernetics, Silver Spring, MD).
Flow Cytometric Analysis
The purity of endothelial cells was evaluated by staining with CD31- FITC and vascular endothelial growth factor receptor 2–FITC monoclonal antibodies. The purity of smooth muscle cells was evaluated by staining with α-smooth muscle actin PE. Isotype-matched irrelevant antibodies were used as negative controls for nonspecific binding. Samples were analyzed on a FACScan (Becton Dickinson) flow cytometer. Events (0.5 × 106) were acquired, and data analysis was performed using Cell-Quest 3.3 software (Becton Dickinson).
Gene Expression
Human PAECs (n = 14) and MVECs (n = 5) were harvested for RNA at passage 5 or 6 by direct disruption in Qiazol (Qiagen, Valencia, CA) and stored at −80°C. RNA was extracted using the miRNeasy kit (Qiagen) according to the recommended protocol. Samples were labeled and hybridized to Illumina HT-12 or Human-Ref8 expression arrays (Illumina, San Diego, CA). A common probe-set for the two arrays was derived, enabling the data to be combined. Analysis was performed using Genespring software (Agilent, Santa Clara, CA).
Results
Morphological Assessment
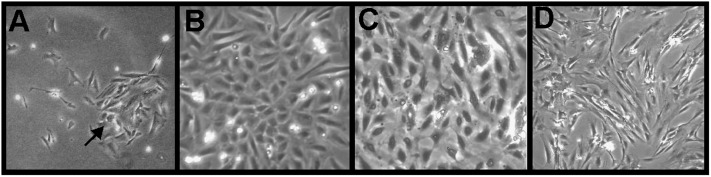
Cells were initially identifiable as endothelial cells according to morphological criteria. Twenty-four hours after the initial seeding, examination by inverted phase contrast microscopy displayed a typical “rosette cobblestone” morphology (Figure 1A). Although some nonendothelial cells were seen in early passage cultures (Figure 1A), the specific heparin-rich growth medium enriches for endothelial cell growth and inhibits contaminant cells quickly. A uniform characteristic cobblestone appearance of pulmonary arterial endothelial cells (Figure 1B) and microvascular endothelial cells (Figure 1C) was seen after passage 5. Culture of pulmonary arterial smooth muscle cells derived from the pulmonary arteries exhibited a characteristic “hill and valley” appearance (Figure 1D).
Figure 1.
Morphology of endothelial cells and smooth muscle cells. Phase-contrast microscopic analysis of primary pulmonary arterial endothelial cells shows a rosette cobblestone morphology (arrow) (A). Confluent pulmonary arterial endothelial cells (B) and microvascular endothelial cells (C) at passage 5 demonstrating the characteristic cobblestone appearance of endothelial cells. Phase-contrast microscopic of primary pulmonary arterial smooth muscle cells (D). Endothelial cells and smooth muscle cells shown in this figure were initiated from pulmonary arterial hypertension (PAH) lungs. There were no morphological differences between endothelial cells or smooth muscle cells derived from PAH or control lungs.
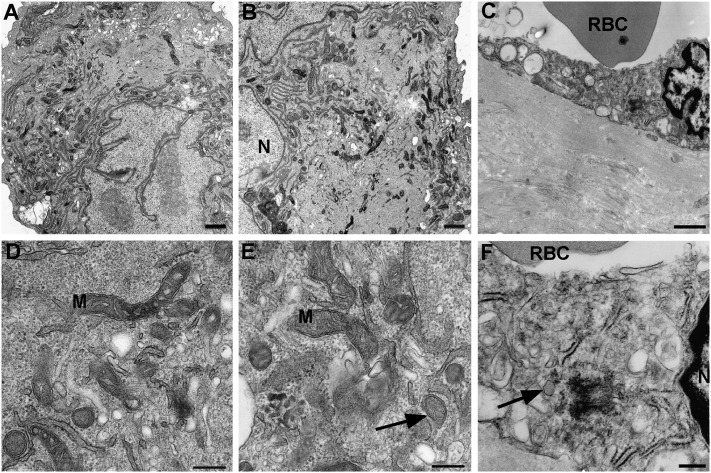
Transmission electron microscopy of PAH cells revealed characteristic features of endothelium, including the oval-shaped bodies described by Weibel and Palade as well as in endothelium in vivo (Figure 2). PAECs from pulmonary hypertension lungs had lower mitochondria numbers as compared with donor lungs (Figure 2) (24).
Figure 2.
Ultrastructure of control and PAH pulmonary arterial endothelial cells and PAH lung tissue. Electromicroscopy of culture control (A, D) and PAH pulmonary arterial endothelial cells (B, E) and PAH lung tissue (C, F). Ultrastructure detail of endothelial cells in vitro (A, B, D, E) and in vivo (C, F) (scale bar: 1 μm). Weibel-Palade bodies in cultured endothelial cell (E) and endothelial cell of lung tissue (F) (arrows) (scale bar: 250 nm). M = mitochondria; N = nucleus; RBC = red blood cells.
Phenotypic Assessment
Protein expression by immunohistochemistry.
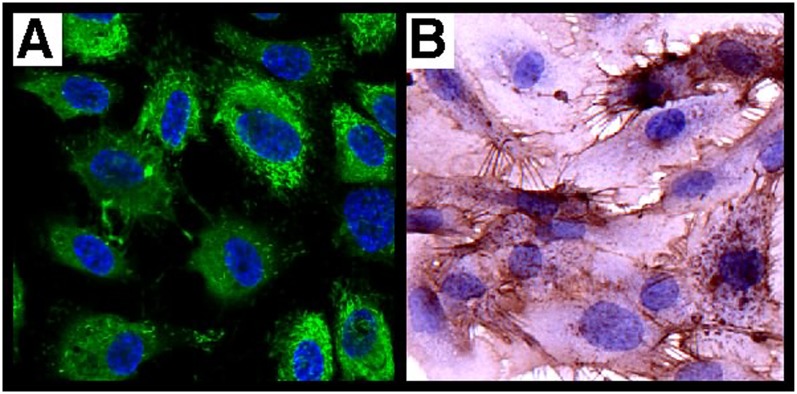
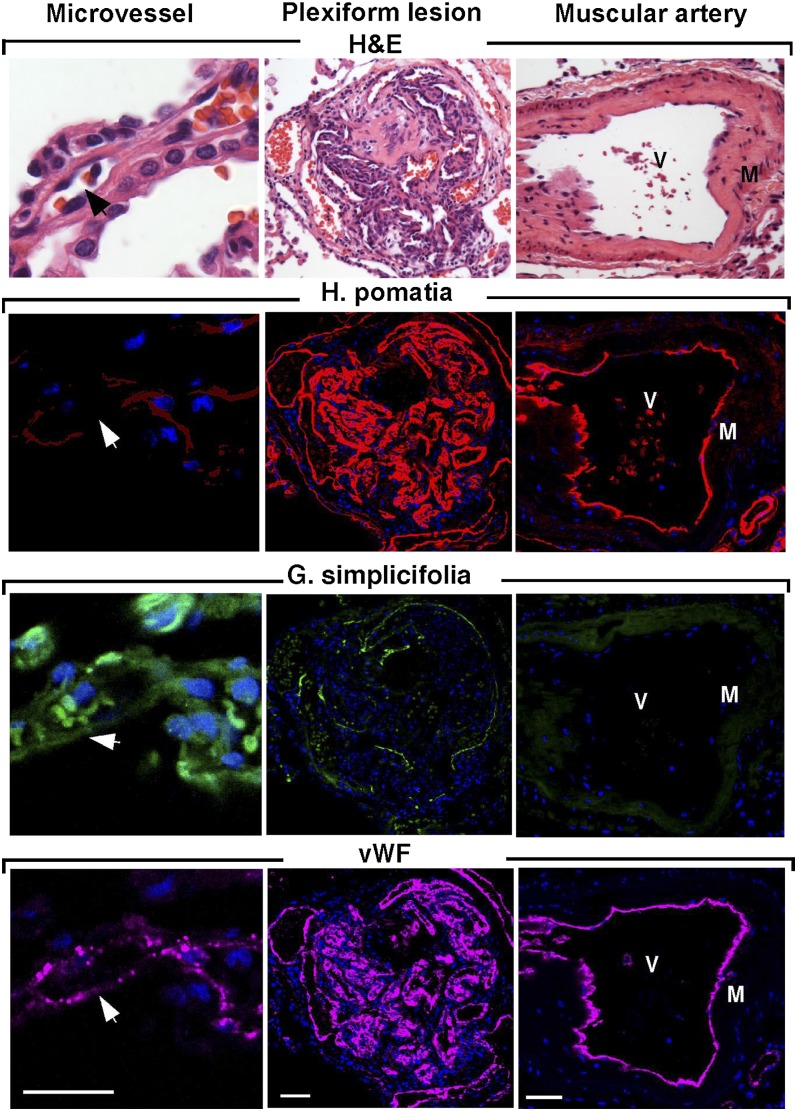
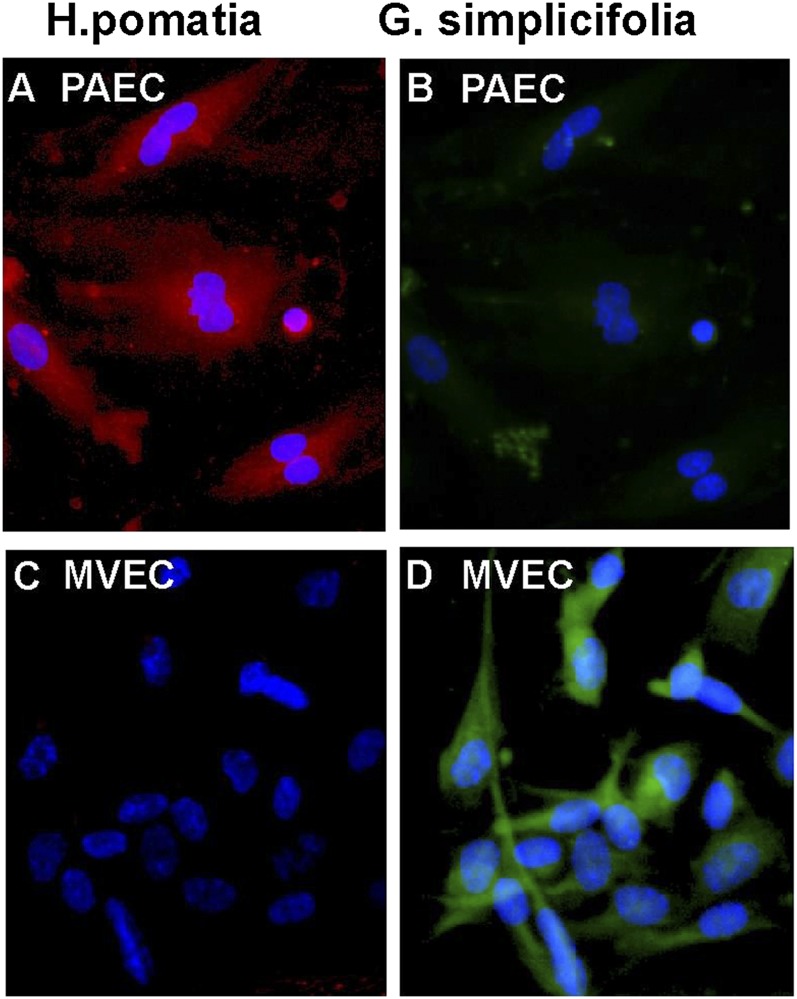
vWF is a classic marker of endothelial cells and is only expressed at significant levels in endothelial cells and platelets (8). PECAM-1 is a universal and highly specific marker of endothelium in tissue sections (29, 30) and isolated cells because it is expressed only on leukocytes (8, 29). In cultured endothelial cells, CD31 was located at intercellular junctions. Analogous to immunohistochemical staining of lungs (22), primary cells were positive for CD31 and showed the characteristic pattern of vWF expression (Figure 3). Lectin binding pattern discriminates between pulmonary arterial cells and microvascular endothelial cells (5). Microvascular endothelium in vivo binds G. simplicifolia lectin, and pulmonary arterial endothelium binds H. pomatia lectin (Figure 4). The majority of endothelial cells in the plexiform lesion of PAH bind H. pomatia, indicating the majority of endothelial cells in the lesion, were more similar to large vessel endothelium. There was a small number of channels in the plexiform lesion lined by endothelial cells that bind G. simplicifolia, indicating a microvascular phenotype (Figure 4). In primary cells isolated from PAH lungs and donor lungs, PAECs bound H. pomatia and MVECs bound G. simplicifolia (Figure 5). These findings indicate success in retrieval of pure populations of PAECs and MVECs that accurately reflect the lectin-binding and protein expression pattern of cells in vivo.
Figure 3.
Phenotype of primary pulmonary arterial endothelial cells. Characterization of pulmonary arterial cells ascertained by morphologic and immunohistochemistry analyses of endothelial markers (CD31, von Willebrand factor). (A) Immunofluorescence for von Willebrand factor (green) and (B) CD31 (brown) by immunohistochemistry. Representative image is from PAH cells. Hence, control pulmonary arterial endothelial cells have the same phenotype.
Figure 4.
Endothelial cell types in vessels and plexiform lesions in PAH. Sequential sections for hematoxylin and eosin (H&E), Helix pomatia (red), Griffonia simplicifolia (green), and vWF (purple). H. pomatia binds to endothelium of muscular artery, whereas G. simplicifolia binds to endothelium of microvessels. Red blood cells also bind G. simplicifolia. White arrows and black arrows indicate capillaries. M = smooth muscle; V = vascular lumen of large muscular artery. Scale bar: 100 μm.
Figure 5.
Lectin binding of microvascular endothelial cells (MVECs) and pulmonary artery endothelial cells (PAECs) in vitro. (A–D) Immunofluorescent H. pomatia (red) binds PAECs in vitro (A) but not MVECs (C). G. simplicifolia (green) binds MVECs in culture (D) but not to PAECs (B). Representative image is from PAH cells. Hence, control PAECs have the same phenotype.
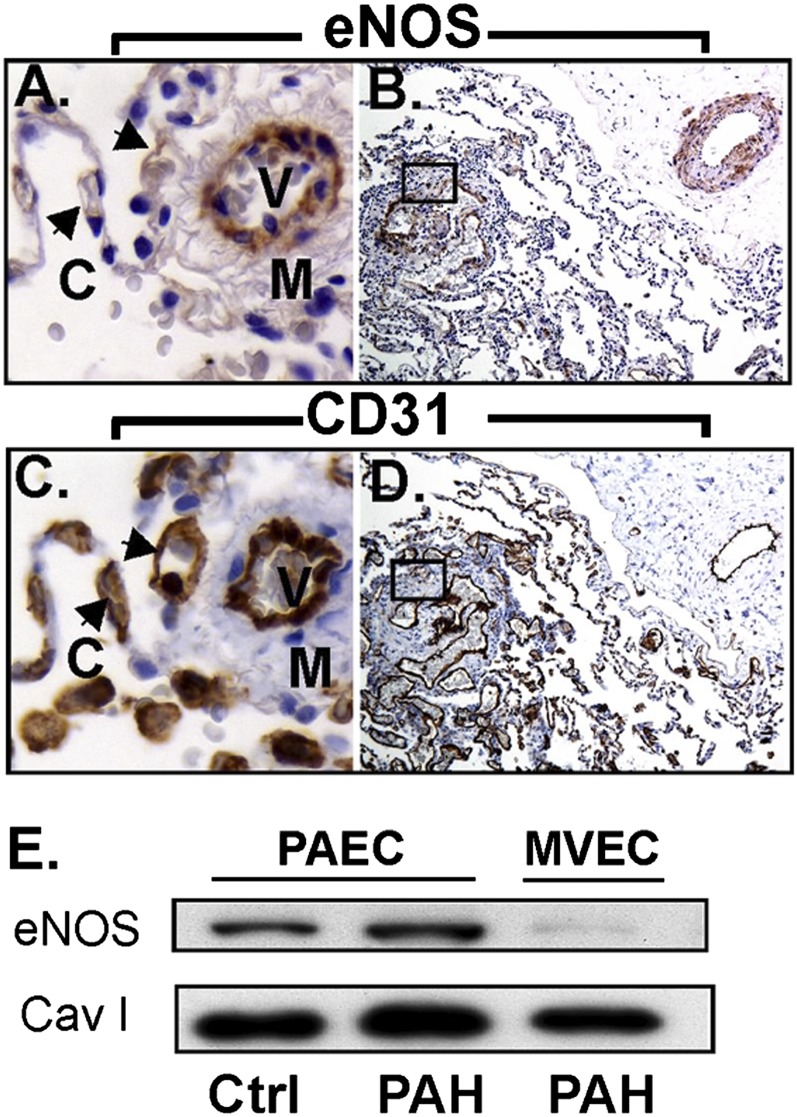
eNOS is capable of synthesizing NO in vascular endothelial cells and plays an important role in the control of vasomotor tone (31–33). Lung biopsy tissue from control and PAH lungs generally shows that vascular endothelial cells of muscular arteries are eNOS positive (Figure 6A). However, eNOS staining in alveolar capillaries is absent, although adjacent muscular vessels express eNOS (Figure 6A). Plexiform lesions are lined with endothelial cells that are mostly positive for eNOS, again suggesting a mixed population of endothelial cells with large and small vessel characteristics (Figure 6B); endothelial cells are confirmed by positivity for CD31(Figures 6C and 6D). Cultured primary endothelial cells express eNOS, whereas the microvascular endothelial cells have low or undetectable eNOS expression (Figure 6E).
Figure 6.
Endothelial nitric oxide synthase (eNOS) expression in PAECs and MVECs. (A, B) Immunohistochemistry shows strong eNOS staining in the muscular vessels where the staining in alveolar capillaries (arrows) is absent. The plexiform lesion (A) is lined with endothelial cells that are mostly positive for eNOS, but some cells are negative. (C, D) Sequential section confirms endothelial cells by CD31 and in plexiform lesions. (E) Western blot analysis shows that control and PAH PAECs express eNOS, whereas MVECs have undetectable or weak expression of eNOS. C = capillaries; M = smooth muscle; V = vessel.
Purity of cells as assessed by flow cytometry.
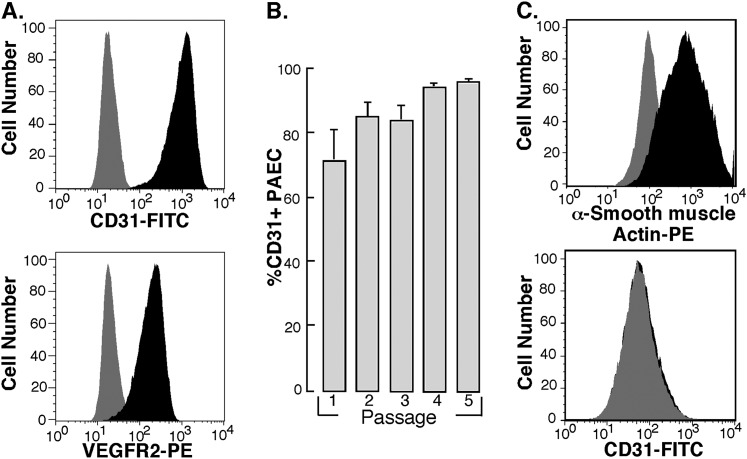
Fluorescence- activated cell sorting (FACS) was used to analyze the purity of cultured endothelial cells. Cultured endothelial cells at passage 5 were positive for cell surface expression of endothelial cell–specific markers CD31 and vascular endothelial growth factor receptor 2 (Figure 7A). After the first passage of harvested cells (passage 1), more than 70% of cells were CD31 positive, and by passage 3 more than 90% of cells were CD31 positive (Figure 7B). The cultured pulmonary arterial smooth muscle cells were positive for α-smooth muscle actin and negative for CD31 (Figure 7C). Furthermore, FACS analysis shows that the cultured endothelial cells were simultaneously negative for α-actin, excluding contamination with smooth muscle cells or fibroblast (data not shown).
Figure 7.
Flow cytometric analysis of endothelial cell surface antigen expression on PAECs. Confluent cell cultures were harvested by trypsinization and stained for cell surface expression of endothelial cell specific markers CD31 and vascular endothelial growth factor receptor 2. (A) Representative profiles for each staining is shown. Gray histograms indicate staining with isotype-matched control antibodies. (B) Purity of primary PAECs, as measured by %CD31-positive cells, increases during passaging of the cells. Cells were more than 95% CD31 positive by passage 4. Passage 1, n = 7; passage 2, n = 14; passage 3, n = 15; passage 4, n = 13; passage 5, n = 31. (C) The smooth muscle cells cultured from pulmonary arteries stained positive for smooth muscle cell α-actin and negative for CD31. Representative profiles for each staining is shown. Gray histograms indicate staining with isotype-matched control antibodies. Representative image is from PAH cells. Control pulmonary arterial endothelial cells have the same phenotype.
Gene-expression profile.
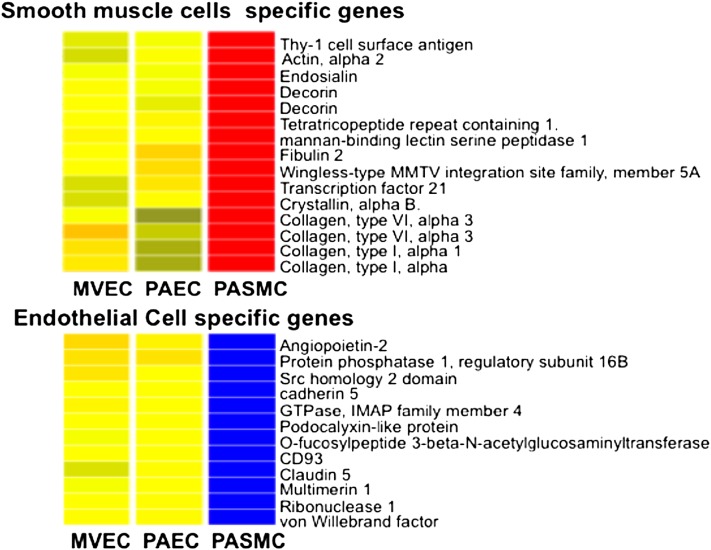
Array analysis confirmed high levels of expression of endothelial markers in PAECs and MVECs, including PECAM1, vWF, tyrosine kinase with immunoglobulin and epidermal growth factor homology domains 1 (TIE1), vascular endothelial cadherin (CDH5), endothelial lipase (LIPG), and apelin. Conversely, smooth muscle markers, such as actin, decorin, multiple collagens, and platelet-derived growth factor receptors A, and B were not expressed in PAECs or MVEC. Subtle differences in gene expression between PAECs and MVEC were detected, but no strongly distinguishing gene signature was detected (Figure 8). Genes differentially expressed between these two cell types include insulin-like growth factor binding protein 5, glycophorin C, and peroxisome proliferator-activated receptor γ. The lack of unique gene expression among PAECs and MVECs suggests that differential lectin binding maybe the best discriminator between these two cell types.
Figure 8.
Gene expression analysis. Hierarchical clustering of gene expression array data from PAECs (n = 14), MVECs (n = 5), and pulmonary artery smooth muscle cells (PASMCs) (n = 4) demonstrates that PAECs and MVECs have closely related gene expression signatures but are distinctly different from PASMCs. Color coding denotes relative gene expression on a continuous scale from blue (lowest expression), through yellow (similar expression levels) to red (highest).
Functional Assessment
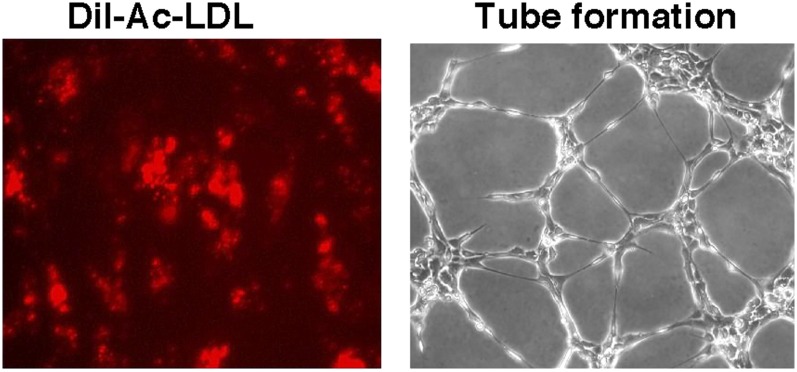
The uptake and metabolism of LDL is a primary function of endothelial cells. Endothelial cells derived from control and PAH lungs are active in Dil-Ac-LDL uptake (Figure 9A), and their pattern of lectin binding reflected lectin binding of vascular sites in vivo. The pattern of lectin binding did not change in cultures over time in passages 5 through 7. The primary endothelial cells are also able to form tube like structures on an extracellular matrix (Figure 9B) (see Movie E1 in the online supplement). Prior work has shown that tube formation is dysregulated in PAH PAECs (22).
Figure 9.
Functional assays. (A) Accumulation of acetylated low-density lipoprotein (Dil-Ac-LDL) by primary PAECs (passage 5). (B) Capillary-like tubule formation produced by primary PAECs 8 hours after plating onto Matrigel. Representative image is from control cells. Hence, PAH PAECs have the same phenotype.
Discussion
The majority of studies involving cultured pulmonary endothelial cells have used animal-derived cells because of the difficulties in isolating and maintaining human pulmonary endothelial cells in culture. Here, we describe methods for successful isolation and culturing of primary PAECs and primary human MVECs from unused donor lungs as well as explanted lungs of pulmonary hypertension origin. The success of this method is based on three major components: (1) early access to lung tissue, (2) initial careful and rapid dissection, and (3) selection of cell population by culture conditions containing growth medium enriched with heparin (25, 26). To identify endothelial cells, many different morphological, ultrastructural, molecular, and biochemical criteria are needed. Characterization of endothelial cells based upon morphology is not selective. The cobblestone appearance of confluent endothelial cells is not unique to endothelial cells but is also a characteristic of cultured mesothelial cells (8). Chung-Welch and colleagues reported that endothelial cells could be distinguished from mesothelial cells, which do not have the ability to form capillary-like tube structures when plated onto a Matrigel (34). The uptake of acetylated LDL has been used to distinguish endothelial cells from fibroblasts, smooth muscle cells, and pericytes (35). Transmission electron microscopy reveals that cultured endothelial cells contained structures resembling discoid bodies, described by Weibel and Palade, as seen as in endothelial cells of the vascular lumen (36, 37).
CD31, vWF, and eNOS are among the markers that are highly specific for endothelium in lung tissue (29, 30). The endothelial cells isolated by this procedure manifest these phenotypic characteristics of endothelial cells found in vivo. The absence of certain markers, such as smooth muscle α-actin, are equally important to rule out contaminating cells in cultures (38, 39). The gene expression array analysis confirms high expression of multiple endothelial markers and the lack of smooth muscle markers; there are 922 genes with a greater than 10-fold difference in expression between the cultured endothelial cells and pulmonary artery smooth muscle cells. Although there were only five paired samples of artery and microvessel cells available for study, there were some differences found in gene expression among endothelial cells from pulmonary artery in comparison to microvessels harvested from the same lung. Overall, 177 genes out of 15,251 total genes evaluated were significantly different between the two cell types, indicating that endothelial cells derived from large and small vessels have variance in expression profiles as previously described (40). The lesser variance seen in this study as compared with the study by Chi and colleagues (40) may be due to the fact that PAECs and MVECs in this study were derived from the same individual, whereas the endothelial cells in the previous study were obtained from commercial sources and different biologic donors.
Lectin binding has previously been used as an effective method of discriminating between macro- and microvascular endothelial cells (5). Our data show that G. simplicifolia selectively interacts with MVECs in vivo and in vitro, whereas PAECs interact with H. pomatia. The majority of endothelial cells in the lesional endothelium of PAH bind H. pomatia and immunostain for eNOS, indicating that the majority of endothelial cells in the plexiform lesion are of pulmonary arterial endothelial origin or have become “arterialized” during the disease process. In either case, the primary human endothelial cells cultured by this method are a valid model system to investigate endothelial pathology in pulmonary hypertension (Table 1). Because this method enriches for endothelial cells based upon selectivity of the culture media, comparison of late passages to very early cultures (passages 1–3) would be confounded by the 10 to 25% contaminating cells. However, in this report and in previous studies, we have confirmed that the cultures from passages that have greater than 95% purity for endothelial cells were representative of cells in the lung. For example, the primary pulmonary arterial cells from pulmonary hypertension lungs have greater proliferation (by BrdU incorporation and Ki-67 nuclear antigen expression), decreased apoptosis upon growth factor withdrawal (by caspase 3 activation and terminal dUTP nick-end labeling), and activation of prosurvival pathways (hypoxia inducible factor and signal transducer and activator of transcription 3) (19, 22, 24). The greater glycolytic metabolism of primary pulmonary arterial cells derived from pulmonary hypertension lungs in vitro mirrors the greater [18F]fluoro-deoxy-d-glucose positron emission tomography uptake values in lungs of patients with pulmonary hypertension as compared with healthy control lungs (24). The low level of NO synthesis by pulmonary arterial cells in culture is in line with the observed low NO in exhaled breath and the low NO products in the bronchoalveolar lavage fluid of patients with PAH (23). Overall, the PAECs provide an accurate representative in vitro model system for study of pulmonary vascular diseases (41–45).
TABLE 1.
CHARACTERISTICS OF PULMONARY ARTERIAL ENDOTHELIAL CELLS ACCURATELY REFLECT ENDOTHELIAL CELLS IN VASCULAR LESIONS OF HUMAN PULMONARY ARTERIAL HYPERTENSION LUNGS IN VIVO
| Characteristics | Pulmonary Arterial Endothelial Cells In Vitro (Ref. No.) | Endothelium of Human Lungs In Vivo (Ref. No.) |
| Proliferation increased | Ki67 (22), BrdU (22) | Ki67 (41–44) |
| Apoptosis-resistance | Caspase 3 (22), Bcl2 (22), MCl1 (22), TUNEL (22) | BAX (45) |
| Metabolic shift | Shift to glycolysis by metabolic measures (24) | Shift to glycolysis by FDG-PET scan (24) |
| Signal transduction activation | HIF (19, 24), pSTAT3 (22) | pSTAT3 (22), HIF (46) |
| Nitric oxide synthesis | Arginase2 (23), and decreased NO synthesis (23) | Increased Arginase2 (23) and decreased NO production (23) |
Definition of abbreviations: BAX = BcL-2 gene family; BrdU = 5-bromo-2'-deoxyuridine; FDG-PET = [18F]fluoro-deoxy-d-glucose positron emission tomography; HIF = hypoxia-inducible factor; MCL1 = induced myeloid leukemia cell differentiation protein; STAT3 = signal transducer and activator of transcription 3; TUNEL, terminal dUTP nick-end labeling.
In summary, we present a method for harvesting and culture of human PAECs and MVECs and provide evidence that these cells accurately represent endothelial cells in the lung. Endothelial cells derived from control or PAH lungs express the same characterization markers (i.e., CD31, vWF, and lectin staining). Cells of multiple passages derived by this method accurately represent the characteristics of cells in lungs of patients with pulmonary hypertension (i.e., proliferation potential, apoptotic susceptibility, metabolism, signal transduction, and NO synthesis) (Table 1). Furthermore, chromosome abnormalities that were discovered in PAECs derived from PAH lungs by this method were stably found in cultured cells (passage 5–6) and were present in the plexiform lesions of primary lung tissue (14). Altogether, the results provide solid molecular evidence of a shared genetic lineage and validity for the concept that cells derived by this method are informative for understanding pulmonary vascular disease in vivo. Thus, the availability and purity of these cells provides a rich resource for molecular, genetic, and biochemical studies on human pulmonary vascular diseases and provides an economical and disease-specific model system for translational human studies.
Supplementary Material
Acknowledgments
The authors thank Mei Yin for ultrastructural analysis; Robert Naples, Michelle Koo, and Laura Peterson for tissue collection; and Fares Masri for the real time movie of in vitro tube formation.
Footnotes
This work was supported by National Institutes of Health grants HL60917 and HL103453.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2011-0416TE on March 15, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Archer S, Rich S. Primary pulmonary hypertension: a vascular biology and translational research “work in progress”. Circulation 2000;102:2781–2791 [DOI] [PubMed] [Google Scholar]
- 2.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980;288:373–376 [DOI] [PubMed] [Google Scholar]
- 3.Ignarro LJ. Nitric oxide-mediated vasorelaxation. Thromb Haemost 1993;70:148–151 [PubMed] [Google Scholar]
- 4.Moncada S. Nitric oxide: discovery and impact on clinical medicine. J R Soc Med 1999;92:164–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.King J, Hamil T, Creighton J, Wu S, Bhat P, McDonald F, Stevens T. Structural and functional characteristics of lung macro- and microvascular endothelial cell phenotypes. Microvasc Res 2004;67:139–151 [DOI] [PubMed] [Google Scholar]
- 6.Ryan US, Clements E, Habliston D, Ryan JW. Isolation and culture of pulmonary artery endothelial cells. Tissue Cell 1978;10:535–554 [DOI] [PubMed] [Google Scholar]
- 7.Catravas JD, Snead C, Dimitropoulou C, Chang AS, Lucas R, Verin AD, Black SM. Harvesting, identification and barrier function of human lung microvascular endothelial cells. Vascul Pharmacol 2010;52:175–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hewett PW, Murray JC. Human microvessel endothelial cells: isolation, culture and characterization. In Vitro Cell Dev Biol Anim 1993;29A:823–830 [DOI] [PubMed] [Google Scholar]
- 9.Visner GA, Staples ED, Chesrown SE, Block ER, Zander DS, Nick HS. Isolation and maintenance of human pulmonary artery endothelial cells in culture isolated from transplant donors. Am J Physiol 1994;267:L406–L413 [DOI] [PubMed] [Google Scholar]
- 10.Johnson AR. Human pulmonary endothelial cells in culture: activities of cells from arteries and cells from veins. J Clin Invest 1980;65:841–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin WJ, II, Kachel DL. Oxygen-mediated impairment of human pulmonary endothelial cell growth: evidence for a specific threshold of toxicity. J Lab Clin Med 1989;113:413–421 [PubMed] [Google Scholar]
- 12.Tuder RM, Marecki JC, Richter A, Fijalkowska I, Flores S. Pathology of pulmonary hypertension. Clin Chest Med 2007;28:23–42 (vii.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watson DR, Mossbrook SS, Johnson MJ. Potential barriers to good nutrition. J Med Assoc Ga 1980;69:841–843 [PubMed] [Google Scholar]
- 14.Aldred MA, Comhair SA, Varella-Garcia M, Asosingh K, Xu W, Noon GP, Thistlethwaite PA, Tuder RM, Erzurum SC, Geraci MW, et al. Somatic chromosome abnormalities in the lungs of patients with pulmonary arterial hypertension. Am J Respir Crit Care Med 2010;182:1153–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asosingh K, Aldred MA, Vasanji A, Drazba J, Sharp J, Farver C, Comhair SA, Xu W, Licina L, Huang L, et al. Circulating angiogenic precursors in idiopathic pulmonary arterial hypertension. Am J Pathol 2008;172:615–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aytekin M, Comhair SA, de la Motte C, Bandyopadhyay SK, Farver CF, Hascall VC, Erzurum SC, Dweik RA. High levels of hyaluronan in idiopathic pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 2008;295:L789–L799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drake KM, Zygmunt D, Mavrakis L, Harbor P, Wang L, Comhair SA, Erzurum SC, Aldred MA. Altered microRNA processing in heritable pulmonary arterial hypertension: an important role for smad-8. Am J Respir Crit Care Med 2011;184:1400–1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farha S, Asosingh K, Xu W, Sharp J, George D, Comhair S, Park M, Tang WH, Loyd JE, Theil K, et al. Hypoxia-inducible factors in human pulmonary arterial hypertension: a link to the intrinsic myeloid abnormalities. Blood 2011;117:3485–3493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fijalkowska I, Xu W, Comhair SA, Janocha AJ, Mavrakis LA, Krishnamachary B, Zhen L, Mao T, Richter A, Erzurum SC, et al. Hypoxia inducible-factor1alpha regulates the metabolic shift of pulmonary hypertensive endothelial cells. Am J Pathol 2010;176:1130–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gangopahyay A, Oran M, Bauer EM, Wertz JW, Comhair SA, Erzurum SC, Bauer PM. Bone morphogenetic protein receptor ii is a novel mediator of endothelial nitric-oxide synthase activation. J Biol Chem 2011;286:33134–33140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masri FA, Comhair SA, Koeck T, Xu W, Janocha A, Ghosh S, Dweik RA, Golish J, Kinter M, Stuehr DJ, et al. Abnormalities in nitric oxide and its derivatives in lung cancer. Am J Respir Crit Care Med 2005;172:597–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masri FA, Xu W, Comhair SA, Asosingh K, Koo M, Vasanji A, Drazba J, Anand-Apte B, Erzurum SC. Hyperproliferative apoptosis-resistant endothelial cells in idiopathic pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 2007;293:L548–L554 [DOI] [PubMed] [Google Scholar]
- 23.Xu W, Kaneko FT, Zheng S, Comhair SA, Janocha AJ, Goggans T, Thunnissen FB, Farver C, Hazen SL, Jennings C, et al. Increased arginase ii and decreased no synthesis in endothelial cells of patients with pulmonary arterial hypertension. FASEB J 2004;18:1746–1748 [DOI] [PubMed] [Google Scholar]
- 24.Xu W, Koeck T, Lara AR, Neumann D, DiFilippo FP, Koo M, Janocha AJ, Masri FA, Arroliga AC, Jennings C, et al. Alterations of cellular bioenergetics in pulmonary artery endothelial cells. Proc Natl Acad Sci USA 2007;104:1342–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoshi H, McKeehan WL. Isolation, growth requirements, cloning, prostacyclin production and life-span of human adult endothelial cells in low serum culture medium. In Vitro Cell Dev Biol 1986;22:51–56 [DOI] [PubMed] [Google Scholar]
- 26.Thornton SC, Mueller SN, Levine EM. Human endothelial cells: use of heparin in cloning and long-term serial cultivation. Science 1983;222:623–625 [DOI] [PubMed] [Google Scholar]
- 27.Kessler S, Rho H, West G, Fiocchi C, Drazba J, de la Motte C. Hyaluronan (ha) deposition precedes and promotes leukocyte recruitment in intestinal inflammation. Clin Transl Sci 2008;1:57–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de La Motte CA, Hascall VC, Calabro A, Yen-Lieberman B, Strong SA. Mononuclear leukocytes preferentially bind via cd44 to hyaluronan on human intestinal mucosal smooth muscle cells after virus infection or treatment with poly(i.C). J Biol Chem 1999;274:30747–30755 [DOI] [PubMed] [Google Scholar]
- 29.Kuzu I, Bicknell R, Harris AL, Jones M, Gatter KC, Mason DY. Heterogeneity of vascular endothelial cells with relevance to diagnosis of vascular tumours. J Clin Pathol 1992;45:143–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Page C, Rose M, Yacoub M, Pigott R. Antigenic heterogeneity of vascular endothelium. Am J Pathol 1992;141:673–683 [PMC free article] [PubMed] [Google Scholar]
- 31.Champion HC, Bivalacqua TJ, Greenberg SS, Giles TD, Hyman AL, Kadowitz PJ. Adenoviral gene transfer of endothelial nitric-oxide synthase (enos) partially restores normal pulmonary arterial pressure in enos-deficient mice. Proc Natl Acad Sci USA 2002;99:13248–13253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fagan KA, McMurtry I, Rodman DM. Nitric oxide synthase in pulmonary hypertension: lessons from knockout mice. Physiol Res 2000;49:539–548 [PubMed] [Google Scholar]
- 33.Steudel W, Ichinose F, Huang PL, Hurford WE, Jones RC, Bevan JA, Fishman MC, Zapol WM. Pulmonary vasoconstriction and hypertension in mice with targeted disruption of the endothelial nitric oxide synthase (nos 3) gene. Circ Res 1997;81:34–41 [DOI] [PubMed] [Google Scholar]
- 34.Chung-Welch N, Patton WF, Yen-Patton GP, Hechtman HB, Shepro D. Phenotypic comparison between mesothelial and microvascular endothelial cell lineages using conventional endothelial cell markers, cytoskeletal protein markers and in vitro assays of angiogenic potential. Differentiation 1989;42:44–53 [DOI] [PubMed] [Google Scholar]
- 35.Voyta JC, Via DP, Butterfield CE, Zetter BR. Identification and isolation of endothelial cells based on their increased uptake of acetylated-low density lipoprotein. J Cell Biol 1984;99:2034–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins: identification by morphologic and immunologic criteria. J Clin Invest 1973;52:2745–2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weibel ER, Palade GE. New cytoplasmic components in arterial endothelia. J Cell Biol 1964;23:101–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diaz-Flores L, Gutierrez R, Varela H, Rancel N, Valladares F. Microvascular pericytes: a review of their morphological and functional characteristics. Histol Histopathol 1991;6:269–286 [PubMed] [Google Scholar]
- 39.Herman IM, D'Amore PA. Microvascular pericytes contain muscle and nonmuscle actins. J Cell Biol 1985;101:43–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chi JT, Chang HY, Haraldsen G, Jahnsen FL, Troyanskaya OG, Chang DS, Wang Z, Rockson SG, van de Rijn M, Botstein D, et al. Endothelial cell diversity revealed by global expression profiling. Proc Natl Acad Sci USA 2003;100:10623–10628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakao S, Taraseviciene-Stewart L, Lee JD, Wood K, Cool CD, Voelkel NF. Vascular endothelial growth factor receptor blockade by su5416 combined with pulsatile shear stress causes apoptosis and subsequent proliferation of apoptosis-resistant endothelial cells. Chest 2005;128(Suppl)610S–611S [DOI] [PubMed] [Google Scholar]
- 42.Sakao S, Taraseviciene-Stewart L, Lee JD, Wood K, Cool CD, Voelkel NF. Initial apoptosis is followed by increased proliferation of apoptosis-resistant endothelial cells. FASEB J 2005;19:1178–1180 [DOI] [PubMed] [Google Scholar]
- 43.Taraseviciene-Stewart L, Kasahara Y, Alger L, Hirth P, Mc Mahon G, Waltenberger J, Voelkel NF, Tuder RM. Inhibition of the vegf receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J 2001;15:427–438 [DOI] [PubMed] [Google Scholar]
- 44.Tuder RM, Groves B, Badesch DB, Voelkel NF. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol 1994;144:275–285 [PMC free article] [PubMed] [Google Scholar]
- 45.Yeager ME, Halley GR, Golpon HA, Voelkel NF, Tuder RM. Microsatellite instability of endothelial cell growth and apoptosis genes within plexiform lesions in primary pulmonary hypertension. Circ Res 2001;88:E2–E11 [DOI] [PubMed] [Google Scholar]
- 46.Tuder RM, Chacon M, Alger L, Wang J, Taraseviciene-Stewart L, Kasahara Y, Cool CD, Bishop AE, Geraci M, Semenza GL, et al. Expression of angiogenesis-related molecules in plexiform lesions in severe pulmonary hypertension: evidence for a process of disordered angiogenesis. J Pathol 2001;195:367–374 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.