Abstract
Lung contusion (LC), commonly observed in patients with thoracic trauma is a leading risk factor for development of acute lung injury/acute respiratory distress syndrome. Previously, we have shown that CC chemokine ligand (CCL)-2, a monotactic chemokine abundant in the lungs, is significantly elevated in LC. This study investigated the nature of protection afforded by CCL-2 in acute lung injury/acute respiratory distress syndrome during LC, using rats and CC chemokine receptor (CCR) 2 knockout (CCR2−/−) mice. Rats injected with a polyclonal antibody to CCL-2 showed higher levels of albumin and IL-6 in the bronchoalveolar lavage and myeloperoxidase in the lung tissue after LC. Closed-chest bilateral LC demonstrated CCL-2 localization in alveolar macrophages (AMs) and epithelial cells. Subsequent experiments performed using a murine model of LC showed that the extent of injury, assessed by pulmonary compliance and albumin levels in the bronchoalveolar lavage, was higher in the CCR2−/− mice when compared with the wild-type (WT) mice. We also found increased release of IL-1β, IL-6, macrophage inflammatory protein-1, and keratinocyte chemoattractant, lower recruitment of AMs, and higher neutrophil infiltration and phagocytic activity in CCR2−/− mice at 24 hours. However, impaired phagocytic activity was observed at 48 hours compared with the WT. Production of CCL-2 and macrophage chemoattractant protein-5 was increased in the absence of CCR2, thus suggesting a negative feedback mechanism of regulation. Isolated AMs in the CCR2−/− mice showed a predominant M1 phenotype compared with the predominant M2 phenotype in WT mice. Taken together, the above results show that CCL-2 is functionally important in the down-modulation of injury and inflammation in LC.
Keywords: lung contusion, macrophage chemoattractant protein-1, CC chemokine ligand-2, CC chemokine receptor 2, inflammation
Clinical Relevance
The study reports on the protective effect of CC chemokine ligand (CCL)-2 in the pathogenesis of acute inflammation secondary to lung contusion (LC). LC is a unique, focal, and direct risk factor for acute lung injury/acute respiratory distress syndrome. The models that we report here involve a focal, direct lung injury characterized by immediate onset of alveolar disruption, hemorrhage, and subsequent acute inflammation. We report that, in the presence of CCL-2, there are major changes in the M1/M2 phenotype of alveolar macrophages. This study allows us to pursue future clinical studies with patients suffering from trauma after LC, whereby chemokines, such as CCL-2, could be used to alter the inflammatory response and mitigate acute inflammation.
Thoracic injury is involved in nearly one-third of all acute trauma admissions (1–4), often including clinically significant lung contusion (LC) with subsequent respiratory deficits. LC is an important independent risk factor for the development of acute lung injury (ALI), acute respiratory distress syndrome (ARDS), and ventilator-associated pneumonia in affected patients (1–4), all of which are associated with high mortality and morbidity (5). The clinical pathophysiology of LC includes hypoxemia, hypercarbia, increased work of breathing, and decreased lung volumes and compliance in association with ventilation–perfusion mismatching, intrapulmonary shunting, edema, and segmental lung damage (1, 6, 7). As 30% of patients with LC progress to ALI/ARDS (1), it is important to delineate the factors responsible for the development of progressive respiratory failure. Several lines of evidence suggest that factors in addition to trauma-induced tissue damage may contribute to respiratory failure in LC (1, 3).
Macrophage chemoattractant protein (MCP)-1/CC chemokine ligand (CCL)-2 is produced by a number of cells in response to inflammatory stimuli, such as IL-1β, -4, -6, and -10, transforming growth factor-β, and LPS (8, 9). There are several reports that indicate the protective nature of CCL-2 in modulating the resolution of acute pulmonary injury in LC. We have shown that elevated levels of CCL-2 in the bronchoalveolar lavage (BAL) are associated with improved oxygenation in LC injury in rats (10). We have also reported increased mortality, persistent inflammation, and decreased granulomatous compartmentalization in CCL-2 knockout mice injured with gastric aspiration (11). Amano and colleagues (12) showed, using a Pseudomonas aeruginosa pneumonia model of lung injury, that administration of CCL-2–neutralizing antibody increased neutrophil infiltration and lung injury and reduced phagocytic activity of alveolar macrophages (AMs) for apoptotic neutrophils (12).
Here, we studied the role of CCL-2 in LC by examining the importance of its interactions with CC chemokine receptor (CCR) 2 receptor by using CCR2−/− mice. In mice, CCR2 is the only receptor for CCL-2, and is a primary receptor for MCP-5, the chemokine that bears the closest similarity to the human homolog of CCL-2 (8). Based on our previous results (11), we hypothesized that animals lacking CCL-2 will have a more severe lung injury with an exaggerated acute inflammatory response after LC. Our results show that, in the absence of the CCR2, the extent of mechanical injury in ALI after LC is worsened and prolonged. In addition, there is aggravation of the inflammatory response, as evidenced by increased BAL levels of albumin, cytokines, and chemokines, as well as increased neutrophil infiltration and decreased macrophage recruitment and activation of M2-type macrophages. The same response was observed in rats injected with anti–CCL-2 antibody. This study provides new information regarding the protective nature of CCL-2/CCR2 signaling in lung injury after LC.
Materials and Methods
Additional experimental details are available in the online supplement.
Animals
Adult male Long-Evans rats (250–300 g; Harlan Sprague-Dawley, Indianapolis, IN), as well as male, age-matched (6–8 wk old), wild-type (WT) (C57/BL6) and CCR2−/− mice (Jackson Laboratories, Bar Harbor, ME) were used in this study. All procedures performed were approved by the Institutional Animal Care and Use Committee at the State University of New York, Buffalo, and the University of Michigan, and complied with state, federal, and National Institutes of Health regulations.
Induction of Isolated LC in Rats
LC was induced in halothane-anesthetized rats using energy equivalent of 2.45 J by a hollow cylindrical weight dropped from a defined height onto a precordial shield that prevented associated cardiac trauma, as previously described (3, 4).
Murine Model for LC
Male, C57/BL6 (20–25 g, 6–8 wk old, bred in-house), along with the CCR2−/− mice, were anesthetized and LC was induced (13) and subsequently modified by our group. Briefly, after induction of anesthesia, the mouse was placed in a left lateral position and, using a cortical contusion impactor, the right chest was struck along the posterior axillary line 1.3 cm above the costal margin using a velocity of 5.8 m/s adjusted to a depth of 10 mm. Mice were then allowed to recover spontaneously. Each experiment was repeated at least three times with three to five animals per group.
Whole Lung Myeloperoxidase Activity
Whole-lung myeloperoxidase (MPO) activity was studied as an added measure of neutrophil-associated pulmonary inflammation. After BAL, rat lungs were excised, and the whole-cell lysate was used to assess in vitro MPO activity, as previously described (14–16).
In Vitro Phagocytosis Assay
After LC, AMs isolated by BAL were plated at 2 × 105 cells/well and cultured overnight in Dulbecco's modified Eagle's medium. Wells were aspirated and replaced with 50 μl serum-free medium. Macrophages were then incubated with FITC-labeled, heat-killed P. aeruginosa. Phagocytosis of FITC-labeled bacteria was measured after quenching of noningested bacteria with trypan blue, as previously described (17–19).
Histopathology
Lung specimens harvested at time of death were fixed in 10% formalin, sectioned, and stained with hematoxylin and eosin. Slides were evaluated by an experienced, blinded pathologist and examined for the presence of interstitial neutrophilic infiltrate, intra-alveolar hemorrhage, and pulmonary septal edema, as described previously (20).
Statistical Analysis
Data are expressed as the means (±SEM). Statistical significance was estimated using one-way ANOVA (GraphPad Prism 5.01; GraphPad Software, San Diego, CA). Individual intergroup comparisons were analyzed using the two-tailed unpaired t test with Welch's correction. A value of P less than 0.05 was considered significant (10, 11).
Results
Anti–CCL-2 Antibodies Aggravate the Severity of Lung Injury at 48 Hours after LC in Rats
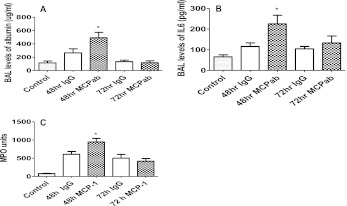
Albumin levels in the BAL were higher in rats given an intraperitoneal injection of polyclonal antibodies against CCL-2 at 48 hours after LC (Figure 1A). Antibody was administered at the time of the initial insult to deplete circulating levels of the cytokine. These rats also showed greater inflammation, as seen in the increased levels of IL-6 in BAL (Figure 1B) and increased levels of the neutrophil-related marker MPO in whole lung tissue (Figure 1C), compared with IgG-injected control animals. These results indicate that CCL-2 is functionally important in LC, and may play a protective role in regulating acute neutrophil-mediated injury in this condition.
Figure 1.
Anti–CC chemokine ligand (CCL)-2 antibody worsens inflammation and injury in rats with LC. Rats received rabbit polyclonal anti–CCL-2 antibody by intraperitoneal injection at the time of lung contusion (LC), and control animals received IgG followed by LC. After LC, rats were killed at 48- and 72-hour time points, and the concentrations of albumin in bronchoalveolar lavage (BAL) (A), IL-6 (B), and whole-lung myeloperoxidase (MPO) (C) were determined by ELISA. Values are presented as means (±SEM; n = 9). Each experiment was repeated at least three times with three animals per group. Paired samples were analyzed using the two-tailed unpaired t test with Welch's correction. *P < 0.05 compared with animals that received IgG.
AMs Are the Predominant Source of CCL-2 in Rats after LC
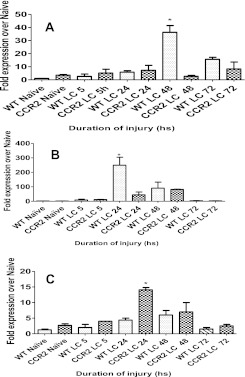
Tissue sections from rats given closed-chest bilateral LC injury at 24, 48, and 72 hours after insult were analyzed for CCL-2 production using specific immunohistochemical staining with anti–CCL-2 antibody. As shown in Figure 2, significant staining for CCL-2 was found primarily in the AMs at 48 hours, when CCL-2 levels in BAL are highest in the injured animals (Figure 2C). In addition, there was also lighter staining for CCL-2 in alveolar epithelial cells. Similar data (Figure 2B) also indicated that immunohistochemical staining for CCL-2 was most prominently localized to the AMs at 24 hours after contusion, another time point at which substantial levels of this chemokine have been shown to be present in BAL from rats with LC. These results indicate that AMs are likely the primary cell of origin for CCL-2 production in the injured lung after LC.
Figure 2.
Immunohistochemistry of lung samples at 24 and 48 hours after LC. Lung tissue samples from rats at 24 and 48 hours after LC were subjected to immunohistochemistry with anti–CCL-2 polyclonal antibody as described in Materials and Methods. Shown are nonspecific IgG antibody staining as control (A), 24 hours after LC (B), and dense staining of alveolar macrophages (AMs) by CCL-2 antibody at 48 hours after LC (C).
CCR2−/− Mice Showed Increased Lung Injury after LC
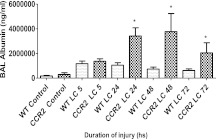
To determine the extent of mechanical injury in the CCR2−/− group versus WT, we measured the pressure–volume (PV) mechanics at different time intervals after LC. For this measurement, LC was induced in the WT and CCR2−/− phenotypes, as described previously here (images of lung after injury shown in Figure 3), and PV measurements were done after different time intervals (5, 24, 48, and 72 h). At all time points, but most notably at 5, 24, and 48 hours, PV measurements indicated that pulmonary compliance and volumes were significantly decreased in the CCR2−/− group relative to the control (WT) (Figure 4). There was a marked increase in the BAL albumin level, an indicator of the extent of permeability injury in the knockout phenotype when compared with the corresponding WT, after 24, 48, and 72 hours of injury due to LC (Figure 5). The maximal effect was at 48 hours (3.5-fold increase versus WT).
Figure 3.
Pulmonary contusion in mice. Explanted, wild-type (WT) mouse lungs, showing uninjured control (A) and lungs 5 hours after contusion (B); the arrow highlights the point of contusion on a representative mouse.
Figure 4.
Pulmonary compliance measurements in WT and CC chemokine receptor (CCR) 2 knockout (CCR−/−) mice after LC. “Quasistatic” closed-chest pressure–volume behavior was measured at 5, 24, 48, and 72 hours after injury in WT and CCR2−/− mice. Points along each curve represent the mean (±SEM) for nine animals at each time point. Each experiment was repeated at least three times with three animals per group. Asterisks represent a significant difference between the groups described in each graph (see main text for details).
Figure 5.
CCR2−/− mice showed elevated albumin levels in the BAL compared with WT mice after LC. After LC, mice were killed at 5-, 24-, 48-, and 72-hour time points and albumin concentrations in the BAL were determined by ELISA, as described in Materials and Methods (mean ± SEM; n = 14). Statistical analysis was performed on data at each time point; paired samples were analyzed using the two-tailed unpaired t test with Welch's correction. *P < 0.05 compared with WT mice and uninjured control animals.
CCR2−/− Mice Exhibited Signs of Increased Lung Inflammation after LC
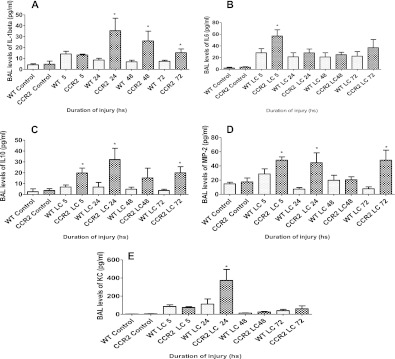
ALI during LC is characterized by an intense inflammatory response, which contributes to the physiological dysfunction in this condition, and this involves production of mediators, such as chemokines and cytokines. To determine if CCL-2 activation has any role in the production of these mediators after LC, we measured the levels of both proinflammatory (IL-1β, IL-6) and anti-inflammatory (IL-10) cytokines, as well as neutrophil chemoattractants, keratinocyte chemoattractant (KC) and macrophage inflammatory protein (MIP)-2. The levels of IL-1β were increased in the mice subjected to LC when compared with corresponding control animals, as has been previously reported (11). There was no significant difference in the levels of IL-1β between the two injury groups at 5 hours after LC, but there was a significant increase in its levels at the 24-, 48-, and 72-hour time points after LC in the CCR2−/− genotype when compared with WT (Figure 6A). IL-6 levels in the BAL were higher in CCR2−/− mice after LC (Figure 6B). Comparing the two groups, CCR2−/− mice showed a significant increase at the 5-hour time point compared with WT. A similar trend was also observed for the anti-inflammatory cytokine, IL-10. Mice subjected to LC had significantly higher levels of this cytokine when compared with corresponding WT control animals (Figure 6C). Levels of MIP-2 and KC were also elevated prominently in CCR2−/− mice (Figures 6D and 6E) when compared with WT, although there were slight variations in the nature of response. Although MIP-2 levels were higher after LC in CCR2−/− mice at 5, 24, 48, and 72 hours after injury, the increase was found to be significant only at 5, 24, and 72 hours. The levels of KC showed an increase at 24 hours after LC, but this was not maintained at 72 hours. These results suggest that CCL-2 plays a role in down-modulating the intensity of acute inflammation after LC.
Figure 6.
CCR2−/− mice showed a marked increase in cytokine response compared with WT after LC. IL1 β (A), IL-6 (B), IL10 (C), macrophage inflammatory protein-2 (D), and keratinocyte chemoattractant (E) levels in the BAL of WT and CCR2−/− mice were analyzed by ELISA at 5, 24, 48, and 72 hours after injury (mean ± SEM; n = 14). Statistical analysis was performed on data at each time point, and intergroup comparisons were made with two-tailed unpaired t test with Welch's correction. *P < 0.05 compared with corresponding WT.
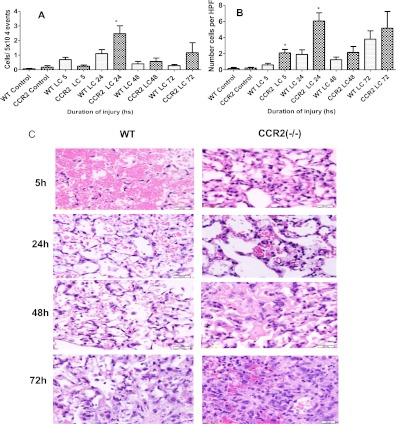
CCR2−/− Mice Showed Increased BAL and Tissue Levels of Neutrophils after LC
Neutrophil accumulation in the lung parenchyma after lung injuries has been reported by others (21–23). We have previously reported that neutrophils aggravate the severity of lung injury due to LC (10, 14). Therefore, we measured the levels of neutrophils in the BAL after sustained LC in both CCR2−/− and WT mice using flow cytometry (Figure 7A). At 5 and 24 hours, there was a significant increase in neutrophil levels in the CCR2−/− group and, at 72 hours, a similar trend was seen with neutrophil counts on cytospin when compared with WT (Figure 7B). Histological examination of CCR2−/− and WT mice 5 hours after injury showed extensive intra-alveolar hemorrhage. At 24 hours, hemorrhage was still significant in both groups, but the CCR2−/− mice had thickened alveolar septa due to congestion, edema, and inflammatory cells in the capillaries. At 48 hours, there was extensive fibrinous, neutrophil-rich exudate in the CCR2−/− mice. At 72 hours, the intensity of the inflammation was greater in the CCR2−/− mice with obliteration of the normal lung. The WT mice had extensive damage, with necrosis, fibrinous exudate, and edema (Figure 7C).
Figure 7.
Neutrophil levels in the BAL were higher in the CCR2−/− mice after LC. Flow cytometric analyses of relative numbers of neutrophils in the BAL at 5, 24, 48, and 72 hours after lung injury based on CD11b and GR1 expression (A). Cytospin analysis of BAL samples collected at 5, 24, 48, and 72 hours after lung injury (B), as described in detail in Materials and Methods. Values are presented as means (±SEM; n = 14). *P < 0.05 compared with corresponding WT. (C) Histopathology of Lung in CCR2−/− and WT mice a 5, 24, 48, and 72 hours after LC are shown at 400× magnification.
Macrophage Recruitment Was Significantly Reduced and Showed a Predominant M1 Phenotype in the CCR2−/−Mice after LC
Activation of tissue macrophages is observed in the lungs in response to a variety of inflammatory stimuli. Macrophage trafficking to the inflammatory sites is regulated largely by CCL-2 (24, 25), and CCL-2/CCR2 pathway is considered to be a major regulator of monocyte/macrophage recruitment in ALI (26). We determined the levels of macrophages in BAL collected from both knockout and WT groups at different time intervals using cytospin technique (Figure 8A). The numbers of macrophages were significantly higher at all time points in the WT mice compared with CCR2−/− mice, but the absolute numbers were most pronounced at the 5-hour time point (65%). This finding was additionally confirmed using flow cytometric analyses in separate experiments (Figures 8B and 8C).
Figure 8.
Recruitment of AMs was significantly less in CCR2−/− mice after LC. (A) Cytospin analysis of BAL samples collected at 5, 24, 48, and 72 hours after lung injury visualized by light microscopy at ×200 magnification. Values are represented as mean and SEM (n = 14). (B) Flow cytometric analyses of relative numbers of monocytes/ macrophages and neutrophils in the BAL at 5, 24, 48, and 72 hours after lung injury based on CD11b and CD11c expression and the presence of MHC II. Values are represented as mean and SEM (n = 14). *P < 0.05 compared with corresponding WT. (C) This is an example of FCM analysis of BAL macrophages and neutrophils after LC. Cells collected by BAL after LC were stained for five -color fluorescence and the macrophages and neutrophils subset (defined by forward and side scatter) was selected for FACScan analysis.
Real-time PCR analysis showed that there was an approximate 25-fold decrease in the levels of arginase-1 (48 h) and FIZZ1 (24 h), the classic M2 marker genes, in the knockout genotype (Figures 9A and 9B) compared with WT after LC. The M1 macrophages were examined for nitric oxide synthase (NOS)-2 expression after LC in WT and CCR2−/− mice. The most striking observation was that the BAL-derived macrophages from CCR2−/− mice significantly increased NOS-2 transcript levels compared with WT mice (Figure 9C). These results demonstrate that, in the absence of CCL-2/CCR2, the AMs undergo a preferential injurious phenotypic differentiation (M1).
Figure 9.
Activation of M2 macrophages is observed more in the WT mice after LC. Quantitative TaqMan PCR analysis of Arg1 (A), FIZZ1 (B), and nitric oxide synthase (NOS)-2 (C) transcript expression in the WT and CCR2−/− mice after 5, 24, 48, and 72 hours of LC, were performed. Data are means (±SEM) from BAL macrophages cultured from five mice at each time point. Statistical analysis was performed on data at each time point using one-way ANOVA. *P < 0.05 compared with corresponding WT.
AM Phagocytic Activity Is Enhanced then Impaired in CCR2−/− Mice after LC
AMs play a crucial role in mediating innate immune responses in the lung (27, 28) and, together with the neutrophils that are recruited to the lungs, they mediate the phagocytic response to gram-negative bacteria. Because increased susceptibility to bacterial infection during lung injuries correlate with defects in AM phagocytosis, we measured ex vivo phagocytosis in an in vitro assay using isolated AMs from different groups. The relative phagocytic activity was significantly higher after LC in the WT mice, showing a twofold increase by 48 hours (Figure 10). The AMs from CCR2−/− mice showed an initial increase in phagocytic activity after LC, which later started to decline, and, by 48 hours, they exhibited significantly less phagocytic ability than their WT counterparts.
Figure 10.
Impaired AM phagocytosis in CCR2−/− mice after LC. Phagocytosis of non–serum opsonized FITC labeled Pseudomonas aeruginosa was assessed using AMs from uninjured mice, as well as injured WT and CCR2−/− mice, as described in Materials and Methods (mean ± SEM; n = 14 per group). For possible differences in AM adherence to tissue culture plate, data were normalized for cell number using a LDH Cytotoxicity Detection Kit (Roche, Indianapolis, IN). Statistical analysis was performed on data at each time point, and paired samples were analyzed using the two-tailed unpaired t test with Welch's correction. *P < 0.05 compared with corresponding WT.
CCL-2 and MCP-5 Levels Were Elevated in the CCR2−/− Mice
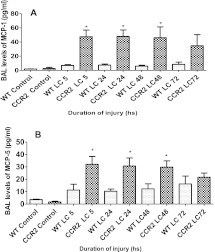
The absence of a chemokine receptor through gene deletion often leads to an abundant increase in the expression of its ligand(s) (29). The chemokines, CCL-2 and MCP-5, are the major ligands of the CCR2. The levels of both of the chemokines were increased in CCR2−/− mice compared with WT mice (Figures 11A and 11B). As reported by us previously (11), CCL-2 levels were higher after LC. However, in CCR2−/− mice, this increase is further exaggerated to show a fivefold difference between the two genotypes at 5, 24, and 48 hours. The BAL MCP-5 level of CCR2−/− mice was significantly higher compared with the WT mice. These results strongly suggest the existence of a feedback loop between CCL-2/MCP-5 and their receptor, CCR2.
Figure 11.
Levels of CCL-2 and macrophage chemoattractant protein (MCP)-5 were higher in the CCR2−/−mice compared with WT mice after LC. The chemokines, CCL-2 (MCP-1) (Figure 5A) and MCP-5 (Figure 5B), were measured in the BAL of WT and CCR2−/− mice at 5, 24, 48, and 72 hours after LC by ELISA, as described in Materials and Methods. Values are presented as means (±SEM; n = 14). Each experiment was repeated at least three times with four to five animals per group. Statistical analysis was performed on data at each time point, and samples were analyzed using the two-tailed unpaired t test with Welch's correction. *P < 0.05 compared with corresponding WT.
Discussion
The present study was conducted to understand the mechanistic role of CCL-2, its receptor, CCR2, and the related murine chemokine, MCP-5, in the initiation, maintenance, and resolution of acute injury after LC. LC is a unique, direct, and focal risk factor for the development of ALI/ARDS in human subjects. The injury, as exhibited by the animal models employed in the current study, is characterized by mechanical tissue trauma and accumulation of blood in the parenchyma, with the subsequent development of acute inflammation. We demonstrate that, in the absence of CCL-2 signaling, lung inflammation, injury, and neutrophil sequestration are increased after LC, whereas macrophage infiltration and phagocytosis were reduced. Several lines of evidence indicate that early elaboration of CCL-2 facilitates the timely resolution of the acute inflammatory response in injured lungs (12, 30–32). LC enhances the mRNA expression for CCR2 in monocytes and interstitial macrophages and for CCL-2 in AMs (33). We have previously reported that the early increases in CCL-2 in the BAL are associated with the timely resolution of acute inflammatory response in isolated pulmonary contusion or gastric aspiration in rats (10). This is the first reported study highlighting the protective effect of CCL-2 in LC.
Inflammatory stimuli from tissue injury with infectious agents and cytokines have been known to induce CCL-2 secretion (34, 35). Studies in other models of ALI have documented that early increases in CCL-2 (as opposed to more chronic increases that may be detrimental) enhance monocyte/macrophage recruitment, activation, and/or the phagocytosis of apoptotic neutrophils (12, 30–32). For instance, CCL-2 has been shown to play a crucial role in the resolution and repair process of bacterial pneumonia by promoting the removal of dying neutrophils and production of hepatocyte growth factor by AMs (12). Our hypothesis on the protective role of CCL-2 in LC is well supported by the data that show worsening of injury after LC in rats after the administration of a polyclonal antibody against CCL-2. Recently, Amano and colleagues (12) showed that administration of anti–MCP-1/CCL2 antibodies aggravated lung injury in P. aeruginosa infection by reducing the number of alveolar neutrophil–ingesting macrophages and hepatocyte growth factor levels in BAL fluid. Our findings identify AMs as the prominent source of CCL-2 production in LC. In response to conditions like hypoxia, CCL-2 can act as a mediator of the systemic inflammatory response elicited by macrophages (36).
Interaction of CCL-2 with CCR2 has been shown to be important in the process of re-epithelialization after lung injury (37). Similar to the observed data in rats, extent of lung injury after LC was aggravated in CCR2−/− mice. Levels of proinflammatory cytokines were elevated in CCR2−/− mice during LC. In addition, IL-10 was increased, which may represent a compensatory attempt to reduce inflammation in the CCR2−/− host. This is highly relevant, because inhibiting inflammatory signals can be protective during lung infections (reviewed in Ref. 38). Similar to this, Okuma and colleagues (32) have demonstrated that CCR2−/− mice showed increased mRNA levels of CCL-2, IL-1b, thioredoxin-1, and inducible NOS in lung tissues compared with WT mice in a hyperoxia-induced tissue injury (32). The inflammatory response in LC, in conjunction with direct tissue injury, has been shown to damage the integrity of the alveolar–capillary membrane barrier function, thereby increasing epithelial cell apoptosis/necrosis (15). We found significantly elevated levels of IL-1β in BAL from CCR2−/− mice, which has been shown to induce apoptosis in other cell types (39–41). In mice subjected to intratracheal LPS treatment, CCR2 deficiency enhanced the apoptosis of the alveolar epithelial cells and increased the permeability injury (42). We also found up-regulation of IL-10 during LC as a result of CCR2 deficiency. This is particularly relevant, as IL-10 overexpression has been shown to induce lung fibrosis in a CCR2/CCL-2–dependent pathway (43).
Previously, we have shown that ALI with LC is neutrophil dependent (10). We have also documented the recruitment and activation of neutrophils and lung tissue macrophages, as well as the production of multiple cytokines and chemokines in LC (15). Chemokines from lung cells stimulate chemotaxis and influence the directional motility of neutrophils (44). Neutrophils are known to be activated, at least in part, via Toll-like receptors (TLRs), such as TLR 2 and TLR 4, in the epithelium (13, 45). Here, we show that the number of neutrophils in the BAL was significantly higher in the CCR2−/− mice after LC. The precise nature of the effect of CCL-2/MCP-5 with respect to neutrophil infiltration in LC remains unclear. However, it is clear that there is diminished recruitment and phagocytic potential of the AMs in CCR2 deficiency. Therefore, it is very likely that, in the presence of CCR2, there is increased potential for macrophage-mediated phagocytosis of neutrophils.
LC is known to promote increased recruitment of AMs to the site of injury, as well as subsequent activation of M2 phenotype (46, 47). In this article, we provide strong evidence to show that this is dependent on CCL-2/CCR2 signaling. CCL-2/CCR2–dependent macrophage infiltration has been shown to be protective in other models of ALI (32). We demonstrate that lung-derived macrophages from CCR2−/− mice exhibited transient M1 activation, as evidenced by increased NOS-2, and these macrophages did not show an increase in arginase-1 and FIZZ-1 expression at any time point after LC. The M1 phenotype (also termed as classically activated) is characterized by increased production of oxidative burst and nitric oxide release, in addition to cytotoxic properties, by their ability to secrete proinflammatory cytokines, such as TNF, IL-1, and IL-6 (48). Conversely the M2 phenotype (also termed as alternatively activated) is associated with decreased production of proinflammatory cytokines and an increased up-regulation of FIZZ-1 and the arginase pathway (48). These responses were transient and were not observed at the 72-hour time point. These observations are similar to those made after bleomycin-induced pulmonary fibrosis and experimental silicosis (48–50). Although persistent presence of M2 phenotype can lead to fibrosis, a more transient presence, as suspected in our models of lung injury with respect to CCL-2 production, has been shown to be protective (49). These results, therefore, indicate that CCL-2/CCR2 signaling is mainly responsible for the protective differentiation to M2 phenotype during the period of acute inflammation in LC.
It is emphasized that the data presented here reflect the role of CCL-2/MCP-5 in the context of acute inflammation in LC. We did not examine the role of this chemokine in later stages of inflammation characterized by subsequent development of pulmonary fibrosis. It is entirely conceivable that CCL-2 is integral to the development of pulmonary fibrosis in late stages of LC as well, as described by several of the coauthors of the current manuscript (48, 51).
CCL-2 is also elevated in BAL, sputum, exhaled breath condensate samples, and bronchiolar epithelium from smokers and patients with chronic obstructive pulmonary disease, and it has been correlated with increased recruitment of inflammatory cells to the airways (52–55). Moreover, higher levels observed at the end of 2 weeks in BAL samples of patients with ARDS denoted a poorer prognosis (8).
In conclusion, the absence of CCL-2 signaling in LC leads to diminished recruitment, activation, phenotypic alteration, and diminished phagocytic ability of the AMs with increased neutrophilic sequestration along with lung inflammation. Few agents that modulate the acute inflammatory response after LC have actually shown a protective, resolution-related effect. Because our findings clearly suggest such a role for CCL-2, understanding the mechanisms of CCL-2/CCR2 signaling in LC may lead to the development of future therapeutic strategies in the resolution of exuberant acute inflammatory response after LC.
Supplementary Material
Acknowledgments
The authors thank Dr. J. Erby Wilkinson, D.V.M., Ph.D., D.A.C.V.P., Associate Professor of Comparative Pathology, University of Michigan, for his valuable help in histological analysis.
Footnotes
This work was supported by National Institutes of Health grant 5R01HL102013-02.
Author Contributions: Conception and design, M.V.S., M.D.B., B.B.M, and K.R.; performed research, M.V.S, B.Y., D.M.-A., and L.O.-F.; analysis and interpretation, M.V.S., B.B.M., and K.R.; drafting the manuscript for important intellectual content, M.V.S., J.D.H., B.A.D., P.R.K., C.M.H., and K.R.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2011-0358OC on January 26, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Cohn SM. Pulmonary contusion: review of the clinical entity. J Trauma 1997;42:973–979 [DOI] [PubMed] [Google Scholar]
- 2.Lewis FR. Thoracic trauma. Surg Clin North Am 1982;62:97–104 [DOI] [PubMed] [Google Scholar]
- 3.Miller PR, Croce MA, Bee TK, Qaisi WG, Smith CP, Collins GL, Fabian TC. ARDS after pulmonary contusion: accurate measurement of contusion volume identifies high-risk patients. J Trauma 2001;51:223–228, discussion 229–230 [DOI] [PubMed] [Google Scholar]
- 4.Miller PR, Croce MA, Kilgo PD, Scott J, Fabian TC. Acute respiratory distress syndrome in blunt trauma: identification of independent risk factors. Am Surg 2002;68:845–850; discussion 850–851 [PubMed] [Google Scholar]
- 5.Li Bassi G, Torres A. Ventilator-associated pneumonia: role of positioning. Curr Opin Crit Care 2011;17:57–63 [DOI] [PubMed] [Google Scholar]
- 6.Oppenheimer L, Craven KD, Forkert L, Wood LD. Pathophysiology of pulmonary contusion in dogs. J Appl Physiol 1979;47:718–728 [DOI] [PubMed] [Google Scholar]
- 7.Van Eeden SF, Klopper JF, Alheit B, Bardin PG. Ventilation–perfusion imaging in evaluating regional lung function in nonpenetrating injury to the chest. Chest 1989;95:632–638 [DOI] [PubMed] [Google Scholar]
- 8.Rose CE, Jr, Sung SS, Fu SM. Significant involvement of CCL2 (MCP-1) in inflammatory disorders of the lung. Microcirculation 2003;10:273–288 [DOI] [PubMed] [Google Scholar]
- 9.Wong LM, Myers SJ, Tsou CL, Gosling J, Arai H, Charo IF. Organization and differential expression of the human monocyte chemoattractant protein 1 receptor gene: evidence for the role of the carboxyl-terminal tail in receptor trafficking. J Biol Chem 1997;272:1038–1045 [DOI] [PubMed] [Google Scholar]
- 10.Raghavendran K, Davidson BA, Woytash JA, Helinski JD, Marschke CJ, Manderscheid PA, Notter RH, Knight PR. The evolution of isolated bilateral lung contusion from blunt chest trauma in rats: cellular and cytokine responses. Shock 2005;24:132–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raghavendran K, Davidson BA, Mullan BA, Hutson AD, Russo TA, Manderscheid PA, Woytash JA, Holm BA, Notter RH, Knight PR. Acid and particulate-induced aspiration lung injury in mice: importance of MCP-1. Am J Physiol Lung Cell Mol Physiol 2005;289:L134–L143 [DOI] [PubMed] [Google Scholar]
- 12.Amano H, Morimoto K, Senba M, Wang H, Ishida Y, Kumatori A, Yoshimine H, Oishi K, Mukaida N, Nagatake T. Essential contribution of monocyte chemoattractant protein-1/C-C chemokine ligand-2 to resolution and repair processes in acute bacterial pneumonia. J Immunol 2004;172:398–409 [DOI] [PubMed] [Google Scholar]
- 13.Hoth JJ, Hudson WP, Brownlee NA, Yoza BK, Hiltbold EM, Meredith JW, McCall CE. Toll-like receptor 2 participates in the response to lung injury in a murine model of pulmonary contusion. Shock 2007;28:447–452 [DOI] [PubMed] [Google Scholar]
- 14.Raghavendran K, Davidson BA, Huebschmann JC, Helinski JD, Hutson AD, Dayton MT, Notter RH, Knight PR. Superimposed gastric aspiration increases the severity of inflammation and permeability injury in a rat model of lung contusion. J Surg Res 2009;155:273–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raghavendran K, Notter RH, Davidson BA, Helinski JD, Kunkel SL, Knight PR. Lung contusion: inflammatory mechanisms and interaction with other injuries. Shock 2009;32:122–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raghavendran K, Davidson BA, Knight PR, Wang Z, Helinski J, Chess PR, Notter RH. Surfactant dysfunction in lung contusion with and without superimposed gastric aspiration in a rat model. Shock 2008;30:508–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ojielo CI, Cooke K, Mancuso P, Standiford TJ, Olkiewicz KM, Clouthier S, Corrion L, Ballinger MN, Toews GB, Paine R, III, et al. Defective phagocytosis and clearance of Pseudomonas aeruginosa in the lung following bone marrow transplantation. J Immunol 2003;171:4416–4424 [DOI] [PubMed] [Google Scholar]
- 18.Aronoff DM, Canetti C, Peters-Golden M. Prostaglandin E2 inhibits alveolar macrophage phagocytosis through an E-prostanoid 2 receptor–mediated increase in intracellular cyclic AMP. J Immunol 2004;173:559–565 [DOI] [PubMed] [Google Scholar]
- 19.Ballinger MN, Paine R, III, Serezani CH, Aronoff DM, Choi ES, Standiford TJ, Toews GB, Moore BB. Role of granulocyte macrophage colony-stimulating factor during gram-negative lung infection with Pseudomonas aeruginosa. Am J Respir Cell Mol Biol 2006;34:766–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoth JJ, Stitzel JD, Gayzik FS, Brownlee NA, Miller PR, Yoza BK, McCall CE, Meredith JW, Payne RM. The pathogenesis of pulmonary contusion: an open chest model in the rat. J Trauma 2006;61:32–44; discussion 44–45 [DOI] [PubMed] [Google Scholar]
- 21.Garcia CC, Russo RC, Guabiraba R, Fagundes CT, Polidoro RB, Tavares LP, Salgado AP, Cassali GD, Sousa LP, Machado AV, et al. Platelet-activating factor receptor plays a role in lung injury and death caused by influenza A in mice. PLoS Pathog 2010;6:e1001171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee JS, Frevert CW, Thorning DR, Segerer S, Alpers CE, Cartron JP, Colin Y, Wong VA, Martin TR, Goodman RB. Enhanced expression of Duffy antigen in the lungs during suppurative pneumonia. J Histochem Cytochem 2003;51:159–166 [DOI] [PubMed] [Google Scholar]
- 23.Abraham E. Neutrophils and acute lung injury. Crit Care Med 2003;31(4 Suppl):S195–S199 [DOI] [PubMed] [Google Scholar]
- 24.Rollins BJ. Monocyte chemoattractant protein 1: a potential regulator of monocyte recruitment in inflammatory disease. Mol Med Today 1996;2:198–204 [DOI] [PubMed] [Google Scholar]
- 25.Boring L, Gosling J, Cleary M, Charo IF. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature 1998;394:894–897 [DOI] [PubMed] [Google Scholar]
- 26.Okuma T, Terasaki Y, Kaikita K, Kobayashi H, Kuziel WA, Kawasuji M, Takeya M. C-C chemokine receptor 2 (CCR2) deficiency improves bleomycin-induced pulmonary fibrosis by attenuation of both macrophage infiltration and production of macrophage-derived matrix metalloproteinases. J Pathol 2004;204:594–604 [DOI] [PubMed] [Google Scholar]
- 27.Vidal F, Mensa J, Martinez JA, Almela M, Marco F, Gatell JM, Richart C, Soriano E, Jimenez de Anta MT. Pseudomonas aeruginosa bacteremia in patients infected with human immunodeficiency virus type 1. Eur J Clin Microbiol Infect Dis 1999;18:473–477 [DOI] [PubMed] [Google Scholar]
- 28.Morrison AJ, Jr, Wenzel RP. Epidemiology of infections due to Pseudomonas aeruginosa. Rev Infect Dis 1984;6:S627–S642 [DOI] [PubMed] [Google Scholar]
- 29.Blease K, Mehrad B, Standiford TJ, Lukacs NW, Gosling J, Boring L, Charo IF, Kunkel SL, Hogaboam CM. Enhanced pulmonary allergic responses to Aspergillus in CCR2−/− mice. J Immunol 2000;165:2603–2611 [DOI] [PubMed] [Google Scholar]
- 30.Winter C, Taut K, Srivastava M, Langer F, Mack M, Briles DE, Paton JC, Maus R, Welte T, Gunn MD, et al. Lung-specific overexpression of CC chemokine ligand (CCL) 2 enhances the host defense to Streptococcus pneumoniae infection in mice: role of the CCL2–CCR2 axis. J Immunol 2007;178:5828–5838 [DOI] [PubMed] [Google Scholar]
- 31.Gomes RN, Figueiredo RT, Bozza FA, Pacheco P, Amancio RT, Laranjeira AP, Castro-Faria-Neto HC, Bozza PT, Bozza MT. Increased susceptibility to septic and endotoxic shock in monocyte chemoattractant protein 1/CC chemokine ligand 2–deficient mice correlates with reduced interleukin 10 and enhanced macrophage migration inhibitory factor production. Shock 2006;26:457–463 [DOI] [PubMed] [Google Scholar]
- 32.Okuma T, Terasaki Y, Sakashita N, Kaikita K, Kobayashi H, Hayasaki T, Kuziel WA, Baba H, Takeya M. MCP-1/CCR2 signalling pathway regulates hyperoxia-induced acute lung injury via nitric oxide production. Int J Exp Pathol 2006;87:475–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seitz DH, Niesler U, Palmer A, Sulger M, Braumuller ST, Perl M, Gebhard F, Knoferl MW. Blunt chest trauma induces mediator-dependent monocyte migration to the lung. Crit Care Med 2010;38:1852–1859 [DOI] [PubMed] [Google Scholar]
- 34.Rollins BJ. Chemokines. Blood 1997;90:909–928 [PubMed] [Google Scholar]
- 35.Yoshimura T, Robinson EA, Tanaka S, Appella E, Kuratsu J, Leonard EJ. Purification and amino acid analysis of two human glioma–derived monocyte chemoattractants. J Exp Med 1989;169:1449–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chao J, Donham P, van Rooijen N, Wood JG, Gonzalez NC. Monocyte chemoattractant protein-1 released from alveolar macrophages mediates the systemic inflammation of acute alveolar hypoxia. Am J Respir Cell Mol Biol 2011;45:53–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Christensen PJ, Du M, Moore B, Morris S, Toews GB, Paine R., III Expression and functional implications of CCR2 expression on murine alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 2004;286:L68–L72 [DOI] [PubMed] [Google Scholar]
- 38.Mizgerd JP. Acute lower respiratory tract infection. N Engl J Med 2008;358:716–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cnop M, Welsh N, Jonas JC, Jorns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes 2005;54:S97–S107 [DOI] [PubMed] [Google Scholar]
- 40.El Btaouri H, Rath G, Morjani H, Schneider C, Petitfrere E, Antonicelli F, Martiny L. Interleukin-1beta–induced apoptosis through adenylyl cyclase and ERK1/2 inhibition in primary cultured thyroid cells. Biochem Biophys Res Commun 2006;339:469–476 [DOI] [PubMed] [Google Scholar]
- 41.Laster SM, Wood JG, Gooding LR. Tumor necrosis factor can induce both apoptic and necrotic forms of cell lysis. J Immunol 1988;141:2629–2634 [PubMed] [Google Scholar]
- 42.Herold S, Tabar TS, Janssen H, Hoegner K, Cabanski M, Lewe-Schlosser P, Albrecht J, Driever F, Vadasz I, Seeger W, et al. Exudate macrophages attenuate lung injury by the release of IL-1 receptor antagonist in gram-negative pneumonia. Am J Respir Crit Care Med 2011;183:1380–1390 [DOI] [PubMed] [Google Scholar]
- 43.Sun L, Louie MC, Vannella KM, Wilke CA, LeVine AM, Moore BB, Shanley TP. New concepts of IL-10–induced lung fibrosis: fibrocyte recruitment and M2 activation in a CCL2/CCR2 axis. Am J Physiol Lung Cell Mol Physiol 2011;300:L341–L353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mizgerd JP. Molecular mechanisms of neutrophil recruitment elicited by bacteria in the lungs. Semin Immunol 2002;14:123–132 [DOI] [PubMed] [Google Scholar]
- 45.Hoth JJ, Wells JD, Brownlee NA, Hiltbold EM, Meredith JW, McCall CE, Yoza BK. Toll-like receptor 4–dependent responses to lung injury in a murine model of pulmonary contusion. Shock 2009;31:376–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Laskin DL, Pendino KJ. Macrophages and inflammatory mediators in tissue injury. Annu Rev Pharmacol Toxicol 1995;35:655–677 [DOI] [PubMed] [Google Scholar]
- 47.Monick MM, Hunninghake GW. Activation of second messenger pathways in alveolar macrophages by endotoxin. Eur Respir J 2002;20:210–222 [DOI] [PubMed] [Google Scholar]
- 48.Trujillo G, O'Connor EC, Kunkel SL, Hogaboam CM. A novel mechanism for CCR4 in the regulation of macrophage activation in bleomycin-induced pulmonary fibrosis. Am J Pathol 2008;172:1209–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Misson P, van den Brule S, Barbarin V, Lison D, Huaux F. Markers of macrophage differentiation in experimental silicosis. J Leukoc Biol 2004;76:926–932 [DOI] [PubMed] [Google Scholar]
- 50.Kannan S, Huang H, Seeger D, Audet A, Chen Y, Huang C, Gao H, Li S, Wu M. Alveolar epithelial type II cells activate alveolar macrophages and mitigate P. aeruginosa infection. PLoS ONE 2009;4:e4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moore BB, Murray L, Das A, Wilke CA, Herrygers AB, Toews GB. The role of CCL12 in the recruitment of fibrocytes and lung fibrosis. Am J Respir Cell Mol Biol 2006;35:175–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Capelli A, Di Stefano A, Gnemmi I, Balbo P, Cerutti CG, Balbi B, usuardi M, Donner CF. Increased MCP-1 and MIP-1beta in bronchoalveolar lavage fluid of chronic bronchitics. Eur Respir J 1999;14:160–165 [DOI] [PubMed] [Google Scholar]
- 53.Ko FW, Lau CY, Leung TF, Wong GW, Lam CW, Hui DS. Exhaled breath condensate levels of 8-isoprostane, growth related oncogene alpha and monocyte chemoattractant protein-1 in patients with chronic obstructive pulmonary disease. Respir Med 2006;100:630–638 [DOI] [PubMed] [Google Scholar]
- 54.Kuschner WG, D'Alessandro A, Wong H, Blanc PD. Dose-dependent cigarette smoking–related inflammatory responses in healthy adults. Eur Respir J 1996;9:1989–1994 [DOI] [PubMed] [Google Scholar]
- 55.Traves SL, Culpitt SV, Russell RE, Barnes PJ, Donnelly LE. Increased levels of the chemokines GROalpha and MCP-1 in sputum samples from patients with COPD. Thorax 2002;57:590–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.