Abstract
Severe asthma is associated with fixed airway obstruction attributable to inflammation, copious luminal mucus, and increased airway smooth muscle (ASM) mass. Paradoxically, studies demonstrated that the hypertrophic and hyperplastic ASM characteristic of severe asthma has reduced contractile capacity. We compared the G-protein–coupled receptor (GPCR)–induced Ca2+ mobilization and expression of GPCRs and signaling proteins related to procontractile signaling in ASM derived postmortem from subjects who died of nonrespiratory causes, with cells from subjects who died of asthma. Despite the increased or comparable expression of contraction-promoting GPCRs (bradykinin B2 or histamine H1 and protease-activated receptor 1, respectively) in asthmatic ASM cells relative to cells from healthy donors, asthmatic ASM cells exhibited reduced histamine-induced Ca2+ mobilization and comparable responses to bradykinin and thrombin, suggesting a postreceptor signaling defect. Accordingly, the expression of regulator of G-protein signaling–5 (RGS5), an inhibitor of ASM contraction, was increased in cultured, asthmatic ASM cells and in bronchial smooth muscle bundles of both human subjects with asthma and allergen-challenged mice, relative to those of healthy human subjects or naive mice. The overexpression of RGS5 impaired the release of Ca2+ to thrombin, histamine, and carbachol, and reduced the contraction of precision-cut lung slices to carbachol. These results suggest that increased RGS5 expression contributes to decreased myocyte shortening in severe and fatal asthma.
Keywords: asthma, bronchial smooth muscle, signal transduction, G-protein–coupled receptors
Clinical Relevance
We show that a negative regulator of G-protein–coupled receptor–mediated airway smooth muscle (ASM) contraction, regulator of G-protein signaling–5 (RGS5), is up-regulated in human asthmatic airways. RGS5 could contribute to a hypocontractile ASM state in severe asthma, leading to fixed airway obstruction.
Chronic asthma is associated with reversible airways obstruction and extensive lung remodeling (1–6). Histological abnormalities typical of severe asthma include bronchial epithelial cell sloughing, mucus cell hyperplasia and mucus hypersecretion, subepithelial basement membrane thickening, increased myocyte number and size, the submucosal deposition of extracellular matrix components, and interstitial edema (3). A substantial body of evidence suggests that airway smooth muscle (ASM) mass is increased in the airways of patients with severe asthma (1, 6, 7).
Compelling studies also indicate that asthmatic ASM is characterized by hyperplasia or hypertrophy, increased synthetic and secretory function, and normal or reduced force-generating capacity (7, 8). Proliferating ASM cells phenotypically manifest a less contractile state in vitro, with reduced contractile protein expression (9). Asthmatic ASM also secretes more cytokines, chemokines, and extracellular matrix–modifying enzymes than does ASM from control samples (10–14). A fixed narrowing of the airways is hypothesized to occur in fatal asthma, which renders patients resistant to bronchodilator therapy (15). Although several studies characterized the molecular components of pathogenic ASM hyperplasia and hypertrophy in asthma (e.g., extracellular regulated kinase [ERK], phosphoinositide-3 kinase, S6 kinase, and glycogen synthase kinase 3 beta) (1, 16), abnormalities in procontractile signaling pathways in asthmatic ASM remain relatively undefined.
Soluble mediators linked to allergen-induced inflammation include histamine, leukotrienes, bradykinin, and thrombin, which evoke ASM contraction by stimulating G-protein–coupled receptor (GPCR) coupled to the activation (GTP binding) of G-protein alpha q (Gαq) (17). Activated Gαq stimulates phospholipase Cβ(PLCβ), which hydrolyzes phosphatidylinositol 4,5-bisphosphate to generate inositol (3,4,5)-trisphosphate (IP3). IP3 elicits the release of Ca2+ from sarco/endoplasmic reticulum by the activation of IP3 receptors. The hydrolysis of GTP by Gαq promotes pathway deactivation through the formation of inactive Gαq–GDP–Gβγ heterotrimers. These upstream phenomena increase the frequency of intracellular Ca2+ oscillations, which induce the Ca2+–calmodulin–dependent protein kinase–mediated activation of myosin light chain kinase (MLCK). The phosphorylation of the myosin light chain on serine 19 by MLCK promotes actin–myosin cross-bridge formation (17).
In this study, we examined the GPCR-induced Ca2+ mobilization and expressed GPCR pathway components in ASM cells cultured from patients with and without asthma. We focused on the expression of regulator of G-protein signaling (RGS) proteins as potential modulators of bronchial contractility in asthma. RGS proteins have emerged as physiologically important components of cellular desensitization to GPCR stimulation by virtue of their ability to accelerate GTP hydrolysis by Gαq, and thereby blunt downstream effector activation (18). Recently, we showed that ASM cells from healthy subjects expressed RGS 2, 3, 4, and 5 (19). Here we found that the expression of RGS5 was increased in ASM cells of patients with asthma, and that RGS5 overexpression inhibited bronchial contraction. RGS5 appears to be a physiologically relevant inhibitor of bronchial smooth muscle shortening in asthma.
Materials and Methods
Additional methods are available in the online supplement.
Subjects
Subjects with asthma and control subjects without asthma were recruited from Leicester, United Kingdom. Subjects with asthma had a consistent history and objective evidence of asthma, as described previously (20) and as outlined in Table 1. Subjects underwent extensive clinical characterization, including video-assisted fiberoptic bronchoscopic examination. This study was approved by the Leicestershire Ethics Committee. All patients gave written, informed consent.
TABLE 1.
CLINICAL AND SPUTUM CHARACTERISTICS OF SUBJECTS UNDERGOING ENDOBRONCHIAL BIOPSY
| Normal | Severe Asthma | |
| Number | 8 | 9 |
| Age, yr, median (range) | 55 (27–61) | 51 (44–57) |
| Male (n) | 4 | 6 |
| Cigarette pack years* | 0 (0) | 0 (0–10) |
| Atopy (n) | 5 | 7 |
| PC20FEV1, mg/ml, median (range) | >16 | 1.2 (0.5–3.3) |
| FEV1 percent predicted* | 105 (5) | 83 (6) |
| FEV1/FVC percentage* | 82 (2) | 72 (4) |
| Inhaled corticosteroids, beclamethasone diproprionate/d, median (range) | 0 | 1,600 (1200–2,000) |
| Oral corticosteroids (n) | 0 | 2 |
| LABA (n) | 0 | 11 |
| Sputum cell counts | ||
| TCC¶ | 2.3 (1.5) | 4.0 (0.7) |
| Eosinophil percentage, median (range) | 0.7 (0.6–0.8) | 8.5 (0.5–45) |
| Neutrophil percentage* | 67 (7) | 48 (10) |
| Macrophage percentage* | 22 (7) | 27 (6) |
Definition of abbreviations: LABA, long-acting β-adrenergic agonists; PC20, provocation concentration producing a 20% fall in FEV1; TCC, total cell count.
Mean (SE).
Cell Culture and Tissues
Human ASM cells and bronchial tissue from healthy donors and individuals with asthma were isolated from lungs obtained postmortem from the National Disease Research Interchange (Philadelphia, PA), following previously established procedures (21). The isolation of ASM was performed in accordance with protocols approved by the Committee on Studies Involving Human Beings at the University of Pennsylvania. ASM cells were maintained in Ham's F-12 medium (Invitrogen, Carlsbad, CA) supplemented with 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, and 25 mM HEPES buffer. Cells in subculture during passages 2–5 were used. Clinical profiles and demographics of analyzed samples (where known) are shown in Table 2. Human embryonic kidney 293T cells (HEK293T cells) were obtained from the American Tissue Type Culture Collection (Manassas, VA) and were cultured in Eagle's minimal essential medium (Invitrogen) supplemented with 10% FBS and antibiotics, as already described.
TABLE 2.
Clinical profiles and demographics of analyzed human samples
| Status | Sex | Age (yr) | Race | Smoking Status | Causes of Death |
| Male | 37 | African American | S | Intracranial hemorrhage | |
| Control | Male | 19 | African American | NS | Closed head injury |
| Male | 50 | White | NS | Intracranial hemorrhage | |
| Male | 49 | Hispanic | S | Cerebrovascular accident | |
| Male | 25 | White | S | Cardiac arrest | |
| Female | 23 | African American | S | Unknown | |
| Female | 19 | African American | NS | Unknown | |
| Male | 21 | White | S | Head trauma | |
| Female | 49 | Hispanic | NS | Unknown | |
| Female | 47 | White | NS | Cerebrovascular accident | |
| Male | 28 | African American | S | Gunshot wound | |
| Male | 28 | Asian | NS | Head trauma | |
| Female | 22 | White | S | Gunshot wound | |
| Female | 24 | White | NS | Cerebrovascular accident | |
| Male | 31 | African American | NS | Anoxia, secondary to asthma attack | |
| Male | 13 | White | NS | Anoxia, secondary to asthma attack | |
| Asthma | Male | 44 | Hispanic | NS | Anoxia, secondary to asthma attack |
| Female | 46 | White | NS | Anoxia, secondary to asthma attack | |
| Female | 15 | Hispanic | NS | Anoxia, secondary to asthma attack | |
| Male | 59 | White | S | Stroke | |
| Female | 48 | White | NS | Anoxia, secondary to asthma attack | |
| Male | 9 | White | NS | Anoxia, secondary to asthma attack | |
| Female | 44 | White | NS | Respiratory arrest | |
| Male | 44 | Hispanic | NS | Anoxia, secondary to asthma attack |
Definition of abbreviations: NS, nonsmoker; S, smoker.
Statistical Analysis
Functional data were analyzed by one-way or two-way ANOVA or the Student t test, using PRISM software (GraphPad, La Jolla, CA). Statistical outliers were determined by Grubb's test. We considered P < 0.05 statistically significant.
Results
Reduced Excitation–Contraction Coupling in Asthmatic ASM
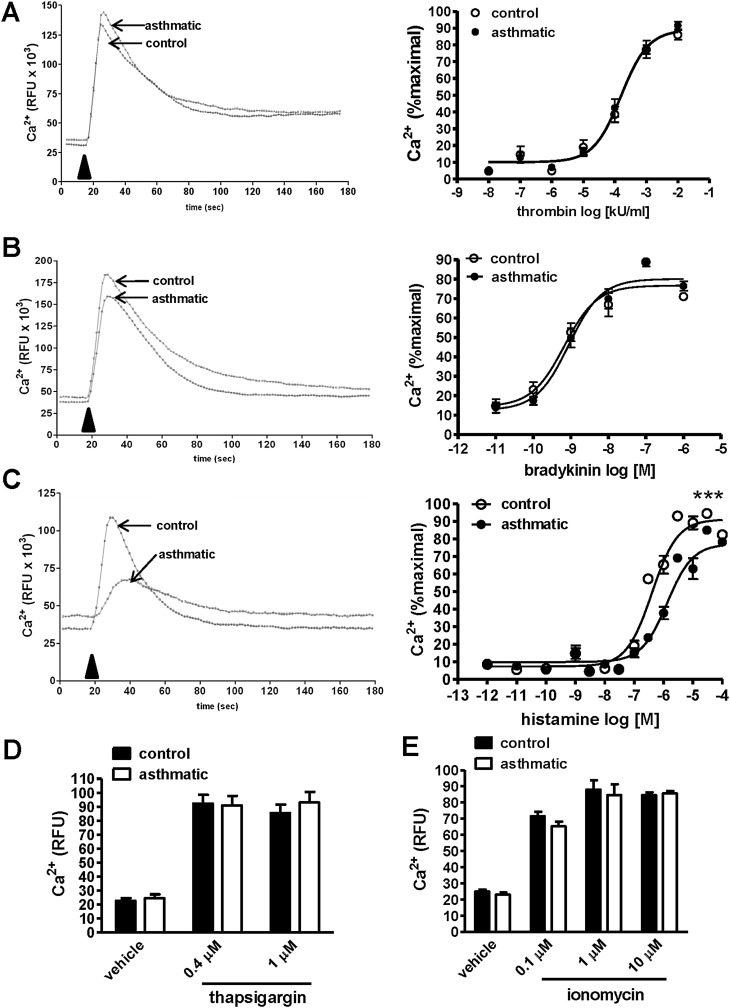
To evaluate signaling pathways modulating myocyte contractility in severe asthma, we cultured ASM cells postmortem from patients who died of respiratory failure because of severe asthma, and compared their functional responses to those from control subjects without asthma. We measured GPCR-induced Ca2+ mobilization after stimulation with substances present at elevated concentrations in asthmatic airways (histamine, bradykinin, and thrombin) (22) (Figures 1A–1C). We incubated cells with a Ca2+-binding fluorophore, and detected intracellular fluorescence over a period of several minutes after stimulation. The addition of agonist led to a rapid increase in intracellular Ca2+, followed by a slower decline in concentration, consistent with IP3-mediated Ca2+ mobilization from intracellular stores (representative kinetic tracings are shown in Figure 1 at left). Although the response to bradykinin and thrombin was similar in asthmatic and nonasthmatic ASM cells (Figures 1A and 1B), histamine induced significantly less Ca2+ flux in asthmatic cells (Figure 1C). These results suggest that ASM cells from subjects with asthma manifest impaired excitation–contraction signaling responses to some but not all GPCR ligands.
Figure 1.
Decreased G-protein–coupled receptor (GPCR)–evoked Ca2+ mobilization in asthmatic airway smooth muscle (ASM). (A–D) ASM cells derived from healthy donors or subjects with asthma were labeled with Ca2+-binding fluorophore, followed by stimulation with increasing concentrations of bradykinin (A), thrombin (B), or histamine (C), and by measurement of intracellular Ca2+ by fluorimetry. Representative kinetic tracings for bradykinin (100 nM), thrombin (10 U/ml), and histamine (1 μM) are shown at left. Arrowheads indicate the time of stimulus addition. Relative fluorescence units (RFU) were normalized to the cell number and percent maximal response to each concentration at right. Graphs represent the mean ± SEM of seven independent experiments performed in quadruplicate, using cells derived from 3–5 individual donors in each group. ***P < 0.0001, least-squares fit, logEC50 and Emax. (D and E) Cytosolic Ca2+ was measured upon stimulation with the indicated concentrations of thapsigargin (D) or ionomycin (E), as already described. The data in D and E represent the mean ± SEM of two experiments performed in quadruplicate, using cells from separate donors.
GPCR and Signaling Protein Expression in Asthmatic ASM
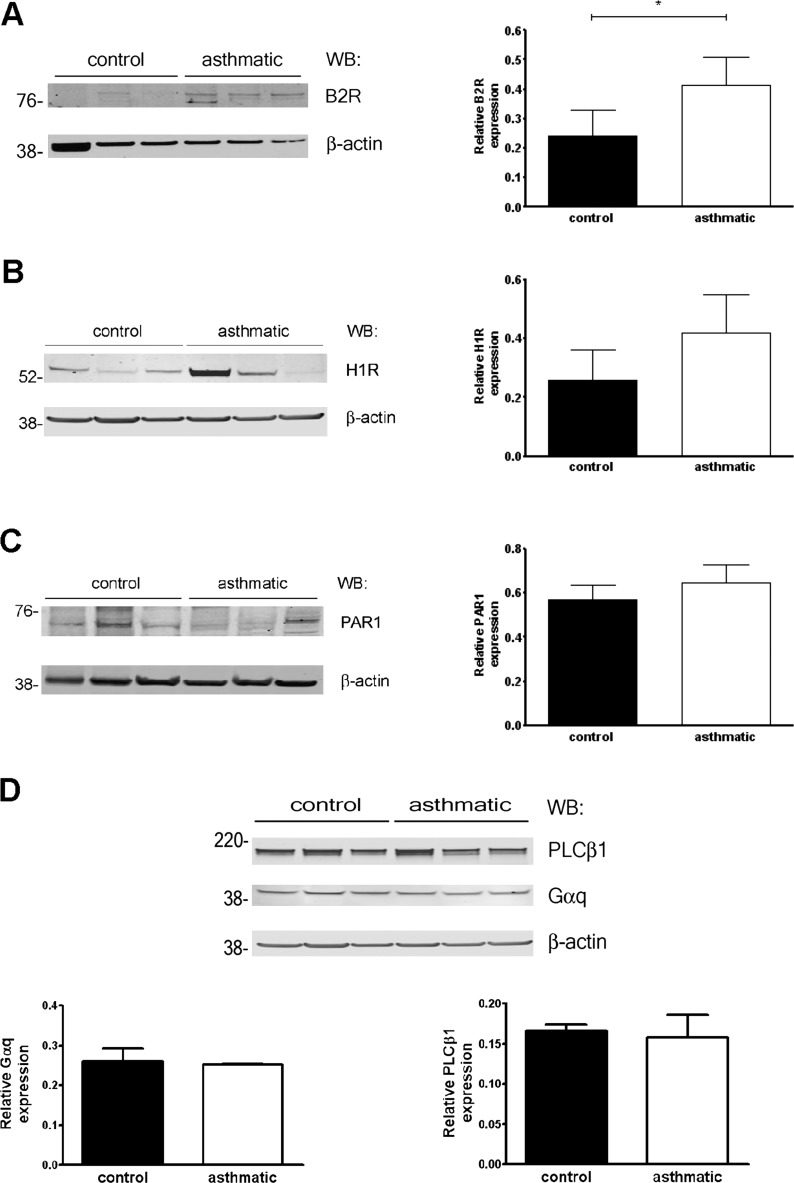
Thapsigargin, which raises intracellular Ca2+ concentrations by blocking the sarco/endoplasmic reticular Ca2+ ATPase (SERCA) pump and depleting endoplasmic reticulum stores, or the Ca2+ ionophore (ionomycin) triggered equivalent Ca2+influx in asthmatic and nonasthmatic ASM cells (Figures 1D and 1E), indicating intact Ca2+ homeostasis mechanisms in asthmatic cells. Alternatively, the selectively reduced responses of asthmatic ASM cells to histamine relative to control samples could have resulted from reduced receptor expression. However, we found increased bradykinin B2 receptor (B2R) expression in asthmatic ASM cells compared with control samples, whereas the expression of histamine H1 receptor (H1R) and thrombin receptors protease-activated receptor 1 (PAR1) was equivalent (Figures 2A–2C). These results suggest that the reduced histamine-induced Ca2+ mobilization in these cells resulted from a postreceptor defect.
Figure 2.
GPCR and signaling protein expression in ASM. (A–C) Expression of bradykinin B2 (A), histamine H1 (B), or PAR1 (C) receptors was determined by immunoblotting. *P = 0.04, Mann-Whitney U test. (D) Relative expression of signaling proteins in control and asthmatic ASM cells was determined by densitometry (normalized to β-actin, n = 3–7 donors/group). WB, Western blot.
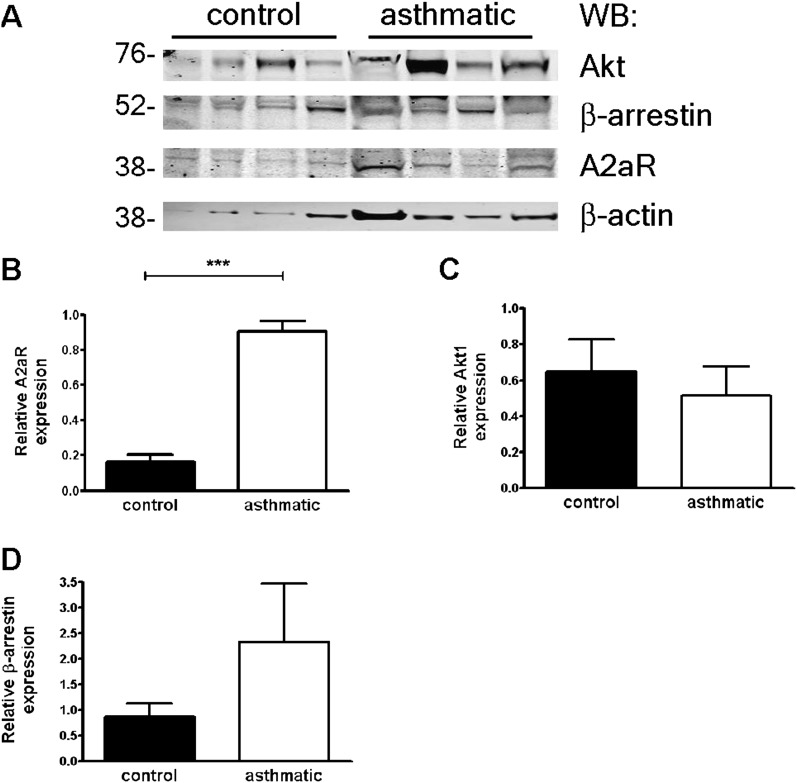
Because the abundance of effectors mediating Ca2+ flux immediately downstream of GPCRs (Gαq and phospholipase C beta 1 [PLCβ1]) was similar in asthmatic and nonasthmatic ASM cells (Figure 2D), we further analyzed gene expression related to GPCR signaling by a quantitative PCR array (a full gene list is provided in Table E1 in the online supplement). Notably, the expression of cell-cycle genes (cyclin D1, E1, and E2), prosurvival factors (Akt1), cytokines (IL-1β and vascular endothelial growth factor [VEGF]), and matrix metalloproteinases (MMP9; an ∼ 30-fold increase) was increased in ASM cells from subjects with asthma relative to control subjects, consistent with the previously described proliferative/synthetic phenotype of asthmatic ASM. Although the expression of several GPCRs and signaling molecules was increased in asthmatic ASM cells compared with control samples (e.g., the adenosine A2a receptor [A2aR], 12-fold; the sphingosine-1–phosphate-1 receptor, 10-fold; and β-arrestin 1/2, 7-fold), we did not detect corresponding significant changes in the expression of these proteins, with the exception of A2a receptors (Figures 3A–3D).
Figure 3.
Protein expression in asthmatic and nonasthmatic ASM cells. (A–D) Relative expression of proteins (adenosine A2a [A2a] receptors, Akt1, and β-arrestin) in control and asthmatic ASM cells was determined by densitometry (mean ± SEM normalized to β-actin, n = 3–7 donors/group). ***P = 0.0001, unpaired t test.
RGS5 Is Up-Regulated in Asthmatic ASM
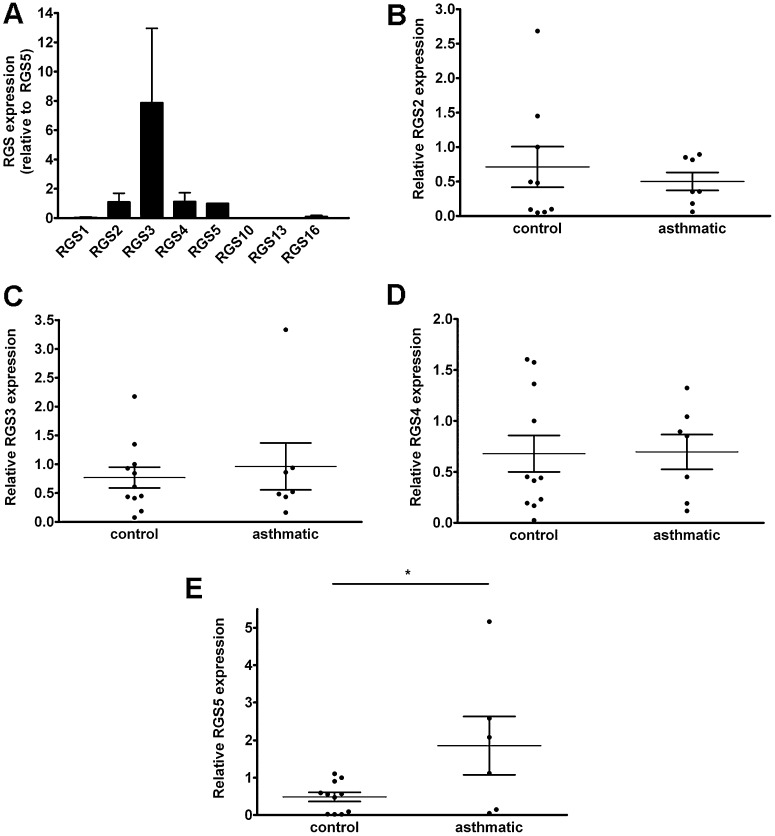
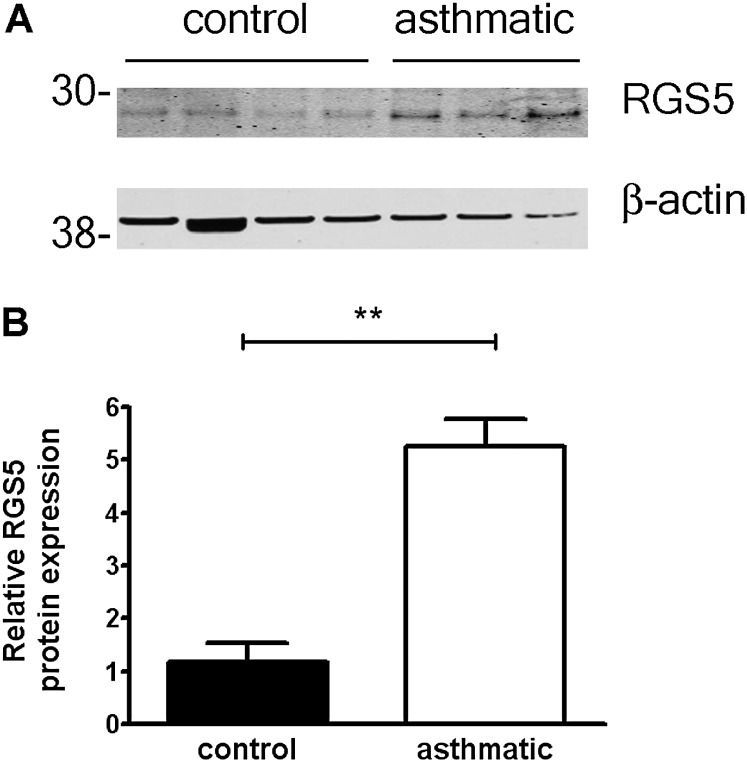
Because our studies thus far failed to reveal changes in procontractile signaling components that could underlie the abnormal histamine response of asthmatic ASM cells, we considered other modulators of GPCR signal transduction pathways. The expression of RGS proteins, which are potent negative regulators of GPCRs, is often dynamically regulated in diseased tissue or in response to environmental stimuli (18). We hypothesized that RGS expression could be altered in asthmatic ASM, and specifically RGS5, which we previously identified as a physiological, relevant modulator of GPCR signaling in healthy ASM cells (19). Similar to control cells, ASM from asthmatic cells predominantly expressed RGS 2–5 (Figure 4A). Because unique primer pairs detected individual RGS gene expressions, relative amounts of each could not be compared by this method. We evaluated the expression of specific RGSs individually by real-time PCR. Although quantities of RGS 2, 3, and 4 were similar in ASM cells from healthy donors and from donors with asthma (Figures 4B–4D), RGS5 mRNA was significantly increased in asthmatic ASM relative to control ASM (Figure 4E). To determine whether RGS5 protein expression was increased in cells from subjects with asthma relative to subjects without asthma, we prepared lysates from cultured ASM cells and analyzed RGS5 quantitatively by immunoblotting. Concentrations of RGS5 were significantly increased in ASM cells from several patients with asthma, compared with control subjects (Figures 5A and 5B).
Figure 4.
Regulator of G-protein signaling (RGS) expression in asthmatic ASM. (A) Expression of RGS mRNAs was determined in ASM from subjects with asthma by real-time PCR (mean ± SEM of seven donors relative to RGS5, set as 1). (B–E) Expression of individual RGS mRNAs in asthmatic and nonasthmatic ASM cells was determined by real-time PCR (mean ± SEM relative to a single control donor, set as 1). *P = 0.03, unpaired t test.
Figure 5.
RGS5 protein is up-regulated in asthmatic ASM cells. (A) Expression of RGS5 in asthmatic and nonasthmatic ASM cells was evaluated by immunoblotting. (B) Relative expression of RGS5 in control and asthmatic ASM cells was determined by densitometry (mean ± SEM of four control donors and three donors with asthma, normalized to β-actin). **P = 0.003, unpaired t test.
Increased RGS5 Expression in the Asthmatic Lung
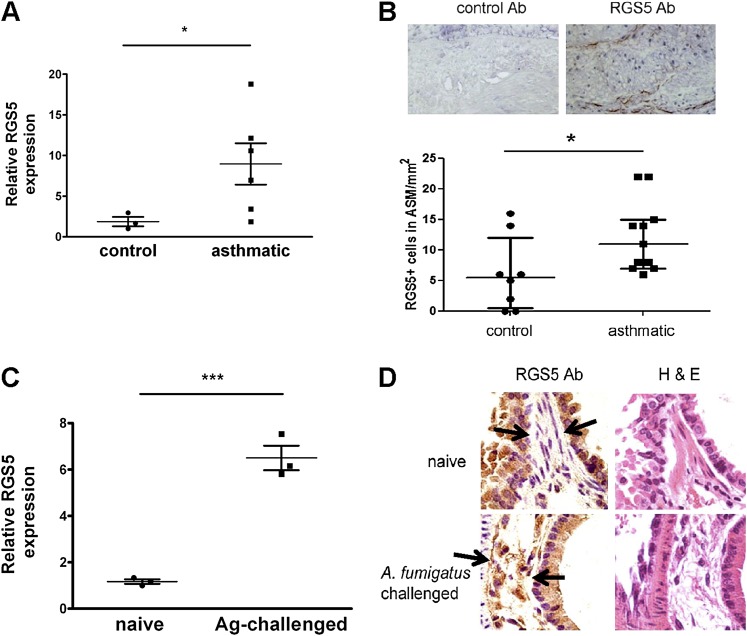
To ascertain whether the changes in amounts of RGS5 in ASM cells from subjects with asthma correlated with the expression of RGS5 in vivo, we measured RGS5 mRNA in bronchial tissue from subjects with asthma and subjects without asthma by quantitative PCR. RGS5 was significantly increased in asthmatic lung tissue relative to control lung tissue (Figure 6A). To determine which cells express RGS5 protein in vivo, we evaluated endobronchial biopsies from subjects with asthma and age-matched healthy control subjects by immunohistochemistry. We obtained samples from patients with severe asthma according to criteria established by the Global Initiative for Asthma (Table 2). RGS5 was expressed predominantly in ASM bundles in situ, and bronchial smooth muscle from subjects with asthma demonstrated significantly increased numbers of RGS5+ ASM cells compared with healthy subjects (Figure 6B). Furthermore, RGS5 mRNA concentrations were 6-fold higher in whole-lung tissue from allergen-sensitized and allergen-challenged mice compared with naive mice (Figure 6C), and RGS5 was detected in lung ASM of challenged mice (Figure 6D). Lung epithelium also stained with the RGS5 antibody in wild-type (WT) mice. However, similar epithelial staining was present in lung sections from in Rgs5−/− mice (Figure E1 in the online supplement). Thus, the expression of RGS5 in murine lungs is restricted to ASM and is up-regulated in vivo in the setting of asthmatic/allergic pulmonary inflammation in both mice and humans.
Figure 6.
Increased RGS5 expression in asthmatic bronchi. (A) RGS5 mRNA expression in bronchial tissue from donors with and without asthma (Table 2) was evaluated by real-time PCR (mean ± SEM of 3–6 donors). *P = 0.04, Mann-Whitney U test. (B) Representative photomicrographs of a bronchial biopsy from a donor with asthma (magnification, ×200), stained with either isotype control antibody (Ab; left) or RGS5 antibody (right), illustrating RGS5+ cells within the ASM bundle (dot-plot of the number of RGS5+ cells/mm2 ASM). Horizontal bars represent the medians and interquartile ranges. *P = 0.028, Mann-Whitney U test. (C) RGS5 mRNA expression in bronchial tissue from PBS-challenged or Af-challenged mice was evaluated by real-time PCR (mean ± SEM of three mice/group). Ag, allergen. ***P = 0.0006, unpaired t test. (D) Paraffin-embedded sections of lungs from naive and allergen (Aspergillus fumigatus)-challenged mice were evaluated by immunohistochemistry, using an RGS5 antibody. Images (magnification, ×40) are derived from a single mouse, representative of three mice/group, and RGS5 staining is brown. H&E, hematoxylin and eosin. Arrows show the sites of RGS5 expression.
RGS5 Overexpression Inhibits Ca2+ Release in ASM and Bronchial Contractions
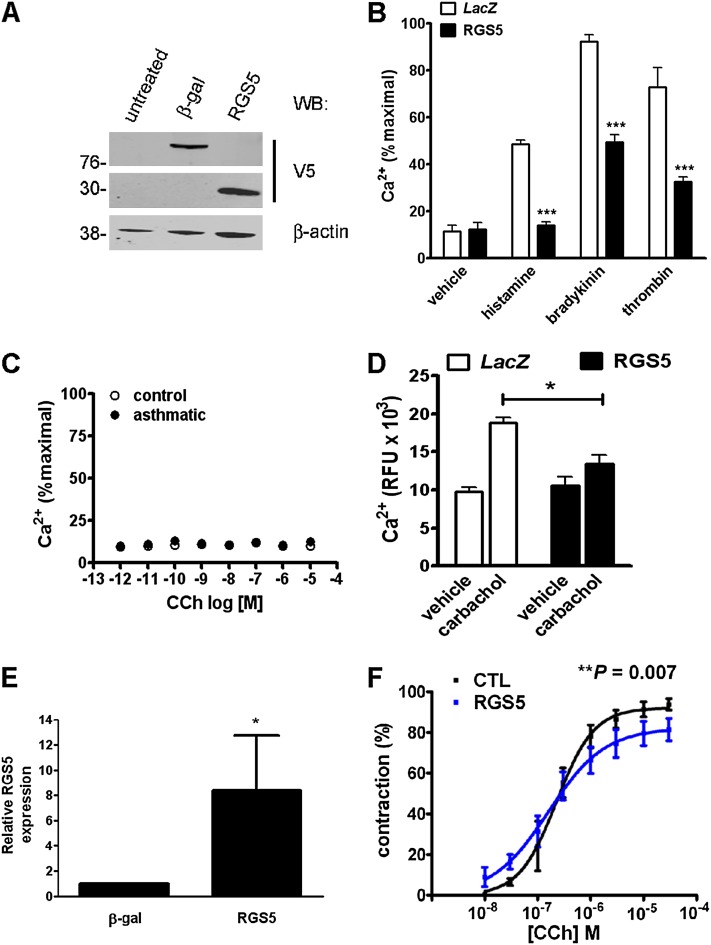
To assess the functional significance of elevated RGS5 expression in the bronchi of subjects with asthma for smooth muscle Ca2+ signaling, we expressed RGS5 in ASM cells from healthy donors by lentiviral transduction. The overexpression of RGS5 in ASM cells (Figure 7A) led to a greater than 50% reduction in Ca2+ mobilization in response to bradykinin, thrombin, and histamine (Figure 7B). Because we could not detect Ca2+ flux in ASM stimulated with carbachol, most likely attributable to the previously described down-regulation of M3 muscarinic receptors in ASM cultures (23) (Figure 7C), we expressed RGS5 in HEK293T cells, which have endogenous M3 receptors. Compared with cells expressing empty vector, RGS5-overexpressing cells showed significantly reduced Ca2+ after stimulation with carbachol (Figure 7D).
Figure 7.
RGS5 inhibits GPCR-induced Ca2+ mobilization and airway contraction. (A and B) ASM derived from healthy donors was left untreated or was transduced with lentivirus encoding β-galactosidase or RGS5. (A) The expression of recombinant proteins was determined by immunoblotting cell lysates with anti-V5 antibody. β-gal, β-galactosidase. (B) Control or lentivirus-transduced cells were stimulated with histamine (10 μM), bradykinin (100 nM), or thrombin (1 U/ml), followed by measurement of intracellular Ca2+ concentrations, as in Figure 1. Data represent the mean ± SEM of two independent experiments performed in quadruplicate, using cells derived from two healthy donors. ***P < 0.001, one-way ANOVA. (C) Human ASM cells were stimulated with various concentrations of carbachol (CCh), followed by measurement of intracellular Ca2+ concentrations by fluorimetry. Graphs represent the mean ± SEM of two independent experiments performed in quadruplicate, using cells derived from two individual donors. (D) HEK293T cells were transfected with plasmids encoding RGS5 or β-galactosidase (control), followed by the measurement of Ca2+ flux after stimulation with carbachol (100 μM) or vehicle alone. Data represent the mean ± SEM of two independent experiments, measured in quadruplicate. ***P < 0.01, two-way ANOVA. (E) Precision-cut human lung slices (PCLS) were prepared as described in Materials and Methods. The expression of RGS5 in slices was determined by quantitative PCR. *P = 0.01, Mann-Whitney U test. (F) PCLS were contracted to various concentrations of carbachol, followed by measurement of airway luminal diameter by microscopy. The data represent mean ± SEM of 3–4 airways in each group, as performed in two independent experiments. CTL, control.
To determine how RGS5 overexpression affected GPCR-evoked contractility, we transduced precision-cut lung slices (PCLS) with lentivirus encoding RGS5, and measured airway luminal narrowing by microscopy. Because our previous work indicated that these lung slices do not contract well to agonists other than carbachol (data not shown) (24), we measured airway contraction in response to carbachol. RGS5 mRNA concentrations were significantly increased in lung slices transduced with RGS5-encoding lentivirus compared with control virus (LacZ) (Figure 7E). The overexpression of RGS5 significantly reduced the maximal contraction of PCLS elicited by carbachol (Figure 7F). In concordance with our previous study using PCLS from RGS5-deficient mice (19), the overexpression of RGS5 did not affect the potency of carbachol (log[EC50], 0.68 ± 0.1 μM versus 0.73 ± 0.14 μM), but reduced the maximal contraction (Emax, 94% ± 3.3% versus 80.2% ± 3.4%; P = 0.007). Thus, RGS5 inhibits the contraction of human bronchi in response to muscarinic receptor activation.
Discussion
Although allergic inflammation has a central function in the establishment and maintenance of airway hyperresponsiveness (AHR) in asthma, only 50% of patients with asthma are atopic (25). Moreover, the degree of inflammation present in asthmatic lungs may not correlate with the degree of AHR (26). Such findings suggest that intrinsic abnormalities in lung structural cells, including ASM, merit distinct consideration in severe or fatal disease. Unfortunately, only a few studies have addressed the excitation–contraction coupling and contractile function of ASM cells because of the difficulties in obtaining ASM cells in the setting of fatal asthma (death from asthma is relatively rare), and because of the lack of adequate techniques to address the contractile function of ASM cells in vitro. Here we studied upstream, receptor-related events leading to cellular contraction (Ca2+ flux) and the expression of components of procontractile GPCR pathways in ASM cells from patients with and without asthma, in an attempt to uncover new or unique therapeutic targets.
Our experiments revealed differences in GPCR-evoked Ca2+ mobilization in asthmatic and nonasthmatic ASM cells. We found reduced peak Ca2+ concentrations after stimulation with histamine, but not bradykinin or thrombin, in asthmatic ASM cells compared with nonasthmatic ASM cells. Our results differ from those of Mahn and colleagues (27), who found reduced responses of asthmatic ASM to bradykinin. They observed that the recovery of Ca2+ concentrations to baseline was delayed in asthmatic cells, which they attributed to depleted sarcoplasmic reticulum Ca2+ stores resulting from decreased SERCA concentrations. In contrast, we detected equivalent responses to the SERCA inhibitor thapsigargin and similar kinetics of Ca2+ recovery after the GPCR stimulation of healthy and asthmatic ASM cells. These results indicate that Ca2+ stores were comparable in our samples. Our study and that of Mahn and colleagues contain the key difference that Mahn and colleagues (27) obtained bronchial specimens from subjects with mild to moderate asthma, whereas we studied ASM cells from bronchi obtained postmortem from patients who died of asthma.
We found that the total expression of receptor-stimulated signaling components such as Gαq and PLCβ1 was equivalent in our ASM samples, whereas amounts of bradykinin B2 and adenosine A2a receptor were significantly increased in asthmatic cells relative to control cells. We are unaware of any extensive comparison of GPCR expression in asthmatic and nonasthmatic ASM cells reported by others, and B2 receptor concentrations were not assessed in the study of Mahn and colleagues (27). Cytokines, including IFN-γ and TGF-β, which are both increased in asthmatic airways (5), up-regulate ASM GPCR expression in cultured ASM cells (cysteinyl leukotriene D1 and B2, respectively) (28, 29). Asthmatic ASM cells also express higher concentrations of C-C chemokine receptor 3, which may mediate migration and cytokine secretion (30). Although the amounts of Gαq were increased in bronchial tissue from allergen-sensitized and challenged mice in a previous study, we observed no changes in Gαq expression in asthmatic human ASM cells compared with control cells. These findings are similar to those of McGraw and colleagues (31), who demonstrated unchanged Gαq concentrations in Gαi2 inhibitory peptide transgenic mice, which demonstrate increased AHR relative to wild-type mice in the absence of allergic inflammation. Currently, the physiological significance of altered G-protein expression in these models has not been clearly established.
Because no obvious reductions in receptor, G-protein, or effector expression could account for the impaired histamine-evoked Ca2+ mobilization in asthmatic ASM cells, we analyzed the expression of other genes that could potentially regulate excitation–contraction pathways. Consistent with the proliferative phenotype of asthmatic cells observed by others (8), the expression of cyclins and antiapoptosis factors (Akt, Elk1/4, and Bcl2) was substantially increased compared with that in control cells. In addition, asthmatic cells displayed a synthetic/secretory phenotype, as evidenced by the up-regulation of cytokines and matrix-modifying enzymes such as MMP9, VEGF, C-C chemokine ligand 2, and connective tissue growth factor, among others, as shown in previous studies (5, 32). We also detected increased expression of adenosine A2a and sphingosine-1–phosphate receptors in asthmatic ASM cells, which were reported by others to affect bronchial smooth muscle tone (33–35), suggesting physiological relevance to asthma.
To sum up most important finding of this study, the expression of RGS5 mRNA and protein was significantly up-regulated in asthmatic ASM compared with nonasthmatic cells and lung tissue. Asthmatic ASM cells displayed reduced histamine responses relative to nonasthmatic ASM cells, but had comparable to responses to bradykinin and thrombin. These results suggest that RGS5 is a regulator of histamine-mediated contractile signaling in asthmatic ASM, but may not couple to bradykinin or PAR1 receptors in these cells. Our previous work demonstrated that short interfering (si)RNA-mediated RGS5 knockdown in healthy ASM cells augmented thrombin responses (19). In a separate study, RGS5 specifically inhibited angiotensin II type 1A receptor–mediated but not muscarinic M3 receptor–mediated ERK activation in vascular smooth muscle cells. In contrast, Anger and colleagues found that RGS5 impaired carbachol-mediated IP3 formation and ERK activation in COS-7 cells expressing M3 receptors (36). Collectively, these results suggest the receptor-selective, and perhaps cell type–specific, regulation of signaling by RGS5 (37).
We also demonstrated that the overexpression of RGS5 inhibited PCLS contraction to carbachol. A current limitation of our PCLS transfection involved the nonselective overexpression/knockdown in the slices, and future studies using lentivirus driven by a smooth muscle–specific promoter will allow us to achieve selective expression in ASM. However, agonist-induced bronchoconstriction is visualized directly, which is mediated by ASM contraction. Although the contribution of factors secreted by epithelial cells or other cells cannot be formally excluded, the sections are aggressively washed immediately before the addition of an agonist, which induces a reduction in airway diameter within minutes. We showed that RGS5 overexpression blunts the Ca2+ signaling evoked by procontractile ligands, including carbachol (Figures 7B and 7D). In addition, because we observed previously that PCLS from Rgs5–/– mice contracted significantly more to carbachol than did airways from WT mice (19), these studies indicate that RGS5 is a physiologically relevant modulator of airway contraction.
We also determined previously that the prolonged exposure of ASM to β-adrenergic agonists down-regulates RGS5 expression (19). Thus, we may be underestimating the extent of RGS5 up-regulation in ASM from patients with severe asthma who died of asthma, given that such patients were probably exposed to sustained high doses of β-agonist. What mechanisms might underlie the increased RGS5 quantities in asthmatic ASM? RGS5 is up-regulated in arterial smooth muscle in models of skin wound–healing and tumor angiogenesis (38), suggesting that extracellular matrix–modifying enzymes and growth factors linked to airway remodeling in asthma also modulate RGS5 expression. Inflammatory cytokines (Th2-related, IL-1, and TNF-α) also directly influence GPCR responsiveness (39). IL-13 increases the Ca2+ responsiveness of ASM to histamine, bradykinin, and acetylcholine (Ach), and the contraction of tracheas to Ach (40). Although our preliminary work indicates that IL-13 does not affect RGS5 expression in ASM (data not shown), numerous cytokines and chemokines modify RGS protein transcription in other cell types (18).
Finally, whether the up-regulation of RGS5 in asthmatic ASM is beneficial or maladaptive remains unclear. Recently, Li and colleagues demonstrated that transgenic mice expressing a cardiac-specific Rgs5 transgene were resistant to hypertrophic cardiomyopathy and fibrosis, whereas Rgs5–/– mice were more sensitive to pressure overload–induced cardiomyopathy than were WT mice (41). The up-regulation of RGS5 could be a compensatory event that protects ASM from chronic hyperstimulation in asthma. Future studies of bronchial contraction in mice with varying amounts of RGS5 expression in models of allergic and nonallergic pulmonary inflammation will be needed to address this and other issues more fully.
Supplementary Material
Footnotes
This study was supported by the Intramural Research Program of the NIAID at the National Institutes of Heath (grant AI000939 LAD to K.M.D., and grants HL5452235, HL543102, ES013505, and HL544157 to R.A.P.), and by the Wellcome Trust and Glaxo Smith Kline (C.E.B.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2011-0110OC on January 26, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Hershenson MB, Brown M, Camoretti-Mercado B, Solway J. Airway smooth muscle in asthma. Annu Rev Pathol 2008;3:523–555 [DOI] [PubMed] [Google Scholar]
- 2.Gil FR, Lauzon AM. Smooth muscle molecular mechanics in airway hyperresponsiveness and asthma. Can J Physiol Pharmacol 2007;85:133–140 [DOI] [PubMed] [Google Scholar]
- 3.Halayko AJ, Amrani Y. Mechanisms of inflammation-mediated airway smooth muscle plasticity and airways remodeling in asthma. Respir Physiol Neurobiol 2003;137:209–222 [DOI] [PubMed] [Google Scholar]
- 4.An SS, Bai TR, Bates JH, Black JL, Brown RH, Brusasco V, Chitano P, Deng L, Dowell M, Eidelman DH, et al. Airway smooth muscle dynamics: a common pathway of airway obstruction in asthma. Eur Respir J 2007;29:834–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tran T, Halayko AJ. Extracellular matrix and airway smooth muscle interactions: a target for modulating airway wall remodelling and hyperresponsiveness? Can J Physiol Pharmacol 2007;85:666–671 [DOI] [PubMed] [Google Scholar]
- 6.Bentley JK, Hershenson MB. Airway smooth muscle growth in asthma: proliferation, hypertrophy, and migration. Proc Am Thorac Soc 2008;5:89–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seow CY, Schellenberg RR, Pare PD. Structural and functional changes in the airway smooth muscle of asthmatic subjects. Am J Respir Crit Care Med 1998;158:S179–S186 [DOI] [PubMed] [Google Scholar]
- 8.Johnson PR, Roth M, Tamm M, Hughes M, Ge Q, King G, Burgess JK, Black JL. Airway smooth muscle cell proliferation is increased in asthma. Am J Respir Crit Care Med 2001;164:474–477 [DOI] [PubMed] [Google Scholar]
- 9.Halayko AJ, Salari H, Ma X, Stephens NL. Markers of airway smooth muscle cell phenotype. Am J Physiol 1996;270:L1040–L1051 [DOI] [PubMed] [Google Scholar]
- 10.Black JL, Roth M. Intrinsic asthma: is it intrinsic to the smooth muscle? Clin Exp Allergy 2009;39:962–965 [DOI] [PubMed] [Google Scholar]
- 11.John AE, Zhu YM, Brightling CE, Pang L, Knox AJ. Human airway smooth muscle cells from asthmatic individuals have CXCl8 hypersecretion due to increased NF-kappa B p65, C/EBP beta, and RNA polymerase II binding to the CXCl8 promoter. J Immunol 2009;183:4682–4692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson PR. Role of human airway smooth muscle in altered extracellular matrix production in asthma. Clin Exp Pharmacol Physiol 2001;28:233–236 [DOI] [PubMed] [Google Scholar]
- 13.Chan V, Burgess JK, Ratoff JC, O'Connor BJ, Greenough A, Lee TH, Hirst SJ. Extracellular matrix regulates enhanced eotaxin expression in asthmatic airway smooth muscle cells. Am J Respir Crit Care Med 2006;174:379–385 [DOI] [PubMed] [Google Scholar]
- 14.Kaur D, Saunders R, Berger P, Siddiqui S, Woodman L, Wardlaw A, Bradding P, Brightling CE. Airway smooth muscle and mast cell-derived CC chemokine ligand 19 mediate airway smooth muscle migration in asthma. Am J Respir Crit Care Med 2006;174:1179–1188 [DOI] [PubMed] [Google Scholar]
- 15.Tliba O, Panettieri RA Jr. Noncontractile functions of airway smooth muscle cells in asthma. Annu Rev Physiol 2009;71:509–535 [DOI] [PubMed] [Google Scholar]
- 16.Liu W, Liang Q, Balzar S, Wenzel S, Gorska M, Alam R. Cell-specific activation profile of extracellular signal–regulated kinase 1/2, Jun N-terminal kinase, and p38 mitogen–activated protein kinases in asthmatic airways. J Allergy Clin Immunol 2008;121:893–902 [DOI] [PubMed] [Google Scholar]
- 17.Deshpande DA, Penn RB. Targeting G protein–coupled receptor signaling in asthma. Cell Signal 2006;18:2105–2120 [DOI] [PubMed] [Google Scholar]
- 18.Bansal G, Druey KM, Xie Z. R4 RGS proteins: regulation of G-protein signaling and beyond. Pharmacol Ther 2007;116:473–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Z, Cooper PR, Damera G, Mukhopadhyay I, Cho H, Kehrl JH, Panettieri RA Jr, Druey KM. {Beta}-agonist associated reduction in RGS5 expression promotes airway smooth muscle hyperresponsiveness. J Biol Chem 2011;286:11444–11455. [DOI] [PMC free article] [PubMed]
- 20.Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med 2002;346:1699–1705 [DOI] [PubMed] [Google Scholar]
- 21.Panettieri RA, Murray RK, DePalo LR, Yadvish PA, Kotlikoff MI. A human airway smooth muscle cell line that retains physiological responsiveness. Am J Physiol 1989;256:C329–C335 [DOI] [PubMed] [Google Scholar]
- 22.Abraham WM, Scuri M, Farmer SG. Peptide and non-peptide bradykinin receptor antagonists: role in allergic airway disease. Eur J Pharmacol 2006;533:215–221 [DOI] [PubMed] [Google Scholar]
- 23.Gosens R, Nelemans SA, Grootte Bromhaar MM, McKay S, Zaagsma J, Meurs H. Muscarinic M3-receptors mediate cholinergic synergism of mitogenesis in airway smooth muscle. Am J Respir Cell Mol Biol 2003;28:257–262 [DOI] [PubMed] [Google Scholar]
- 24.Ressmeyer AR, Larsson AK, Vollmer E, Dahlen SE, Uhlig S, Martin C. Characterisation of guinea pig precision-cut lung slices: comparison with human tissues. Eur Respir J 2006;28:603–611 [DOI] [PubMed] [Google Scholar]
- 25.Beasley R, Pekkanen J, Pearce N. Has the role of atopy in the development of asthma been over-emphasized? Pediatr Pulmonol 2001;149–150 [PubMed] [Google Scholar]
- 26.Crimi E, Spanevello A, Neri M, Ind PW, Rossi GA, Brusasco V. Dissociation between airway inflammation and airway hyperresponsiveness in allergic asthma. Am J Respir Crit Care Med 1998;157:4–9 [DOI] [PubMed] [Google Scholar]
- 27.Mahn K, Hirst SJ, Ying S, Holt MR, Lavender P, Ojo OO, Siew L, Simcock DE, McVicker CG, Kanabar V, et al. Diminished sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) expression contributes to airway remodelling in bronchial asthma. Proc Natl Acad Sci USA 2009;106:10775–10780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amrani Y, Moore PE, Hoffman R, Shore SA, Panettieri RA Jr. Interferon-gamma modulates cysteinyl leukotriene receptor–1 expression and function in human airway myocytes. Am J Respir Crit Care Med 2001;164:2098–2101 [DOI] [PubMed] [Google Scholar]
- 29.Kim JH, Jain D, Tliba O, Yang B, Jester WF, Jr, Panettieri RA, Jr, Amrani Y, Pure E. TGF-beta potentiates airway smooth muscle responsiveness to bradykinin. Am J Physiol Lung Cell Mol Physiol 2005;289:L511–L520 [DOI] [PubMed] [Google Scholar]
- 30.Joubert P, Lajoie-Kadoch S, Labonte I, Gounni AS, Maghni K, Wellemans V, Chakir J, Laviolette M, Hamid Q, Lamkhioued B. CCR3 expression and function in asthmatic airway smooth muscle cells. J Immunol 2005;175:2702–2708 [DOI] [PubMed] [Google Scholar]
- 31.McGraw DW, Elwing JM, Fogel KM, Wang WC, Glinka CB, Mihlbachler KA, Rothenberg ME, Liggett SB. Crosstalk between Gi and Gq/Gs pathways in airway smooth muscle regulates bronchial contractility and relaxation. J Clin Invest 2007;117:1391–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simcock DE, Kanabar V, Clarke GW, Mahn K, Karner C, O'Connor BJ, Lee TH, Hirst SJ. Induction of angiogenesis by airway smooth muscle from patients with asthma. Am J Respir Crit Care Med 2008;178:460–468 [DOI] [PubMed] [Google Scholar]
- 33.Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J. International union of pharmacology: XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev 2001;53:527–552 [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenfeldt HM, Amrani Y, Watterson KR, Murthy KS, Panettieri RA Jr, Spiegel S. Sphingosine-1–phosphate stimulates contraction of human airway smooth muscle cells. FASEB J 2003;17:1789–1799 [DOI] [PubMed] [Google Scholar]
- 35.Roviezzo F, Di Lorenzo A, Bucci M, Brancaleone V, Vellecco V, De Nardo M, Orlotti D, De Palma R, Rossi F, D'Agostino B, et al. Sphingosine-1–phosphate/sphingosine kinase pathway is involved in mouse airway hyperresponsiveness. Am J Respir Cell Mol Biol 2007;36:757–762 [DOI] [PubMed] [Google Scholar]
- 36.Anger T, Klintworth N, Stumpf C, Daniel WG, Mende U, Garlichs CD. RGS protein specificity towards Gq- and Gi/o-mediated ERK 1/2 and AKT activation, in vitro. J Biochem Mol Biol 2007;40:899–910 [DOI] [PubMed] [Google Scholar]
- 37.Wang Q, Liu M, Mullah B, Siderovski DP, Neubig RR. Receptor-selective effects of endogenous RGS3 and RGS5 to regulate mitogen-activated protein kinase activation in rat vascular smooth muscle cells. J Biol Chem 2002;277:24949–24958 [DOI] [PubMed] [Google Scholar]
- 38.Gu S, Cifelli C, Wang S, Heximer SP. RGS proteins: identifying new gaps in the understanding of blood pressure regulation and cardiovascular function. Clin Sci (Lond) 2009;116:391–399 [DOI] [PubMed] [Google Scholar]
- 39.Amrani Y, Panettieri RA Jr. Modulation of calcium homeostasis as a mechanism for altering smooth muscle responsiveness in asthma. Curr Opin Allergy Clin Immunol 2002;2:39–45 [DOI] [PubMed] [Google Scholar]
- 40.Tliba O, Deshpande D, Chen H, Van Besien C, Kannan M, Panettieri RA, Jr, Amrani Y. IL-13 enhances agonist-evoked calcium signals and contractile responses in airway smooth muscle. Br J Pharmacol 2003;140:1159–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li H, He C, Feng J, Zhang Y, Tang Q, Bian Z, Bai X, Zhou H, Jiang H, Heximer SP, et al. Regulator of G protein signaling 5 protects against cardiac hypertrophy and fibrosis during biomechanical stress of pressure overload. Proc Natl Acad Sci USA 2010;107:13818–13823 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.