Abstract
We previously reported that plasminogen activator inhibitor (PAI)-1 deficiency prevents collagen deposition in the airways of ovalbumin (OVA)-challenged mice. In this study, we explored the therapeutic utility of blocking PAI-1 in preventing airway remodeling, using a specific PAI-1 inhibitor, tiplaxtinin. C57BL/6J mice were immunized with intraperitoneal injections of OVA on Days 0, 3, and 6. Starting on Day 11, mice were challenged with phosphate-buffered saline or OVA by nebulization three times per week for 4 weeks. Tiplaxtinin was mixed with chow and administered orally from 1 day before the phosphate-buffered saline or OVA challenge. Lung tissues were harvested after challenge and characterized histologically for infiltrating inflammatory cells, mucus-secreting goblet cells, and collagen deposition. Airway hyperresponsiveness was measured using whole-body plethysmography. Tiplaxtinin treatment significantly decreased levels of PAI-1 activity in bronchoalveolar lavage fluids, which indicates successful blockage of PAI-1 activity in the airways. The number of infiltrated inflammatory cells was reduced by tiplaxtinin treatment in the lungs of the OVA-challenged mice. Furthermore, oral administration of tiplaxtinin significantly attenuated the degree of goblet cell hyperplasia and collagen deposition in the airways of the OVA-challenged mice, and methacholine-induced airway hyperresponsiveness was effectively reduced by tiplaxtinin in these animals. This study supports our previous findings that PAI-1 promotes airway remodeling in a murine model of chronic asthma, and suggests that PAI-1 may be a novel target of treatment of airway remodeling in asthma.
Keywords: asthma, airway remodeling, collagen deposition, plasminogen activator inhibitor-1, tiplaxtinin
Clinical Relevance
In this study, we show that a plasminogen activator inhibitor (PAI)-1 inhibitor, tiplaxtinin, significantly reduced the bronchoalveolar lavage fluid levels of active PAI-1, the degree of inflammation, airway remodeling, and airway hyperresponsiveness in a murine model of chronic asthma. These data suggest that PAI-1 plays a key role in promoting airway remodeling in mice, and may be a potential therapeutic target for treatment of airway remodeling in asthma.
Allergic asthma is now the most common chronic disease of children, and one of the most common respiratory diseases in adults. The hallmark of asthma is chronic airway inflammation with multiple pulmonary pathologies, including airway hyperresponsiveness (AHR), eosinophilic infiltration, mucus hypersecretion, and subepithelial fibrosis (1, 2).
Plasminogen activator inhibitor (PAI)-1 is a member of the serine protease inhibitor gene family, and the major physiologic inhibitor of the serine proteases, urokinase-type plasminogen activator (uPA) and tissue-type plasminogen activator (tPA). Because uPA and tPA play important roles in damping tissue matrix deposition, high levels of PAI-1 lead to excess extracellular matrix formation (3). Under normal conditions, PAI-1 is present in plasma and tissues at low concentrations. Elevated levels of PAI-1 are often observed in a variety of pathologic conditions and clinical settings, such as infection, stroke, myocardial infarction, diabetes, obesity, sepsis, and cancers (4, 5). We previously reported that the levels of PAI-1 were elevated in the airways of a murine model of chronic asthma, and that PAI-1 deficiency was associated with reduced airway fibrosis in these mice (6). We also demonstrated that PAI-1 expression was elevated in the airways of patients with fatal asthma, and the 4G allele of PAI-1, which is associated with high plasma levels of PAI-1, was preferentially transmitted from parents with asthma to their children (7). In addition, our recent data showed that elevated levels of plasma PAI-1 were associated with a decline of lung function in subjects with asthma (8). Other studies have also shown that PAI-1 levels in induced sputum samples from subjects with asthma were increased compared with healthy control subjects (9, 10). Furthermore, intra-airway administration of small interfering RNA against PAI-1 attenuated not only AHR and airway remodeling, but also the degree of eosinophilic airway inflammation, in murine models of acute and chronic asthma (10). These studies suggest that PAI-1 may play important roles in the pathogenesis of asthma by promoting airway inflammation, remodeling, and AHR.
In this study, we treated ovalbumin (OVA)-challenged mice with tiplaxtinin, a specific PAI-1 inhibitor, to test whether inhibition of PAI-1 is able to block antigen-induced airway fibrosis and further test the role of PAI-1 in airway remodeling in asthma.
Materials and Methods
Mice and Mouse Model of Chronic Allergic Asthma
C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME) were divided into four groups; phosphate-buffered saline (PBS) only (group 1); PBS + tiplaxtinin (group 2); OVA only (group 3); and OVA + tiplaxtinin (group 4) (n = 8 mice/group). Mice were sensitized via three intraperitoneal injections (on Days 0, 3, and 6) of 50 μg/0.1 ml chicken OVA (grade V, ≥98% pure; Sigma, St. Louis, MO). After sensitization, the mice were exposed to aerosolized PBS or OVA (10 mg/15 ml OVA in PBS) for 20 min/d on 3 d/wk for 4 weeks, beginning from the 11th day of the study (11). Tiplaxtinin (generously supplied by Wyeth Research [Collegeville, PA]) was mixed with regular chow and administrated orally to groups 2 and 4 at a dose of 5 mg/day, from 1 day before challenge until Day 36. At 24 hours after the last challenge, bronchoalveolar lavage (BAL) and lung tissues were collected. The whole experimental process is summarized in Figure 1. All experimental procedures were performed according to the requirements of the Animal Care and Ethics Committee of Northwestern University.
Figure 1.
Experimental protocol for oral administration of tiplaxtinin in chronic asthma model. Tiplaxtinin was administered from Day 10 to Day 36 after the first ovalbumin (OVA) sensitization. On Day 36, bronchoalveolar lavage (BAL) was performed, airway hyperresponsiveness (AHR) was measured, and lungs were removed. Detailed procedures are described in Materials and Methods.
Bronchoalveolar Lavage Fluid
BAL fluid (BALF) was collected by cannulating the upper part of the trachea, followed by lavage with 1 ml of 1% BSA in PBS. Lavage samples from each mouse were kept on ice until they were centrifuged and stored at −80°C.
Lung Histopathology and Morphometry
Lungs were fixed in 10% paraformaldehyde and embedded in paraffin. Sections (5 μm) were stained with hematoxylin and eosin for evaluation of lung inflammation and peribroncheal eosinophil infiltration or periodic acid-Schiff (PAS) for enumeration of goblet cells (12). Goblet cell hyperplasia was assessed by determining the percentage of PAS-positive cells/bronchial basal lamina in 10 sites, as measured by ImageJ image analysis software (http://rsbweb.nih.gov/ij; National Institutes of Health, Bethesda, MD) (13). Gomori trichrome stain was used to evaluate collagen deposition. Histological assessments were made by an investigator who was blinded to the treatment conditions.
PAI-1 Activity Assay
PAI-1 activity in BALF was determined by a commercial ELISA (Molecular Innovations, Southfield, MI). The lower limit of detection for this assay was 0.02 ng/ml.
Measurement of AHR
AHR was measured using whole-body plethysmography (Buxco, Wilmington, NC), which has been recently validated for measuring lung function in murine asthma models by other investigators (13, 14). The enhanced minute pause (Penh) value was derived from bronchoconstriction-induced changes in box pressure during expiration and changes in box pressure during inspiration. Mice were exposed to nebulized PBS for 2 minutes, and then to increasing concentrations of nebulized methacholine (0–20 mg/ml), by use of an ultrasonic nebulizer. Penh values measured during this period were averaged and expressed as absolute Penh values.
Statistical Analysis
All data points represent the mean (±SEM) for groups of individual mice. Analyses were performed with GraphPad Instat software (GraphPad, San Diego, CA). A two-tailed unpaired Student's t test was used to determine statistical significance. A P value less than 0.05 was considered significant.
Results
Effect of Tiplaxtinin on Active PAI-1 Level
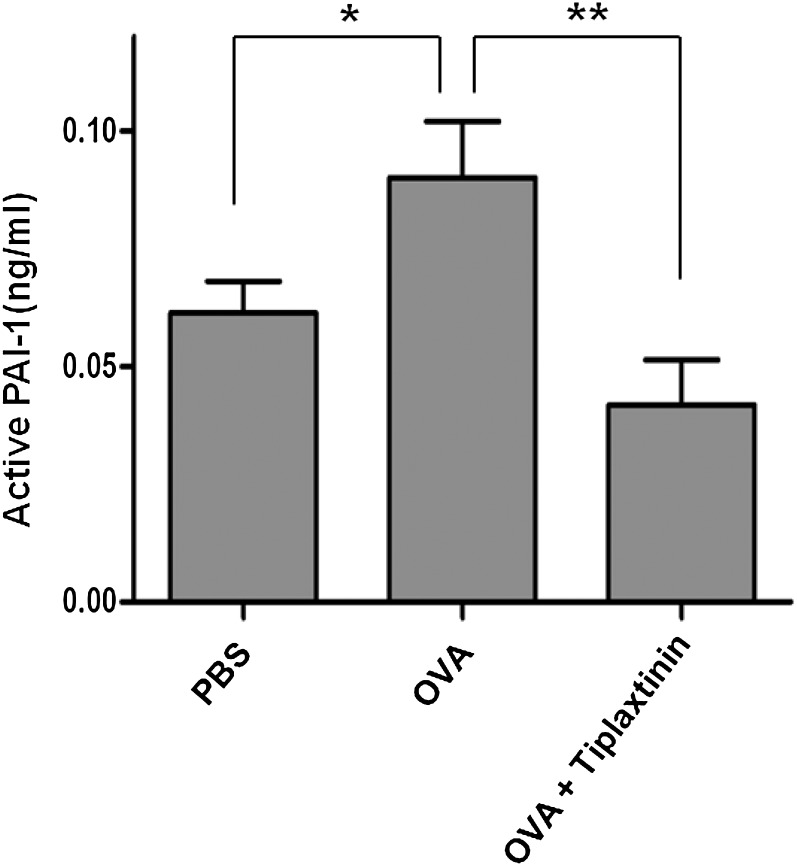
In a previous study, PAI-1 production was found to be increased in lung tissue and BALF of OVA-challenged mice, and knockout of PAI-1 protected the mice from tissue remodeling (6, 10). In parallel with a test of the ability of the inhibitor to prevent antigen-induced remodeling (see subsequent text), we examined whether orally administered tiplaxtinin would affect levels of active PAI-1 in BALF of OVA-challenged mice using ELISA. As shown in Figure 2, levels of active PAI-1 in BALFs from OVA-challenged mice were increased approximately 30% compared with those from PBS-challenged mice (0.061 ± 0.007 versus 0.091 ± 0.011 ng/ml; n = 8 mice/group; P < 0.05). Tiplaxtinin treatment in OVA-challenged mice reduced levels of active PAI-1 approximately 50% compared with untreated OVA-challenged mice (0.091 ± 0.011 versus 0.043 versus 0.011 ng/ml; n = 8 mice/group; P < 0.01).
Figure 2.
Effect of tiplaxtinin treatment on levels of active plasminogen activator inhibitor (PAI)-1 in BAL fluid (BALF) samples from phosphate-buffered saline (PBS)– or OVA-challenged mice. OVA + tiplaxtinin mice were treated with tiplaxtinin during OVA challenge and levels of active PAI-1 were assessed by ELISA. Data are shown as mean ± SEM (n = 8 mice/group). *P < 0.05 and **P < 0.01 between groups.
Effect of Tiplaxtinin on Lung Inflammation and Infiltrating Eosinophils
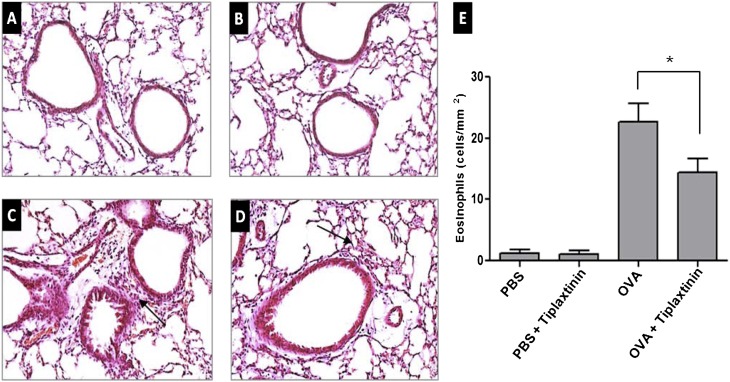
The inflammatory nature of asthma can be visualized in the OVA-challenged lung, and is characterized by eosinophilia. To determine whether tiplaxtinin reduces lung inflammation and eosinophil infiltration, we evaluated the effect of tiplaxtinin treatment in OVA-challenged mice on overall lung inflammation histologically with hematoxylin and eosin staining (Figures 3A–3D). The recruitment of inflammatory cells in OVA-challenged mice was observed in the peribronchial and perivascular areas (Figure 3C). Tiplaxtinin administration significantly reduced the inflammatory cell recruitment (Figure 3D) and the number of eosinophils (Figure 3E) in OVA-challenged mice (n = 8; P < 0.05). As shown in Figure 3E, the numbers of inflammatory cells in lung tissues of OVA-challenged mice were significantly increased compared with PBS-challenged mice (n = 8; P < 0.005). No significant effect of tiplaxtinin treatment in PBS-challenged mice was observed.
Figure 3.
Effect of tiplaxtinin treatment on histopathologic changes in the lung tissues of PBS- or OVA-challenged mice. Lung tissues were fixed with 10% paraformaldehyde, sectioned, and stained with hematoxylin and eosin (H&E) (A–D; representative figures from each group) (original magnification: 200×). Mice were nebulized with PBS (A and B) or OVA (C and D), and treated with tiplaxtinin during the nebulization (B and D). Arrows indicate infiltrated inflammation cells. To quantify peribroncheal allergic inflammation, eosinophils were counted by microscopy (100 μm × 100 μm area) (E). The data in the histogram (E) are means ± SEM (n = 8). *P < 0.05 between groups.
Effect of Tiplaxtinin on Goblet Cell Hyperplasia
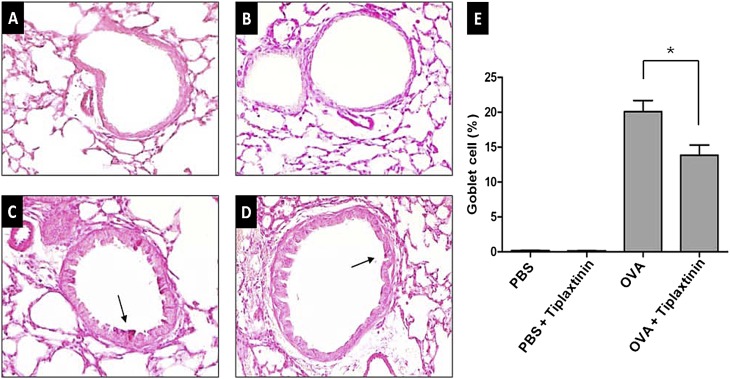
Airway tissue remodeling is thought to result from chronic repetitive injury to the airway wall caused by airway inflammation. It is characterized by increased goblet cell hyperplasia. Lung sections from mice were stained with PAS (Figures 4A–4D). The number of goblet cells, which were quantified as a percentage of PAS-positive cells per linear length of basement membrane, were compared between PBS-challenged and OVA-challenged mice. The number of goblet cells were significantly lower in OVA-challenged mice with tiplaxtinin administration compared with OVA-challenged mice without tiplaxtinin treatment (n = 8 mice/group, P < 0.005) (Figure 4E).
Figure 4.
Effect of tiplaxtinin treatment on goblet cell hyperplasia in the lung tissues of PBS- or OVA-challenged mice (A–D; representative figures from each group). Goblet cell hyperplasia was quantified in percentage of periodic acid-Schiff (PAS)–positive cells (indicated by arrows)/length of bronchial basal membrane by microscopy and Image J (E, right panel). Mice were nebulized with PBS (A and B) or OVA (C and D), and treated with tiplaxtinin during the nebulization (B and D). The data in the histogram (E) are means ± SEM (n = 8). *P < 0.005 between groups.
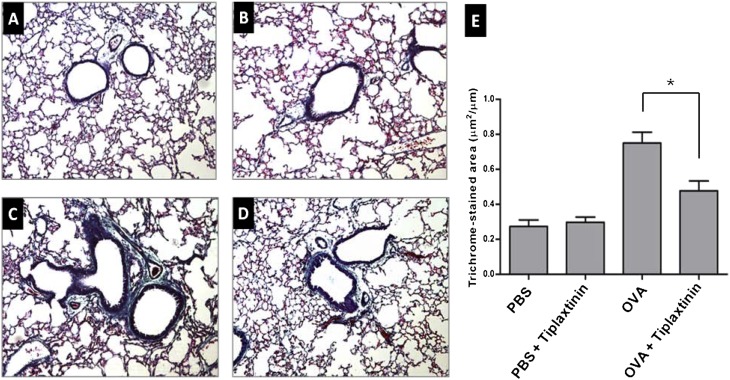
Effect of Tiplaxtinin on Lung Collagen Deposition
Increased collagen deposition is a hallmark of airway remodeling due to prolonged inflammation in chronic asthma. To evaluate the degree of collagen deposition, lung sections from mice were stained with Gomori trichrome (Figures 5A–5D). As shown in Figure 5E, the accumulation of collagen in lung tissues was markedly increased in the OVA-challenged mice compared with PBS-challenged mice (n = 8 mice/group; P < 0.005). Mice treated with tiplaxtinin during the challenge phase demonstrated remarkable inhibition of the collagen deposition compared with OVA-challenged mice without tiplaxtinin treatment (Figure 5E) (n = 8 mice/group; P < 0.05).
Figure 5.
Effect of tiplaxtinin treatment on collagen deposition. For assessment of collagen deposition, tissue sections of lungs from mice were stained with Gomori trichrome (left panels) and quantified in trichrome-stained area/length of bronchial basal membrane by microscopy and Image J (E, right panel). Mice were nebulized with PBS (A and B) or OVA (C and D), and treated with tiplaxtinin during the nebulization (B and D). The data in the histogram (E) are means ± SEM (n = 8). *P < 0.05 between groups.
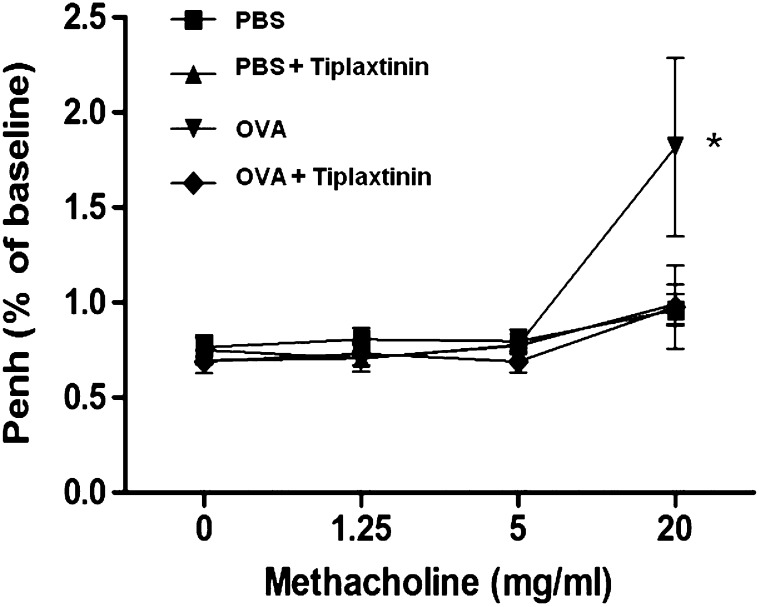
Effect of Tiplaxtinin on AHR to Methacholine
The findings presented in Figures 2–4 suggest that the PAI-1 inhibitor, tiplaxtinin, suppresses allergic inflammation and collagen deposition. Therefore, we further examined the physiologic effect of tiplaxtinin on AHR. The Penh value of OVA-challenged mice was increased, as assessed by methacholine administration (20 mg/ml) (Figure 6). However, this increased Penh response to methacholine challenge in OVA-challenged mice was effectively reduced by tiplaxtinin administration (n = 8 mice/group; P < 0.05)
Figure 6.
Effect of tiplaxtinin on AHR in PBS- or OVA-challenged mice. The AHR to methacholine was measured by use of the Buxco apparatus, and was reported as change in enhanced minute pause (Penh). Data are means ± SEM (n = 8 mice/group). *P < 0.05 versus OVA-challenged mice with tiplaxtinin treatment.
Discussion
Previously, we and others demonstrated that deletion of PAI-1 reduced collagen deposition in the airways of a murine model of chronic asthma (6, 10). In the present study, we confirmed that PAI-1 plays an important role in airway remodeling in a murine model of chronic asthma using a specific PAI-1 inhibitor. Moreover, this study suggests that PAI-1 could be a potential therapeutic target in a strategy to diminish the airway remodeling in asthma.
Tiplaxtinin, the most studied small molecule PAI-1 inhibitor, is an indole oxoacetic acid. The therapeutic potential of tiplaxtinin has been reported in obesity, diabetes, and thrombosis (15–18). Tiplaxtinin decreased the expression of peroxisome proliferator–activated receptor-γ and leptin in cultured adipocytes (15). Acute oral administration of tiplaxtinin decreased thrombosis in a rat carotid artery model (17) and it effectively protected against l-NAME–induced thrombosis (18). However, the antiasthmatic effects of tiplaxtinin have not yet been investigated. In the present study, we report that tiplaxtinin was capable of inhibiting airway PAI-1 activity, lung inflammation, goblet cell hyperplasia, collagen deposition, and AHR in an OVA-challenged murine model of chronic asthma. Although the inhibitory effect of tiplaxtinin against all of these parameters was partial, PAI-1 activity in vivo was completely shut off by tiplaxtinin, such that levels of active PAI-1 in OVA-challenged mice with tiplaxtinin treatment were lower than those in PBS-challenged mice. This suggests that PAI-1 might not be the only contributor to airway inflammation or remodeling.
Our study shows that PAI-1 inhibition by tiplaxtinin decreased the degree of eosinophilic airway inflammation and AHR in an OVA-challenged model of chronic asthma. Miyamoto and colleagues (10) reported that intra-airway administration of small interfering RNA against PAI-1 reduced eosinophilic airway inflammation and AHR in a murine model of acute asthma. They suggest that elevation of hepatocyte growth factor by PAI-1 inhibition may be a mechanism of attenuation in airway inflammation and AHR in their model of acute asthma. Sejima and colleagues (19) also showed that deletion of PAI-1 shifted the immune response from a T helper (Th) 2 to a Th1 type in an OVA-challenged nasal allergy model. Based on these studies, we speculate that inhibition of PAI-1 by tiplaxtinin may reduce eosinophilic airway inflammation via attenuation of Th2 immune response or increased hepatocyte growth factor production. Together with decreased matrix deposition, reduction of airway inflammation by tiplaxtinin may contribute to the attenuation of AHR in our study. Interestingly, although tiplaxtinin modestly inhibited leukocyte recruitment and matrix deposition, it completely inhibited the increase in airway reactivity, indicating a nonlinear relationship between these parameters. This result also suggests that PAI-1 may have some previously unsuspected influence on the function of airway smooth muscle or nerves.
Goblet cell hyperplasia and collagen deposition, structural changes in the airway wall, are now thought to be key components in the pathophysiology of asthma (6, 20). Airway remodeling is characterized by thickening of the lamina reticularis, with deposition of collagen and other extracellular matrix proteins leading to subepithelial fibrosis, and increased airway goblet cells causing mucus hypersecretion. Our results show that tiplaxtinin significantly decreased OVA-induced collagen deposition and hyperplasia of goblet cells in lung tissues of treated mice, and these data are consistent with our previous data on OVA treatment of PAI-1–deficient mice (6).
The current understanding of airway fibrosis is that chronic inflammation injures the airways and generates fibrotic changes, perhaps during an excessive repair response. Current asthma therapies (e.g., corticosteroids) are successful in treating allergic inflammation. However, there is a lack of supporting evidence of reversal of airway fibrosis with optimal control of inflammation by corticosteroids (21, 22). Moreover, despite widespread use of anti-inflammatory treatments, recalcitrant asthma with airway remodeling is a major, ongoing challenge in asthma management. This suggests that there are noninflammatory mechanisms that possibly coexist with inflammatory processes in generating airway fibrosis, and may be important therapeutic targets in patients with recalcitrant asthma.
In this study, we show that a PAI-1 inhibitor, tiplaxtinin, significantly reduced the BALF levels of active PAI-1, the degree of inflammation, airway remodeling, and AHR in a murine model of chronic asthma. These data suggest that PAI-1 plays a key role in promoting airway remodeling in mice, and may be a potential therapeutic target for treatment of airway remodeling in asthma.
Supplementary Material
Acknowledgments
The authors thank Dr. David L. Crandal for kindly providing the plasminogen activator inhibitor-1 inhibitor, tiplaxtinin.
Footnotes
This work was supported by the Bazley Trust Fund, American Heart Association grant AHA 11SDG7590063, and National Heart, Lung, and Blood Institute/National Institutes of Health/Department of Health and Human Services grant R01 HL051387.
Originally Published in Press as DOI: 10.1165/rcmb.2011-0369OC on February 9, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Orihara K, Dil N, Anaparti V, Moqbel R. What's new in asthma pathophysiology and immunopathology? Expert Rev Respir Med 2010;4:605–629 [DOI] [PubMed] [Google Scholar]
- 2.Shum BO, Rolph MS, Sewell WA. Mechanisms in allergic airway inflammation—lessons from studies in the mouse. Expert Rev Mol Med 2008;27:10–15 [DOI] [PubMed] [Google Scholar]
- 3.Beier JI, Kaiser JP, Guo L, Martínez-Maldonado M. Plasminogen activator inhibitor-1 deficient mice are protected from angiotensin II–induced fibrosis. Arch Biochem Biophys 2011;510:19–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown NJ. Therapeutic potential of plasminogen activator inhibitor-1 inhibitors. Ther Adv Cardiovasc Dis 2010;4:315–324 [DOI] [PubMed] [Google Scholar]
- 5.Mengele K, Napieralski R, Magdolen V, Reuning U, Gkazepis A, Sweep F, Brünner N, Foekens J, Harbeck N, Schmitt M. Characteristics of the level-of-evidence-1 disease forecast cancer biomarkers uPA and its inhibitor PAI-1. Expert Rev Mol Diagn 2010;10:947–962 [DOI] [PubMed] [Google Scholar]
- 6.Oh CK, Ariue B, Alban RF, Shaw B, Cho SH. PAI-1 promotes extracellular matrix deposition in the airways of a murine asthma model. Biochem Biophys Res Commun 2002;294:1150–1160 [DOI] [PubMed] [Google Scholar]
- 7.Cho SH, Hall IP, Wheatley A, Dewar J, Abraha D, Del MJ, Lee H, Oh CK. Possible role of the 4G/5G polymorphism of the plasminogen activator inhibitor 1 gene in the development of asthma. J Allergy Clin Immunol 2001;108:212–214 [DOI] [PubMed] [Google Scholar]
- 8.Cho SH, Kang J, Lyttle C, Harris K, Daley B, Grammer L, Avila P, Kumar R, Schleimer R. Association of elevated plasminogen activator inhibitor 1 levels with diminished lung function in patients with asthma. Ann Allergy Asthma Immunol 2011;106:371–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xio W, Hsu YP, Ishizaka A, Kirikae T, Moss RB. Sputum cathelicidin, urokinase plasminogen activation system components, and cytokines discriminate cystic fibrosis, COPD, and asthma inflammation. Chest 2005;128:2316–2326 [DOI] [PubMed] [Google Scholar]
- 10.Miyamoto S, Hattori N, Senoo T, Onari Y, Lwamoto H, Kanehara M, Ishikawa N, Fusitaka K, Haruta Y, Murai H, et al. Intra-airway administration of small interfering RNA targeting plasminogen activator inhibitor-1 attenuates allergic asthma in mice. Am J Physiol Lung Cell Mol Physiol 2011;301:L908–L916 [DOI] [PubMed] [Google Scholar]
- 11.Olmez D, Babayigit A, Uzuner N, Erbil G, Karaman O, Yilmaz O, Cetin EO, Ozogul C. Efficacy of sulphasalazine on lung histopathology in a murine model of chronic asthma. Exp Lung Res 2008;34:501–511 [DOI] [PubMed] [Google Scholar]
- 12.Munroe ME, Businga TR, Kline JN, Bishop GA. Anti-inflammatory effects of the neurotransmitter agonist Honokiol in a mouse model of allergic asthma. J Immunol 2010;185:5586–5597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lundequist A, Nallamshetty SN, Xing W, Feng C, Laidlaw TM, Uematsu S, Akira S, Boyce JA. Prostaglandin E(2) exerts homeostatic regulation of pulmonary vascular remodeling in allergic airway inflammation. J Immunol 2010;184:433–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu SY, Wang WZ, Yen CL, Tsai MY, Yang PW, Wang JY, Ho CY, Shieh CC. Leukocyte nicotinamide adenine dinucleotide phosphate–reduced oxidase is required for isocyanate-induced lung inflammation. J Allergy Clin Immunol 2011;127:1014–1023 [DOI] [PubMed] [Google Scholar]
- 15.Crandall DL, Quinet EM, El AS, Hreha AL, Leik CE, Savio DA, Vague IJ, Alessi MC. Modulation of adipose tissue development by pharmacological inhibition of PAI-1. Arterioscler Thromb Vasc Biol 2006;26:2209–2215 [DOI] [PubMed] [Google Scholar]
- 16.Lijnen HR, Alessi MC, Van Hoef B, Collen D, Juhan-Vague I. On the role of plasminogen activator inhibitor-1 in adipose tissue development and insulin resistance in mice. J Thromb Haemost 2005;6:1174–1179 [DOI] [PubMed] [Google Scholar]
- 17.Hennan JK, Morgan GA, Swillo RE, Antrilli TM, Mugford C, Vlasuk GP. Effect of tiplaxtinin (PAI-039), an orally bioavailable PAI-1 antagonist, in a rat model of thrombosis. J Thromb Haemost 2008;6:1558–1564 [DOI] [PubMed] [Google Scholar]
- 18.Smith LH, Dixon JD, Stringham JR, Eren M, Elokdah H, Crandall DL, Washington K, Vaughan DE. Pivotal role of PAI-1 in a murine model of hepatic vein thrombosis. Blood 2006;107:132–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sejima T, Madoiwa S, Mimuro J, Sugo T, Okada K, Ueshima S, Matsuo O, Ishida T, Ichimura K, Sakata Y. Protection of plasminogen activator inhibitor-1–deficient mice from nasal allergy. J Immunol 2005;174:8135–8143 [DOI] [PubMed] [Google Scholar]
- 20.Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma: from bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med 2000;161:1720–1745 [DOI] [PubMed] [Google Scholar]
- 21.Adcock IM, Ford PA, Bhavsar P, Ahmad T, Chung KF. Steroid resistance in asthma: mechanisms and treatment options. Curr Allergy Asthma Rep 2008;8:171–178 [DOI] [PubMed] [Google Scholar]
- 22.Laitinen LA, Laitinen A, Haahtela T. A comparative study of the effects of an inhaled corticosteroid, budesonide, and a beta 2-agonist, terbutaline, on airway inflammation in newly diagnosed asthma: a randomized, double-blind, parallel-group controlled trial. J Allergy Clin Immunol 1992;90:32–42 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.