Abstract
Recent data link vitamin A and its retinoid metabolites to the regulation of adipogenesis, insulin sensitivity, and glucose homeostasis. Retinoid metabolism is tightly controlled by an enzymatic network in which retinaldehyde dehydrogenases (Aldh1–3) are the rate-limiting enzymes that convert retinaldehyde to retinoic acid. Aldh1a1-deficient mice are protected from diet-induced obesity and hence diabetes. Here we investigated whether Aldh1a1 and the retinoid axis regulate hepatic glucose and lipid metabolism independent of adiposity. The impact of Aldh1a1 and the retinoid pathway on glucose homeostasis and lipid metabolism was analyzed in hepatocytes in vitro and in chow-fed, weight-matched Aldh1a1-deficient vs. wild-type (WT) mice in vivo. Aldh1a1-deficient mice displayed significantly decreased fasting glucose concentrations compared with WT controls as a result of attenuated hepatic glucose production. Expression of key gluconeogenic enzymes as well as the activity of Forkhead box O1 was decreased in Aldh1a1-deficient vs. WT livers. In vitro, retinoid or cAMP agonist stimulation markedly induced gluconeogenesis in WT but not Aldh1a1-deficient primary hepatocytes. Aldh1a1 deficiency increased AMP-activated protein kinase α activity, decreased expression of lipogenic targets of AMP-activated protein kinase α and significantly attenuated hepatic triacylglycerol synthesis. In metabolic cage studies, lean Aldh1a1-deficient mice manifested enhanced oxygen consumption and reduced respiratory quotient vs. WT controls, consistent with increased expression of fatty acid oxidation markers in skeletal muscle. Taken together, this work establishes a role for retinoid metabolism in glucose homeostasis in vivo and for Aldh1a1 as a novel determinant of gluconeogenesis and lipid metabolism independent of adiposity.
Retinoids are vitamin A metabolites with diverse, essential biological functions, including cell cycle progression, differentiation, embryogenesis, reproduction, vision, and immunity (1–3). Retinoids are also implicated in the pathogenesis of cancer, obesity, diabetes, and cardiovascular disease (4, 5). Retinoids exert these actions largely by activating the retinoic acid receptor (RAR) and the retinoid X receptor (RXR), nuclear hormone receptors that regulate gene expression (2, 6). RXR can homodimerize or heterodimerize with RAR. RXR is also an obligatory heterodimeric partner for other nuclear receptors, including the peroxisome proliferator-activated receptors (PPAR) important for glucose homeostasis, fatty acid oxidation, and adipogenesis (1, 2).
All-trans retinoic acid (ATRA) is a bona fide in vivo ligand for RAR whereas 9-cis-retinoic acid (9-cis-RA) activates RXR and RAR in vitro and was recently reported to function in vivo in the pancreas (7–9). The biological impact of these retinoids establishes the importance of understanding their generation. Dietary vitamin A, or retinol, is first oxidized to retinaldehyde (Rald) by a family of alcohol dehydrogenases and retinol dehydrogenases. Subsequently, retinaldehyde dehydrogenases (Aldhs) irreversibly oxidize Rald to RA, the rate-limiting step of RA formation (2, 6, 10). Recent work establishes that specific components of the vitamin A pathway, including retinoids, retinoid-binding proteins, and the distinct enzymes involved in retinoid metabolism have unique cellular effects (2, 6, 11). In development, retinoids are classic diffusible morphogens, controlling temporal and spatial patterns as a function of Aldh expression (3). In adult metabolism, previous studies documented that selective RXR agonists (rexinoids) decrease hyperglycemia and hyperinsulinemia (12, 13), whereas pharmacological RXR inhibition reportedly ameliorates type 2 diabetes in mice (14). However, endogenous pathways of retinoid metabolism regulating glucose homeostasis, including hepatic gluconeogenesis, remain poorly understood.
Recently, we reported that Rald itself and the enzymes involved in its conversion to RA were present and differentially regulated in rodent fat. Moreover, deficiency of the Aldh1a1 isoform protected mice from diet-induced obesity and diabetes (15). However, given the markedly lower body weight in high-fat-fed Aldh1a1-deficient vs. wild-type (WT) mice, it remained unclear whether Aldh1a1 directly regulated glucose independent of body weight.
We demonstrate here that lean, chow-fed Aldh1a1-deficient mice have lower fasting glucose levels than weight-matched WT mice. In investigating mechanisms underlying this effect, we found that Aldh1a1 deficiency modulates expression of the key gluconeogenic mediators glucose 6 phosphatase (G6Pase) and phosphoenolpyruvate carboxykinase (PEPCK) as evident in hepatocyte models in vitro and hepatic gluconeogenesis in vivo. These Aldh1a1 effects appear to result from decreased RA levels and altered RAR/RXR activation. Decreased fasting glucose levels and hepatic gluconeogenesis in Aldh1a1 deficiency were coupled to increased fatty acid oxidation and lower plasma triacylglycerol concentrations. These findings provide evidence for retinoid metabolism as a determinant of hepatic gluconeogenesis in vivo and identify Aldh1a1 as the specific Aldh isoform responsible for coordinating glucose homeostasis and fatty acid oxidation independent of body mass and adiposity.
Materials and Methods
Animals
C57BL/6J WT mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Aldh1a1-deficient (Aldh1a1−/−) mice were provided by G. Duester and backcrossed to C57Bl/6J background for more than 20 generations. Mice were kept on a standard chow diet with a vitamin A content of 15I U/g, had free access to food and water except as indicated, and were handled according to Harvard Medical School Institutional Animal Care and Use Committee guidelines.
Reagents
All media were obtained from Invitrogen (Carlsbad, CA); collagen type I, d-glucose, bovine insulin, forskolin, all-trans retinaldehyde, and all-trans RA were obtained from Sigma (St. Louis, MO). HX531 was kindly provided by Dr. Hiroyuki Kagechika (University of Tokyo, Tokyo, Japan).
Nuclear magnetic resonance and tissue mass
Body fat, lean mass, and free fluid were measured (under 2 min) using a nuclear magnetic resonance analyzer (Minispec LF90II; Bruker Optics, Billerica, MA). Conscious mice were placed individually into the measuring tube with a minimal restraint device. Inguinal white adipose tissue (SWAT), gonadal white adipose tissue (GWAT), and liver were dissected and immediately weighed.
Glucose, insulin, and pyruvate tolerance tests
Glucose and insulin tolerance tests were performed on mice fasted for 12 h and 6 h, respectively. Mice were injected ip either with d-glucose (Sigma, 1 g/kg body weight) or recombinant human regular insulin (1 U/kg body weight; Novolin R; Novo Nordisk, Bagsværd, Denmark), and blood glucose concentrations were measured periodically. For pyruvate tolerance test mice, were starved for 24 h and then injected ip with pyruvate (Invitrogen) at 2 g/kg body weight, and blood glucose concentrations were repeatedly measured up to 125 min after injection.
Hyperinsulinemic-euglycemic clamp studies
Experiments were performed in conscious, unrestrained mice fitted with intravenous catheters as previously described (16, 17). Briefly, after a 5-h fast, mice underwent a 120-min tracer equilibration phase (t = −120 to 0 min) followed by a 120-min experimental period (t = 0 to 120 min). At t = −120, a bolus infusion of [3-3H] glucose (2 μCi) was given, followed by a 0.05 μCi/min infusion for 2 h. At t = −10 min, basal serum measurements of insulin, glucose, and glucose turnover were taken. The insulin clamp started at t = 0 with a prime-continuous infusion (16 mU/kg bolus, followed by 2.5 mU/kg/min) of human insulin (Novo Nordisk). The [3-3H]glucose infusion was increased to 0.075 μCi/min for the remainder of the experiment to minimize changes in specific activity. Euglycemia (120∼130 mg/dl) was maintained during the clamp by measuring blood glucose every 10 min starting at t = 0 and infusing 50% glucose at variable rates. Glucose-specific activity was serially determined (t = 80, 90, 100, and 120 min) during steady state of glucose infusion. Blood insulin concentrations were determined at t = −10 and 120 min.
Tissue-specific glucose uptake.
To estimate insulin-stimulated glucose uptake in individual tissues, a bolus injection of [1-14C]-2-deoxyglucose ([14C]2DG; 10 μCi, PerkinElmer, Waltham, MA) was given at t = 120 min during steady-state conditions. [14C]2DG is a nonmetabolizable glucose analog transported into cells where it is phosphorylated to [14C]2DG-6-phosphate ([14C]2DGP) and subsequently trapped. Plasma [14C]2DG radioactivity was measured at 2, 5, 10, 15, and 25 min after the injection. At the end of the experiment, GWAT, SWAT, interscapular brown adipose tissue (BAT), and musculus (M) gastrocnemius were collected and immediately frozen in liquid nitrogen for subsequent tissue [14C]2DGP radioactivity assessment.
Sample analysis.
Blood glucose was measured via Accu-Check glucometer (Roche, Manheim, Germany). Plasma insulin was measured using rat/mouse insulin ELISA kits (Linco Research, Inc., St. Charles, MO). For [3-3H]glucose and [1-14C]2DG radioactivity measurements, plasma samples were deproteinized and counted by liquid scintillation (LS6500 Multi-purpose Scintillation Counter, Beckman Coulter, Brea, CA) on a dual-channel 3H/14C separation program. For [14C]2DGP measurement, tissues were homogenized in 0.5% perchloric acid, and the supernatants were neutralized with potassium hydroxide before total tissue counts were measured.
Indirect calorimetry
Indirect calorimetric measurements were performed in WT and Aldh1a1−/− mice (12–14 wk old) as described elsewhere (18, 19). Briefly, oxygen consumption (VO2) and carbon dioxide production (VCO2) were measured using the Comprehensive Laboratory Animal Monitoring System (Columbus Instruments, Columbus, OH), an integrated open-circuit calorimeter. Mice were individually housed in sealed chambers with free access to food and water. Animals were acclimatized for 48 h before experiments began. Analyses were performed for 48–72 h at ambient temperature (23 C) and 12-h dark-light cycles. VO2 and VCO2 were sampled sequentially. Respiratory quotient (RQ) was calculated as VCO2/VO2. The Percent Relative Cumulative Frequency was used for VO2 and RQ analysis (19).
Lipoprotein profiling
Plasma from 6-h fasted mice was used for lipoproteins analysis by HPLC using molecular sieve columns (Skylight Biotech, Akita, Japan).
Triacylglycerol production assay
After a 4-h fast, mice were injected iv with the lipase inhibitor tyloxapol (Triton WR1339; 500 mg/kg body weight). Blood was sampled before and periodically after injection. Triacylglycerol were measured enzymatically (L-Type TG Kit, Wako Chemicals USA, Richmond, VA).
Primary hepatocyte cultures
Mouse hepatocytes were isolated using modification of the two-step hepatic portal vein perfusion method (20). Briefly, after anesthetization the portal vein and inferior vena cava were exposed. A 24-gauge catheter was inserted into the portal vein, and the liver was perfused with liver perfusion medium (2 ml/min) followed by collagenase-dispase perfusion (7 ml/min) via peristaltic pump. The liver was then aseptically removed and hepatocytes released and filtered (70-μm nylon cell strainer). Cells were washed and 90–95% cell viability was assessed using trypan blue staining. Hepatocytes were plated in Williams' E growth medium (Invitrogen) on collagen-coated culture dishes. After recovery (24 h), cells were stimulated in serum-free DMEM as indicated.
Reverse transcription and gene expression
Total RNA was extracted (Rneasy; Qiagen, Hilden, Germany), DNase treated (Qiagen), and RNA (2 μg) reverse transcribed. Gene expression, normalized to 36B4 and glyceraldehyde-3-phosphate dehydrogenase, was analyzed by quantitative real-time RT-PCR in 96-well plates using a MyiQ cycler (Bio-Rad Laboratories, Hercules, CA). Primer sequences are available upon request.
Immunoblotting
Homogenized liver tissue or primary mouse hepatocytes were lysed in radioimmune precipitation assay buffer (Boston Bioproducts, Boston, MA). Standard Western blot techniques were performed using specific antibodies against phospho-Forkhead box O1 (Foxo1), total Foxo1, phospho-AMPKα (Cell Signaling Technology, Danvers, MA), PEPCK, G6Pase, and glyceraldehyde-3-phosphate dehydrogenase (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Proteins were detected by chemiluminescence (GE HealthCare Amersham, Amersham, UK).
Statistics
All data are given as means ± sem. Genotypes were compared by unpaired two-tailed Student's t test. A P value of 0.05 or less was considered statistically significant.
Results
Lean Aldh1a1-deficient mice manifest decreased fasting glucose levels
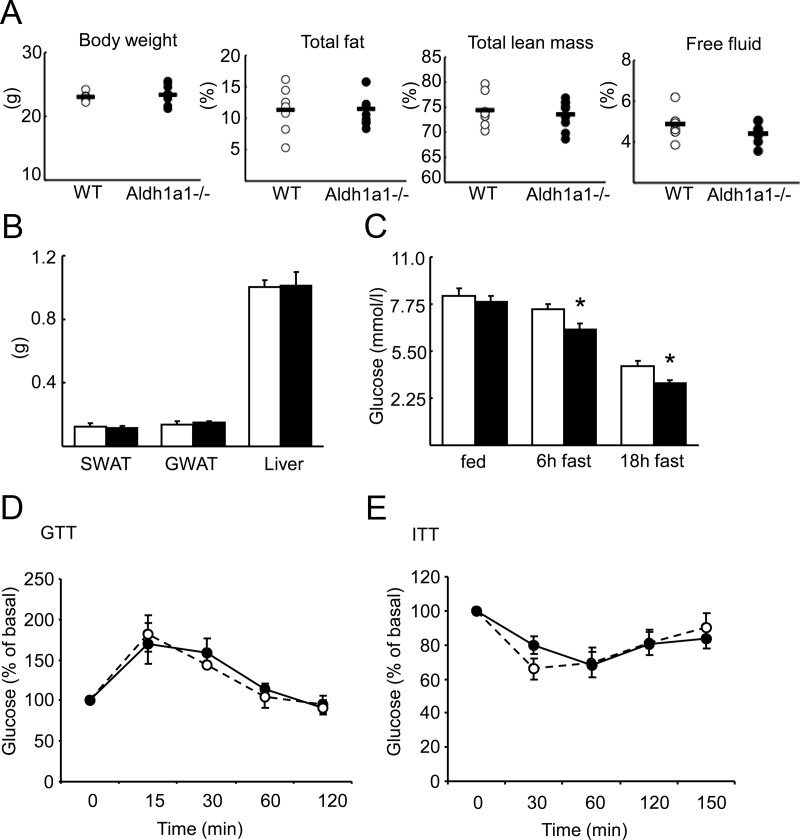
To test the role of Aldh1a1 in glucose homeostasis in the absence of weight or body fat differences, WT and Aldh1a1−/− female mice were fed a standard chow diet for 12–14 wk before body composition analysis. Both genotypes demonstrated similar body weight, fat mass, lean mass, and free fluid content, SWAT, and GWAT as well as liver mass (Fig. 1, A and B). Although random fed blood glucose levels were similar between groups, Aldh1a1−/− mice manifested significantly lower glucose concentrations after 6 and 18 h of fasting compared with WT mice (Fig. 1C). In contrast, neither plasma insulin concentrations nor glucose or insulin tolerance differed between Aldh1a1−/− vs. WT mice (Supplemental Fig. 1A and Fig. 1, D and E, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org).
Fig. 1.
Aldh1a1 deficiency decreases fasting glucose. A, Twelve to 14-wk-old weight matched WT (open circle) and Aldh1a1−/− mice (solid circle) on a normal chow diet were analyzed for body composition using nuclear magnetic resonance spectroscopy. B, SWAT, GWAT, and liver weights were measured immediately after dissection (WT, white bars; Aldh1a1−/−, black bars) C, Blood glucose concentrations were determined in WT and Aldh1a1−/− mice at random fed state and after 6 h and 18 h fasting. D and E, Glucose (GTT) and insulin tolerance tests (ITT) were performed in WT (dashed line) and Aldh1a1−/− mice (solid line). Percent of basal glucose during GTT and ITT is given (n = 8/group; *, P < 0.05).
Aldh1a1 deficiency inhibits hepatic glucose production
To further characterize this change in fasting glucose levels, we performed pyruvate tolerance test (PTT) and hyperinsulinemic-euglycemic clamp studies in chow-fed WT and Aldh1a1−/− mice. During a PTT, pyruvate is used as a substrate for glucose production. Whereas plasma glucose rose significantly in WT animals after ip pyruvate injection as expected, in Aldh1a1−/− mice, significantly less pyruvate was converted into glucose (Fig. 2A), indicative of reduced hepatic gluconeogenesis. Hyperinsulinemic-euglycemic clamp studies revealed similar glucose infusion rate (Fig. 2B) and insulin concentrations (Fig. 2C) in both genotypes, establishing that Aldh1a1 does not alter insulin sensitivity. However, hepatic glucose production at baseline and under clamp conditions was markedly reduced in Aldh1a1−/− vs. WT mice (Fig. 2D), consistent with the prior PTT results. Insulin did not further suppress glucose production (Fig. 2E). Compared with WT mice, Aldh1a1 deficiency decreased glucose turnover and clearance, as well as glycolysis, but did not alter glycogen synthesis (Fig. 2F). To evaluate whether factors other than decreased hepatic glucose production contributed to reduced fasting glucose levels in Aldh1a1−/− mice, we measured insulin-stimulated glucose uptake in GWAT, SWAT, BAT, and M. gastrocnemius. No difference between genotypes was detected in these tissues (Fig. 2G). Taken together, these data strongly implicate Aldh1a1 in the regulation of hepatic gluconeogenesis and fasting glucose response.
Fig. 2.
Aldh1a1−/− mice display reduced hepatic glucose production. A, Pyruvate tolerance test was performed in WT (dashed line) and Aldh1a1−/− mice (solid line). Blood glucose concentrations after ip pyruvate injection and area under the curve are given. B–G, Hyperinsulinemic-euglycemic clamp studies were performed in WT (white bars) and Aldh1a1−/− mice (black bars). Glucose infusion rate (B), plasma insulin concentrations (C), and hepatic glucose production (D) were measured at baseline and during clamp conditions. Insulin-mediated suppression of hepatic glucose production was assessed (E), and whole-body glucose turnover, glycolysis, glucose clearance, and glycogen synthesis (F) were determined under basal and clamp conditions. G, Tissue-specific glucose uptake was analyzed by enrichment of phosphorylated [14C]2DG in GWAT, SWAT, M. gastrocnemius, and BAT (n = 8/group; *, P < 0.05).
Expression of specific gluconeogenic mediators is altered in Aldh1a1 deficiency
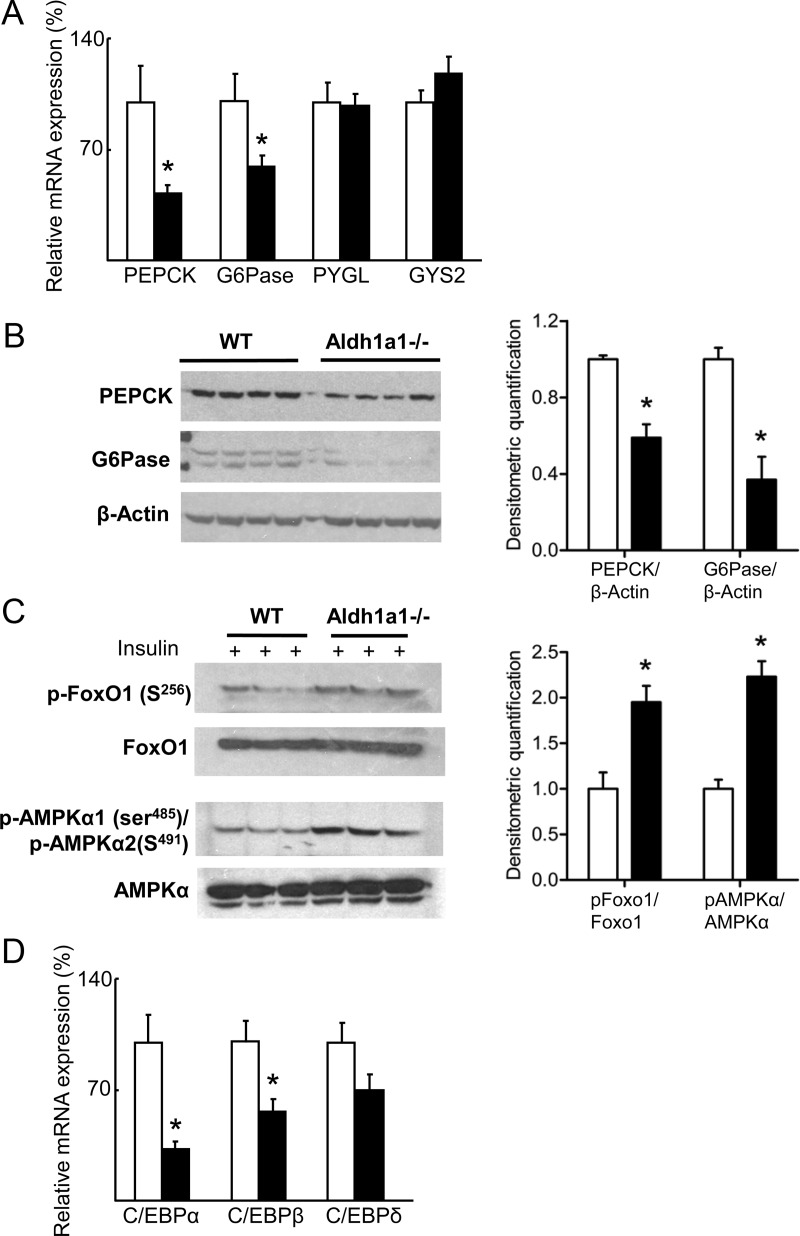
Given decreased hepatic gluconeogenesis as a function of Aldh1a1 deficiency, we next analyzed expression of the key hepatic gluconeogenic enzymes G6Pase and PEPCK. Both G6Pase and PEPCK were markedly decreased in Aldh1a1−/− vs. WT livers (Fig. 3, A and B). In contrast, glycogen phosphorylase and glycogen synthase mRNA expression were unchanged between genotypes (Fig. 3A). Forkhead box O1 (FOXO1) and AMP-activated protein kinase α (AMPKα) are known regulators of hepatic glucose production. FOXO1 induces G6Pase and PEPCK expression but is inactivated by phosphorylation (21). In contrast, serine phosphorylation activates AMPKα, which then inhibits glucose production and lipogenesis while increasing fatty acid oxidation (22). Phosphorylation of both FOXO1 and AMPKα was increased in livers of Aldh1a1−/− mice after ip insulin injection (Fig. 3C). Activated AMPK suppresses expression of CCAAT enhancer-binding protein (C/EBP), which binds to and activates the PEPCK promoter. Hepatic expression of C/EBPα and β was significantly repressed in Aldh1a1−/− mice whereas C/EBPδ was decreased but did not reach statistical significance (Fig. 3D). Thus, in the genetic absence of Aldh1a1, coordinated regulation of Foxo1 and AMPKα action decreases G6Pase and PEPCK expression.
Fig. 3.
Aldh1a1 deficiency inhibits gluconeogenic pathways. A, Hepatic gene expression of PEPCK, G6Pase, glycogen phosphorylase (PYGL), and glycogen synthase 2 (GYS2) was analyzed in WT (white bars) and Aldh1a1−/− mice (black bars). B, Western blot analysis of hepatic PEPCK and G6Pase expression. C, WT and Aldh1a1−/− mice were injected ip with insulin at 5 U/kg body weight. Subsequently, p-Foxo1 (serine 256), total Foxo1, p-AMPKα1 (serine 485)/p-AMPKα2 (serine 491), and total AMPKα were evaluated by immunoblot in liver lysates. D, Hepatic gene expression analysis of C/EBPα, β and δ (n = 6/group; *, P < 0.05).
Retinoids promote hepatic gluconeogenesis by inducing PEPCK and G6Pase expression in an Aldh1a1-dependent manner
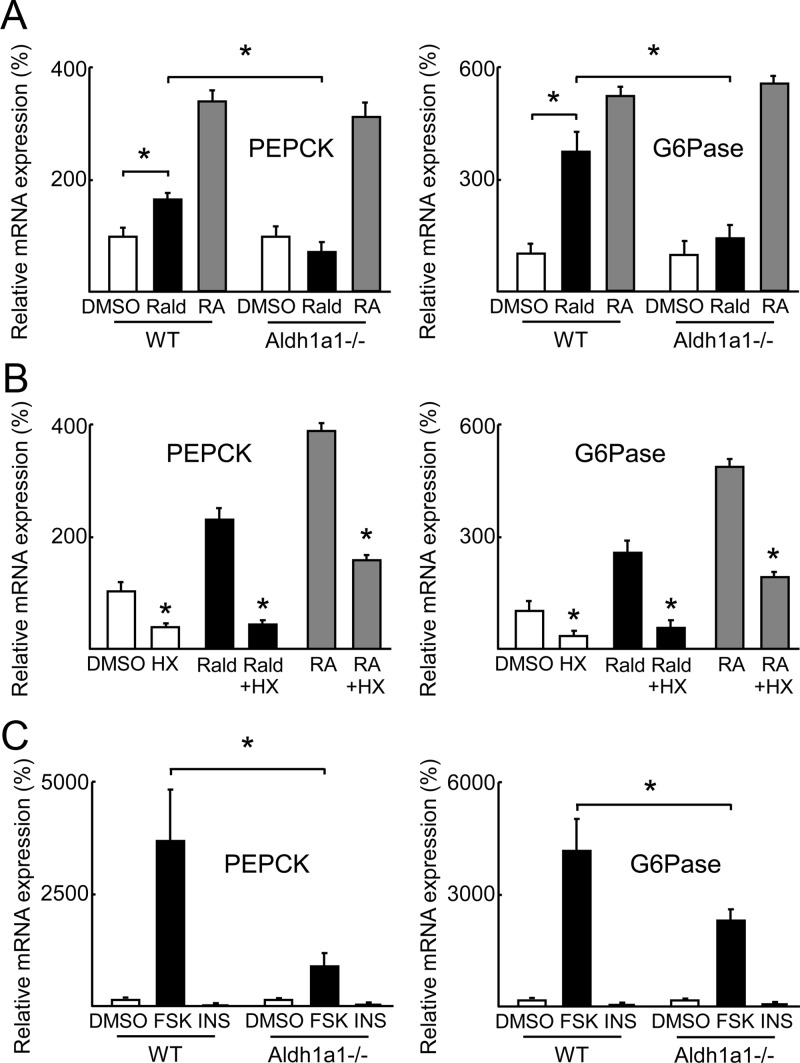
Prior work has established that Aldh1a1-deficient mice have increased Rald and decreased all-trans-RA (ATRA) levels (23). To study directly retinoid transcriptional effects relevant for gluconeogenesis, primary hepatocytes isolated from Aldh1a1−/− and WT mice were stimulated with Rald and ATRA (1 μm, 24 h) before PEPCK and G6Pase mRNA expression was analyzed. Both Rald and ATRA significantly induced PEPCK and G6Pase mRNA expression in WT hepatocytes (Fig. 4A). In contrast, in Aldh1a1−/− hepatocytes Rald failed to induce PEPCK and G6Pase expression whereas ATRA stimulation still increased expression of these two gluconeogenic enzymes (Fig. 4A). Given the role of Aldh1a1 in converting Rald to RA and subsequent retinoid receptor activation, we next investigated retinoid receptor-dependent induction of PEPCK and G6Pase expression. We stimulated primary WT hepatocytes with Rald or ATRA in the absence or presence of the established RXR antagonist HX531 (24). Both Rald and ATRA induced PEPCK and G6Pase expression in primary WT hepatocytes as before, but this effect was significantly blunted in the presence of HX531 (Fig. 4B). Consistent with these findings, expression of the RA-metabolizing enzyme cytochrome P450 26a1 (Cyp26a1) was 10-fold less in livers from Aldh1a1−/− vs. WT mice (Supplemental Fig. 1B). Because expression of Cyp26a1 is known to be strongly induced by RA (25), this finding supports decreased RA levels in Aldh1a1-deficient livers.
Fig. 4.
Retinoids and their regulation by Aldh1a1 determine hepatic gluconeogenesis. Primary hepatocytes were isolated from 12- to 14-wk-old WT and Aldh1a1−/− mice. A and B, Cells were stimulated with vehicle [dimethylsulfoxide (DMSO)], indicated retinoids (1 μm), and/or RXR antagonist HX531 (1 μ m) for 24 h before measuring PEPCK and G6Pase mRNA expression. C, WT primary hepatocytes were stimulated with forskolin (FSK, 1 nm), insulin (INS, 30 nm), or vehicle (DMSO) for 4 h before PEPCK and G6Pase mRNA expression. Data represent four independent experiments. *, P < 0.05.
Given these changes in gluconeogenic enzyme expression, we next considered effects of known fasting-induced proximal pro-gluconeogenic signals. Fasting increases cAMP release in response to β-adrenergic signaling and glucagon secretion (26, 27). As such, we investigated cAMP-induced gluconeogenesis in primary Aldh1a1−/− vs. WT hepatocytes using the synthetic cAMP agonist forskolin (27). PEPCK and G6Pase mRNA were induced markedly in WT hepatocytes after forskolin treatment, but significantly less so in Aldh1a1-deficient hepatocytes (Fig. 4C). These data identify Aldh1a1 as a key determinant of hepatic gluconeogenesis through retinoid receptor-mediated effects on PEPCK and G6Pase expression.
Aldh1a1 deficiency results in repressed triacylglycerol synthesis
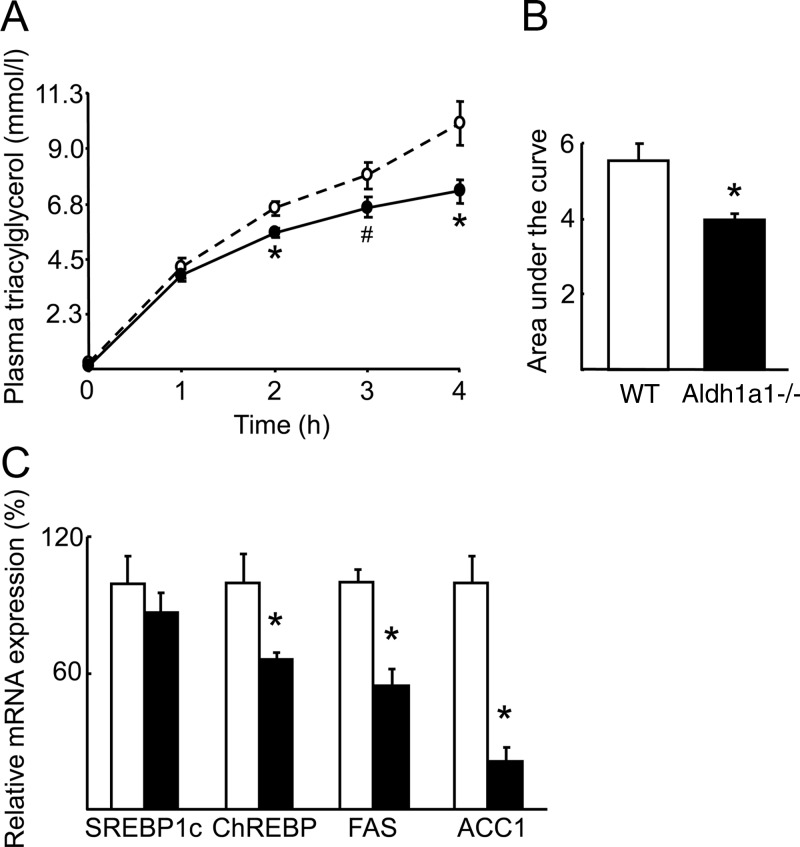
In addition to gluconeogenesis, AMPKα and FOXO1 also regulate lipogenesis, very low density lipoprotein (VLDL) secretion, and fatty acid turnover. Hence, the modulation of AMPKα and FOXO1 activity seen in Aldh1a1 deficiency suggests that this retinoid-metabolizing enzyme might be involved in coupling fasting glucose levels to lipoprotein metabolism independent of body mass or adiposity. After a 6-h fast, Aldh1a1−/− mice had significantly lower concentrations of total plasma triacylglycerol as well as VLDL- and low-density lipoprotein-triacylglycerol fractions compared with WT mice (Table 1). In Aldh1a1−/− animals, high-density lipoprotein-cholesterol concentrations were significantly increased whereas other cholesterol fractions were unchanged, resulting in increased total cholesterol levels (Table 1). To further investigate these changes, hepatic triacylglycerol production rate was measured in WT and Aldh1a1−/− mice using ip injection of tyloxapol (Triton WR1339), a lipolysis inhibitor, followed by serum triacylglycerol measurement over the subsequent 4-h period. Tyloxapol increased triacylglycerol production in Aldh1a1-deficient mice 28% less compared with weight-matched WT mice (Fig. 5, A and B).
Table 1.
Lipid profile
| Parameter (mg/dl) | WT | Aldh1a1−/− |
|---|---|---|
| Total cholesterol | 88.3 ± 1.2 | 120.7 ± 8.2a |
| CM cholesterol | 0.29 ± 0.03 | 0.41 ± 0.06 |
| VLDL cholesterol | 2.44 ± 0.13 | 3.04 ± 0.24 |
| LDL cholesterol | 12.8 ± 0.8 | 13.5 ± 1.0 |
| HDL cholesterol | 72.8 ± 1.0 | 103.7 ± 7.5a |
| Total triglycerides | 33.1 ± 1.5 | 20.0 ± 2.5a |
| CM triglycerides | 2.21 ± 0.27 | 2.03 ± 0.51 |
| VLDL triglycerides | 14.3 ± 1.0 | 9.0 ± 1.0a |
| LDL triglycerides | 14.7 ± 1.0 | 7.3 ± 0.4a |
| HDL triglycerides | 1.87 ± 0.17 | 1.70 ± 0.23 |
| Total cholesterol | 88.3 ± 1.2 | 120.7 ± 8.2a |
| CM cholesterol | 0.29 ± 0.03 | 0.41 ± 0.06 |
| VLDL cholesterol | 2.44 ± 0.13 | 3.04 ± 0.24 |
| LDL cholesterol | 12.8 ± 0.8 | 13.5 ± 1.0 |
| HDL cholesterol | 72.8 ± 1.0 | 103.7 ± 7.5a |
| Total triglycerides | 33.1 ± 1.5 | 20.0 ± 2.5a |
| CM triglycerides | 2.21 ± 0.27 | 2.03 ± 0.51 |
Serum lipoprotein concentrations were measured in 12- to 14-wk-old WT and Aldh1a1−/− mice after 6 h fasting by HPLC. Data are expressed as mean ± sem (n = 8/group). CM, Chylomicrons.
P < 0.05.
Fig. 5.
Aldh1a1 deficiency inhibits hepatic triacylglycerol synthesis. A, Hepatic triacylglycerol production was determined in WT (dashed line) and Aldh1a1−/− mice (solid line) after ip tyloxapol (Triton WR1339) injection. B, Area under the curve quantification of hepatic triacylglycerol production. C, Gene expression of key lipogenic factors was analyzed in livers of WT (white bars) and Aldh1a1−/− mice (black bars) (n = 6/group; *, P < 0.05; #, P = 0.08). ACC1, acetyl-coA carboxylase 1; FAS, fatty acid synthase; SREBP, sterol regulatory element-binding protein.
In considering molecular mechanisms for reduced VLDL secretion in Aldh1a1 deficiency, both sterol-regulatory element-binding-protein 1c and carbohydrate response element binding protein (ChREBP) are known lipogenic factors inhibited by AMPK activation (28, 29). Whereas hepatic sterol-regulatory element-binding-protein 1c mRNA expression was unaltered by Aldh1a1 deficiency, ChREBP expression was significantly reduced in Aldh1a1−/− vs. WT livers (Fig. 5C). ChREBP and AMPK have opposing effects on the transcriptional regulation and activity of the lipogenic enzymes fatty acid synthase and acetyl-CoA carboxylase 1 (30), both of which were repressed in mice lacking Aldh1a1 (Fig. 5C). These results are consistent with enhanced AMPK activity (Fig. 3C) and decreased ChREBP (Fig. 5C) expression in Aldh1a1-deficient livers.
Aldh1a1 controls energy substrate utilization in vivo
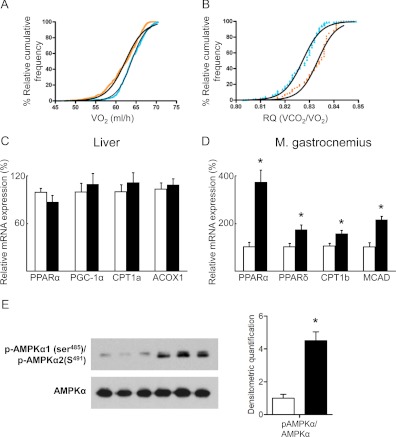
The combination of decreased hepatic gluconeogenesis and lower fasting glucose and triacylglycerol levels suggests that Aldh1a1 deficiency promotes fatty oxidation to maintain energy balance in the setting of decreased glucose levels. To test this, metabolic cage studies were performed in lean weight-matched WT vs. Aldh1a1−/− mice. Aldh1a1−/− mice exhibited increased oxygen consumption (VO2, Fig. 6A) and significantly decreased respiratory quotient (VCO2/VO2, Fig. 6B) compared with WT mice, consistent with increased fatty acid oxidation. Although enzymes and mediators of fatty acid oxidation like acyl coA oxidase 1, carnitine palmitoyltransferase 1, PPARγ coactivator 1α, and PPARα were unchanged in Aldh1a1−/− livers (Fig. 6C); in skeletal muscle, carnitine palmitoyltransferase 1, medium chain acyl-coA dehydrogenase, PPARα, and PPARδ were all significantly increased compared with WT mice (Fig. 6D). Consistently, AMPKα phosphorylation was enhanced in Aldh1a1-deficient vs. WT skeletal muscle (Fig. 6E). These data indicate that both reduced hepatic lipogenesis and enhanced fatty acid oxidation in muscle underlies the altered lipid profile in Aldh1a1 deficiency.
Fig. 6.
Aldh1a1 controls energy balance by regulating fatty acid oxidation. Indirect calorimetry was performed in WT (orange line) and Aldh1a1−/− mice (blue line) (12–14 wk of age, n = 5/group) A, O2 consumption (VO2) and (B) respiratory quotient (VCO2/VO2) curves after percent relative cumulative frequency analyses are given [EC50: 63.87 vs. 62.34 (P < 0.001) for VO2,; and 0.8272 vs. 0.8334 (P < 0.001) for VCO2/VO2, respectively]. Gene expression of fatty acid oxidation markers was analyzed in liver (C) and gastrocnemius muscle (D) of WT (white bars) and Aldh1a1−/− mice (black bars). E, Immunoblot analyses of p-AMPKα1 (serine 485)/p-AMPKα2 (serine 491) and total AMPKα in M. gastrocnemius of WT and Aldh1a1−/− mice (n = 6/group; *, P < 0.05). ACOX, Acyl coA oxidase; CPT, carnitine palmitoyltransferase; MCAD, medium chain acyl-coA dehydrogenase PGC, PPAR coactivator; RQ, respiratory quotient.
Discussion
Although 70 years have elapsed since increased hepatic vitamin A content was first demonstrated in patients with type 2 diabetes (31), distinct roles for retinoids in glucose homeostasis, lipid metabolism, and adiposity have remained obscure. We demonstrate here that deficiency of Aldh1a1, one of three Aldh isoforms responsible for converting Rald into RA, results in decreased fasting glucose independent of body mass or adiposity. Aldh1a1 deficiency is characterized by defective hepatic gluconeogenesis, with decreased hepatic expression of the key gluconeogenic regulators PEPCK and G6Pase. Aldh1a1 deficiency also exerts a coordinated effect on increased fatty acid oxidation, decreased circulating triacylglycerol levels, and altered respiratory quotient.
Vitamin A, an essential micronutrient, plays crucial, distinct biological roles through metabolites that modulate nuclear receptor activity and subsequent transcription (1, 2). ATRA is a known RAR ligand in vivo and in vitro, whereas 9-cis-RA mainly activates RXR (7, 8). Consistent with their fundamental importance, retinoid concentrations are tightly controlled during embryogenesis and throughout the lifespan of most organisms (32). A complex network exists to control the levels of specific retinoids in different tissues at different times, involving the action of enzymes that govern retinoid formation (6, 10).
Retinol is converted by alcohol dehydrogenases to Rald. Aldhs then catalyze Rald oxidation to RA, the rate-limiting, irreversible step in RA formation (10). Recent studies highlight how specific Aldh isoforms exert distinct biological responses, despite their approximately 70% amino acid homology. Aldh1a2 is essential for retinal development whereas Aldh1a1-deficient mice lack retinal abnormalities. Aldh1a2 is also implicated in lung and myocardial development whereas Aldh1a3 expression occurs in specific locations during facial and gut development (33, 34). Aldh isoforms also play unique roles outside of development, as seen in the immune system. Aldh isoforms differ in dendritic cells, including those of splenic vs. bone marrow origin. Aldh1a2 is the main form in mesenteric lymph nodes and splenic dendritic cells; Aldh1a1 appears to be most important in Peyer's patch dendritic cells (35, 36).
Despite the importance of RA and retinoid receptors in metabolism, little has been known about Aldh function in the liver. Aldh1a1 is the predominant hepatic isoform (Supplemental Fig. 1C). The data presented here establish that Aldh1a1 helps to maintain fasting glucose levels through hepatic gluconeogenesis. Aldh1a1-deficient mice display lower fasting glucose concentrations vs. WT controls (Fig. 1C) independent of body mass, insulin sensitivity, or glucose tolerance (Fig. 1, D and E, and Fig. 2, B and C). Hepatic glucose production and gluconeogenic enzyme expression were significantly repressed in Aldh1a1−/− mice (Fig. 2, A and D, and Fig. 3, A and B). Importantly, no difference was seen in Aldh1a2 or Aldh1a3 hepatic expression levels in this model (Supplemental Fig. 1C), arguing for a specific role of Aldh1a1 in these responses. Together, these data strongly implicate Aldh1a1 as a key determinant of hepatic gluconeogenesis.
Aldh1a1 deficiency protects mice from diet-induced obesity. Those studies suggested that Rald itself might regulate adipogenesis in this model (15). However, the Aldh1a1-mediated regulation of hepatic gluconeogenesis presented here suggests decreased RA levels and subsequent diminished RXR and/or RAR activation as accounting for the observed effects on glucose homeostasis (Figs. 1–3). Distinct retinoid responses in different tissues are well documented, albeit not fully understood. Variable expression patterns of retinoid receptor isomers and retinoid-binding proteins as well as local differences in retinoid formation contribute to tissue-specific retinoid responses (10, 37, 38). For example, treatment with synthetic RXR agonists suppressed skeletal and cardiac muscle but not adipose tissue lipoprotein lipase activity (39).
RA formation in vivo is tightly regulated, and changes in RA tissue concentrations can be subtle and thus difficult to detect (40). However, even modest shifts in RA tissue concentrations can significantly alter RA target gene expression (41). The decreased expression of the RA-regulated target gene Cyp26a1 in Aldh1a1−/− livers is consistent with lower RA levels in this model (Supplemental Fig. 1B).
RA is also a potent regulator of gluconeogenic enzyme expression as demonstrated here (Fig. 4, A and B). However, Rald stimulation in cells lacking Aldh1a1, which would otherwise convert Rald to RA, had no impact on PEPCK or G6Pase expression (Fig. 4A). Such findings are consistent with prior data demonstrating decreased hepatic glycogen content and PEPCK expression during global vitamin A deficiency, although underlying mechanisms for this effect were previously unknown (42, 43). Importantly, RA induction of PEPCK and G6Pase was almost completely abolished in the presence of the RXR antagonist HX531 (Fig. 4B). RXR can either homodimerize or heterodimerize with RAR, PPARγ, or other nuclear receptors. The PEPCK promoter does contain a putative RAR response element (RARE) (44). Because HX531 is a pan-retinoid receptor antagonist (24), perturbation of RAR/RXR heterodimer formation could account for the loss of RA effects seen in HX531-treated primary hepatocytes (Fig. 4B).
In terms of distal mechanisms involved in Aldh1a1 control of fasting glucose, the second messenger cAMP potently induces gluconeogenesis during fasting (26, 27). Aldh1a1-deficient primary hepatocytes had diminished cAMP-mediated induction of gluconeogenic enzyme expression (Fig. 4C), implicating retinoid metabolism in cAMP regulation of fasting gluconeogenesis. Interestingly, in prior reports RA sensitized leukemia cells and human bronchial epithelial cells to the action of cAMP (45, 46). Moreover, cAMP-dependent stimulation of PEPCK gene expression requires adequate hepatic RA levels (43). Thus, a lack of RA-mediated gluconeogenic induction via cAMP may contribute to fasting hypoglycemia in lean Aldh1a1−/− mice.
Retinoids also regulate lipid metabolism (12, 14, 47). Compared with weight-matched, lean WT mice, Aldh1a1-deficient mice are characterized by reduced plasma triacylglycerol levels (Table 1), enhanced oxygen consumption, and increased expression of fatty acid oxidation genes in skeletal muscle (Fig. 6). Given the systemic hypoglycemia seen in the fasting state (Fig. 1), it is possible that Aldh1a1 deficiency drives a systemic change in metabolites that prompts the mice to preferentially use fatty acids, particularly in skeletal muscle, to maintain energy balance. In addition to increased fatty acid oxidation, attenuated lipogenesis (Fig. 5) seems to contribute to the improved lipid profile in Aldh1a1−/− mice. Rexinoids are RXR-selective ligands that have been reported to ameliorate glucose metabolism in diabetics mainly due to improved insulin sensitivity. However, the pursuit of rexinoids as insulin sensitizers has been limited by hypertriglyceridemia (47, 48). Given retinoid-mediated increases in hepatic lipogenesis, retinoid signaling may promote nonalcoholic fatty liver disease. Indeed, nonalcoholic fatty liver disease patients have increased Aldh expression, suggesting enhanced RA formation (49). RA-mediated RARγ activation also augmented hepatic cannabinoid receptor-1 expression, promoted lipogenesis, and reduced fatty acid oxidation (50). Interestingly, 13-cis-RA treatment in acne patients increased triacylglycerol and glucose levels (51). Thus, Aldh1a1 inhibition may be a possible therapeutic target for modulating glucose production and hypertriglyceridemia.
Taken together, our findings establish Aldh1a1 as the specific Aldh isoform responsible in vivo for integrating retinoid effects on fasting glucose levels, hepatic gluconeogenesis, and lipogenesis independent of body mass or adiposity. In so doing, these studies uncover how Aldh1a1, the rate-limiting enzyme in RA formation, helps coordinate energy balance. These results add Aldh1a1 action to the growing evidence for how specific aspects of retinoid metabolism can regulate systemic responses.
Supplementary Material
Acknowledgments
We thank Dr. Hiroyuki Kagechika for kindly providing HX531.
This study was supported by Grants HL048743, 5P30DK057521, and AR054604–03S1 (to J.P.), Nutrition Obesity Research Center Grants DK 089503 and MDRTC DK 020572 (to C.F.B.), and Grant J3107-B19 from the Austrian Science Fund (to F.W.K.).
Disclosure Summary: The authors have nothing to disclose. F.W.K. wrote manuscript and researched data., G.O. wrote manuscript and researched data., S.N. researched data and contributed to discussion., J.D.B. researched data and contributed to discussion., H.W. and P.L. researched data., C.F.B. contributed to discussion and reviewed manuscript., N.R.Q. researched data and reviewed manuscript., G.D. kindly provided the Aldh1a1-deficient mouse and reviewed manuscript., J.P. contributed to discussion, wrote and edited manuscript.
Footnotes
- Aldh
- retinaldehyde dehydrogenase
- AMPK
- AMP-activated protein kinase
- ATRA
- all-trans retinoic acid
- BAT
- brown adipose tissue
- C/EBP
- CCAAT enhancer-binding protein
- ChREBP
- carbohydrate response element binding protein
- [14C]2DG
- [1-14C]-2-deoxyglucose
- Foxo1
- forkhead box O1
- G6Pase
- glucose 6 phosphatase
- GWAT
- gonadal white adipose tissue
- M
- musculus
- PEPCK
- phosphoenolpyruvate carboxykinase
- PPAR
- peroxisome proliferator-activated receptor
- PTT
- pyruvate tolerance test
- RA
- retinoic acid
- Rald
- retinaldehyde
- RAR
- retinoic acid receptor
- RXR
- retinoid X receptor
- SWAT
- inguinal white adipose tissue
- VLDL
- very low density lipoprotein
- WT
- wild type.
References
- 1. Mark M, Ghyselinck NB, Chambon P. 2006. Function of retinoid nuclear receptors: lessons from genetic and pharmacological dissections of the retinoic acid signaling pathway during mouse embryogenesis. Annu Rev Pharmacol Toxicol 46:451–480 [DOI] [PubMed] [Google Scholar]
- 2. Ziouzenkova O, Plutzky J. 2008. Retinoid metabolism and nuclear receptor responses: new insights into coordinated regulation of the PPAR-RXR complex. FEBS Lett 582:32–38 [DOI] [PubMed] [Google Scholar]
- 3. Niederreither K, Dollé P. 2008. Retinoic acid in development: towards an integrated view. Nat Rev Genet 9:541–553 [DOI] [PubMed] [Google Scholar]
- 4. Altucci L, Leibowitz MD, Ogilvie KM, de Lera AR, Gronemeyer H. 2007. RAR and RXR modulation in cancer and metabolic disease. Nat Rev Drug Discov 6:793–810 [DOI] [PubMed] [Google Scholar]
- 5. Villarroya F, Iglesias R, Giralt M. 2004. Retinoids and retinoid receptors in the control of energy balance: novel pharmacological strategies in obesity and diabetes. Curr Med Chem 11:795–805 [DOI] [PubMed] [Google Scholar]
- 6. Ross AC. 1993. Overview of retinoid metabolism. J Nutr 123:346–350 [DOI] [PubMed] [Google Scholar]
- 7. Ahuja HS, Szanto A, Nagy L, Davies PJ. 2003. The retinoid X receptor and its ligands: versatile regulators of metabolic function, cell differentiation and cell death. J Biol Regul Homeost Agents 17:29–45 [PubMed] [Google Scholar]
- 8. Germain P, Chambon P, Eichele G, Evans RM, Lazar MA, Leid M, De Lera AR, Lotan R, Mangelsdorf DJ, Gronemeyer H. 2006. International Union of Pharmacology. LXIII. Retinoid X receptors. Pharmacol Rev 58:760–772 [DOI] [PubMed] [Google Scholar]
- 9. Kane MA, Folias AE, Pingitore A, Perri M, Obrochta KM, Krois CR, Cione E, Ryu JY, Napoli JL. 2010. Identification of 9-cis-retinoic acid as a pancreas-specific autacoid that attenuates glucose-stimulated insulin secretion. Proc Natl Acad Sci USA 107:21884–21889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Duester G, Mic FA, Molotkov A. 2003. Cytosolic retinoid dehydrogenases govern ubiquitous metabolism of retinol to retinaldehyde followed by tissue-specific metabolism to retinoic acid. Chem Biol Interact 143–144:201–210 [DOI] [PubMed] [Google Scholar]
- 11. Perlmann T. 2002. Retinoid metabolism: a balancing act. Nat Genet 31:7–8 [DOI] [PubMed] [Google Scholar]
- 12. Mukherjee R, Davies PJ, Crombie DL, Bischoff ED, Cesario RM, Jow L, Hamann LG, Boehm MF, Mondon CE, Nadzan AM, Paterniti JR, Jr, Heyman RA. 1997. Sensitization of diabetic and obese mice to insulin by retinoid X receptor agonists. Nature 386:407–410 [DOI] [PubMed] [Google Scholar]
- 13. Li X, Hansen PA, Xi L, Chandraratna RA, Burant CF. 2005. Distinct mechanisms of glucose lowering by specific agonists for peroxisomal proliferator activated receptor gamma and retinoic acid X receptors. J Biol Chem 280:38317–38327 [DOI] [PubMed] [Google Scholar]
- 14. Yamauchi T, Waki H, Kamon J, Murakami K, Motojima K, Komeda K, Miki H, Kubota N, Terauchi Y, Tsuchida A, Tsuboyama-Kasaoka N, Yamauchi N, Ide T, Hori W, Kato S, Fukayama M, Akanuma Y, Ezaki O, Itai A, Nagai R, Kimura S, Tobe K, Kagechika H, Shudo K, Kadowaki T. 2001. Inhibition of RXR and PPARγ ameliorates diet-induced obesity and type 2 diabetes. J Clin Invest 108:1001–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ziouzenkova O, Orasanu G, Sharlach M, Akiyama TE, Berger JP, Viereck J, Hamilton JA, Tang G, Dolnikowski GG, Vogel S, Duester G, Plutzky J. 2007. Retinaldehyde represses adipogenesis and diet-induced obesity. Nat Med 13:695–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim JK, Michael MD, Previs SF, Peroni OD, Mauvais-Jarvis F, Neschen S, Kahn BB, Kahn CR, Shulman GI. 2000. Redistribution of substrates to adipose tissue promotes obesity in mice with selective insulin resistance in muscle. J Clin Invest 105:1791–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ayala JE, Bracy DP, McGuinness OP, Wasserman DH. 2006. Considerations in the design of hyperinsulinemic-euglycemic clamps in the conscious mouse. Diabetes 55:390–397 [DOI] [PubMed] [Google Scholar]
- 18. Simonson DC, DeFronzo RA. 1990. Indirect calorimetry: methodological and interpretative problems. Am J Physiol 258:E399–E412 [DOI] [PubMed] [Google Scholar]
- 19. Riachi M, Himms-Hagen J, Harper ME. 2004. Percent relative cumulative frequency analysis in indirect calorimetry: application to studies of transgenic mice. Can J Physiol Pharmacol 82:1075–1083 [DOI] [PubMed] [Google Scholar]
- 20. Klaunig JE, Goldblatt PJ, Hinton DE, Lipsky MM, Chacko J, Trump BF. 1981. Mouse liver cell culture. I. Hepatocyte isolation. In Vitro 17:913–925 [DOI] [PubMed] [Google Scholar]
- 21. Altomonte J, Richter A, Harbaran S, Suriawinata J, Nakae J, Thung SN, Meseck M, Accili D, Dong H. 2003. Inhibition of Foxo1 function is associated with improved fasting glycemia in diabetic mice. Am J Physiol Endocrinol Metab 285:E718–E728 [DOI] [PubMed] [Google Scholar]
- 22. Kola B, Grossman AB, Korbonits M. 2008. The role of AMP-activated protein kinase in obesity. Front Horm Res 36:198–211 [DOI] [PubMed] [Google Scholar]
- 23. Molotkov A, Duester G. 2003. Genetic evidence that retinaldehyde dehydrogenase Raldh1 (Aldh1a1) functions downstream of alcohol dehydrogenase Adh1 in metabolism of retinol to retinoic acid. J Biol Chem 278:36085–36090 [DOI] [PubMed] [Google Scholar]
- 24. Takahashi B, Ohta K, Kawachi E, Fukasawa H, Hashimoto Y, Kagechika H. 2002. Novel retinoid X receptor antagonists: specific inhibition of retinoid synergism in RXR-RAR heterodimer actions. J Med Chem 45:3327–3330 [DOI] [PubMed] [Google Scholar]
- 25. Zhang Y, Zolfaghari R, Ross AC. 2010. Multiple retinoic acid response elements cooperate to enhance the inducibility of CYP26A1 gene expression in liver. Gene 464:32–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nordlie RC, Foster JD, Lange AJ. 1999. Regulation of glucose production by the liver. Annu Rev Nutr 19:379–406 [DOI] [PubMed] [Google Scholar]
- 27. Zhang EE, Liu Y, Dentin R, Pongsawakul PY, Liu AC, Hirota T, Nusinow DA, Sun X, Landais S, Kodama Y, Brenner DA, Montminy M, Kay SA. 2010. Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat Med 16:1152–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kawaguchi T, Osatomi K, Yamashita H, Kabashima T, Uyeda K. 2002. Mechanism for fatty acid “sparing” effect on glucose-induced transcription: regulation of carbohydrate-responsive element-binding protein by AMP-activated protein kinase. J Biol Chem 277:3829–3835 [DOI] [PubMed] [Google Scholar]
- 29. Ferré P, Azzout-Marniche D, Foufelle F. 2003. AMP-activated protein kinase and hepatic genes involved in glucose metabolism. Biochem Soc Trans 31:220–223 [DOI] [PubMed] [Google Scholar]
- 30. Hardie DG, Scott JW, Pan DA, Hudson ER. 2003. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett 546:113–120 [DOI] [PubMed] [Google Scholar]
- 31. Moore T. 1937. Vitamin A and carotene: the vitamin A reserve of the adult human being in health and disease. Biochem J 31:155–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Duester G. 2008. Retinoic acid synthesis and signaling during early organogenesis. Cell 134:921–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Niederreither K, Fraulob V, Garnier JM, Chambon P, Dollé P. 2002. Differential expression of retinoic acid-synthesizing (RALDH) enzymes during fetal development and organ differentiation in the mouse. Mech Dev 110:165–171 [DOI] [PubMed] [Google Scholar]
- 34. Song L, Li Y, Wang K, Wang YZ, Molotkov A, Gao L, Zhao T, Yamagami T, Wang Y, Gan Q, Pleasure DE, Zhou CJ. 2009. Lrp6-mediated canonical Wnt signaling is required for lip formation and fusion. Development 136:3161–3171 [DOI] [PubMed] [Google Scholar]
- 35. Manicassamy S, Ravindran R, Deng J, Oluoch H, Denning TL, Kasturi SP, Rosenthal KM, Evavold BD, Pulendran B. 2009. Toll-like receptor 2-dependent induction of vitamin A-metabolizing enzymes in dendritic cells promotes T regulatory responses and inhibits autoimmunity. Nat Med 15:401–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Molenaar R, Knippenberg M, Goverse G, Olivier BJ, de Vos AF, O'Toole T, Mebius RE. 2011. Expression of retinaldehyde dehydrogenase enzymes in mucosal dendritic cells and gut-draining lymph node stromal cells is controlled by dietary vitamin A. J Immunol 186:1934–1942 [DOI] [PubMed] [Google Scholar]
- 37. Haugen BR, Larson LL, Pugazhenthi U, Hays WR, Klopper JP, Kramer CA, Sharma V. 2004. Retinoic acid and retinoid X receptors are differentially expressed in thyroid cancer and thyroid carcinoma cell lines and predict response to treatment with retinoids. J Clin Endocrinol Metab [Erratum (2008) 93:4553] 89:272–280 [DOI] [PubMed] [Google Scholar]
- 38. Colbert MC, Linney E, LaMantia AS. 1993. Local sources of retinoic acid coincide with retinoid-mediated transgene activity during embryonic development. Proc Natl Acad Sci USA 90:6572–6576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Davies PJ, Berry SA, Shipley GL, Eckel RH, Hennuyer N, Crombie DL, Ogilvie KM, Peinado-Onsurbe J, Fievet C, Leibowitz MD, Heyman RA, Auwerx J. 2001. Metabolic effects of rexinoids: tissue-specific regulation of lipoprotein lipase activity. Mol Pharmacol 59:170–176 [DOI] [PubMed] [Google Scholar]
- 40. Kane MA, Chen N, Sparks S, Napoli JL. 2005. Quantification of endogenous retinoic acid in limited biological samples by LC/MS/MS. Biochem J 388:363–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Balmer JE, Blomhoff R. 2002. Gene expression regulation by retinoic acid. J Lipid Res 43:1773–1808 [DOI] [PubMed] [Google Scholar]
- 42. Wolf G, Lane MD, Johnson BC. 1957. Studies on the function of vitamin A in metabolism. J Biol Chem 225:995–1008 [PubMed] [Google Scholar]
- 43. Shin DJ, McGrane MM. 1997. Vitamin A regulates genes involved in hepatic gluconeogenesis in mice: phosphoenolpyruvate carboxykinase, fructose-1,6-bisphosphatase and 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. J Nutr 127:1274–1278 [DOI] [PubMed] [Google Scholar]
- 44. Shin DJ, Odom DP, Scribner KB, Ghoshal S, McGrane MM. 2002. Retinoid regulation of the phosphoenolpyruvate carboxykinase gene in liver. Mol Cell Endocrinol 195:39–54 [DOI] [PubMed] [Google Scholar]
- 45. Zhao Q, Tao J, Zhu Q, Jia PM, Dou AX, Li X, Cheng F, Waxman S, Chen GQ, Chen SJ, Lanotte M, Chen Z, Tong JH. 2004. Rapid induction of cAMP/PKA pathway during retinoic acid-induced acute promyelocytic leukemia cell differentiation. Leukemia 18:285–292 [DOI] [PubMed] [Google Scholar]
- 46. Aggarwal S, Kim SW, Cheon K, Tabassam FH, Yoon JH, Koo JS. 2006. Nonclassical action of retinoic acid on the activation of the cAMP response element-binding protein in normal human bronchial epithelial cells. Mol Biol Cell 17:566–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lalloyer F, Pedersen TA, Gross B, Lestavel S, Yous S, Vallez E, Gustafsson JA, Mandrup S, Fiévet C, Staels B, Tailleux A. 2009. Rexinoid bexarotene modulates triglyceride but not cholesterol metabolism via gene-specific permissivity of the RXR/LXR heterodimer in the liver. Arterioscler Thromb Vasc Biol 29:1488–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Martin G, Poirier H, Hennuyer N, Crombie D, Fruchart JC, Heyman RA, Besnard P, Auwerx J. 2000. Induction of the fatty acid transport protein 1 and acyl-CoA synthase genes by dimer-selective rexinoids suggests that the peroxisome proliferator-activated receptor-retinoid X receptor heterodimer is their molecular target. J Biol Chem 275:12612–12618 [DOI] [PubMed] [Google Scholar]
- 49. Ashla AA, Hoshikawa Y, Tsuchiya H, Hashiguchi K, Enjoji M, Nakamuta M, Taketomi A, Maehara Y, Shomori K, Kurimasa A, Hisatome I, Ito H, Shiota G. 2010. Genetic analysis of expression profile involved in retinoid metabolism in non-alcoholic fatty liver disease. Hepatol Res 40:594–604 [DOI] [PubMed] [Google Scholar]
- 50. Mukhopadhyay B, Liu J, Osei-Hyiaman D, Godlewski G, Mukhopadhyay P, Wang L, Jeong WI, Gao B, Duester G, Mackie K, Kojima S, Kunos G. 2010. Transcriptional regulation of cannabinoid receptor-1 expression in the liver by retinoic acid acting via retinoic acid receptor-γ. J Biol Chem 285:19002–19011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Heliövaara MK, Remitz A, Reitamo S, Teppo AM, Karonen SL, Ebeling P. 2007. 13-cis-Retinoic acid therapy induces insulin resistance, regulates inflammatory parameters, and paradoxically increases serum adiponectin concentration. Metabolism 56:786–791 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.