Abstract
GnIH was first identified in avian species, and there is now strong evidence that it is operant in mammals as an inhibitor of reproduction. Mammalian gonadotropin-inhibitory hormone (GnIH)-3 is encoded by the RFRP gene in neurons of the dorsomedial nucleus. These neurons project to the median eminence, predicting a role as a secreted neurohormone and regulation of the pituitary gonadotropes. To determine whether GnIH-3 is a secreted neurohormone, we measured its concentration in hypophyseal portal blood in ewes during the nonbreeding (anestrous) season and during the luteal and follicular phases of the estrous cycle in the breeding season. Paired portal and jugular blood samples were collected and plasma prepared for RIA using an ovine GnIH-3 antibody. Pulsatile GnIH-3 secretion was observed in the portal blood of all animals. Mean GnIH-3 pulse amplitude and pulse frequency was higher during the nonbreeding season. GnIH-3 was virtually undetectable in peripheral blood plasma. There was a lack of association between secretory pulses of GnIH-3 (portal) and LH (peripheral). To determine the role of secreted GnIH-3, we examined its effects on GnRH-stimulated LH secretion in hypothalamo-pituitary-disconnected ewes; a significant reduction in the LH response to GnRH was observed. Finally, to identify cellular targets in the pituitary, the expression of GnIH receptor [G protein-coupled receptor 147 (GPR147)] in fractions enriched for gonadotropes somatotropes, and lactotropes was examined; expression was observed in each cell type. These data show GnIH-3 is secreted into portal blood to act on pituitary gonadotropes, reducing the action of GnRH.
Reproduction depends on the coordinated control of hormones within the hypothalamic-pituitary-gonadal (HPG) axis. The hypothalamic peptide GnRH is secreted into the hypophyseal portal system and stimulates the synthesis and secretion of the pituitary gonadotropins LH and FSH. Although long sought, a hypothalamic factor that inhibits the HPG axis was first identified and characterized only in the last decade. This factor, discovered in avian species, was named gonadotropin-inhibitory hormone (GnIH) (1), and there is now evidence for a similar factor operant in mammals (2, 3).
Originally named RF-amide-related peptide (RFRP) (4), GnIH peptides are encoded by the RFRP gene, which is located in neurons of the dorsomedial hypothalamic nucleus and the ventral region of the paraventricular nucleus in mice, rats, hamsters and sheep; (2–5). In most mammals, two peptides (RFRP-1 and RFRP-3) are encoded, but RFRP-3 (herein termed GnIH-3) has been the major focus in mammalian research, because this peptide appears to be most genetically related to avian GnIH, although the role of other GnIH peptides cannot be excluded. A significant body of evidence indicates GnIH-3 as a regulatory neuropeptide in mammalian species (for review see Refs. 6–8). Thus, central administration of GnIH-3 reduces basal LH concentrations in hamsters (3, 9) and rats (10, 11) and at the time of the GnRH/LH surge in rats (12). This effect appears most likely to be due to a direct input to GnRH neurons, because GnIH terminals are found in close apposition to GnRH neurons in rats (10), hamsters (3), sheep (13, 14), nonhuman primates (15, 16), and humans (17), and GnIH-3 treatment inhibited the firing rate of GnRH neurons in mouse brain slices (18, 19).
On the other hand, GnIH terminals are seen in the neurosecretory zone of the median eminence in hamsters, sheep, and primates (2, 3, 15–17, 20), predisposing action at the pituitary level. Peripheral administration of GnIH-3 inhibits LH secretion in rats (21) and sheep (2), although in the former, GnIH terminals do not appear to reach the secretory zone of the median eminence and is therefore unlikely to have a hypophysiotropic role (22). A strong case can be made for a pituitary site of action of GnIH-3 in sheep, because the peptide reduces GnRH-stimulated LH secretion from pituitary cells in vitro (2) and blocks GnRH-stimulated increase in LHβ and FSHβ mRNA as well as the phosphorylation of ERK expression in gonadotropes (23). Most recently, we have shown that systemic infusion of GnIH-3 blocks the estrogen-induced LH surge in ovariectomized ewes, strongly suggesting a pituitary site of action (24), although the possibility that GnIH-3 crosses the blood-brain barrier cannot be excluded. A reduction in expression of GnIH mRNA in the ewe hypothalamus in the preovulatory period is consistent with a permissive lowering of negative tone on the reproductive axis, in conjunction with a rise in GnRH secretion at this time (25).
If GnIH-3 is a bona fide regulator of pituitary gonadotropes, then secretion into the hypophyseal portal blood is a prerequisite. Accordingly, we aimed to measure concentrations in the portal blood of sheep, using a newly developed RIA. Furthermore, we determined whether GnIH-3 can attenuate GnRH-stimulated LH secretion in vivo using the ovine hypothalamo-pituitary disconnection (HPD) model (26, 27). Finally, we measured the level of expression of the GnIH receptor (GPR147) in gonadotrope-enriched fractions of pituitary cells.
Materials and Methods
Animals
All experimental procedures were conducted according to the guidelines established by the Australian Prevention of Cruelty to Animals Act under a protocol approved by the Monash University, School of Biomedical Sciences, Animal Ethics Committee. Corriedale ewes of similar age (3–4 yr old) and weight (50–60 kg) were housed under natural lighting and brought into the Monash University Sheep Facility (Werribee, Victoria, Australia) when required. For the use of gonad-intact animals, estrous cycles were synchronized by im injection of 125 μg of a synthetic luteolysin (Cloprostenol, Estrumate; Pitman-Moore, Sydney, New South Wales, Australia). For cell culture, ewes were euthanized either 24 h after injection (follicular phase) or on d 10 of the ensuing estrous cycle (luteal phase) and the stage of cycle was confirmed by examination of ovaries for the presence or absence of ovarian follicles or corpora lutea.
Experiment 1: determination of GnIH in the hypophyseal portal circulation
Ewes were prepared for hypophyseal portal blood sampling by implantation of a needle that was directed at the portal vessels on the anterior surface of the pituitary gland as previously described (28, 29). Studies were undertaken during the nonbreeding season (September, n = 6) and during the breeding season (April) with animals in luteal and follicular phases of the estrous cycle (n = 4 per group). Approximately 1 wk after surgery, one external jugular vein was cannulated and kept patent with heparinized saline for measurement of peripheral LH concentration. Portal blood samples and paired jugular samples were collected every 10 min for 6 h. Portal blood samples were collected onto ice, into tubes containing 50 μl aprotinin (1.5 mg/ml) and 50 μl bacitracin (5 mm) (Sigma-Aldrich, Castle Hill, New South Wales, Australia). In nonbreeding-season animals, GnIH-3 (VPNLPQRF-NH2, 100 μg) was administered after 6 h of collection using the jugular line to confirm that our extraction procedure was appropriate and to measure half-life of the peptide in plasma. Portal sampling continued for another 60 min, and samples were included as GnIH-3 assay positive control. Plasma was harvested immediately from jugular and portal samples and frozen at −20 C until assayed.
Experiment 2: effect of GnIH on the pituitary in vivo
The in vivo action of GnIH-3 at the level of the pituitary gland was determined using HPD ewes. All HPD animals were bilaterally ovariectomized at least 1 month before the experiment, and the HPD surgery was performed as previously described (26, 27). The latter procedure removes all neural inputs to the median eminence, but gonadotropin secretion can be restored by iv administration of GnRH in a pulsatile fashion. Approximately 1 wk after HPD surgery, the animals were housed in single pens, and one external jugular vein was cannulated for administration (iv) of 250-ng pulses (over 5 min) of GnRH every 2 h. GnRH was diluted in heparinized saline (50 IU/liter) and delivered iv using an infusion pump as previously described (30). Animals received GnRH replacement for 7 d (to produce fully functional gonadotropes), after which time GnIH-3 treatment began. At 0530 h, animals were disconnected from infusion pumps, and remaining GnRH pulses (0600, 0800, 1000, 1200, 1400, 1600, 1800, and 2000 h) were delivered through the jugular cannula manually. At 1130, 1530, and 1930 h, an infusion of GnIH-3 (VPNLPQRF-NH2, 100-μg loading dose and 400-μg infusion over 1 h,) or vehicle (n = 8 per group) was administered via an additional jugular cannula (to overlap the GnRH pulses at 1200, 1600, and 2000 h). Blood samples were collected at −5, +5, +10, +15, +20, +30, and +55 min after GnRH pulses. GnRH pulses coinciding with GnIH-3 treatment were delivered at three doses (200, 100, and 50 ng) in a randomized order. Blood was also collected at the same times for the 1000-, 1400-, and 1800-h GnRH pulses (250 ng, as an internal control). All blood samples were collected into heparinized tubes and plasma harvested for LH RIA.
Experiment 3: quantitative RT-PCR of GPR147 mRNA in pituitary cells
Pituitary cells were collected from gonad-intact ewes (n = 5), dissociated, and passed through Percoll gradients to provide fractions enriched for gonadotropes, somatotropes, and lactotropes, as described previously (30–32). GPR147 mRNA quantitative RT-PCR was performed using total RNA extracted from pituitary cell fractions using the TRIzol reagent method (Invitrogen, Sydney, New South Wales, Australia). First-strand cDNA was synthesized under the manufacturer's protocol (Applied Biosystems, Melbourne, Victoria, Australia). Resultant cDNA was used as a template for PCR primers designed against the GPR147 cDNA sequence (GenBank accession number XM_002698905). The GPR147 nested primers used were sense outer primers 5′-GCG AGA ATG GAA GTG ATG CT and 3′-GGC CAG GTT GAG GAT AAA CA and inner primers 5′-TGC AAA CCT CAC CTT CTC CT and 3′-TGA AGC AGA CCA GCG TAT TG. Quantitative PCR was performed in 20-μl reaction volumes using the Realplex 4 from Eppendorf with RealMaster Mix with SYBR solution (Eppendorf AG, Hamburg, Germany). The regular 119-bp GPR147 PCR product was extracted (Geneclean II kit; Q-BIOgene, Irvine, CA), quantified by spectrophotometry, and then used to generate a standard curve via serial dilutions. The PCR cycling conditions included an initial denaturation at 95 C for 2 min, followed by 60 cycles at 95 C for 15 sec, 62 C for 60 sec, and 72 C for 30 sec. Fluorescence values were analyzed, and a standard curve was constructed using the Realplex 4 software. Melting-curve analysis showed a single GPR147 PCR product, and this was confirmed by gel electrophoresis (119-bp product, data not shown). Whole hypothalami (extending from the preoptic area to the mammillary bodies) were collected at the time of pituitary dissection in ewes, and additional whole pituitary glands were also collected and mRNA extracted from fragments and used as positive control for GPR147 mRNA RT-PCR. All PCR data were normalized to the geometric mean of three housekeeping genes: β2-microglobulin, β-actin, and cyclophilin transcript. The cellular content (gonadotropes, somatotropes, and lactotropes) of each Percoll fraction was confirmed before quantitative RT-PCR in an aliquot of cells by immunocytochemistry (Table 1) and RT-PCR as previously described (30).
Table 1.
Confirmation of cellular content of pituitary Percoll fractions
| Percoll fraction | Immunolocalization |
||
|---|---|---|---|
| GH | LH | PRL | |
| Somatotrope (%) | 62 ± 6 | 6 ± 3 | 11 ± 2 |
| Gonadotrope (%) | 9 ± 1 | 45 ± 3 | 17 ± 3 |
| Lactotrope (%) | 4 ± 1 | 6 ± 2 | 50 ± 4 |
Cellular content was determined by immunocytochemistry in an aliquot of cells using primary antibodies against GH, LH, and prolactin (PRL) as previously described (30). Data are mean ± sem (n = 5).
LH RIA
Plasma LH concentrations were measured in duplicate, using the method of Lee et al. (33) with NIH-oLH-S18 as standard. Assay results were calculated using the program of Burger et al. (34). Assay sensitivity was 0.1 ng/ml, and the intraassay coefficient of variation was less than 10% over the range of 0.2–8.1 ng/ml.
GnIH-3 RIA
GnIH-3 concentration was assessed in experiment 1 in both jugular and portal plasma samples. Samples were extracted with acidified methanol (30). Plasma concentrations were measured in duplicate, with 125I mono-iodinated GnIH-3/human RFRP-3(Tyr) as tracer and GnIH-3/human RFRP-3(Tyr) as standard. The GnIH-3 primary antibody was raised in guinea pig against ovine/human RFRP-3 (VPNLPQRF-NH2; Antibodies Australia, Melbourne, Victoria, Australia). Assay sensitivity was 0.3 pg/ml, and the intraassay coefficient of variation was less than 10% over the range of 1.0–24 pg/ml. Complete GnIH-3 RIA details can be found in the Supplemental Materials and Methods (published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). Assay cross-reactivity was assessed using 50–10,000 pg/ml concentrations of the following peptides: GnRH, TRH, GHRH, somatostatin (all from Auspep Pty. Ltd., Parkville, Victoria, Australia), prolactin-releasing peptide, α-MSH, neuropeptide Y, quail GnIH, RFRP-1, RFRP-2, human kisspeptin-10, ovine kisspeptin-10, neuropeptide AF (NPAF), NPFF, and pyroglutamylated RFamide peptide-43 (all from Phoenix Pharmaceuticals, Belmont, CA). Cross-reactivity was observed only for quail GnIH (<1%), RFRP-1 (<1%), and NPAF (15%) (data not shown). It is unlikely that NPAF is secreted into the portal circulation because terminals expressing neuropeptides NPAF and NPFF are not found in the median eminence (35).
Data analysis
For GnIH-3 detection in the hypophyseal portal system, a pulse analysis of the GnIH-3 and LH data was performed based on the method described for GnRH (36, 37). The half-life of GnIH-3 was determined using the formula (half-life = T × log2/log (B/E), where T is time, B is starting concentration, and E is end concentration). Comparisons between GnIH-3 and LH pulses were determined using a method previously described (38). Because GnIH is proposed to inhibit LH release from the pituitary, GnIH-3 pulses were considered to be paired with LH pulses if a GnIH-3 pulse occurred at the same time or within 10 min of an LH pulse. Data from each reproductive phase were assessed by one-way ANOVA. Additionally, total GnIH-3 pulse data were compared with randomly mismatched LH data from another animal to serve as controls. Statistical significance was assessed by a Student's t test. In addition, three mathematically independent statistical approaches were applied to examine GnIH-3-LH synchrony: cross-correlation analysis, discrete peak coincidence analysis, and cross-approximate entropy (ApEn) (39, 40). Cross-correlation analysis detects lag-specific linear relationships between successively paired sample measurements; discrete peak coincidence testing requires a priori pulse identification followed by statistical concordance analysis; cross-ApEn quantifies lag-independent and nonlinear bihormonal synchrony (40). For the in vivo HPD experiments, differences were initially assessed by two-way (repeated measures) ANOVA. Area under the curve (Sigma-Plot version 9.0) was calculated for the 60 min after each GnRH pulse, and differences between GnIH-3 and saline treatment were compared using one-way ANOVA.
Results
GnIH-3 is present in the hypophyseal portal circulation
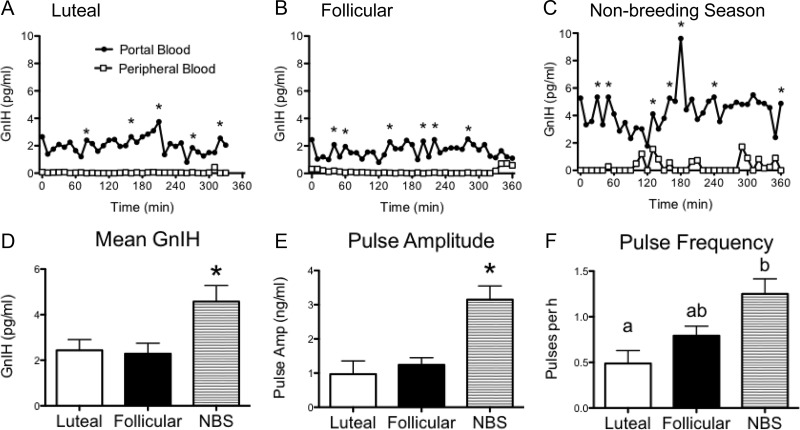
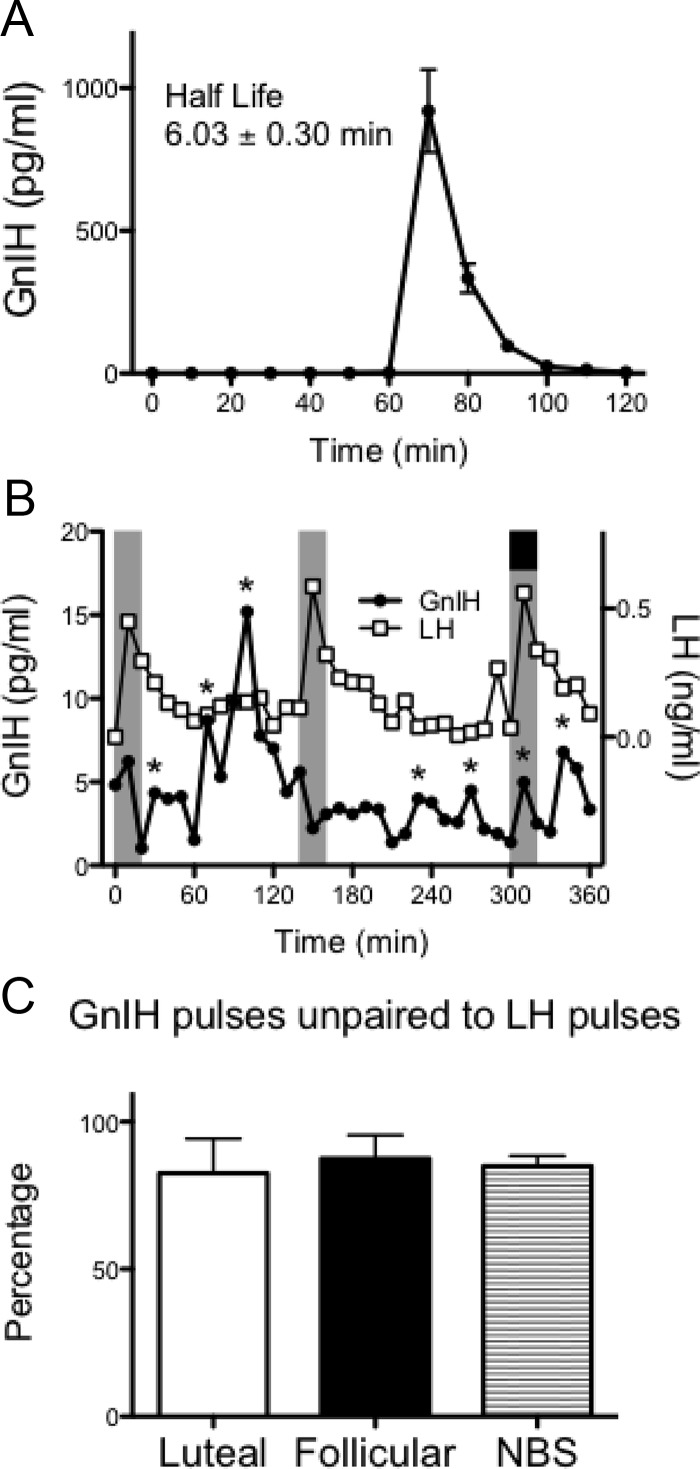
GnIH-3 was detected in the portal blood of all ewes, and the secretion appeared episodic (Fig. 1, A–C). Importantly, GnIH-3 concentrations were virtually undetectable in peripheral blood, with no pulses evident (Fig. 1, A–C). During the nonbreeding season, the concentration of GnIH-3 appeared greater compared with the breeding season (Fig. 1, A–C). Quantitative assessment revealed greater mean GnIH-3 (74%), GnIH-3 pulse amplitude (3.3-fold), and GnIH-3 pulse frequency (2.6-fold) during the nonbreeding season compared with the luteal phase of the breeding season (all P < 0.05, Fig. 1, D–F). Peripheral injections of GnIH-3 in nonbreeding-season ewes caused a rapid elevation of detectable GnIH-3 in the hypophyseal portal system and revealed the half-life of GnIH-3 in portal blood was 6.03 ± 0.30 min (Fig. 2A).
Fig. 1.
Plasma concentrations of GnIH in the ovine hypophyseal portal system are greater during the nonbreeding season. A–C, GnIH concentrations in the portal system (●) and in peripheral blood (□) in representative animals during the breeding season (luteal and follicular phase of the estrous cycle) and during the nonbreeding (anestrous) season. Pulses of GnIH are indicated by asterisks. D–F, Mean GnIH, GnIH pulse amplitude, and GnIH pulse frequency in luteal, follicular, and nonbreeding-season (NBS) animals (n = 4–6 per group). Data are the mean ± sem; *, P < 0.05. For pulse frequency, values without common notations differ significantly at P < 0.05.
Fig. 2.
Pharmacokinetics of GnIH within the hypophyseal portal system and GnIH/LH pulse relationship in the ewe. A, Nonbreeding season ewes were administered 100 μg GnIH (60 min) into the jugular vein, and portal sampling continued for 1 h. Data are the mean ± sem (n = 6). The half-life of GnIH in the portal blood was 6.03 ± 0.30 min. B, Representative GnIH (●) and LH (□) concentrations from a nonbreeding season animal. Pulses of GnIH are indicated by asterisks. Pulses of LH are indicated by the shaded columns. A GnIH pulse paired with an LH pulse is indicated by a black box. C, The percentage of GnIH pulses unpaired (not associated) with LH pulses in luteal, follicular, and nonbreeding-season (NBS) animals (n = 4–6 per group). Data are the mean ± sem.
The majority of GnIH-3 pulses were not paired with LH pulses (Fig. 2B), and this did not differ among reproductive phases (Fig. 2C). Overall, GnIH-3 pulses were unpaired (not associated) with LH pulses 85 ± 2% of the time. However, statistical analysis indicated that this association (or lack of association) was no different from that seen with random chance (82 ± 2%; P = 0.56). Similarly, cross-correlation analysis showed no negative correlation between GnIH-3 and LH. Discrete peak coincidence testing showed exact concordance of detected GnIH-3 and LH peaks was not significant, indicating neither a low nor a high number of coincident pulses. Finally, cross-ApEn shows highly random GnIH-3 pulses but consistently better-organized LH, with no significant coupling of patterns between GnIH-3 and LH.
Effect of GnIH on the pituitary in vivo
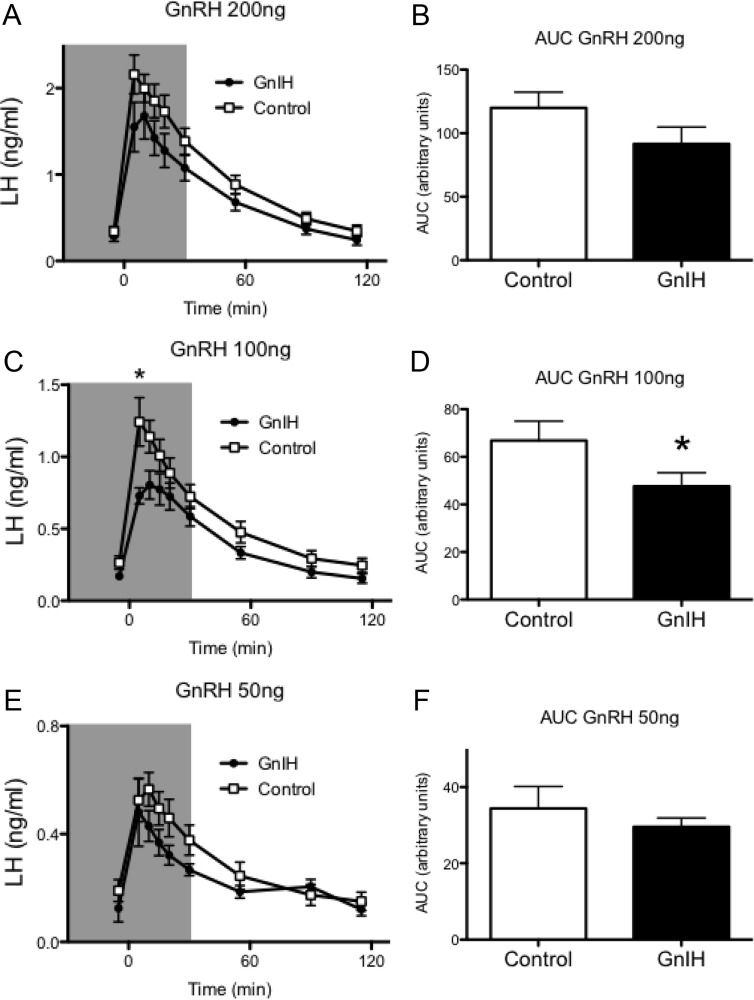
When paired with a 100-ng dose of GnRH, infusion of GnIH-3 significantly reduced the LH response in HPD ewes (P < 0.05, Fig. 3C). Analyses of the area under the curve showed a 29% reduction in the LH response to 100 ng GnRH when animals were treated with GnIH-3 compared with control (P < 0.05, Fig. 3D). No significant effect of GnIH-3 was seen when paired with 50- and 200-ng doses of GnRH, although a trend for reduced LH response was observed (Fig. 3, A, B, E, and F).
Fig. 3.
GnIH inhibits the LH response to GnRH in vivo. A, C, and E, Plasma concentrations of LH in HPD ewes treated with GnIH (●, 400 μg, shaded area) or saline control (□) and GnRH (200, 100, and 50 ng at time zero). *, P < 0.05. B, D, and F, Area under the curve (AUC) analyses revealed a significant decrease in the LH response in GnIH-treated animals to the 100-ng dose of GnRH. Data are the mean ± sem; n = 8 per group. *, P < 0.05.
GPR147 expression in pituitary gonadotropes
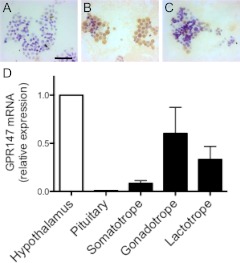
GPR147 mRNA was detectable by RT-PCR in cellular fractions of ovine pituitary cells enriched for gonadotropes (Fig. 4). Quantitative analysis revealed that the level of expression in gonadotropes was statistically similar (but tended to be greater) to that of fractions enriched for somatotropes and lactotropes. The level of GPR147 mRNA expression in gonadotrope fractions was far greater than that seen in the whole pituitary but was comparable with the expression in the whole hypothalamus (Fig. 4D).
Fig. 4.
GPR147 mRNA in pituitary gonadotropes. A–C, Representative photomicrographs of GH (A), LH (B), and prolactin (C) immunostaining (brown) in a gonadotrope-enriched pituitary cell fraction. Cells were counterstained with cresyl violet. D, Relative levels of GPR147 mRNA in cellular fractions of ovine pituitary cells enriched for somatotropes, gonadotropes, and lactotropes as well as the whole pituitary and hypothalamus. Data are the mean ± sem and are normalized to the geometric mean of three housekeeping genes and then expressed as a proportion of hypothalamic expression.
Discussion
GnIH has emerged as an important regulator of reproduction, yet the precise level at which it operates within the HPG axis is not well defined. We now demonstrate that GnIH-3 is secreted into the ovine hypophyseal portal system, enabling action on the pituitary gonadotropes. Whereas the peptide was readily detectable in hypophyseal portal plasma, with our newly developed RIA, it was undetectable in peripheral blood plasma, indicating that it is a secreted neurohormone. Moreover, the secretory pattern of GnIH-3 was pulsatile, displaying greater pulse frequency and amplitude in the nonbreeding season; this substantiates a role as at least one factor causative in seasonal quiescence of reproduction. GnIH-3 secreted into the portal system is highly likely to regulate gonadotropin secretion because we saw an inhibition of the GnRH response in vivo, and pituitary gonadotropes were found to express the GnIH receptor GPR147.
The role of GnIH-3 as a hypophysiotropic hormone has, until now, been an issue of some controversy. GnIH-3-producing cells localized within the dorsomedial hypothalamic nucleus send neuronal projections to a range of hypothalamic nuclei (for review see Refs. 6–8), suggesting a role for GnIH-3 in the regulation of physiological homeostatic functions. Moreover, GnIH-3 terminals have been visualized within the neurosecretory zone of the median eminence, predisposing a hypophysiotropic action, in hamsters, sheep, and primates (2, 3, 16, 17, 20), but others have disputed this, at least for the rat (22). Nevertheless, iv administration of GnIH-3 reduces plasma LH secretion in rats (21). In ovariectomized ewes, peripheral administration of GnIH-3 inhibits the pulsatile release of LH (2), and GnIH-3 reduces GnRH-stimulated LH secretion in primary pituitary cultures in a dose-dependent manner (2). Moreover, GnIH-3 eliminates the GnRH-stimulated mobilization of intracellular calcium in pituitary cell cultures (2) as well as inhibiting GnRH stimulation of LHβ mRNA and phosphorylation of ERK (23), mandatory for LH release/synthesis. The present data show unequivocally that GnIH-3 is present in the hypophyseal portal circulation of sheep. Whether the same is true for other species remains to be determined. GnIH-3 is likely to act on the pituitary gonadotropes in the nonhuman and human primate because terminals expressing GnIH peptide are detectable in the external zone of the median eminence in these species (16, 17).
Mean GnIH-3 concentrations, pulse amplitude, and pulse frequency were higher during the nonbreeding season than in the breeding season. Consistent with a suppressive role in anestrus, GnIH-3 protein expression in the hypothalamus was higher during the nonbreeding season (14), and GnIH mRNA expression was higher in ewes maintained on artificial long-day photoperiods (replicating the natural nonbreeding season) compared with ewes held at short-day photoperiod (20). Whether this change in expression reflects an increase in the number or intensity of GnIH terminals in the median eminence is yet to be determined; however, it is worth noting there is an increase in the number of terminal projections from GnIH neurons to GnRH neurons in the nonbreeding season (14).
During hypophyseal portal sampling, we administered GnIH-3 peptide to animals to assess the reliability of our GnIH-3 RIA. Peripheral treatment with 100 μg GnIH-3 resulted in a predictable rise in measurable GnIH-3 in the portal blood, which was approximately 200 times greater than the endogenous concentration measured during the nonbreeding season. This also enabled us to estimate the half-life of the peptide in the portal circulation. To this end, we show the peptide is cleared from the circulation extremely quickly. This could explain inconsistencies with GnIH-3 treatment effects (and the lack of effect) in past in vivo experiments where a single bolus of GnIH-3 was administered and most likely rapidly cleared from the circulation (12, 21).
Our data show a significant reduction in the LH response to GnRH in GnIH-3-treated HPD ewes. This inhibition was seen only when 100 ng GnRH was administered to animals, with effects at higher (200 ng) or lower (50 ng) doses of GnRH failing to reach significance. At the higher dose of GnRH, GnIH-3 appears insufficient to prevent the secretion of LH. At the lower dose of GnRH, LH may not have been stimulated to a degree where a discernible reduction (due to GnIH-3) could be achieved. This modest effect in vivo is consistent with data from the rat (22). Moreover, there was no direct relationship between GnIH-3 pulses and LH pulses, leading us to believe GnIH is not a dynamic regulator of the pulsatile release of LH. GnIH-3 pulses were not noted at the time of LH pulses or between LH pulses, which we would predict given the proposed role of GnIH-3. Given the overall increase in GnIH output during the nonbreeding season, it is likely GnIH-3 acts as a modulator of the activity of the pituitary to GnRH pulses. In this sense, it may create a set-point of negative tone at the level of the pituitary gonadotrope. In addition, our data also show the GnIH-3 receptor, GPR147, is expressed on lactotropes and somatotropes. Thus, GnIH may act at the pituitary to influence the GH and/or prolactin axes.
It seems most likely that GnIH-3 inhibits the reproductive system through action on the GnRH neurons as well as the gonadotropes. Regarding the former, GnIH terminal beds are located in the POA, septum, and diagonal band of Broca (16), where the majority of GnRH neurons are found. Indeed, a large percentage of GnRH neurons (40–80%) possess GnIH terminal appositions in the mouse, rat, hamster (3), sheep (13, 14), and primate (15, 16). Moreover, GnIH-3 receptors have recently been located in GnRH neurons in hamsters (9) and mice (41). Central (intracerebroventricular) administration of GnIH reduces plasma LH secretion in hamsters (3) and rats (10, 11) presumably by direct action on GnRH neurons, although similar data were not recently forthcoming in sheep, despite central administration of a GnIH-3 receptor antagonist stimulating LH secretion (42). To further confirm this mode of action, GnIH treatment inhibits both activation of GnRH neurons (examined by neuronal expression of the early gene Fos) in rats (12) and the firing rate of a significant number of GnRH neurons in mouse brain slices (18, 19). Alternately, recent data in the hamster suggest that, under certain conditions, GnIH-3 stimulates the gonadotropic axis (9, 43). These data add another level of complexity to the peptide and indicate that its function depends on the species and the physiological status of the animal model.
We conclude that GnIH-3 is secreted into the hypophyseal portal blood in sheep and acts on the pituitary gonadotropes to oppose the action of GnRH. GnIH-3 secretion appears to be pulsatile, and pulse frequency and amplitude are increased during the nonbreeding season. Importantly, pituitary gonadotropes express the GnIH-3 receptor GPR147, and GnIH-3 can inhibit the GnRH response in vivo in the isolated pituitary. Overall, our data unequivocally prove GnIH to be a secreted neuroendocrine hormone.
Supplementary Material
Acknowledgments
We thank Bruce Doughton, Lynda Morrish, and Elaine Chase for animal care, and we are grateful to Alexandra Rao, Alda Pereira, and Sofie Saleh for their technical assistance.
This work was supported by the National Health and Medical Research Council of Australia Project Grants 606538 and 1024346 and National Institutes of Health Grant AG31763. J.T.S. is supported by an Australian Research Council Future Fellowship, FT0990986.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ApEn
- Approximate entropy
- GPR147
- G protein-coupled receptor 147
- GnIH
- gonadotropin-inhibitory hormone
- HPD
- hypothalamo-pituitary-disconnection
- HPG
- hypothalamic-pituitary-gonadal
- NPAF
- neuropeptide AF
- RFRP
- RF-amide-related peptide.
References
- 1. Tsutsui K, Saigoh E, Ukena K, Teranishi H, Fujisawa Y, Kikuchi M, Ishii S, Sharp PJ. 2000. A novel avian hypothalamic peptide inhibiting gonadotropin release. Biochem Biophys Res Commun 275:661–667 [DOI] [PubMed] [Google Scholar]
- 2. Clarke IJ, Sari IP, Qi Y, Smith JT, Parkington HC, Ubuka T, Iqbal J, Li Q, Tilbrook A, Morgan K, Pawson AJ, Tsutsui K, Millar RP, Bentley GE. 2008. Potent action of RFamide-related peptide-3 on pituitary gonadotropes indicative of a hypophysiotropic role in the negative regulation of gonadotropin secretion. Endocrinology 149:5811–5821 [DOI] [PubMed] [Google Scholar]
- 3. Kriegsfeld LJ, Mei DF, Bentley GE, Ubuka T, Mason AO, Inoue K, Ukena K, Tsutsui K, Silver R. 2006. Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proc Natl Acad Sci USA 103:2410–2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hinuma S, Shintani Y, Fukusumi S, Iijima N, Matsumoto Y, Hosoya M, Fujii R, Watanabe T, Kikuchi K, Terao Y, Yano T, Yamamoto T, Kawamata Y, Habata Y, Asada M, Kitada C, Kurokawa T, Onda H, Nishimura O, Tanaka M, Ibata Y, Fujino M. 2000. New neuropeptides containing carboxy-terminal RFamide and their receptor in mammals. Nat Cell Biol 2:703–708 [DOI] [PubMed] [Google Scholar]
- 5. Ukena K, Tsutsui K. 2001. Distribution of novel RFamide-related peptide-like immunoreactivity in the mouse central nervous system. Neurosci Lett 300:153–156 [DOI] [PubMed] [Google Scholar]
- 6. Smith JT, Clarke IJ. 2010. Gonadotropin inhibitory hormone function in mammals. Trends Endocrinol Metab 21:255–260 [DOI] [PubMed] [Google Scholar]
- 7. Tsutsui K, Bentley GE, Bedecarrats G, Osugi T, Ubuka T, Kriegsfeld LJ. 2010. Gonadotropin-inhibitory hormone (GnIH) and its control of central and peripheral reproductive function. Front Neuroendocrinol 31:284–295 [DOI] [PubMed] [Google Scholar]
- 8. Khan AR, Kauffman AS. 2012. The role of kisspeptin and RFamide-related peptide-3 neurones in the circadian-timed preovulatory luteinising hormone surge. J Neuroendocrinol 24:131–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ubuka T, Inoue K, Fukuda Y, Mizuno T, Ukena K, Kriegsfeld LJ, Tsutsui K. 2012. Identification, expression, and physiological functions of Siberian hamster gonadotropin-inhibitory hormone. Endocrinology 153:373–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Johnson MA, Tsutsui K, Fraley GS. 2007. Rat RFamide-related peptide-3 stimulates GH secretion, inhibits LH secretion, and has variable effects on sex behavior in the adult male rat. Horm Behav 51:171–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pineda R, Garcia-Galiano D, Sanchez-Garrido MA, Romero M, Ruiz-Pino F, Aguilar E, Dijcks FA, Blomenröhr M, Pinilla L, van Noort PI, Tena-Sempere M. 2010. Characterization of the inhibitory roles of RFRP3, the mammalian ortholog of GnIH, in the control of gonadotropin secretion in the rat: in vivo and in vitro studies. Am J Physiol Endocrinol Metab 299:E39–E46 [DOI] [PubMed] [Google Scholar]
- 12. Anderson GM, Relf HL, Rizwan MZ, Evans JJ. 2009. Central and peripheral effects of RFamide-related peptide-3 on luteinizing hormone and prolactin secretion in rats. Endocrinology 150:1834–1840 [DOI] [PubMed] [Google Scholar]
- 13. Qi Y, Oldfield BJ, Clarke IJ. 2009. Projections of RFamide-related peptide-3 neurones in the ovine hypothalamus, with special reference to regions regulating energy balance and reproduction. J Neuroendocrinol 21:690–697 [DOI] [PubMed] [Google Scholar]
- 14. Smith JT, Coolen LM, Kriegsfeld LJ, Sari IP, Jaafarzadehshirazi MR, Maltby M, Bateman K, Goodman RL, Tilbrook AJ, Ubuka T, Bentley GE, Clarke IJ, Lehman MN. 2008. Variation in kisspeptin and RFamide-related peptide (RFRP) expression and terminal connections to gonadotropin-releasing hormone neurons in the brain: a novel medium for seasonal breeding in the sheep. Endocrinology 149:5770–5782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smith JT, Shahab M, Pereira A, Pau KY, Clarke IJ. 2010. Hypothalamic expression of KISS1 and gonadotropin inhibitory hormone genes during the menstrual cycle of a non-human primate. Biol Reprod 83:568–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ubuka T, Lai H, Kitani M, Suzuuchi A, Pham V, Cadigan PA, Wang A, Chowdhury VS, Tsutsui K, Bentley GE. 2009. Gonadotropin-inhibitory hormone identification, cDNA cloning, and distribution in rhesus macaque brain. J Comp Neurol 517:841–855 [DOI] [PubMed] [Google Scholar]
- 17. Ubuka T, Morgan K, Pawson AJ, Osugi T, Chowdhury VS, Minakata H, Tsutsui K, Millar RP, Bentley GE. 2009. Identification of human GnIH homologs, RFRP-1 and RFRP-3, and the cognate receptor, GPR147 in the human hypothalamic pituitary axis. PLoS One 4:e8400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ducret E, Anderson GM, Herbison AE. 2009. RFamide-related peptide-3, a mammalian gonadotropin-inhibitory hormone ortholog, regulates gonadotropin-releasing hormone neuron firing in the mouse. Endocrinology 150:2799–2804 [DOI] [PubMed] [Google Scholar]
- 19. Wu M, Dumalska I, Morozova E, van den Pol AN, Alreja M. 2009. Gonadotropin inhibitory hormone inhibits basal forebrain vGluT2-gonadotropin-releasing hormone neurons via a direct postsynaptic mechanism. J Physiol 587:1401–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dardente H, Birnie M, Lincoln GA, Hazlerigg DG. 2008. RFamide-related peptide and its cognate receptor in the sheep: cDNA cloning, mRNA distribution in the hypothalamus and the effect of photoperiod. J Neuroendocrinol 20:1252–1259 [DOI] [PubMed] [Google Scholar]
- 21. Murakami M, Matsuzaki T, Iwasa T, Yasui T, Irahara M, Osugi T, Tsutsui K. 2008. Hypophysiotropic role of RFamide-related peptide-3 in the inhibition of LH secretion in female rats. J Endocrinol 199:105–112 [DOI] [PubMed] [Google Scholar]
- 22. Rizwan MZ, Porteous R, Herbison AE, Anderson GM. 2009. Cells expressing RFamide-related peptide-1/3, the mammalian gonadotropin-inhibitory hormone orthologs, are not hypophysiotropic neuroendocrine neurons in the rat. Endocrinology 150:1413–1420 [DOI] [PubMed] [Google Scholar]
- 23. Sari IP, Rao A, Smith JT, Tilbrook AJ, Clarke IJ. 2009. Effect of RF-amide-related peptide-3 on luteinizing hormone and follicle-stimulating hormone synthesis and secretion in ovine pituitary gonadotropes. Endocrinology 150:5549–5556 [DOI] [PubMed] [Google Scholar]
- 24. Clarke IJ, Smith JT, Henry BA, Oldfield BJ, Stefanidis A, Millar RP, Sari IP, Chng K, Fabre-Nys C, Caraty A, Ang BT, Chan L, Fraley GS. 25 January 2012. Gonadotropin-inhibitory hormone is a hypothalamic peptide that provides a molecular switch between reproduction and feeding. Neuroendocrinology 10.1159/000332822 [DOI] [PubMed] [Google Scholar]
- 25. Clarke IJ. 1995. Evidence that the switch from negative to positive feedback at the level of the pituitary gland is an important timing event for the onset of the preovulatory surge in LH in the ewe. J Endocrinol 145:271–282 [DOI] [PubMed] [Google Scholar]
- 26. Clarke IJ, Cummins JT, de Kretser DM. 1983. Pituitary gland function after disconnection from direct hypothalamic influences in the sheep. Neuroendocrinology 36:376–384 [DOI] [PubMed] [Google Scholar]
- 27. Clarke IJ, Cummins JT, Findlay JK, Burman KJ, Doughton BW. 1984. Effects on plasma luteinizing hormone and follicle-stimulating hormone of varying the frequency and amplitude of gonadotropin-releasing hormone pulses in ovariectomized ewes with hypothalamo-pituitary disconnection. Neuroendocrinology 39:214–221 [DOI] [PubMed] [Google Scholar]
- 28. Clarke IJ. 2002. Two decades of measuring GnRH secretion. Reprod Suppl 59:1–13 [PubMed] [Google Scholar]
- 29. Clarke IJ, Cummins JT. 1985. Increased gonadotropin-releasing hormone pulse frequency associated with estrogen-induced luteinizing hormone surges in ovariectomized ewes. Endocrinology 116:2376–2383 [DOI] [PubMed] [Google Scholar]
- 30. Smith JT, Rao A, Pereira A, Caraty A, Millar RP, Clarke IJ. 2008. Kisspeptin is present in ovine hypophysial portal blood but does not increase during the preovulatory luteinizing hormone surge: evidence that gonadotropes are not direct targets of kisspeptin in vivo. Endocrinology 149:1951–1959 [DOI] [PubMed] [Google Scholar]
- 31. Roh SG, Doconto M, Feng DD, Chen C. 2006. Differential regulation of GHRH-receptor and GHS-receptor expression by long-term in vitro treatment of ovine pituitary cells with GHRP-2 and GHRH. Endocrine 30:55–62 [DOI] [PubMed] [Google Scholar]
- 32. Wu D, Chen C, Zhang J, Bowers CY, Clarke IJ. 1996. The effects of GH-releasing peptide-6 (GHRP-6) and GHRP-2 on intracellular adenosine 3′,5′-monophosphate (cAMP) levels and GH secretion in ovine and rat somatotrophs. J Endocrinol 148:197–205 [DOI] [PubMed] [Google Scholar]
- 33. Lee VW, Cumming IA, de Kretser DM, Findlay JK, Hudson B, Keogh EJ. 1976. Regulation of gonadotrophin secretion in rams from birth to sexual maturity. I. Plasma LH, FSH and testosterone levels. J Reprod Fertil 46:1–6 [DOI] [PubMed] [Google Scholar]
- 34. Burger HG, Lee VW, Rennie GC. 1972. A generalized computer program for the treatment of data from competitive protein-binding assays including radioimmunoassays. J Lab Clin Med 80:302–312 [PubMed] [Google Scholar]
- 35. Kivipelto L, Majane EA, Yang HY, Panula P. 1989. Immunohistochemical distribution and partial characterization of FLFQPQRFamidelike peptides in the central nervous system of rats. J Comp Neurol 286:269–287 [DOI] [PubMed] [Google Scholar]
- 36. Clarke IJ. 1988. Gonadotrophin-releasing hormone secretion (GnRH) in anoestrous ewes and the induction of GnRH surges by oestrogen. J Endocrinol 117:355–360 [DOI] [PubMed] [Google Scholar]
- 37. Smith JT, Li Q, Yap KS, Shahab M, Roseweir AK, Millar RP, Clarke IJ. 2011. Kisspeptin is essential for the full preovulatory LH surge and stimulates GnRH release from the isolated ovine median eminence. Endocrinology 152:1001–1012 [DOI] [PubMed] [Google Scholar]
- 38. Keen KL, Wegner FH, Bloom SR, Ghatei MA, Terasawa E. 2008. An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology 149:4151–4157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Clarke I, Moore L, Veldhuis J. 2002. Intensive direct cavernous sinus sampling identifies high-frequency, nearly random patterns of FSH secretion in ovariectomized ewes: combined appraisal by RIA and bioassay. Endocrinology 143:117–129 [DOI] [PubMed] [Google Scholar]
- 40. Veldhuis JD, Johnson ML. 1994. Testing pulse detection algorithms with simulations of episodically pulsatile substrate, metabolite, or hormone release. Methods Enzymol 240:377–415 [DOI] [PubMed] [Google Scholar]
- 41. Poling MC, Kim J, Dhamija S, Kauffman AS. 2012. Development, sex steroid regulation, and phenotypic characterization of RFamide-related peptide (Rfrp) gene expression and RFamide receptors in the mouse hypothalamus. Endocrinology 153:1827–1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Caraty A, Blomenrohr M, Vogel GM, Lomet D, Briant C, Beltramo M. 27 January 2012. RF9 powerfully stimulates gonadotrophin secretion in the ewe: evidence for a seasonal threshold of sensitivity. J Neuroendocrinol 10.1111/j.1365-2826.2012.02283.x [DOI] [PubMed] [Google Scholar]
- 43. Ancel C, Bentsen AH, Sébert ME, Tena-Sempere M, Mikkelsen JD, Simonneaux V. 2012. Stimulatory effect of RFRP-3 on the gonadotrophic axis in the male Syrian hamster: the exception proves the rule. Endocrinology 153:1352–1363 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.