Abstract
The incidence of idiopathic pulmonary fibrosis (IPF) increases with age. The mechanisms that underlie the age-dependent risk for IPF are unknown. Based on studies that suggest an association of IPF and γherpesvirus infection, we infected young (2–3 mo) and old (≥18 mo) C57BL/6 mice with the murine γherpesvirus 68. Acute murine γherpesvirus 68 infection in aging mice resulted in severe pneumonitis and fibrosis compared with young animals. Progressive clinical deterioration and lung fibrosis in the late chronic phase of infection was observed exclusively in old mice with diminution of tidal volume. Infected aging mice showed higher expression of transforming growth factor-β during the acute phase of infection. In addition, aging, infected mice showed elevation of proinflammatory cytokines and the fibrocyte recruitment chemokine, CXCL12, in bronchoalveolar lavage. Analyses of lytic virus infection and virus reactivation indicate that old mice were able to control chronic infection and elicit antivirus immune responses. However, old, infected mice showed a significant increase in apoptotic responses determined by in situ terminal deoxynucleotidyl transferase dUTP nick end labeling assay, levels of caspase-3, and expression of the proapoptotitc molecule, Bcl-2 interacting mediator. Apoptosis of type II lung epithelial cells in aging lungs was accompanied by up-regulation of endoplasmic reticulum stress marker, binding immunoglobulin protein, and splicing of X-box–binding protein 1. These results indicate that the aging lung is more susceptible to injury and fibrosis associated with endoplasmic reticulum stress, apoptosis of type II lung epithelial cells, and activation of profibrotic pathways.
Keywords: idiopathic pulmonary fibrosis, lung, aging, γherpesvirus, endoplasmic reticulum stress
Clinical Relevance
Idiopathic pulmonary fibrosis is predominantly a disease of older adults. No data are available concerning the age-related mechanisms involved in the high susceptibility to lung injury and fibrosis. We found that aging type II alveolar epithelial cells have sustained endoplasmic reticulum stress that causes severe apoptosis.
Idiopathic pulmonary fibrosis (IPF) is characterized by progressive destruction of the normal architecture of the lung. Its cause is unknown, and there is not a proven effective therapy other than lung transplantation. The prevalence of IPF increases with age, with most patients aged more than 60 years (1, 2). IPF remains a progressive, irreversible, and lethal disease with a median survival from 3 to 5 years after the diagnosis. The cellular and molecular pathways that drive the pathogenesis of IPF are not fully delineated. However, several clinical studies have found DNA or protein from the human γherpesvirus, Epstein-Barr virus (3–6) in 40–70% of IPF cases (3–6). We have established a mouse model of progressive pulmonary fibrosis using the murine γherpesvirus (MHV) 68, a virus closely related to Epstein-Barr virus (7). We have shown that γherpesvirus infection causes persistent virus infection, injury of lung epithelial cells, and pulmonary fibrosis in IFN-γR−/− mice with similar features to the human disease. Progressive fibrosis was not found in virus-infected wild-type mice (8–13).
Aging is an important risk factor for the development of IPF. The process of aging is complex, multifactorial, and is associated with alterations in the physiological responses to injury and repair. There are structural and functional age-related changes in the lung even in the absence of disease. In addition, there is a propensity for oxidative stress, proinflammatory responses, and apoptosis in stress conditions (14, 15). One of the potential mechanisms involves alterations in the homeostasis of the endoplasmic reticulum (ER), predisposing to ER stress. ER stress results from misfolding of proteins, and leads to up-regulation of a signaling pathway called the ER stress response or the unfolded protein response (UPR). The most evolutionary conserved UPR signaling pathways existing in mammals is the activation of the inositol-requiring enzyme 1 (IRE1) α and β. This activation results in the excision of a 26-bp fragment from the mRNA encoding the transcription factor, X-box–binding protein 1 (XBP1), by an unconventional splicing event that generates XBP1s a potent inducer of a subset of UPR target genes (16). UPR is characterized by the induction of chaperones, such as BiP (binding immunoglobulin protein), degradation of misfolded proteins, and attenuation of protein translation. Notably, prolonged and severe ER stress triggers apoptosis (17). ER stress and UPR activation are common features of the alveolar epithelium in familial and sporadic IPF (18, 19).
Our data support that virus-induced lung injury in aging mice increases ER stress responses in type II lung epithelial cells, resulting in severe apoptosis and activation of profibrotic pathways.
Materials and Methods
A detailed description of methodologies is provided in the online supplement.
Animals and Animal Treatment
Young (2–3 mo) and old (≥18 mo) C57BL/6 mice were inoculated intranasally with 1 × 105 plaque-forming units (pfu) of MHV68, as we have described previously (10). Infected mice were maintained in a biosafety level 2 (BSL2) facility.
Histopathology, Immunofluorescence, and Immunohistochemistry
After mice were killed, lungs were perfused with 4% paraformaldehyde (10) or Optimal Cutting Temperature Tissue-Tek Compound (Torrance, CA). Sections from paraffin blocks were stained with hematoxylin and eosin and Masson trichrome to determine histopathological changes and fibrosis. Morphometric analyses were performed as described previously (9). Immunohistochemistry analyses were performed using anti-XBP1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Dual immunofluorescence staining was performed using methods outlined previously (18, 20).
Histopathological Score
A 0- to 4-point scale was used as follow: 0 = normal lung architecture; 1 = lymphocytic infiltrates in perivascular, peribronchial, and subpleural areas, but not fibrosis; 2 = lymphocytic infiltrates and perivascular and peribronchial fibrosis; 3 = lymphocytic infiltrates and fibrotic thickening of the interalveolar septa; 4 = lymphocytic infiltrates, presence of foamy macrophages, formation of multiple fibrotic foci, and fibrotic thickening of the pleura. Because of the patchy pathology of the infected lungs, the score for 10 random fields was recorded and the highest score found was assigned for each individual specimen.
Hydroxyproline Assay
Hydroxyproline content in whole mouse lung was used to quantify lung collagen content and was measured colorimetrically by a method described previously (21).
Western Blot Analyses
Extracts from lung tissue samples and cell cultures were prepared as previously described (9). Western blotting for BiP (Millipore, Billerica, MA) was performed, and then the blot was reprobed with an antiserum against β-actin (Santa Cruz Biotechnology) as a loading control. Western blots were quantified using NIH ImageJ software (v.1.43; National Institutes of Health, Bethesda, MD).
Quantitative Real-Time PCR Analysis
Total DNA and RNA were extracted from lung tissue using DNeasy and RNeasy kits, respectively (Qiagen, Valencia, CA), according to manufacturer's recommendations. Real-time RT-PCR was performed using SYBR Green and primers specific for the genes of interest and normalized using 18S RNA and RPL19 genes. Quantitative PCR for MHV68 DNA was performed as described previously (13) using open reading frame (ORF) 50 primers and glyceraldehyde 3-phosphate dehydrogenase as housekeeping gene.
Cytokine and Chemokine Expression
Mouse IL-6, IFN-γ, IL-10, and monocyte chemotactic protein 1 levels were measured in bronchoalveolar lavage (BAL) fluid using a multiplex bead immunoassay (Linco, St. Charles, MI) according to the manufacturer's recommendations, whereas transforming growth factor (TGF)-β was determined with a single-analyte ELISA (Qiagen). CXCL12 was measured in lung whole-cell extracts using an ELISA kit (R&D Systems, Minneapolis, MN).
Statistical Analyses
Data were plotted and statistically analyzed using InStat 3 and Prism 5 (GraphPad Software, San Diego, CA). Differences among groups were assessed using one-way ANOVA and between pairs using Student's t test. Results are presented as means (±SEM). Significant differences have P values less than 0.05.
Results
Virus-Induced Lung Fibrosis in Aging C57BL/6 Mice
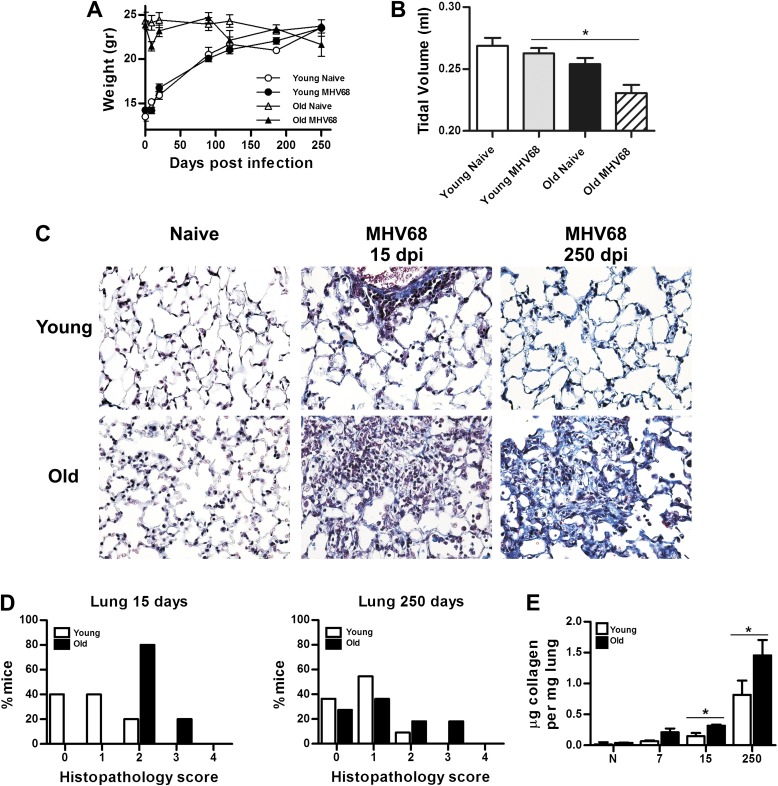
Our previous studies showed that young (2 mo old) C57BL/6 mice infected with γherpesvirus control rapid lytic virus replication during the acute phase of infection (Days 7–15) and develop mild pneumonitis that completely reverses by Day 25 after infection (10). To determine whether aging results in a differential response to herpesvirus infection, we infected old (≥18 mo old) C57BL/6 mice. In an examination of weight loss with time, infection of old mice resulted in a steep decline in weight that peaked around 15 days after infection (dpi), recovered to the starting weights by 90 dpi, and then declined again at 120 dpi without recovering over the remaining 130-day time course (Figure 1A). In sharp contrast, young, virus-infected mice never experienced weight loss during the course of the acute or chronic phase of infection, and remained nearly indistinguishable from their noninfected counterparts (Figure 1A). At Day 250 after infection, lung function was measured using a whole-body plethysmograph. Old C57BL/6 animals showed a significant reduction in tidal volume, corresponding to a restrictive pulmonary defect in lung function (Figure 1B).
Figure 1.
Severe clinical disease and fibrosis in murine γherpesvirus (MHV) 68–infected aging mice. (A) Weight loss data are presented as total body weight. More severe illness was observed in aging infected mice (number of mice: 5–13 per group and time point). Data are representative of three different experiments. (B) Lung function determined by the measurement of tidal volume using a whole-body plethysmograph. Measurements were performed at Day 120 after infection (number of animals: 3–5 per group; *P < 0.05). (C) Masson trichrome staining of lung sections from 3-month-old (young) and >18-mo-old (old) C57BL/6 mice infected with MHV68 at indicated times after infection. Collagen deposition is shown in blue. Notice loss of normal lung architecture of the lung in old infected mice associated with higher interstitial collagen deposition. Young mice only show collagen deposition around blood vessels at Day 250 after infection. (D) Semiquantitative morphometric analysis of lung histopathology in virus-infected young and old mice at the indicated time after infection. Old infected mice showed higher pathology scores corresponding to pneumonitis and thickening of the interalveolar septa compared with young animals (n = 5–8). (E) Normalized lung collagen content based on hydroxyproline microplate assay at 7, 15, and 250 days after infection (dpi) (*P < 0.05; n = 3).
Lungs were analyzed for collagen deposition by Masson trichrome staining at 15 dpi (immediately after the acute phase of infection) and at 250 dpi (late in the chronic period of infection). Compared with naive and young mice, old (≥18 mo old) C57BL/6 mice infected with γherpesvirus developed more severe pneumonitis with collagen deposition indicated by blue staining at 15 dpi that persisted as a patchy interstitial fibrosis during the late chronic phase in 30% of the old, infected mice (Figure 1C). Overall, there was only a mild degree of lung pathology in young mice infected with MHV68 at 15 and 250 dpi as compared with the pathology scores of old, infected mice (Figure1D). Lung fibrosis was also assessed by collagen content by measuring levels of OH-proline (Figure 1E). We found significantly higher levels of OH-proline in aging mice at 15 and 250 dpi. Taken together, these data provided evidence for increased virus-induced lung fibrosis in aging mice.
Control of Lytic and Latent MHV68 Infection with Age
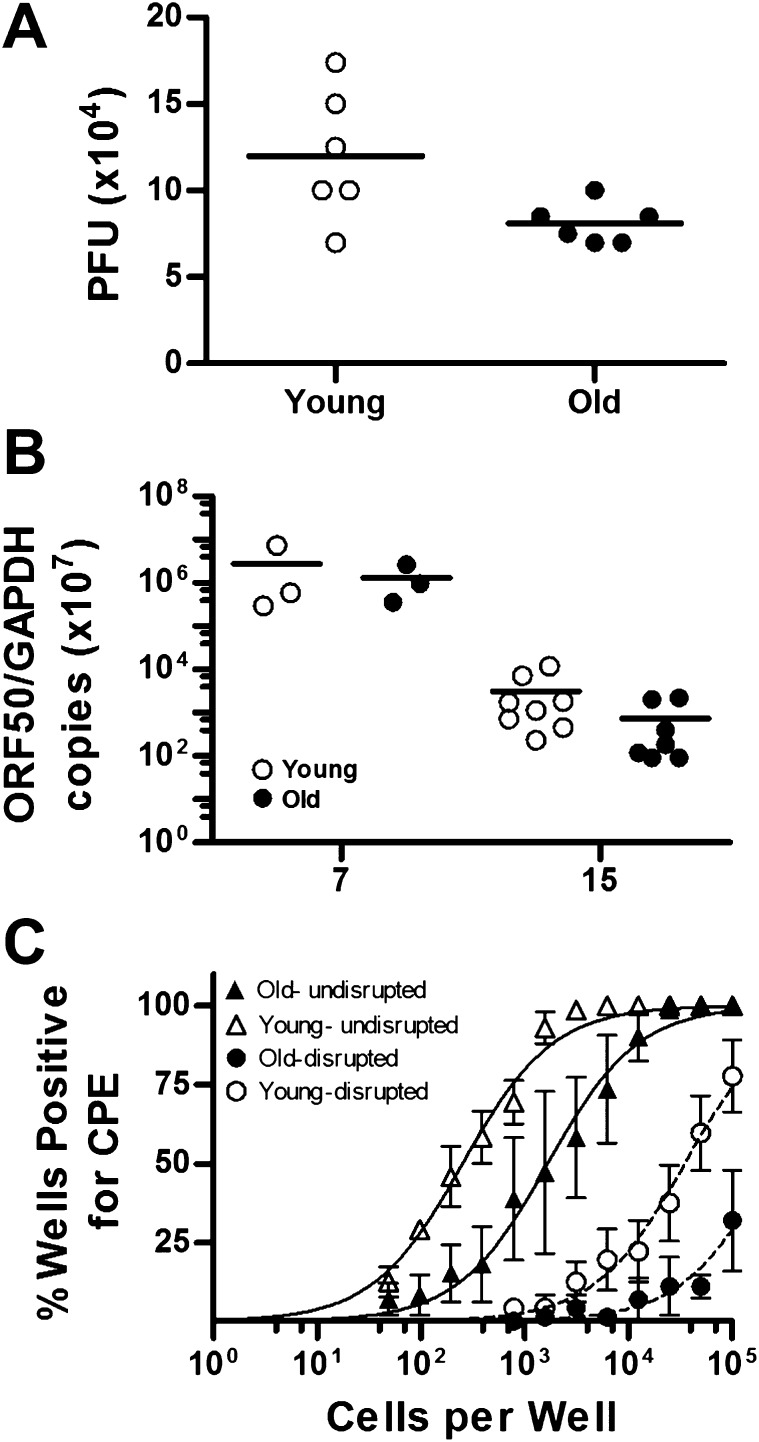
To determine whether there is an age-associated decline in the ability to control lytic and persistent infection, we monitored virus replication during the acute phase of infection at 7 dpi, a period of peak replication in the lung after intranasal infection. Viral titer of the lung was comparable between young and old mice at this time point (Figure 2A). The level of latent virus was measured at 7 and 15 dpi in the lung using a quantitative PCR assay that allows determination of a latency-associated gene product (Figure 2B). We found that latent virus in the lungs at 7 and 15 dpi was similar in young and old mice. In addition, we analyzed virus reactivation and the presence of preformed infectious virus in spleens by a sensitive limiting dilution reactivation assay upon explant at 15 dpi. Virus reactivation was nearly 10-fold lower in aging mice in comparison with their young counterparts (Figure 2C). Preformed infectious virus detected in the disrupted splenocytes was also appreciably lower in the aged mice. We failed to detect lytic virus in the lung or spleen late during chronic infection at 250 dpi.
Figure 2.
Control of chronic virus infection in young and aging mice. (A) Acute replication in the lungs of young and old C57BL/6 mice at Day 7 after infection. Lungs were harvested, disrupted, and titered on NIH 3T12 cells by plaque assay. Data are shown as log10 titer, and the bar indicates the geometric mean titer. (B) Quantitative PCR analysis of virus load of individual lungs from MHV68-infected young and old C57BL/6 mice at 7 and 15 dpi. Viral load was determined as the levels of viral DNA (targeting the using open reading frame [ORF] 50 region) normalized by cellular DNA (targeting glyceraldehyde 3-phosphate dehydrogenase). Bars represent geometric mean. (C) Reactivation of MHV68 in spleens from infected young and old mice was assessed using standard limiting dilution analyses at 15 dpi (n = 3–4 mice). Serial dilutions of bulk intact or disrupted splenocytes were plated on monolayers of mouse embryonic fibroblasts (MEFs). The presence of reactivating virus was determined by the presence of cytopathic effect (CPE). CPE observed in the disrupted splenocytes indicate preformed infectious virus. Symbols represent the mean percentage of wells positive for CPE (±SEM). Curve fit lines were derived from nonlinear regression analysis. PFU, plaque-forming units.
Because T cell responses are important contributors to controlling MHV68 infection, we determined the frequency of MHV68-specific CD8 T cells in lungs of naive and virus-infected mice during the acute (11 d) and chronic (30 d) phase of infection using major histocompatibility complex class I tetramers specific for two well characterized MHV68 epitopes, p56 (ORF6487–495) and p79 (ORF61524–531). The data showed a similar percentage of p56- and p79-specific CD8 T cells in young and old mice (see Figure E1A in the online supplement). In addition, we determined the percentage of vβ4-CD8 T cells, which have been shown to be driven by the M1 latency gene (22). We found similar expansion of these CD8 T cells in spleens at 30 dpi (Figure E1B). These data suggest that aging mice can control chronic MHV68 infection, and that the fibrotic response to infection in old mice is not due to impaired antiviral responses.
High Levels of Proinflammatory Cytokines and Chemokines in MHV68-Infected Aging Mice in the Acute Phase of Infection
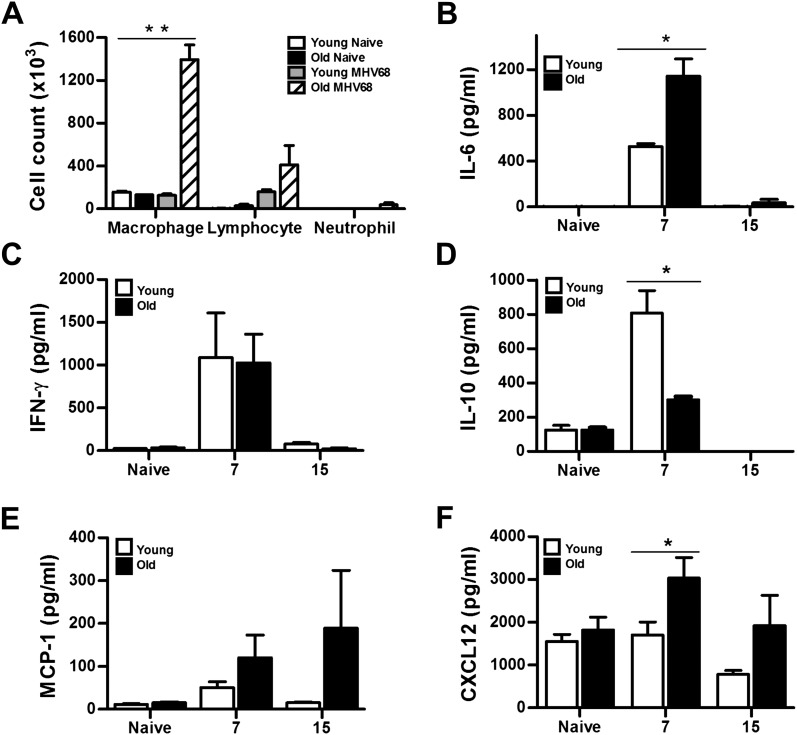
To characterize the inflammatory response in infected aging mice, we performed differential counts from BAL of young and old mice at 15 dpi. Similar numbers of cells were found between naive young and naive old animals (Figure 3A). After infection, higher number of macrophages and lymphocytes were found in aging mice compared with young animals; however, the relative percentage of lymphocytes in the young infected mice was higher than their old infected counterparts (54.7 versus 30.2%).
Figure 3.
Higher levels of proinflammatory cytokines and chemokines in MHV68-infected aging mice. (A) Differential cell counts in the bronchoalveolar lavage (BAL) of naive and infected young and old mice at 15 dpi. (**P < 0.01; n = 8). (B–F) IL-6, IFN-γ, IL-10, and MCP-1 levels were measured in BAL fluid from naive and MHV68-infected young and old mice at the indicated time points after infection, whereas CXCL12 was measured in lung tissue. Bars represent means (±SEM) (*P < 0.05; n = 5 per group).
In BAL, we found up-regulation of both IL-6 and IFN-γ in aging and young mice at 7 dpi although significantly higher levels of IL-6 were found in old animals (Figures 3B and 3C). In contrast, levels of the anti-inflammatory cytokine, IL-10, were significantly higher in young animals (Figure 3D). We also compared levels of the chemokines, monocyte chemotactic protein 1 (Figure 3E) and CXCL12 (Figure 3F), in the BAL and lung tissue, respectively. These two chemokines have been associated with the recruitment of fibrocytes. We found high levels of both chemokines in infected lungs of aging mice at Days 7 and 15 after infection. However, determination of fibrocyte recruitment to the infected lungs by FACS using CD45+ CXCR4+ collagen1+ markers showed similar percentage of fibrocytes in lungs of young and old mice (Figure E2). These results suggest that increased fibrosis observed in MHV68-infected, aging mice is independent of fibrocyte recruitment.
Age-Related Changes in TGF-β Expression
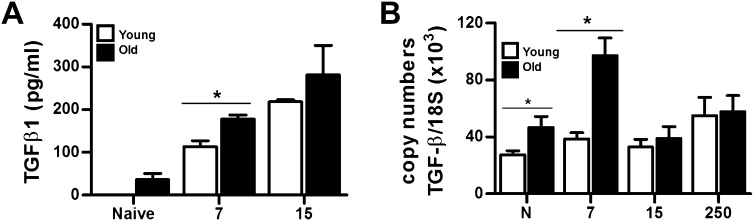
Our previous studies in IFN-γR–deficient mice have shown that MHV68 infection induces up-regulation and activation of the profibrotic factor, TGF-β1. We found a higher concentration of active TGF-β1 in BAL of naive and acutely infected aging mice (Figure 4A). In concordance, quantitative RT-PCR analyses of total lung lysates showed that naive and 7 dpi–infected, aging mice have higher levels of TGF-β transcripts (Figure 4B). Chronic infected young and old mice show similar high levels of TGF-β transcripts (Figure 4B). Our data suggest that aging lungs up-regulate TGF-β during the acute phase of virus infection.
Figure 4.
High expression of transforming growth factor (TGF)-β in naive and virus-infected aging mice. (A) Active TGF-β levels were measured in BAL fluid from naive and MHV68 infected young and old mice at 7 and 15 dpi (n = 3). (B) Quantitative RT-PCR analysis for TGF-β transcripts in the lungs of naive and infected young and old mice at different time point after infection (*P < 0.05; n = 4).
Apoptosis of Lung Epithelial Cells in Infected, Aging Mice
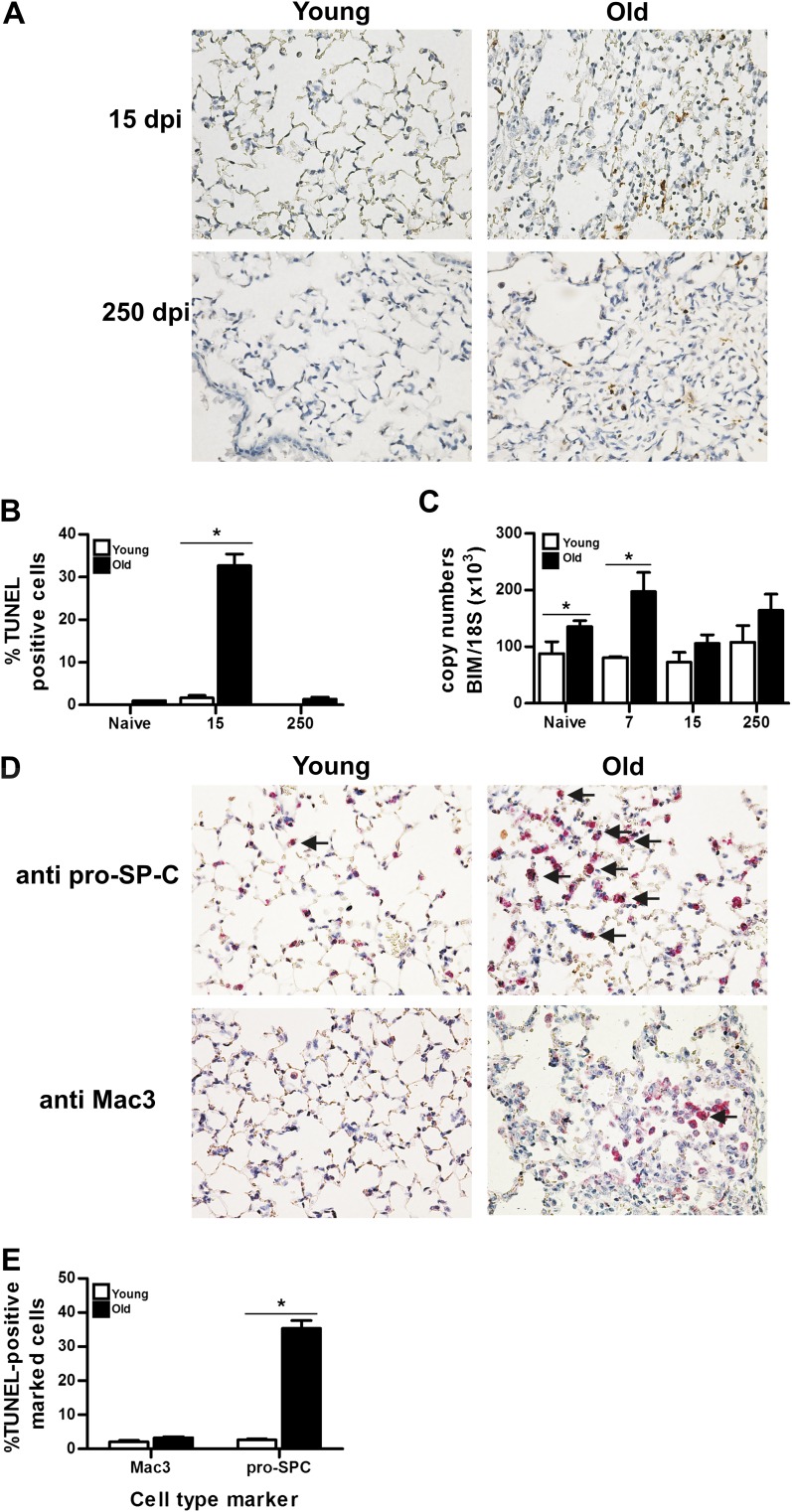
Several lines of evidence imply that apoptosis may play a role in the aging process and the age-related functional declines of multicellular organisms. To determine the apoptotic response in young and old mice infected with MHV68, we performed in situ terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay in lung slides from naive and infected mice at 15 and 250 dpi. Naive young and aging mice showed low numbers of apoptotic cells, but the frequency of apoptotic cells increased dramatically after infection, especially in aging mice (Figure 5A). Semiquantitative analyses of the signal from the in situ TUNEL assay showed significant elevation of the apoptotic responses after infection in lung samples from aging mice (Figure 5B). Apoptosis can be triggered by members of the Bcl-2 protein family, such as Bcl-2 interacting mediator (Bim). By quantitative RT-PCR, we found higher expression of Bim in naive and infected, aging lungs compared with samples from young animals (Figure 5C). Injury of lung epithelial cells is believed to be critical for the initiation of the fibrotic process in the lung. In addition, a potential role for apoptotic macrophages in pulmonary inflammation and fibrosis has been reported (23). To determine the type of cells undergoing apoptosis, we performed TUNEL assay and staining using anti-CD107b (Mac-3) and anti–pro–surfactant C antibodies as markers of macrophages and type II lung epithelial cells, respectively (Figure 5D). Numerous TUNEL-positive type II lung epithelial cells (pro–surfactant C positive) were present in infected lungs from aging mice, whereas only few apoptotic cells were Mac3 positive. Less abundant apoptotic type II lung epithelial cells were found in lungs of infected, young animals (Figure 5E).
Figure 5.
Apoptosis in type II epithelial cells from infected old C57BL/6 mice. (A) Representative images from in situ terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay in lung sections from young and old mice at 15 and 250 dpi. Magnification, 40×. (B) Semiquantitative analyses in high-power field show a greater percentage of TUNEL positive cells in old infected mice (n = 3). (C) Quantitative RT-PCR analysis for Bim transcripts in the lungs of naive and infected young and old mice at different time point after infection (n = 4). (D) Macrophage and type II lung epithelial cell death was evaluated in paraffin-embedded tissue sections from 15 dpi using TUNEL assay (brown). Macrophages and type II lung epithelial cells were identified using Mac3 and pro–surfactant-C antibodies (red). Higher number of apoptotic type II lung epithelial cells (arrows) was found in old infected mice. (E) Semi-quantitative analyses in high-power field show a greater percentage of double stained cells (TUNEL positive nucleus + cell type marker) in old infected mice (n = 4). SP-C, surfactant protein-C.
Activation of ER Stress Responses in Lung Epithelial Cells from Infected, Aging Mice
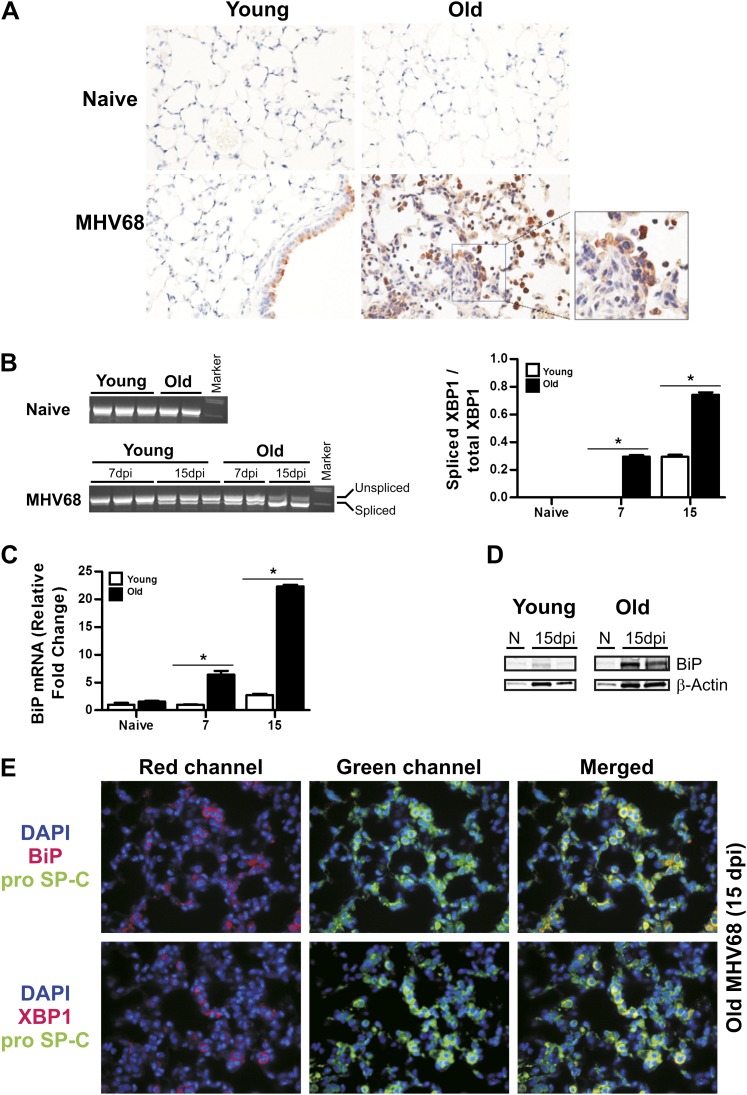
Susceptibility to lung injury and apoptosis has been associated with ER stress responses in the lung. The transcription factor, XBP1s is a key component of the ER stress response in lung epithelial cells (24). We analyzed the XBP1 expression in lungs from uninfected and infected young and old mice by immunohistochemistry analysis. No signal was observed in naive young and old mice. At 15 dpi, airway epithelial cells from infected, young mice showed XBP1-positive signal, whereas old infected mice had extensive staining in lung epithelial and inflammatory cells (Figure 6A). In conditions of ER stress, XBP1 mRNA undergoes IRE1-dependent splicing that permits translation of the biologically active XBP1 protein. The ratio of the spliced isoform to total XBP1 mRNA can be used as a marker of the IRE1-mediated ER stress response (13). We found predominance of unspliced XBP1 in naive young and old mice (Figure 6B). After infection, splicing of XBP1 was evident at 15 dpi in young mice; however, old animals showed evidence of ER stress earlier, at 7 dpi, with a further increase by 15 dpi (Figure 6B). ER stress also induces expression of the chaperone BiP. RT-PCR analyses showed that lungs from old, infected mice have higher expression of BiP compared with young animals (Figure 6C). Immunoblot analysis confirmed higher protein levels in the aging lung (Figure 6D). To determine if markers of ER stress were expressed in type II lung epithelial cells from aging mice, we performed coimmunostaining for type II lung epithelial cells (anti–pro–surfactant protein C, green) and XBP1 or BiP (red). Colocalization of type II lung epithelial cell markers and ER stress markers were observed in infected lungs from aging mice at 15 dpi (Figure 6E) and persisted at high levels at 250 dpi (Figure E3). These data provide evidence that type II lung epithelial cells in aging mice have lower resistance to ER stress responses after virus infection, and that this process is accompanied by increased apoptosis.
Figure 6.
Endoplasmic reticulum (ER) stress in lung epithelial cells from infected old C57BL/6 mice. (A) Immunohistochemistry analyses of X-box–binding protein (XBP) 1 expression in lung sections of young and old mice at Day 15 of mock or MHV68 infection. Notice abundant positive staining in old MHV68-infected mice. (B) Determination of mRNA levels of unspliced and spliced XBP1 in lung samples of naive and MHV68-infected mice at Days 7 and 15 after infection. Ratio of spliced versus total XBP1 mRNA of samples is shown on the right. (C) Quantitative RT-PCR analysis for binding immunoglobulin protein (BiP) transcripts in the lungs of naive and infected young and old mice at different time point after infection (n = 3–4; *P < 0.001). (D) Immunoblot assay of lung lysates from naive (N) and infected young and old C57BL/6 mice at the indicated time point after infection using anti-BiP antibody. Blot was stripped and reprobed with an anti–β-actin antibody as a loading control. (E) Dual immunofluorescent staining in lung sections of aging mice infected at 15 dpi for pro–SP-C (green) and the markers of ER stress BiP (red) and XBP1 (red). Yellow cells in the merge column indicate type II cells supporting ER stress. Nuclei were visualized by 4′,6-diamidino-2-phenylindole staining (blue). SP-C, surfactant protein-C.
Discussion
The incidence and prevalence of IPF increase noticeably with age. A recent study showed that prevalence of IPF increased 50- to 60-fold when comparing adults less than 35 years of age with those over 75 years of age (1, 2). However, the pathogenic mechanisms involved in the higher susceptibility of aging individuals to lung fibrosis are unknown. Based on several studies that show detection of viral proteins and/or viral genome of herpesvirus in the lungs of patients with IPF, we have established a murine model of MHV68-induced lung fibrosis. Here, we examined whether aging in C57BL/6 mice is sufficient to increase the susceptibility to lung fibrosis after herpesvirus infection. We found that, compared with infected young C57BL/6 mice, which develop only a mild, reversible pneumonitis, infected old C57BL/6 mice have more severe and progressive inflammation and collagen deposition. The severity of the virus-induced lung pathology in aging mice was associated with a dramatic increase of ER stress markers and apoptosis of type II lung epithelial cells with subsequent fibrosis.
Aging has been associated with impaired immune responses that might have an effect in the control of acute virus infection. Less is known of chronic, persistent, and latent virus infections, such as MHV68 infection. Previous studies have shown that aging mice can control acute and latent MHV68 infection with optimal maintenance of functional virus-specific CD8 T cells (25). Similarly, our studies found that de novo infected, old mice do not have deficiencies in virus control. We found no significant differences in virus replication and load in the lung, and no evidence for increased viral load in the spleen. We report that aging mice exhibited normal IFN-γ production and induction of virus-specific CD8 T cells. Moreover, aging mice show higher percentage of effector and central CD8 memory phenotype T cells (Figure E4) in the lungs before and after infection that might contribute to immune responses and maintenance of low levels of viral persistence in the lung.
Alteration of innate immune responses have also been described during aging that include the propensity for increased production of proinflammatory mediators (24). We found that aged, MHV68-infected mice expressed higher levels of proinflammatory cytokines during the acute phase of infection associated with elevated CXCL12, a recruiting chemokine for fibrocytes. Using the bleomycin-induced lung injury model and accelerated senescence mice, we have observed similarly high levels of CXCL12 after injury in senescence mice (14, 26, 27). Those studies showed a senescence-related change in the bone marrow cell population characterized by increased proportion of fibrocytes in the bone marrow and a diminution of mesenchymal stem cells. In addition to this profibrotic mechanism, the susceptibility to fibrosis in aging mice can be related to high levels of TGF-β. Our data indicate that there are age-related changes in the levels of TGF-β transcripts and protein in naive and acutely infected mice. However, differences between young and old infected mice are no longer obvious when young mice reach 250 dpi, a time point at which young mice have reached almost 1 year of age.
Previous studies have demonstrated the importance of the cross-talk between fibroblasts and epithelial cells in the control of the profibrotic responses in the lung (28). Our data suggest that aging lungs are more susceptible to ER stress and injury of lung epithelial cells. Abnormal function of the ER might lead to an evolutionarily conserved cell stress response, the UPR, which is aimed to compensate for the damage, but can eventually trigger cell death if the ER dysfunction is persistent or severe. Three distinct UPR signaling pathways exist in mammalian cells, including IRE1α and -β, pancreatic ER kinase, and activating transcription factor 6 (29). Activation of IRE1 results in an unconventional splicing event that generates XBP1s (30). We have found that aging mice exposed to MHV68 infection have persistent and progressively increasing levels of XBPs in lung epithelial cells and inflammatory infiltrates, as well as high levels of BiP. Markers of ER stress have been found in alveolar type II cells from lungs of patients with IPF. Moreover, epithelial cells from both IPF and chronic obstructive pulmonary disease lungs undergo apoptosis, as evidenced by caspase-3 activation and Bcl-2–associated X protein dimerization, but only IPF specimens show activation of ER stress responses (19). ER stress in IPF lungs has also been associated with altered surfactant protein processing and herpesvirus infection. The association of herpesvirus infection with ER stress and IPF was evaluated in patients with familial IPF, including family members with the surfactant protein C mutation L188Q, individuals with familial interstitial pneumonia without surfactant C protein mutations, and individuals with sporadic IPF. In these studies, herpesvirus protein expression was found in alveolar epithelial cells from 15/23 patients with IPF, and colocalized with UPR markers in alveolar epithelial cells from these patients (18). More recently, a mouse model with transgenic expression of the surfactant C protein mutation, L188Q, in alveolar epithelial cells demonstrated induction of ER stress, but lung fibrosis was only identified in the presence of a profibrotic stimulus (23). These data suggest that patients with alterations in the processing of surfactant C protein and susceptibility to ER stress have predisposition to fibrosis after a second hit such as herpesvirus infection.
Our studies support the concept that the susceptibility to ER stress increases during aging. ER stress initially stimulates an adaptive UPR to promote cellular survival, whereas, in the case of persistent, chronic stress, UPR can trigger apoptotic cell death program (31, 32). The aging process contains abundant characteristics that might affect the ER stress response (e.g., increased oxidative stress, disturbance in calcium homeostasis, misfolding and aggregation of proteins, and impairment in global protein synthesis) (33–35). All these age-related changes imply that aged cells might be more vulnerable to ER stressors, leading to apoptosis. Thus, similar to patients with alterations in the processing of surfactant C protein, aging individuals exposed to injury may have vulnerable epithelial cells that are prone to ER stress, resulting in increased proapoptotic and profibrotic signals. Moreover, ER stress might induce inflammatory responses by production of reactive oxygen species, release of calcium from the ER, activation of the transcription factor, NF-κB, and mitogen-activated protein kinase pathway. Our studies show that type II lung epithelial cells are particularly sensitive to ER stress in the aging lung. Recently, ER stress has been associated with epithelial–mesenchymal transition of type II lung epithelial cells (36, 37). Further studies will be required to determine if increased ER stress in aging lungs has a role in epithelial–mesenchymal transition and fibroblast accumulation by this pathway.
In summary, our studies show increased susceptibility during aging to lung injury by herpesvirus infection, resulting in pulmonary fibrosis. We show that the propensity to disrepair in old animals is unrelated to control of chronic virus infection. Our studies suggest that aging individuals develop persistent and more severe ER stress responses than younger counterparts, causing exacerbated apoptosis and fibrosis. These studies confirm that there are age-related changes in the physiological responses to injury, and that the understanding of these mechanisms might result in better therapeutic approaches for IPF.
Supplementary Material
Acknowledgments
The authors thank Kathleen S. R. Grey and Benson Yee Hin Cheng for technical assistance.
Footnotes
This work was supported by the American Heart Association (AHA) grant 09BGIA2110115 (A.L.M.), National Institutes of Health (NIH) grant 1 P30 AR058910-01 (A.L.M.), R01 HL090851 (D.S.), NIH RO1 HL 085317 (T.S.B.), NIH R01 HL105479 (W.E.L.), Vanderbilt Clinical Translational Science Award (CTSA) grant NIH NCRR UL1 RR024975 (W.E.L.), American Foundation for Aging Research (AFAR) grant (M.R.), and the research support from Vascular Medicine Institute, the Institute for Transfusion Medicine, and the Hemophilia Center of Western Pennsylvania (A.L.M.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2011-0224OC on January 6, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Raghu G, Nyberg F, Morgan G. The epidemiology of interstitial lung disease and its association with lung cancer. Br J Cancer 2004;91 Suppl 2:S3–S10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castriotta RJ, Eldadah BA, Foster WM, Halter JB, Hazzard WR, Kiley JP, King TE, Jr, Horne FM, Nayfield SG, Reynolds HY, et al. Workshop on idiopathic pulmonary fibrosis in older adults. Chest 2010;138:693–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stewart JP, Egan JJ, Ross AJ, Kelly BG, Lok SS, Hasleton PS, Woodcock AA. The detection of Epstein-Barr virus DNA in lung tissue from patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1999;159(4 Pt 1):1336–1341 [DOI] [PubMed] [Google Scholar]
- 4.Egan JJ, Woodcock AA, Stewart JP. Viruses and idiopathic pulmonary fibrosis. Eur Respir J 1997;10:1433–1437 [DOI] [PubMed] [Google Scholar]
- 5.Tang YW, Johnson JE, Browning PJ, Cruz-Gervis RA, Davis A, Graham BS, Brigham KL, Oates JA, Jr, Loyd JE, Stecenko AA. Herpesvirus DNA is consistently detected in lungs of patients with idiopathic pulmonary fibrosis. J Clin Microbiol 2003;41:2633–2640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelly BG, Lok SS, Hasleton PS, Egan JJ, Stewart JP. A rearranged form of Epstein-Barr virus DNA is associated with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2002;166:510–513 [DOI] [PubMed] [Google Scholar]
- 7.Nash AA, Dutia BM, Stewart JP, Davison AJ. Natural history of murine gamma-herpesvirus infection. Philos Trans R Soc Lond B Biol Sci 2001;356:569–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mora AL, Torres-Gonzalez E, Rojas M, Corredor C, Ritzenthaler J, Xu J, Roman J, Brigham K, Stecenko A. Activation of alveolar macrophages via the alternative pathway in herpesvirus-induced lung fibrosis. Am J Respir Cell Mol Biol 2006;35:466–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mora AL, Torres-Gonzalez E, Rojas M, Xu J, Ritzenthaler J, Speck SH, Roman J, Brigham K, Stecenko A. Control of virus reactivation arrests pulmonary herpesvirus–induced fibrosis in IFN-gamma receptor–deficient mice. Am J Respir Crit Care Med 2007;175:1139–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mora AL, Woods CR, Garcia A, Xu J, Rojas M, Speck SH, Roman J, Brigham KL, Stecenko AA. Lung infection with gamma-herpesvirus induces progressive pulmonary fibrosis in Th2-biased mice. Am J Physiol Lung Cell Mol Physiol 2005;289:L711–L721 [DOI] [PubMed] [Google Scholar]
- 11.Krug LT, Moser JM, Dickerson SM, Speck SH. Inhibition of NF-kappaB activation in vivo impairs establishment of gammaherpesvirus latency. PLoS Pathog 2007;3:e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pozharskaya V, Torres-Gonzalez E, Rojas M, Gal A, Amin M, Dollard S, Roman J, Stecenko AA, Mora AL. Twist: a regulator of epithelial–mesenchymal transition in lung fibrosis. PLoS One 2009;4:e7559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krug LT, Torres-Gonzalez E, Qin Q, Sorescu D, Rojas M, Stecenko A, Speck SH, Mora AL. Inhibition of NF-kappaB signaling reduces virus load and gammaherpesvirus-induced pulmonary fibrosis. Am J Pathol 2010;177:608–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mora AL, Rojas M. Aging and lung injury repair: a role for bone marrow derived mesenchymal stem cells. J Cell Biochem 2008;105:641–647 [DOI] [PubMed] [Google Scholar]
- 15.Selman M, Rojas M, Mora AL, Pardo A. Aging and interstitial lung diseases: unraveling an old forgotten player in the pathogenesis of lung fibrosis. Semin Respir Crit Care Med 2010;31:607–617 [DOI] [PubMed] [Google Scholar]
- 16.Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 2002;415:92–96 [DOI] [PubMed] [Google Scholar]
- 17.Kregel KC, Zhang HJ. An integrated view of oxidative stress in aging: Basic mechanisms, functional effects, and pathological considerations. Am J Physiol Regul Integr Comp Physiol 2007;292:R18–R36 [DOI] [PubMed] [Google Scholar]
- 18.Lawson WE, Crossno PF, Polosukhin VV, Roldan J, Cheng DS, Lane KB, Blackwell TR, Xu C, Markin C, Ware LB, et al. Endoplasmic reticulum stress in alveolar epithelial cells is prominent in IPF: association with altered surfactant protein processing and herpesvirus infection. Am J Physiol Lung Cell Mol Physiol 2008;294:L1119–L1126 [DOI] [PubMed] [Google Scholar]
- 19.Korfei M, Ruppert C, Mahavadi P, Henneke I, Markart P, Koch M, Lang G, Fink L, Bohle RM, Seeger W, et al. Epithelial endoplasmic reticulum stress and apoptosis in sporadic idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2008;178:838–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanjore H, Xu XC, Polosukhin VV, Degryse AL, Li B, Han W, Sherrill TP, Plieth D, Neilson EG, Blackwell TS, et al. Contribution of epithelial-derived fibroblasts to bleomycin-induced lung fibrosis. Am J Respir Crit Care Med 2009;180:657–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown S, Worsfold M, Sharp C. Microplate assay for the measurement of hydroxyproline in acid-hydrolyzed tissue samples. Biotechniques 2001;30:38–40, 42 [DOI] [PubMed] [Google Scholar]
- 22.Evans AG, Moser JM, Krug LT, Pozharskaya V, Mora AL, Speck SH. A gammaherpesvirus-secreted activator of Vbeta4+ CD8+ T cells regulates chronic infection and immunopathology. J Exp Med 2008;205:669–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang L, Antonini JM, Rojanasakul Y, Castranova V, Scabilloni JF, Mercer RR. Potential role of apoptotic macrophages in pulmonary inflammation and fibrosis. J Cell Physiol 2003;194:215–224 [DOI] [PubMed] [Google Scholar]
- 24.Lawson WE, Cheng DS, Degryse AL, Tanjore H, Polosukhin VV, Xu XC, Newcomb DC, Jones BR, Roldan J, Lane KB, et al. Endoplasmic reticulum stress enhances fibrotic remodeling in the lungs. Proc Natl Acad Sci U S A 2011;108:10562–10567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaw AC, Joshi S, Greenwood H, Panda A, Lord JM. Aging of the innate immune system. Curr Opin Immunol 2010;22:507–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman J, Brigham KL. Bone marrow–derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol 2005;33:145–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu J, Gonzalez ET, Iyer SS, Mac V, Mora AL, Sutliff RL, Reed A, Brigham KL, Kelly P, Rojas M. Use of senescence-accelerated mouse model in bleomycin-induced lung injury suggests that bone marrow–derived cells can alter the outcome of lung injury in aged mice. J Gerontol A Biol Sci Med Sci 2009;64:731–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Selman M, Pardo A. Idiopathic pulmonary fibrosis: an epithelial/fibroblastic cross-talk disorder. Respir Res 2002;3:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu J, Kaufman RJ. From acute er stress to physiological roles of the unfolded protein response. Cell Death Differ 2006;13:374–384 [DOI] [PubMed] [Google Scholar]
- 30.American Thoracic Society Idiopathic pulmonary fibrosis: Diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med 2000;161(2 Pt 1):646–664 [DOI] [PubMed] [Google Scholar]
- 31.Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov 2008;7:1013–1030 [DOI] [PubMed] [Google Scholar]
- 32.Schroder M. Endoplasmic reticulum stress responses. Cell Mol Life Sci 2008;65:862–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature 2000;408:239–247 [DOI] [PubMed] [Google Scholar]
- 34.Tavernarakis N. Ageing and the regulation of protein synthesis: a balancing act? Trends Cell Biol. 2008;18:228–235 [DOI] [PubMed] [Google Scholar]
- 35.Puzianowska-Kuznicka M, Kuznicki J. The ER and ageing II: calcium homeostasis. Ageing Res Rev 2009;8:160–172 [DOI] [PubMed] [Google Scholar]
- 36.Zhong Q, Zhou B, Ann DK, Minoo P, Liu Y, Banfalvi A, Krishnaveni MS, Dubourd M, Demaio L, Willis BC, et al. Role of endoplasmic reticulum stress in epithelial–mesenchymal transition of alveolar epithelial cells: effects of misfolded surfactant protein. Am J Respir Cell Mol Biol 2011;45:498–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanjore H, Cheng DS, Degryse AL, Zoz DF, Abdolrasulnia R, Lawson WE, Blackwell TS. Alveolar epithelial cells undergo epithelial-to-mesenchymal transition in response to endoplasmic reticulum stress. J Biol Chem 2011;286:30972–30980 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.