Abstract
Mycoplasma pneumoniae causes acute and chronic lung infections in humans, leading to a variety of pulmonary and extrapulmonary sequelae. Of the airway complications of M. pneumoniae infection, M. pneumoniae–associated exacerbation of asthma and pediatric wheezing are emerging as significant sources of human morbidity. However, M. pneumoniae products capable of promoting allergic inflammation are unknown. Recently, we reported that M. pneumoniae produces an ADP-ribosylating and vacuolating toxin termed the community-acquired respiratory distress syndrome (CARDS) toxin. Here we report that naive mice exposed to a single dose of recombinant CARDS (rCARDS) toxin respond with a robust inflammatory response consistent with allergic disease. rCARDS toxin induced 30-fold increased expression of the Th-2 cytokines IL-4 and IL-13 and 70- to 80-fold increased expression of the Th-2 chemokines CCL17 and CCL22, corresponding to a mixed cellular inflammatory response comprised of a robust eosinophilia, accumulation of T cells and B cells, and mucus metaplasia. The inflammatory responses correlate temporally with toxin-dependent increases in airway hyperreactivity characterized by increases in airway restriction and decreases in lung compliance. Furthermore, CARDS toxin–mediated changes in lung function and histopathology are dependent on CD4+ T cells. Altogether, the data suggest that rCARDS toxin is capable of inducing allergic-type inflammation in naive animals and may represent a causal factor in M. pneumoniae–associated asthma.
Keywords: Mycoplasma pneumoniae, asthma, rCARDS toxin, eosinophilia, T cell
Clinical Relevance
Mycoplasma pneumoniae has been associated with human asthma for decades, but a M. pneumoniae product responsible for this observation has been lacking. In this study we provide evidence that a single exposure to rCARDS toxin is sufficient to cause asthma-like disease in mice. These data are significant because this work facilitates the mechanistic analysis of M. pneumoniae–associated asthma in humans and permits the dissection of the causal role of CARDS toxin in human disease.
Mycoplasma pneumoniae is a common human bacterial pathogen that causes acute and chronic infections of the respiratory tract and extrapulmonary pathology (1, 2). With the exception of mycoplasma adherence to the host epithelium, molecular mechanisms of virulence associated with the pathogenesis of M. pneumoniae infection are not well understood (1, 3). M. pneumoniae is predominantly an extracellular pathogen that binds to respiratory epithelial cells using a polarized tip organelle (1, 3–5). Interaction of M. pneumoniae with the respiratory epithelium results in significant cytopathology in cell culture and in vivo (4, 5). Previously, the cytopathology was attributed in part to the cytotoxic effects of hydrogen peroxides produced by M. pneumoniae (3). However, recently, we identified an ADP-ribosylating and vacuolating toxin produced by M. pneumoniae that is capable of inducing cytopathology in vitro and in vivo and that reproduces the infectious process (6–9).
The community-acquired respiratory distress syndrome (CARDS) toxin encoded by the MPN372 gene was functionally identified as a human surfactant protein A binding protein (7). Upon further investigation, we discovered that CARDS toxin possesses structurally and functionally important regions of identity to the pertussis toxin S1 protein. Furthermore, highly purified rCARDS toxin causes extensive dose-dependent cytopathology in mammalian cell and organ culture, suggesting that it contributes directly to the cytopathic effects observed during infection (6). These observations were extended in vivo where dose-dependent vacuolization and cytotoxicity of mouse and baboon bronchiolar and tracheal epithelium were observed after a single exposure to rCARDS toxin (6, 8). A single exposure to rCARDS toxin induces many of the pathological features associated with M. pneumoniae infection (8). An interesting aspect of M. pneumoniae pathogenesis emerging from rodent studies is that disease severity appears to be linked to the amount of CARDS toxin produced (10, 11).
During infection, the underlying host immune environment affects the nature of the resulting immune response and the progression and extent of disease pathogenesis. A number of studies have highlighted the importance of IL-12 and IFN-γ and Th-1 type T-cell responses during the pathogenesis of M. pneumoniae infection in mouse models of pneumonia (8, 12–14). However, if the host is sensitized to allergen before infection, M. pneumoniae can worsen asthma-like disease in mouse models, leading to airway remodeling, mucus metaplasia, and changes in pulmonary function (15, 16). In the sensitized mouse, M. pneumoniae infection leads to the generation of Th-2 type allergic inflammation (15–19), providing a provocative correlation to human disease where M. pneumoniae infection is strongly linked to pediatric wheezing and acute exacerbations of asthma in adults (19–25).
Given the emerging role of CARDS toxin in the pathogenesis of M. pneumoniae–associated disease, we investigated the impact of recombinant CARDS (rCARDS) toxin exposure on pulmonary inflammation in naive mice. Here, we report that a single exposure to rCARDS toxin results in potent allergic-type pulmonary inflammation characterized by T-cell dependence, eosinophilia, mucus metaplasia, airway hyperreactivity, and production of Th-2–type cytokines and chemokines.
Materials and Methods
Detailed methods are provided in the online supplement.
rCARDS Toxin
rCARDS toxin was produced as described (6, 7, 9), and bioactivity was determined by vacuolization of HeLa cells (6, 8) with carrier fluid (CF) as a control.
Animals
BALB/cJ mice from Jackson Laboratory (Bar Harbor, ME) were maintained in an Association for Assessment and Accreditation of Laboratory Animal Care–approved facility in accordance with Institutional Biosafety Committee and Institutional Animal Use and Care Committee protocols established at the University of Texas Health Sciences Center at San Antonio.
Bronchoalveolar Lavage Fluid and Cellular Differentials
To obtain bronchoalveolar fluid (BALF), bronchoalveolar lavage was performed as detailed (8, 26). Cells in the BALF were washed and counted before sedimentation onto microscope slides using a cytospin 2 centrifuge (Shandon; Thermo, Waltham, MA). Slides were stained with Wright Giemsa (Diff-stain; IMEB Inc., San Marcos, CA), and the relative abundance of neutrophils, eosinophils, monocytes/macrophages, and lymphocytes was enumerated. Results are representative of two independent experiments with 5 to 10 animals per group per time point.
Cytokine Analysis
ELISA was used to determine concentrations of eotaxin, CCL17, and CCL22. BALF samples, 5 to 10 per time point, were used in sandwich ELISAs (R&D Systems, Minneapolis, MN). Samples were assayed in duplicate with each experiment done twice.
Quantitative Real-Time PCR
RNA was isolated from lungs of mice treated with 700 pmol of rCARDS toxin or CF as described (27). Relative changes in mRNA expression were determined by the ΔΔCT method using actin normalization. Data are representative of at least two experiments with three to five mice per treatment per time point per experiment.
Histopathology and Immunohistochemistry
After intranasal instillation of rCARDS toxin, lungs were harvested at 1, 4, 7, 14, 21, and 56 days after inoculation. Tissues were processed and embedded in paraffin, from which 4-μm sections were cut and stained with hematoxylin and eosin (H&E) and periodic acid-Schiff (PAS). Eosinophils were detected using a monoclonal antibody against eosinophil major basic protein (MBP) Clone MT-14.7 (Lee Laboratory, Mayo Clinic, Phoenix/Scottsdale, AZ). Complete images of control and rCARDS toxin–treated lungs were obtained digitally using the Aperio Scanscope XT (Aperio, Vista, CA). Printed images of lungs from both study groups were graded for disease severity using a panel of standards (28, 29).
CD4 T-Cell Depletion and Flow Cytometry
To deplete CD4+ T cells, mice were injected intraperitoneally with 250 μg of purified rat anti-CD4 antibody 1 day before rCARDS toxin exposure and then every third day after exposure (30, 31). Purified irrelevant rat IgG served as a negative control. Spleens from mice in each treatment group were dissected, and single-cell suspensions were made before blocking Fc receptors. Cells were stained for surface marker expression and analyzed on a FACS Aria by excluding dead cells and gating on the singlet lymphocyte population using light scattering.
Airway Function
Changes in mouse airway function after rCARDS toxin exposure were determined by invasive pulmonary measurements using the Flexivent system (Scireq, Montreal, PQ, Canada). Animals were treated with 700 pmol of rCARDS toxin or CF, and indicators of airway hyperreactivity (AHR), including airway resistance and compliance, were measured after aerosolized methacholine challenge on Days 4 and 7 after exposure. Data are composites of three or four experiments (12–16 mice per group).
Results
rCARDS Toxin Exposure Results in Mucus Metaplasia
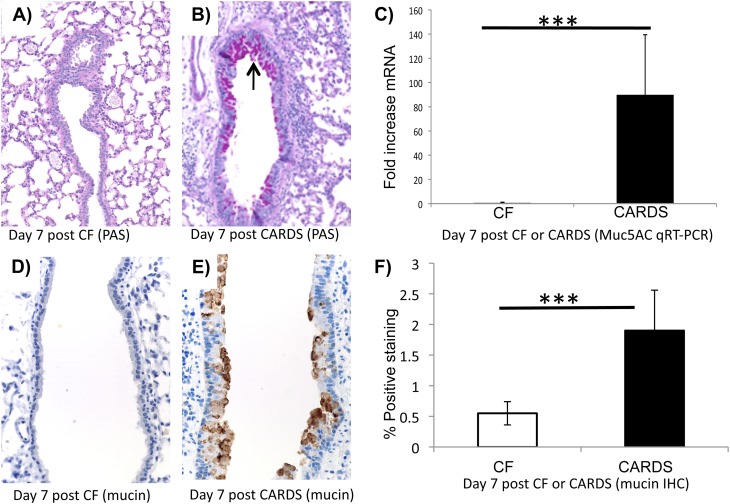
A hallmark of M. pneumoniae infection and allergic lung inflammation is increased mucus production (32, 33). Previously, it was reported that M. pneumoniae lipoproteins induce mucin expression in the lungs in a TLR2-dependent manner (32). We investigated the possibility that rCARDS toxin could promote airway mucus metaplasia histologically. Mice exposed to 700 pmol of rCARDS toxin intranasally produced substantially more mucus as determined by the bright pink PAS staining on Days 4 and 7 after exposure compared with control mice (Figures 1A and 1B, only Day 7 shown). To further test the differences in lung mucus production 7 days after toxin exposure, we evaluated changes in the expression of the major mucin gene, Muc5AC, by quantitative real-time (qRT)-PCR. There was a significant (P < 0.005) 85-fold increase in Muc5AC mRNA in the lungs of mice treated with rCARDS toxin versus animals treated with CF as a control (Figure 1C). Quantification of Muc5AC immunochemistry (34) using the Aperio digital pathology system revealed a significant (P < 0.0006) increase in Muc5AC staining in response to rCARDS toxin exposure relative to the CF control mice (Figures 1D–1F).
Figure 1.
Recombinant Community-Acquired Respiratory Distress Syndrome (rCARDS) toxin–induced mucus metaplasia as detected by periodic acid Schiff (PAS) staining and quantitative real-time (qRT)-PCR. (A) Section of carrier fluid (CF)-treated lung stained with PAS at 7 days after exposure. (B) Section of rCARDS toxin–treated lung stained with PAS 7 days after exposure. Bright pink staining (black arrow) indicates the sites of mucus production. (C) Detection of Muc5AC mRNA by quantitative real-time PCR 7 days after exposure to rCARDS toxin or CF (***P < 0.005). (D and E) Sections of mouse lungs 7 days after exposure from CF or rCARDS toxin–treated animals, respectively, stained for mucin. Sections are stained with a chicken antimouse mucin antibody. (F) Quantification of the antimucin staining using the Aperio system. Data are presented as average percent positive staining ±SD. Sections from eight mice in each group were analyzed (***P < 0.0006). Original magnification of histology images: ×20.
rCARDS Toxin Induces a Mixed Eosinophilic–Lymphocytic Inflammation
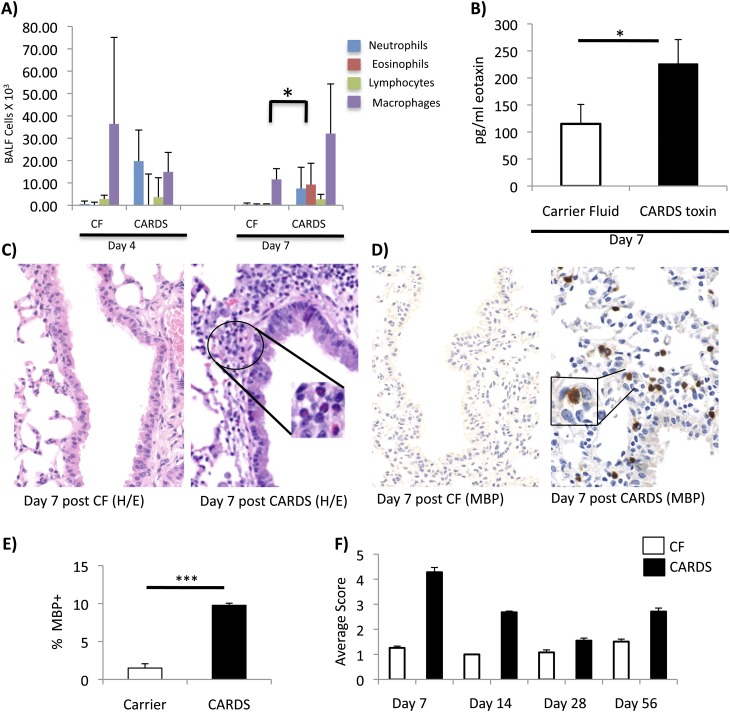
To investigate the cellular composition of the pulmonary response to rCARDS toxin exposure, we performed differential analysis on the cellular components of the BALF. The cellular composition of the BALF aftert rCARDS toxin exposure was dynamic (Figure 2A). Initially, there was a neutrophilia present in the first 4 days after exposure that transitioned into a significant (P < 0.05) eosinophilia at Day 7 after exposure.
Figure 2.
rCARDS toxin–induced inflammatory changes in the lung. (A) Differentials of the cellular component of the bronchoalveolar lavage fluid 4 and 7 days after exposure to rCARDS toxin or CF. There is a significant (*P < 0.05) eosinophilia on Day 7 after exposure to rCARDS toxin. (B) rCARDS toxin–mediated increased expression of eotaxin in the BALF relative to CF control mice (*P< 0.05). (C) rCARDS toxin–dependent peribronchiolar eosinophilia as detected by H&E staining (original magnification: ×40). Inset shows high-magnification image of the eosinophils (original magnification: ×60). (D) rCARDS toxin–dependent peribronchiolar eosinophilia as detected by immunohistochemistry for eosinophil major basic protein (MBP). Positive staining is indicated by brown color (original magnification: ×40). Inset shows high-magnification image of the eosinophils (original magnification: ×60). (E) Quantification of the bronchiolar MBP immunohistochemistry using the Aperio system on Day 7 after toxin exposure (***P = 0.006). (F) Quantification of differences in average histological scores among treatment groups at Days 7, 14, 28, and 56 after toxin exposure. Scores from four blinded investigators were averaged and based on a graded scale of 1 to 5, which represented histological changes ranging from least severe to most severe. Differences between rCARDS toxin treatment and CF controls were significant at all time points (P = 0.001), and there was good agreement among evaluators (κ = 0.94–0.96).
We previously reported that exposure to 700 pmol of rCARDS toxin leads to development of extensive perivascular and peribronchiolar inflammatory lesions (8). Given the pronounced eosinophilia of the BALF on Day 7 after exposure, we extended these studies to investigate the presence of eotaxin within the BALF. There was a significant (P < 0.05) increase in eotaxin in the BALF on Day 7 after toxin exposure (Figure 2B). At 7 days after exposure to 700 pmol of rCARDS toxin, Balb/cJ mice developed characteristic peribronchiolar and perivascular inflammatory lesions (Figure 2C). The lesions were compact and contained different types of cells. Within the lesions were multiple eosinophils with characteristic bilobed nuclei and bright pink cytoplasm (Figure 2C, high magnification). As observed in the bronchiolar wall in Figure 2D, rCARDS toxin–induced inflammatory lesions contained many eosinophils based on MBP immunoreactivity. To quantitatively evaluate differences in eosinophilia after rCARDS toxin treatment, we used the Aperio digital pathology system to distinguish medium- and high-intensity MBP+ staining. There was a highly significant (P < 0.006) increase in eosinophils in the rCARDS toxin–treated mice relative to control animals at 7 days after exposure (Figure 2E). Furthermore, exposure to rCARDS toxin resulted in the infiltration of eosinophils into the bronchiolar and alveolar walls, which is consistent and reminiscent of acute allergic responses (Figure 2C and data not shown) (33, 35, 36).
Although peak histopathological score occurred at 7 days after toxin exposure (Figure 2F), M. pneumoniae infection of the mouse is known to have a prolonged impact on the pulmonary compartments (13). To evaluate the course of pathological changes after a single exposure to rCARDS toxin, additional mice were treated with 700 pmol of rCARDS or CF intranasally, and lungs were harvested 14, 28, and 56 days after exposure. The mixed lymphocytic and eosinophilic mural infiltratrates persisted at the 14-day study period, but eosinophils were not evident in the perivascular lymphoid aggregates that dominated the 28- and 56-day lesions. The extent and severity of gross histological changes among the treatment groups were evaluated using the panel of standards method, with a grade 1 score being least severe and grade 5 score being most severe (28, 29). Images of the graded standards used in this analysis are presented Figures E2A through E2E in the online supplement. Tissues were evaluated without identifiers by four investigators. Animals treated with rCARDS toxin had a higher average histopathology score (2- to 4-fold) relative to control mice at each time point out to 56 days after exposure (Figure 2F). Consistent with data shown in Figures 1 and 2A through 2E, animals exposed to rCARDS toxin exhibited the most severe pathology (grade 4.3 ± 0.19) 7 days after exposure, but, even on Day 56 after exposure, 83% of the animals had moderate changes in pathology (grade 2.7 ± 0.14). The differences between rCARDS toxin–treated animals and CF control animals were significant (P < 0.001) at all time points, and all four blinded investigators were in good agreement with each other (κ = 0.94–0.96). In addition, pathology of the lungs of animals treated with rCARDS toxin for 14, 28, and 56 days was highly significantly different (P < 0.0001) when compared with animals treated with rCARDS toxin for 7 days. Dunn's multiple comparison tests indicated that the panel of standards scores of the rCARDS toxin–treated animals at all time points were significantly different from each other (P < 0.05), except for the Day 14 versus Day 56 comparisons. Altogether, these data suggest that exposure to CARDS toxin can have prolonged and chronic impact on lung histopathology.
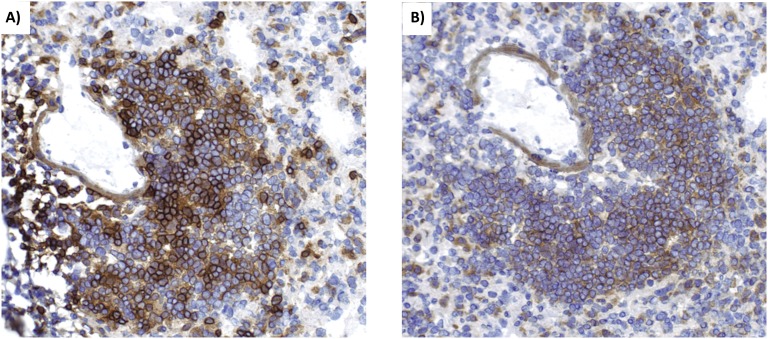
Eosinophils are not the only immune cells present in the CARDS toxin–induced lesions. Immunohistochemistry (IHC) also revealed the presence of CD4+ cells and CD19+ cells, suggesting a mixed lymphocytic lesion containing T and B lymphocytes, respectively (Figure 3).
Figure 3.
rCARDS toxin–induced lesions contain T cells and B cells. Lymphocytic inflammation appears in lungs of mice 7 days after treatment with rCARDS toxin. These lesions are predominantly composed of CD4+ cells (A) and CD19-positive cells (B), indicating the presence of T and B cells, respectively. Original magnification: ×20.
rCARDS Toxin Exposure Leads to Increased Pulmonary Expression of IL-4, IL-13, CCL17, and CCL22
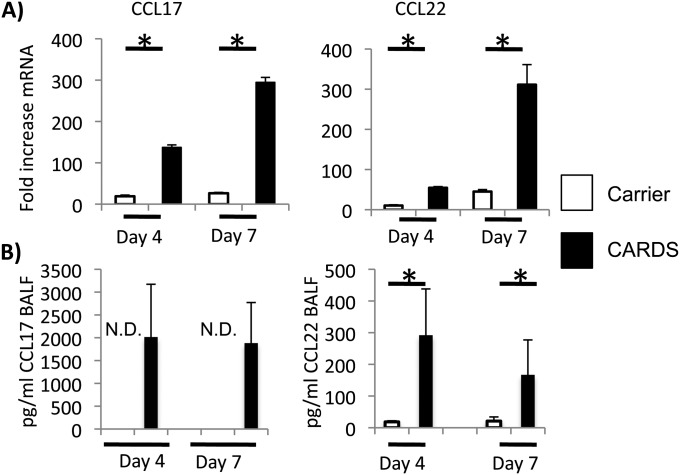
During asthma-like allergic responses, CD4+ T cells of the Th-2 phenotype can be recruited to the site of inflammation by the chemokines CCL17 and CCL22. Expression of CCL17 and CCL22 was significantly and robustly induced at Days 4 and 7 after exposure to rCARDS toxin. There was a 70- to 80-fold increases in CCL17 and CCL22 mRNA as determined by qRT-PCR at 4 and 7 days after toxin exposure relative to animals treated with CF (Figure 4A), which correlated with detectable levels of chemokine in the BALF (Figure 4B).
Figure 4.
rCARDS toxin–dependent chemokine production. Mice were treated with CF or 700 pmol of rCARDS toxin. On Days 4 and 7 after exposure, bronchoalveolar lavage fluid (BALF) was obtained, and RNA was extracted from the lungs. (A) qRT-PCR analysis of CCL17 and CCL22 mRNA expression. White bars represent the CF-treated group; black bars represent the rCARDS toxin–treated group. Data represent an average of two independent experiments with 8 to 10 mice per time point and are presented as fold increase relative to nontreated control mice and normalized to actin expression using the ΔΔCT method (*P < 0.05). (B) Concentration of CCL17 and CCL22 protein in the BALF. ELISA-determined protein concentrations in the BALF. White bars represent the CF-treated group; black bars represent the rCARDS toxin–treated group. Data were obtained from the same animals used in A. Statistical significance could not be determined for the expression of CCL17 because the animals treated with CF had no detectable (N.D.) chemokine (*P < 0.05).
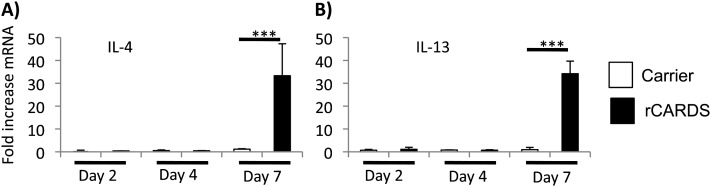
Increases in CCL17 and CCL22 suggest the recruitment of Th-2 T cells after rCARDS toxin exposure. During allergic responses, Th-2 cells often release the effector cytokines IL-4 and IL-13 to promote allergic inflammation. qRT-PCR determined that there was an approximately 30-fold increase in IL-4 and IL-13 mRNA expression in the lungs of mice treated with rCARDS toxin for 7 days relative to CF control mice (Figure 5). Using ELISA, we were unable to detect IL-4 or IL-13 in the BALF at Days 4 or 7 after toxin exposure (data not shown).
Figure 5.
rCARDS toxin–dependent cytokine production. Mice were treated with CF or 700 pmol of rCARDS toxin, and RNA was extracted from the lungs on Days 2, 4, and 7 after exposure. qRT-PCR analysis of IL-4 (A) and IL-13 (B) mRNA expression is shown. White bars represent the CF-treated group; black bars represent the rCARDS toxin–treated group. Data are an average of two independent experiments with 8 to 10 mice per time point and are presented as fold increase relative to nontreated control mice and normalized to actin expression using the ΔΔCT method (***P < 0.005).
rCARDS Toxin Exposure Leads to AHR Characterized by Increased Airway Resistance and Diminished Lung Compliance
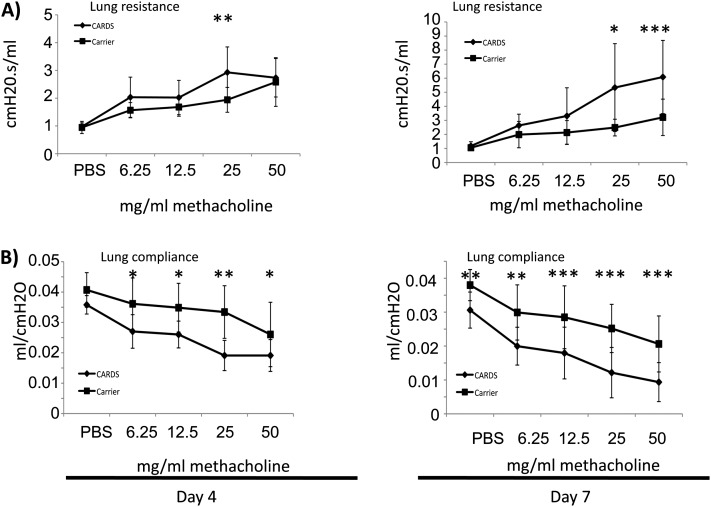
Increased mucus production and accumulation of eosinophils and Th-2 T cells in the lungs are consistent with allergic inflammation and physiologically often correlate with changes in lung function. Naive mice exposed to rCARDS toxin show significantly (P < 0.05) increased airway resistance and decreased lung compliance after methacholine challenge indicative of AHR (Figure 6). Differences were apparent at 4 days after toxin exposure but were more robust at Day 7. Many mice continued to have impaired lung function out to 14 days after toxin exposure, and most returned to baseline by Day 28 after exposure (data not shown).
Figure 6.
rCARDS toxin–mediated changes in pulmonary function. BALB/cJ mice were treated with CF or 700 pmol of rCARDS toxin, and changes in pulmonary function were measured using the Flexivent system at 4 and 7 days after exposure. (A) Pulmonary obstruction as measured by changes in airway resistance after a nebulized methacholine dose escalation. (B) Changes in lung compliance after a nebulized methacholine dose escalation. Diamonds represent mice treated with rCARDS toxin; squares represent mice treated with CF. Data are an average of two independent experiments with 8 to 12 animals per time point. Data are presented as the mean ± SD (*P < 0.05; **P < 0.005; ***P < 0.0005).
Lung Dysfunction and Inflammatory Pathologies Are Dependent on CD4+ Cells
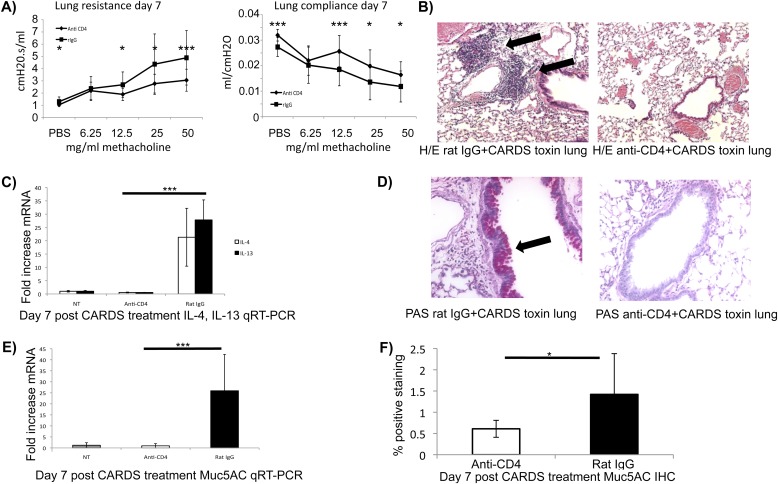
Exposure of naive mice to rCARDS toxin leads to the development of allergic-like inflammation characterized by increased AHR, eosinophilia, mucus metaplasia, Th-2 cytokines/chemokine expression, and lymphocytic peribronchiolar cellular inflammation containing T cells and B cells. Altogether, these data suggest a Th-2–type inflammatory response reminiscent of allergic asthma (33, 37). To test the role of CD4+ cells in the pathogenesis of CARDS toxin–mediated inflammation, we depleted CD4+ T cells before exposure to rCARDS toxin as we have previously described (31) and evaluated lung function, histopathology, and expression of IL-4, IL-13, and Muc5AC mRNA after treatment. To ensure that the CD4+ T-cell depletion was effective, we evaluated the splenic T-cell populations by flow cytometry. As expected, animals treated with the anti-CD4 antibody had almost complete ablation of CD3+, CD4+ cells relative to nontreated mice or mice treated with an irrelevant rat IgG (Figure E3). These data suggest that the CD4+ T-cell depletion was effective. Changes in lung function (airway resistance and compliance) after CARDS toxin treatment were dependent on the presence of CD4+ cells (Figure 7A). Mice treated with irrelevant control antibodies and rCARDS toxin had significant (P < 0.05 to P < 0.008) increases in AHR characterized by increased airway resistance and decreases in lung compliance (Figure 7A) compared with mice treated with CD4+ cell–depleting antibodies and rCARDS toxin. These data suggest a causal link between CD4+ T cells and the development of airway dysfunction after rCARDS toxin exposure in naive mice.
Figure 7.
Dependence of rCARDS toxin–mediated changes in pulmonary function and histopathology on CD4+ T cells. BALB/cJ mice were injected intraperitoneally with 250 μg of rat anti-CD4 IgG antibody or 250 μg of irrelevant rat IgG 1 day before inoculation with 700 pmol rCARDS toxin. Mice were treated again with rat anti-CD4 or irrelevant rat IgG on Day 3 after rCARDS toxin exposure. (A) rCARDS toxin–dependent changes in airway resistance were measured by Flexivent. Animals lacking CD4+ T cells have significantly reduced changes in airway resistance and compliance. Diamonds represent mice treated with rCARDS toxin and rat anti-CD4 IgG; squares represent mice treated with rCARDS and irrelevant rat IgG. Data represent the mean ± SD from two independent experiments with a total of 10 to 15 mice per treatment group (*P < 0.02; ***P ≤ 0.002). (B) Mice treated with anti-CD4 antibodies do not develop peribronchiolar and perivascular inflammatory lesions characteristic of rCARDS toxin exposure (8). Note the large lymphoid aggregates in the rat IgG control lungs (black arrows) that are absent in the anti-CD4–treated animals. (C) Depletion of CD4+ T cells abolishes rCARDS toxin–induced expression of IL-4 and IL-13 mRNA in the lungs as determined by qRT-PCR (***P < 0.005). (D) Mice treated with anti-CD4 antibodies produce less mucus at the bronchiolar epithelium. Note the bright pink staining in the rat IgG control mice (black arrow) that is absent in the anti-CD4–treated mice. (E) Decreased Muc5AC mRNA expression in anti-CD4–treated animals was determined by qRT-PCR (***P < 0.005). (F) Anti-CD4–treated mice have less mucin protein in the lungs after rCARDS treatment compared with rat IgG–treated animals. Quantification of the antimucin staining using the Aperio system is shown. Data are presented as average percent positive staining ±SD. Sections from eight mice in each group were analyzed (*P = 0.03). Data from all other experiments are representative of two independent experiments with 10 to 15 mice. Original magnification of histology images: ×20.
The reduction in CARDS toxin–mediated AHR after depletion of CD4+ T cells could suggest that CD4+ cells participate in other aspects of CARDS toxin–mediated inflammation. To test this possibility, we investigated changes in histopathology after depletion of CD4+ T cells before rCARDS toxin exposure. Depletion of CD4+ cells leads to a marked decrease in the characteristic CARDS toxin–induced perivascular and peribronchiolar inflammatory lesions (Figure 7B) (8). Given the induction of Th-2–associated inflammatory cytokines (IL-4 and IL-13) after rCARDS toxin exposure, we investigated the expression of these cytokines in the CD4+ T-cell–depleted mice. Depletion of CD4+ T cells abolished IL-4 and IL-13 mRNA expression in rCARDS-treated mice (Figure 7C). rCARDS toxin–induced mucus metaplasia was also abolished in the CD4+ T-cell–depleted mice as determined by PAS staining (Figure 7D), Muc5AC IHC, and Muc5AC qRT-PCR (Figure 7E). Mice treated with rat IgG and CARDS toxin had significantly more mucin in the airways (P = 0.03) than the mice treated with anti-CD4 antibodies and CARDS toxin (Figure 7F) as determined by IHC and quantification on the Aperio system. These data are in good agreement with the PAS staining and qRT-PCR analysis, strongly suggesting that the CARDS toxin–induced mucus metaplasia is linked to the presence of CD4+ T cells. Altogether, these data suggest that CD4+ cells are central to rCARDS toxin–mediated pathogenesis in naive mice.
Discussion
M. pneumoniae infection causes a protracted but typically self-limiting pneumonia and can lead to a number of sequellae in humans (1). In addition to pneumonia, one of the most significant pulmonary manifestations of M. pneumoniae exposure is pediatric wheezing and acute exacerbation of asthma, suggesting that the pathogen has evolved mechanisms that modulate pulmonary inflammation (18, 19, 21–25, 38, 39). However, a definitive link between any specific M. pneumoniae product and allergic inflammation or asthma has been lacking. M. pneumoniae produces a number of factors that could affect the inflammatory response, including lipoproteins that activate the innate immune receptor TLR2 and reactive peroxides that cause tissue damage (3, 32). Yet, none of the previously described molecules is directly linked to Th-2–type inflammation. If animals are sensitized to allergen before infection, a strong and prolonged Th-2 response is generated (15). These data suggest that M. pneumoniae manipulates the host depending on the underlying immune environment, and data presented in the current study suggest that CARDS toxin is capable of promoting allergic-like inflammation in mice.
A striking and fundamentally important difference between the current study and other studies involving infection of sensitized mice is that rCARDS toxin induces allergic-type inflammation in naive mice. A single dose of rCARDS is sufficient to cause an eosinophilia, increased mucus production, AHR, and the expression of the Th-2 cytokines and chemokines IL-4, IL-13, CCL17, and CCL22. CCL17 and CCL22 function as chemoattractants for Th-2 T cells through engagement of the CCR4 receptor on the surface of these and other cells (37, 40). It is likely that CCL17- and CCL22-dependent recruitment of T cells leads to the increased expression of the effector cytokines IL-4 and IL-13 observed after rCARDS toxin exposure. Indeed, depletion of CD4+ T cells abolishes rCARDS toxin–induced IL-4 and IL-13 mRNA expression in the lungs. Altogether, these data strongly suggest that CARDS toxin directly contributes to a Th-2–dominant pulmonary inflammation. The induction of Th-2 inflammation by CARDS toxin is noteworthy because identification of a M. pneumoniae virulence factor responsible for asthma-like inflammation has been elusive. However, what is becoming clearer is the link between M. pneumoniae infection, CARDS toxin activity, and asthma (6, 8, 10, 11, 15, 17, 22, 23). Recently, we reported a case series where we detected the prolonged presence (up to18 mo) of CARDS toxin protein in the respiratory secretions of a subset of patients with refractory asthma (41). These data are in good agreement with the current literature where emerging experimental and clinical data strongly links M. pneumoniae and M. pneumoniae–associated virulence factors to wheezing and exacerbation asthma (19, 21–25, 39, 42, 43). Using mouse models of M. pneumoniae infection, it was shown that if mice are sensitized to allergen before infection, then the mice develop Th-2–dominated inflammation associated with airway remodeling and AHR (15, 16). In the current study, we report that rCARDS toxin treatment leads to eosinophilic inflammation, which is a hallmark of allergic cellular inflammation. In agreement with this observation, recently, the importance of mast cells in controlling M. pneumoniae infection was demonstrated, which is relevant because mast cells are another major effector cell during allergic inflammation (44). Together, these studies demonstrate the ability of M. pneumoniae to manipulate the pulmonary immune compartment under Th-1 and Th-2 inflammatory conditions and implicate a role for cells associated with allergy (eosinophils and mast cells) in the pathogenesis of disease. In this study, we show that a single exposure to rCARDS toxin is sufficient to cause prolonged allergic inflammation, with changes in pulmonary inflammation (perivascular lymphocytic lesions) observed up to 56 days after exposure in 83% of the mice. As with allergic asthma, the inflammatory response contributes to severe changes in respiratory physiology. After rCARDS toxin exposure, there is a significant increase in airway resistance that correlates temporally with peak inflammatory changes and a corresponding decrease in lung compliance that are linked to CD4+ T cells. The ability of rCARDS toxin to promote allergic inflammation may have a profound impact on the exacerbation of human asthma. A direct role for CARDS toxin in human asthma has not been proven, but it would not be unreasonable to predict a role for CARDS toxin in human lung disease (41, 45).
Recently, using a mouse model, we reported that CARDS toxin expression is induced in the lung and that the amounts of CARDS toxin produced vary between clinical isolates of M. pneumoniae (10, 11). This study also showed that disease severity in the mouse, as determined by lung function analysis and cytokine production, correlated positively with high levels of toxin, suggesting that human clinical disease severity might be strain dependent and associated with the amount of CARDS toxin produced.
Although many aspects of CARDS toxin biology and the role of this toxin in M. pneumoniae–associated pathologies are just emerging, the data presented in this study indicate that CARDS toxin can potently promote Th-2 inflammation and play a central role in shaping the immune environment of the lung leading to pulmonary pathology.
Supplementary Material
Acknowledgments
MBP antibodies were kindly provided by Drs. James and Nancy Lee from the Mayo clinic. Chicken anti-mouse mucin antibodies were a kind gift from Dr. Samuel Ho from the San Diego Veterans Administration hospital. The authors thank Brandon Guin for the production and purification of rCARDS toxin and Dr. Ben Daniels for assistance with flow cytometry. Flow cytometry data and analysis were performed in the UTHSCSA flow cytometry core facility partially supported by a National Cancer Institute grant P30 CA 54174.
Footnotes
This work was supported by National Institutes of Health grants AI 070412 and P30 CA 54,174 and by the Kleberg foundation.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2011-0135OC on January 26, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Waites KB, Talkington DF. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev 2004;17:697–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baseman JB, Tully JG. Mycoplasmas: Sophisticated, reemerging, and burdened by their notoriety. Emerg Infect Dis 1997;3:21–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tryon VV, Baseman JB. Pathogenic determinants and mechanisms. : Maniloff J, McElhaney RN, Finch LR, Baseman JB, Mycoplasmas: molecular biology and pathogenesis.Washington, DC: American Society for Microbiology; 1992. pp. 457–471 [Google Scholar]
- 4.Krause DC. Mycoplasma pneumoniae cytadherence: unravelling the tie that binds. Mol Microbiol 1996;20:247–253 [DOI] [PubMed] [Google Scholar]
- 5.Baseman JB, Reddy SP, Dallo SF. Interplay between mycoplasma surface proteins, airway cells, and the protean manifestations of mycoplasma-mediated human infections. Am J Respir Crit Care Med 1996;154:S137–S144 [DOI] [PubMed] [Google Scholar]
- 6.Kannan TR, Baseman JB. Adp-ribosylating and vacuolating cytotoxin of Mycoplasma pneumoniae represents unique virulence determinant among bacterial pathogens. Proc Natl Acad Sci USA 2006;103:6724–6729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kannan TR, Provenzano D, Wright JR, Baseman JB. Identification and characterization of human surfactant protein a binding protein of Mycoplasma pneumoniae. Infect Immun 2005;73:2828–2834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hardy RD, Coalson JJ, Peters J, Chaparro A, Techasaensiri C, Cantwell AM, Kannan TR, Baseman JB, Dube PH. Analysis of pulmonary inflammation and function in the mouse and baboon after exposure to Mycoplasma pneumoniae CARDS toxin. PLoS ONE 2009;4:e7562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson C, Kannan TR, Baseman JB. Cellular vacuoles induced by Mycoplasma pneumoniae CARDS toxin originate from rab9-associated compartments. PLoS ONE 2011;6:e22877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kannan TR, Musatovova O, Balasubramanian S, Cagle M, Jordan JL, Krunkosky TM, Davis A, Hardy RD, Baseman JB. Mycoplasma pneumoniae community acquired respiratory distress syndrome toxin expression reveals growth phase and infection-dependent regulation. Mol Microbiol 2010;76:1127–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Techasaensiri C, Tagliabue C, Cagle M, Iranpour P, Katz K, Kannan TR, Coalson JJ, Baseman JB, Hardy RD. Variation in colonization, adp-ribosylating and vacuolating cytotoxin, and pulmonary disease severity among Mycoplasma pneumoniae strains. Am J Respir Crit Care Med 2010;182:797–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fonseca-Aten M, Rios AM, Mejias A, Chavez-Bueno S, Katz K, Gomez AM, McCracken GH, Jr, Hardy RD. Mycoplasma pneumoniae induces host-dependent pulmonary inflammation and airway obstruction in mice. Am J Respir Cell Mol Biol 2005;32:201–210 [DOI] [PubMed] [Google Scholar]
- 13.Hardy RD, Jafri HS, Olsen K, Hatfield J, Iglehart J, Rogers BB, Patel P, Cassell G, McCracken GH, Ramilo O. Mycoplasma pneumoniae induces chronic respiratory infection, airway hyperreactivity, and pulmonary inflammation: a murine model of infection-associated chronic reactive airway disease. Infect Immun 2002;70:649–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardy RD, Jafri HS, Olsen K, Wordemann M, Hatfield J, Rogers BB, Patel P, Duffy L, Cassell G, McCracken GH, et al. Elevated cytokine and chemokine levels and prolonged pulmonary airflow resistance in a murine Mycoplasma pneumoniae pneumonia model: a microbiologic, histologic, immunologic, and respiratory plethysmographic profile. Infect Immun 2001;69:3869–3876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chu HW, Honour JM, Rawlinson CA, Harbeck RJ, Martin RJ. Effects of respiratory Mycoplasma pneumoniae infection on allergen-induced bronchial hyperresponsiveness and lung inflammation in mice. Infect Immun 2003;71:1520–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chu HW, Rino JG, Wexler RB, Campbell K, Harbeck RJ, Martin RJ. Mycoplasma pneumoniae infection increases airway collagen deposition in a murine model of allergic airway inflammation. Am J Physiol Lung Cell Mol Physiol 2005;289:L125–L133 [DOI] [PubMed] [Google Scholar]
- 17.Chu HW, Breed R, Rino JG, Harbeck RJ, Sills MR, Martin RJ. Repeated respiratory Mycoplasma pneumoniae infections in mice: effect of host genetic background. Microbes Infect 2006;8:1764–1772 [DOI] [PubMed] [Google Scholar]
- 18.Martin RJ, Chu HW, Honour JM, Harbeck RJ. Airway inflammation and bronchial hyperresponsiveness after Mycoplasma pneumoniae infection in a murine model. Am J Respir Cell Mol Biol 2001;24:577–582 [DOI] [PubMed] [Google Scholar]
- 19.Martin RJ, Kraft M, Chu HW, Berns EA, Cassell GH. A link between chronic asthma and chronic infection. J Allergy Clin Immunol 2001;107:595–601 [DOI] [PubMed] [Google Scholar]
- 20.Blasi F. Atypical pathogens and respiratory tract infections. Eur Respir J 2004;24:171–181 [DOI] [PubMed] [Google Scholar]
- 21.Johnston SL, Martin RJ. Chlamydophila pneumoniae and Mycoplasma pneumoniae: a role in asthma pathogenesis? Am J Respir Crit Care Med 2005;172:1078–1089 [DOI] [PubMed] [Google Scholar]
- 22.Kraft M, Cassell GH, Henson JE, Watson H, Williamson J, Marmion BP, Gaydos CA, Martin RJ. Detection of Mycoplasma pneumoniae in the airways of adults with chronic asthma. Am J Respir Crit Care Med 1998;158:998–1001 [DOI] [PubMed] [Google Scholar]
- 23.Kraft M, Cassell GH, Pak J, Martin RJ. Mycoplasma pneumoniae and Chlamydia pneumoniae in asthma: effect of clarithromycin. Chest 2002;121:1782–1788 [DOI] [PubMed] [Google Scholar]
- 24.Lieberman D, Printz S, Ben-Yaakov M, Lazarovich Z, Ohana B, Friedman MG, Dvoskin B, Leinonen M, Boldur I. Atypical pathogen infection in adults with acute exacerbation of bronchial asthma. Am J Respir Crit Care Med 2003;167:406–410 [DOI] [PubMed] [Google Scholar]
- 25.Seggev JS, Lis I, Siman-Tov R, Gutman R, Abu-Samara H, Schey G, Naot Y. Mycoplasma pneumoniae is a frequent cause of exacerbation of bronchial asthma in adults. Ann Allergy 1986;57:263–265 [PubMed] [Google Scholar]
- 26.Bubeck SS, Cantwell AM, Dube PH. Delayed inflammatory response to primary pneumonic plague occurs in both outbred and inbred mice. Infect Immun 2007;75:697–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Handley SA, Dube PH, Miller VL. Histamine signaling through the H(2) receptor in the peyer's patch is important for controlling Yersinia enterocolitica infection. Proc Natl Acad Sci USA 2006;103:9268–9273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bell RE, Kuehl TJ, Coalson JJ, Ackerman NB, Jr, Null DM, Jr, Escobedo MB, Yoder BA, Cornish JD, Nalle L, Skarin RM, et al. High-frequency ventilation compared to conventional positive-pressure ventilation in the treatment of hyaline membrane disease in primates. Crit Care Med 1984;12:764–768 [DOI] [PubMed] [Google Scholar]
- 29.Delemos RA, Coalson JJ, Gerstmann DR, Null DM, Jr, Ackerman NB, Escobedo MB, Robotham JL, Kuehl TJ. Ventilatory management of infant baboons with hyaline membrane disease: the use of high frequency ventilation. Pediatr Res 1987;21:594–602 [DOI] [PubMed] [Google Scholar]
- 30.Dube PH, Revell PA, Chaplin DD, Lorenz RG, Miller VL. A role for IL-1 alpha in inducing pathologic inflammation during bacterial infection. Proc Natl Acad Sci USA 2001;98:10880–10885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhong Y, Cantwell A, Dube PH. Transforming growth factor {beta} and CD25 are important for controlling systemic dissemination following Yersinia enterocolitica infection of the gut. Infect Immun 2010;78:3716–3725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chu HW, Jeyaseelan S, Rino JG, Voelker DR, Wexler RB, Campbell K, Harbeck RJ, Martin RJ. Tlr2 signaling is critical for Mycoplasma pneumoniae-induced airway mucin expression. J Immunol 2005;174:5713–5719 [DOI] [PubMed] [Google Scholar]
- 33.Hamid Q, Tulic M. Immunobiology of asthma. Annu Rev Physiol 2009;71:489–507 [DOI] [PubMed] [Google Scholar]
- 34.Shekels LL, Lyftogt C, Kieliszewski M, Filie JD, Kozak CA, Ho SB. Mouse gastric mucin: cloning and chromosomal localization. Biochem J 1995;311:775–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holt PG, Strickland DH, Bosco A, Jahnsen FL. Pathogenic mechanisms of allergic inflammation: atopic asthma as a paradigm. Adv Immunol 2009;104:51–113 [DOI] [PubMed] [Google Scholar]
- 36.Jacobsen EA, Ochkur SI, Lee NA, Lee JJ. Eosinophils and asthma. Curr Allergy Asthma Rep 2007;7:18–26 [DOI] [PubMed] [Google Scholar]
- 37.Afshar R, Medoff BD, Luster AD. Allergic asthma: a tale of many t cells. Clin Exp Allergy 2008;38:1847–1857 [DOI] [PubMed] [Google Scholar]
- 38.Sutherland ER, Martin RJ. Asthma and atypical bacterial infection. Chest 2007;132:1962–1966 [DOI] [PubMed] [Google Scholar]
- 39.Tang LF, Shi YC, Xu YC, Wang CF, Yu ZS, Chen ZM. The change of asthma-associated immunological parameters in children with Mycoplasma pneumoniae infection. J Asthma 2009;46:265–269 [DOI] [PubMed] [Google Scholar]
- 40.Panina-Bordignon P, Papi A, Mariani M, Di Lucia P, Casoni G, Bellettato C, Buonsanti C, Miotto D, Mapp C, Villa A, et al. The c–c chemokine receptors CCR4 and CCR8 identify airway T cells of allergen-challenged atopic asthmatics. J Clin Invest 2001;107:1357–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peters J, Singh H, Brooks EG, Diaz J, Kannan TR, Coalson JJ, Baseman JG, Cagle M, Baseman JB. Persistence of CARDS toxin-producing Mycoplasma pneumoniae in refractory asthma. Chest 2011;140:401–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gil JC, Cedillo RL, Mayagoitia BG, Paz MD. Isolation of Mycoplasma pneumoniae from asthmatic patients. Ann Allergy 1993;70:23–25 [PubMed] [Google Scholar]
- 43.Hou AC, Lu Y, Sha L, Liu LG, Shen J, Xu Y. (T(h1) and T(h2) cells in children with Mycoplasma pneumonia). Zhonghua Er Ke Za Zhi 2003;41:652–656 [PubMed] [Google Scholar]
- 44.Michels NM, Chu HW, LaFasto SC, Case SR, Minor MN, Martin RJ. Mast cells protect against airway Mycoplasma pneumoniae under allergic conditions. Clin Exp Allergy 2010;40:1406–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muir MT, Cohn SM, Louden C, Kannan TR, Baseman JB. Novel toxin assays implicate Mycoplasma pneumoniae in prolonged ventilator course and hypoxemia. Chest 2011;139:305–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.