Abstract
Although estrogens are neuroprotective in young adult animal models of stroke, clinical trials demonstrate that estrogens increase the incidence and severity of stroke in aged women. We have previously shown that experimental stroke pathophysiology differs between young adult and aged rats. The aim of this study was to determine the effects of 17β-estradiol after middle cerebral artery occlusion and reperfusion in young adult and aged female rats. Focal embolic stroke was performed by middle cerebral artery occlusion with fibrin clot followed by reperfusion with iv human recombinant tissue plasminogen activator. Histological and functional outcomes were measured at 24 h after middle cerebral artery occlusion with fibrin clot. Aged rats treated with 17β-estradiol had significantly increased infarct volumes compared with placebo-treated aged rats. Young adult rats treated with 17β-estradiol had significantly decreased infarct volumes and improved functional outcome compared with ovariectomized young adult rats. Our results suggest that 17β-estradiol may act in an age-dependent manner in the postischemic rat brain. In young adult rats, it is neuroprotective; chronic treatment with 17β-estradiol during aging leads to worsened ischemic brain injury in aged female rats.
The Women's Health Initiative (WHI) showed for the first time in a large-scale clinical trial that hormone replacement therapy (HRT) was linked to higher incidence of stroke (1). Meta-analyses of the WHI and other smaller clinical trials demonstrate that women taking estrogens experience greater stroke severity (2, 3). These findings contradict data from prior animal studies indicating a neuroprotective role of estrogens (4–12) and raise concerns with regard to the safe and appropriate use of HRT.
Aging may be a contributing factor accounting for the contrast between animal studies and clinical trial findings. Previous studies from our laboratory underscore the importance of creating clinically relevant models of disease, which in the case of ischemic stroke includes the use of aged animals. Ischemic stroke in aged rats is characterized by increased ischemia/reperfusion injury, earlier disruptions of the blood-brain barrier, exacerbated neuronal degeneration, higher mortality, and reduced functional outcome (13–15).
In the present study, we compared the effects of estradiol (E2) administration on ischemia/reperfusion injury and functional recovery after an experimental stroke between young adult and aged female rats. Using a model of transient ischemic stroke with reperfusion, we confirmed the findings from previous studies showing that E2 acts as a neuroprotectant in young adult female rats. However, chronic E2 administration in aged female rats led to worsened ischemic brain injury and increased mortality after an experimental stroke. We also demonstrate that E2 treatment in aged female rats acts to reduce postischemic astrocyte reactivity and decreases the expression of estrogen receptor (ER)-α. The results presented in this study parallel the findings of the WHI in a rat model of ischemic stroke and reperfusion and suggest a potential age-related difference in the effects of E2 on stroke.
Materials and Methods
Chemicals and animals
Isoflurane was purchased from Halocarbon (River Edge, NJ). Human recombinant tissue plasminogen activator (tPA) was gifted from Genentech (South San Francisco, CA). Pellets containing E2 or placebo (1.5 mg; 90 d release) were purchased from Innovative Research of America (Sarasota, FL). Sixty-five female Sprague Dawley rats [3 months (n = 20) and 9 months (n = 45)] were purchased from Hilltop Animal Laboratory (Scottdale, PA) and housed under 12-h light, 12-h dark conditions with food and water provided ad libitum. All procedures were in accordance with guidelines set forth by the West Virginia University Animal Care and Use Committee.
Treatment protocol
Rats were randomly divided, within age groups, into three treatment groups. E2 rats received bilateral ovariectomy and were implanted with E2 pellets. Intact rats received a sham ovariectomy and were implanted with placebo pellets. Ovariectomized (OVX) rats received bilateral ovariectomy and were implanted with placebo pellet. E2 administration to 3-month-old rats lasted for 30 d and pellets in 9-month-old rats were replaced every 90 d until rats reached 18 months of age. Whole blood was collected from rats at 4 and 18 months of age and allowed to clot at room temperature for 90 min before centrifugation at 2000 × g for15 min. Serum was transferred into clean tubes and samples were shipped to University of Virginia Ligand Core (Charlottesville, VA) for analysis. Serum E2 was measured in duplicate using [I125]RIA with reported detection limits of 5–6000 pg/ml (Beckman Coulter, Inc., Brea, CA).
All rats underwent a transient ischemic stroke by occlusion of the right middle cerebral artery (MCAO) with tPA (5 mg/kg; iv) reperfusion (14). Young adult intact rats were confirmed to be in metestrus at the time of MCAO. After anesthesia induction with isoflurane (4% induction, 2% maintenance) and sterile preparation, a craniotomy was performed for placement of laser Doppler blood flow monitoring. Access to the middle cerebral artery (MCA) through the internal carotid artery was achieved by ligating and cutting the external carotid artery and using it for insertion of a modified PE10 tubing catheter containing a fibrin clot. Fibrin clot was collected from a blood donor animal 1 d before surgery and cleansed of excess red blood cells immediately before insertion. After verification of catheter placement in the proximal MCA by a drop in laser Doppler recording, a clot was injected. Ischemia was defined by a perfusion drop across the MCA territory of greater than 80% as determined by laser Doppler. Ischemia lasted 2 h, and during the entire procedure, rats received 0.9% saline through femoral iv at a rate of 1 ml/h. Reperfusion was achieved by iv injection of tPA (5 mg/kg) into a saline pump with an initial 30% bolus followed by injection of the remaining dose over 30 min. Successful reperfusion was denoted as a return to greater than 80% of baseline perfusion rate by 30 min after tPA administration. Rats not achieving these standards for depth and duration of ischemia as well as reperfusion were excluded from the study. At 24 h after MCAO, functional assessment was measured by an investigator blinded to the treatment using the modified Neurological Severity Score (mNSS) (16).
Determination of infarct volume and neuropathology
At 24 h after MCAO, rats were anesthetized with isoflurane and transcardially perfused with 100 ml of 0.9% saline, and brains were removed and sliced coronally in 2-mm intervals. Sections were incubated with 2% 2,3,5-triphenyltetrazolium chloride (TTC) for 15 min at 37 C for determination of infarct volume using methods previously described (17). After TTC staining, brain slices were fixed in 10% neutral-buffered formalin overnight. Formalin-fixed brain sections were embedded in paraffin, cut into 8-μm sections, and mounted onto slides. Mounted sections were labeled for reactive astrocytes using antiglial fibrillary acidic protein (GFAP; 1:1000; Dako, Glostrup, Denmark), microglia using antiionic calcium binding protein (Iba-1; 1:500; Wako, Richmond, VA), and ERα using anti-ERα (1:100; Abcam, Cambridge, MA). Secondary antibody for GFAP, Iba-1, and ERα was biotinylated antirabbit IgG (1:500; Vector Laboratories, Burlingame, CA). Immunolabeling was visualized by horseradish peroxidase avidin D (1:1000; Vector Laboratories) coupled with 3,3′ diaminobenzidine chromagen kit (Vector Laboratories). Microscopic analysis of immunostained sections was performed using an Olympus AX70 Provis microscope (Center Valley, PA). Optical densitometry was performed on every fifth section from the infarcted region using Image J (U.S. National Institutes of Health, Bethesda, MD).
Statistical analysis
Data are presented as mean ± se. Statistical significance in measured and calculated parameters was determined using one-way ANOVA with Tukey's post hoc analyses. Changes in weight gain were assessed using one-way ANOVA with repeated measures. Differences in mortality were assessed using χ2 analysis. Level of significance was set at P < 0.05.
Results
Confirmation of three distinct treatment groups in 18-month-old rats
Serum E2 levels were measured from blood collected before MCAO (Table 1). In aged rats, the three treatment groups could be differentiated by serum E2 levels. Aged E2 rats had elevated serum E2 levels compared with intact rats and OVX rats. In addition, periodic weighing of aged rats showed a significant increase in weight in aged OVX rats compared with that measured in intact and E2 counterparts (Table 2). Serum E2 levels in young adult rats showed that OVX rats had a significant reduction in serum E2 compared with both E2 and intact rats (Table 2). No difference in serum E2 was measured between young adult E2 and young adult intact rats.
Table 1.
Pre-MCAO serum E2 and mortality at 24 h after MCAO
| Serum E2 (pg/ml) | Mortality (%) | |
|---|---|---|
| Young adult intact | 18.1 ± 3.2 | 0 |
| Young adult OVX | 6.3 ± 0.7a,b | 17a,b |
| Young adult E2 | 25.8 ± 1.5 | 0 |
| Aged intact | 16.4 ± 3.0 | 40 |
| Aged OVX | 5.2 ± 0.2 | 33 |
| Aged E2 | 36.6 ± 3.2a,c | 60c |
P < 0.05 as compared with intact.
P < 0.05 as compared with E2.
P < 0.05 as compared with OVX.
Table 2.
Weight over time of aged female rats
| Weight (g) |
||||
|---|---|---|---|---|
| Before | 12 months | 15 months | 18 months | |
| Intact | 342 ± 14 | 361 ± 7 | 398 ± 13 | 400 ± 13 |
| OVX | 347 ± 12 | 474 ± 16a, b | 500 ± 16a, b | 540 ± 14a, b |
| E2 | 352 ± 11 | 377 ± 9 | 396 ± 9 | 423 ± 27 |
OVX, Ovariectomized; E2, estrogen-treated.
P < 0.05 as compared with intact.
P < 0.05 as compared with E2.
E2 is neuroprotective in 4-month-old rats after transient ischemia and reperfusion
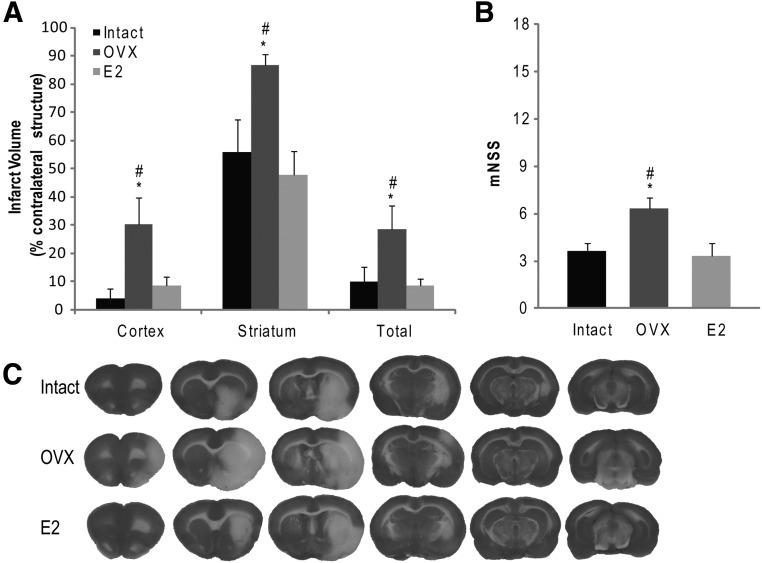
In young adult rats, E2 or placebo administration lasted for 30 d at which time rats underwent MCAO with tPA reperfusion (n = 6/group). Consistent with prior studies, our results demonstrated a significant reduction in cortical (P = 0.030) and striatal (P = 0.018) infarct volumes at 24 h after MCAO compared with young adult OVX rats. No difference in cortical (P = 0.728) or striatal (P = 0.997) infarct volumes were measured between young adult E2 and young adult intact rats. Young adult E2 rats demonstrated a significant improvement in functional outcome, as measured by mNSS compared with young adult OVX rats (P = 0.028) (Fig. 1B). Of the 20 young adult rats in the study, two rats died during MCAO surgery without confirmed infarction and were excluded from further analysis. All remaining young adult rats met the ischemia/reperfusion criteria and were included in the study. The mortality rate from ischemic stroke in young adult rats was 17% in the OVX group and 0% in both the intact and E2 groups (Table 2).
Fig. 1.
Lack of endogenous or supplemented estrogen leads to increased infarct volume in young adult rats. A, Young adult OVX rats demonstrated increased infarct size after MCAO with tPA reperfusion compared with both intact and E2 counterparts. Cortical, striatal, and total infarct size were all significantly increased in young adult OVX rats compared with both young adult intact and young adult E2 rats. B, Lack of endogenous or supplemented estrogen led to worsened functional outcome in young adult rats. Young adult OVX rats showed higher scores on the mNSS, indicative of worsened functional outcome. There was no significant difference in mNSS scores between intact and E2 rats. C, Representative TTC-stained brain slices show the larger infarct area in young adult OVX rats compared with intact and E2 counterparts. *, P < 0.05 compared with intact; #, P < 0.05 compared with E2.
E2 exacerbates ischemic brain injury in 18-month-old rats after ischemia and reperfusion
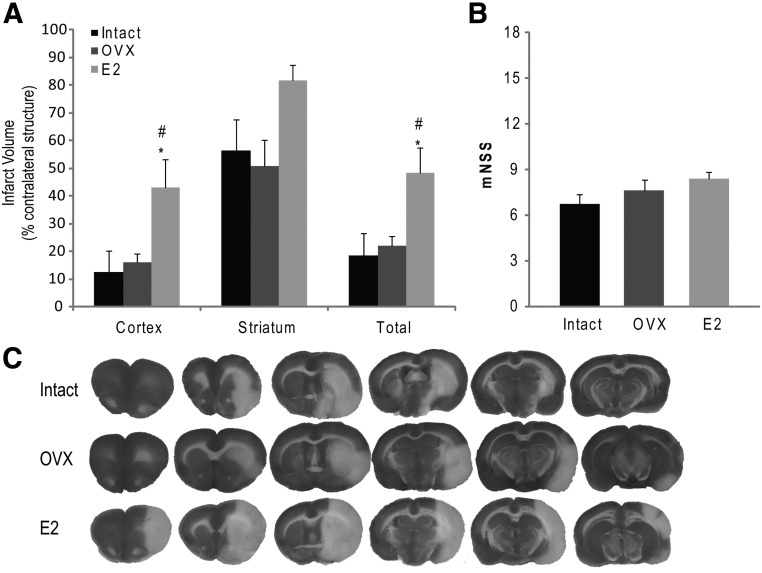
In aged rats, we measured a significant increase in total infarct volume in E2 rats compared with OVX rats (P = 0.041) and intact rats (P = 0.028) (Fig. 2A), with the greatest difference in infarction volume in the cortex. No difference in striatal infarct volume between the treatment groups was measured. Assessment of functional outcome by mNSS showed no difference in neurological score between treatment groups at 24 h after MCAO (Fig. 2B). Of the 45 aged rats in the study, three died before reaching 18 months of age, five were excluded from the analysis for not meeting ischemia/reperfusion inclusion criteria. Of the remaining 37 rats, nine E2, five OVX, and six intact rats met the inclusion criteria for ischemia/reperfusion but died before 24 h (n = 6 per treatment group used for infarct volume analysis). A significant increase in the mortality rate was observed in the aged E2 rats compared with the aged OVX rats (P = 0.0285) (Table 2).
Fig. 2.
Estrogen treatment leads to increased cortical and total infarct volume in aged rats. A, E2 rats demonstrate increased cortical and total infarct after MCAO with tPA reperfusion. There was no significant difference in striatal infarct volume between treatment groups. B, E2 treatment did not significantly alter functional outcome. Aged E2 rats did not show worsened functional outcome despite having increased cortical and total infarct volume. C, Representative brain slices from intact and E2-treated rats demonstrating a significant increase in infarcted tissue in E2 rats. C, Representative TTC-stained brain slices show greater infarct area in aged E2 rats compared with intact and OVX counterparts. *, P < 0.05 compared with intact; #, P < 0.05 compared with OVX.
E2 reduces astrogliosis after ischemia and reperfusion
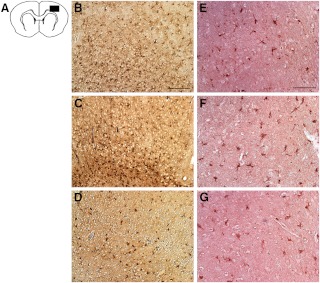
Reactive astrocytes were labeled using GFAP and were most prevalent in ipsilateral striatum and cortex in all aged treatment groups. Compared with aged intact and OVX rats, E2 rats showed reduced astrocyte abundance in the ipsilateral hemisphere (Fig. 3). Optical densitometry of GFAP stained sections confirmed significantly less GFAP in sections from E2 rats compared to that from both Intact (P < 0.001)and OVX (P < 0.001) (Fig. 4). There was no significant difference between sections from Intact and OVX rats (P = 0.633). Abundance of microglia was similar in all rats, with microglia in the ipsilateral cortex displaying the phagocytic phenotype (Fig. 3 and 4). No morphological differences in microglia were observed between the aged E2, OVX, and intact groups.
Fig. 3.
Neurodegeneration and reduced astrogliosis in the ischemic hemisphere of aged E2 rats. Postischemic reactive astrogliosis was observed using anti-GFAP and revealed reduced astrogliosis in the ischemic cortex of aged E2 rats (D) compared with both intact (B) and OVX counterparts (D). Inflammatory response was assessed in the ischemic hemisphere with immunostaining of microglia. No effect of treatment was observed in microglia abundance in the ischemic cortex (D–F). Scale bar, 100 μm.
Fig. 4.
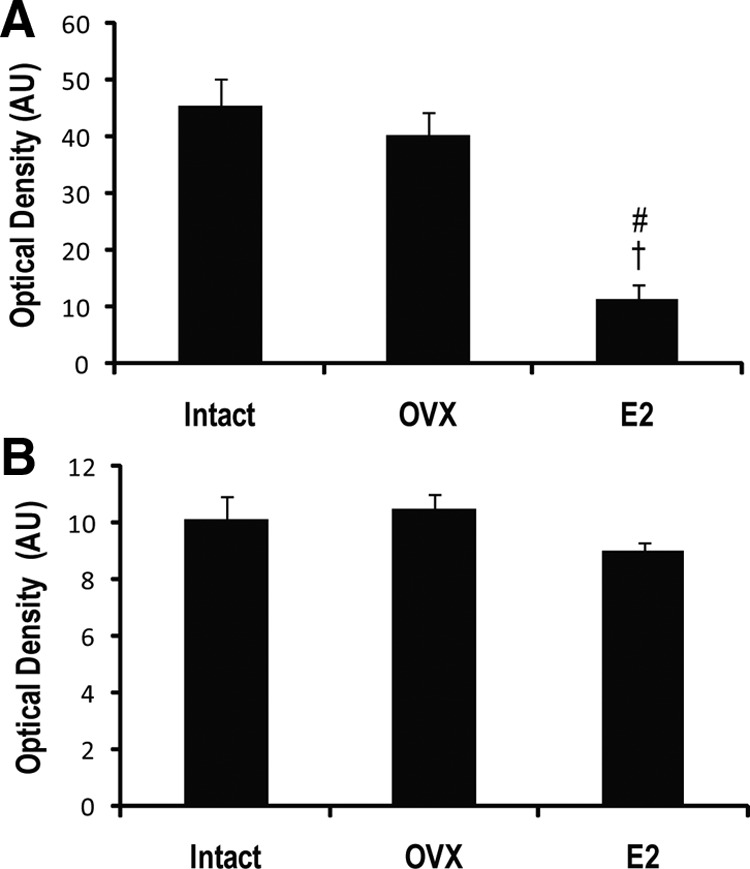
Lower density of GFAP immunostaining in aged E2 rats. A, Optical densitometry confirmed a significantly reduced density of GFAP immunostaining in ischemic cortex of aged E2 rats compared with both intact and OVX counterparts. B, No significant differences in immunostaining density of Iba-1 were observed in the ischemic cortex of the aged treatment groups. *, P < 0.001 compared with intact; †, P < 0.001 as compared with OVX.
Chronic E2 supplementation leads to decreased ERα expression
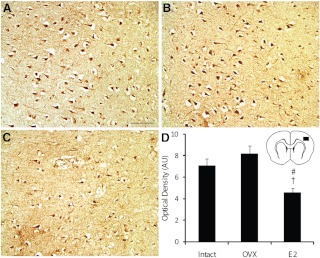
Previous studies have suggested that estrogen-mediated neuroprotection in young adult animals is mediated by ERα. To determine whether a correlation exists between ERα expression or whether it may play a role in the deleterious effects of E2 in the aged female rat brain, we immunolabeled brain sections with ERα antibody (Fig. 5). In all brain sections studied, ERα immunoreactivity was restricted to the infarcted hemisphere. No ERα immunoreactivity was observed in the contralateral hemisphere. In the ischemic cortex, aged E2 rats had reduced numbers of ERα-labeled cells compared with both aged intact and aged OVX rats (Fig. 5D). In the ischemic striatum, no differences in the number of ERα-labeled cells between treatment groups were observed (data not shown).
Fig. 5.
Estrogen supplementation leads to reduced ERα in aged female rats. ERα-positive cells are more abundant in the ischemic cortex of intact (A) and OVX rats (B) compared with E2 rats (C). The area of the cortex represented in photomicrographs is indicated. Optical densitometry demonstrates significantly less ERα immunoreactivity in the E2 rats (D). *, P < 0.01 compared with intact; #, P < 0.01 compared with OVX. Scale bar, 50 μm.
Discussion
The present study showed that female rats treated with E2 through the transition to reproductive senescence and into advanced age have worsened stroke injury and increased mortality after experimental stroke injury. Previous reports have shown that E2 administered before experimental stroke is neuroprotective in young adult rats. Using sc implanted time-release E2 pellets, we confirmed neuroprotection by E2 in young adult rats using a 2 h embolic occlusion of the middle cerebral artery with tPA reperfusion. In contrast, female rats ovariectomized at 9 months of age and administered similar E2 pellets until 18 months of age experienced higher mortality within 24 h of stroke injury. E2 rats surviving 24 h after MCAO showed increased infarct volume (most pronounced in the cortex). Previous reports using younger senescent rats and an endothelin-1 induced occlusion of the MCA have shown similar results (18). This first part of our study builds on those findings by implementing a longer E2 treatment regimen, older female rats, and a clinically relevant thromboembolic stroke model, and supports the concept that E2 effects on the postischemic brain differ over the life span.
In the second part of our study, we investigated potential mechanisms of estrogenic effects on our aged, postischemic rat brains, specifically, postischemic astrocyte activation, and ERα expression. Estrogenic effects on postischemic astrocyte response have been studied using both traditional immunohistochemistry (19) and biophotonic/bioluminescent imaging (20) and demonstrate marked reductions in GFAP-positive cells in animals treated with E2 (21). These prior studies have shown neuroprotective effects of estrogen along with alterations in GFAP. Using GFAP immunostaining in our postischemic aged rat brains, we also found fewer GFAP-positive cells in the ischemic hemisphere of aged E2 rats compared with intact and OVX counterparts. In our study, the reduction in GFAP immunostaining was accompanied by worsened infarct in aged E2 rats. One potential explanation for this discrepancy is an age-related change in the effects of estrogen after ischemia. The specific pathway mediating these effects has not been elucidated. Although the role of reactive astrocytes in the injured brain remains unclear, the absence of GFAP protein in knockout mice leads to higher mortality and an altered astrocyte response to a penetrating brain lesion (22). The increased ischemic brain injury in our E2 rats and the subsequent reduction in GFAP-positive astrocytes suggest a potentially beneficial role of reactive astrocytes in ischemic stroke pathophysiology.
Previous studies have investigated ER, particularly ERα, in the response to stroke injury. Evidence exists that ERα is responsible for neuroprotective effects of estrogens in young adult rats (8, 23). ERα knockout mice administered E2 show a complete reversal of its neuroprotective effects, whereas no differences in E2-mediated neuroprotection are noted in ERβ knockout mice, indicating a prominent role of ERα in the neuroprotective action of E2 (24, 25). Chronic E2 supplementation may result in reductions in estrogen receptors that, in ischemic conditions, may have deleterious effects. To determine whether chronic E2 administration alters the expression levels of ERα in aged rats, we immunostained brain sections from our aged rat groups using ERα antibody. Worsened ischemic injury in E2-treated rats was accompanied by reduced ERα expression. Further studies are needed to determine whether there is a causal relationship between increased infarct volume and decreased ERα expression in aged E2-treated rats.
This study used a number of approaches to improve our understanding of estrogenic effects on focal ischemic stroke by more accurately modeling the clinical presentation. First, E2 rats received treatment starting at 9 months of age and physiological E2 levels were maintained until 18 months of age, thereby avoiding any prolonged period of hypoestrogenicity. Using this approach, we were able to investigate what has become known as the timing of initiation hypothesis, which claims that a prolonged period of hypoestrogenicity following reproductive senescence leads to irreversible vascular changes that are not mimicked in ovariectomized animals immediately supplemented with E2 (26–28). According to proponents of this hypothesis, HRT could be used safely if treatment begins close to the time of reproductive senescence onset. However, our results show that E2 administration initiated before reproductive senescence and continued into advanced age offered no neuroprotection and, in fact, led to higher mortality and increased infarct size after MCAO.
In addition to the clinically relevant E2 treatment paradigm in this study, our model of stroke also accounts for the effects of age on stroke outcome. Our laboratory and others have repeatedly demonstrated that ischemic stroke injury and recovery in aged rats is markedly different from that in younger animal models of ischemic injury (14, 15, 29), similar to the age-related differences in stroke outcome observed in patients (30). By using 18-month-old rats, we are able to more accurately represent the clinical population at greatest risk for and accounting for the majority of stroke patients. In addition, this study used a method of focal cerebral ischemia implementing embolic occlusion of the middle cerebral artery with tissue plasminogen activator reperfusion to more closely model the clinical manifestation of thromboembolic stroke treated with tPA. Although age-related differences in the postischemic response to E2 therapy have been reported previously, the present study is the first to use a prolonged E2 treatment regimen combined with a fibrin clot model of MCAO with tPA reperfusion.
Despite the clinical relevance of our model, there are several caveats to be considered in the present study. Aged OVX rats had significantly higher weight at time of MCAO compared with both intact and E2 rats (Table 1). Increased adiposity in OVX rats could potentially alter physiological parameters such as vascular tone, which were not accounted for in this study. This difference in weight may also have resulted in alterations in circulating levels of estrone and E2 due to conversion of androstenedione and testosterone in adipose tissue (31). However, levels of E2 measured from blood collected at time the animals were euthanized demonstrated E2 levels below the detectable range in OVX rats, indicating a negligible influence of peripheral conversion. Local conversion of steroid hormones to E2 by the enzyme aromatase may also play a role in stroke pathophysiology (32) but was not measured in this study. Local aromatase expression has been shown to increase after neural injury; thus, future experiments may involve postischemic quantification of this enzyme (32, 33). Despite differences in infarct size, aged rats showed no significant differences in functional outcome after MCAO as measured by the mNSS. This result may be due to limitations of that assessment in aged rats. Although useful in young adult rats, the mNSS requires significant motor coordination to perform many of the subtests in the assessment. Furthermore, due to the high mortality in the aged E2 rats, both infarct volumes and mNSS scores may be underestimated by a survivor bias.
An additional consideration arises from the use of time-release pellets for hormone administration. Upon implantation, serum E2 levels reportedly increase rapidly followed by a slow decline in hormone level, which differs from the more constant hormone levels achieved by oral supplementation in women. Likewise, the E2 used in the laboratory setting differs from commonly prescribed hormone therapy. Recent reviews suggest that differing methods of estrogen administration in animal studies may result in unpredicted levels of the hormone, which are often times supraphysiological. An analysis of past studies demonstrates that supraphysiological levels of E2 enhance the experimental neural injury, whereas physiological levels of E2 exert neuroprotective action, thus emphasizing the need for highly regulated experimental parameters (34).
Conclusion
In the present study, we demonstrate deleterious effects of E2 treatment in an aged animal model of ischemic stroke, which parallel the findings of the WHI (1). We also report that worsened stroke injury in aged female rats was accompanied by down-regulation of ERα. Although further investigation is warranted to gain a greater understanding of the mechanism(s) of estrogenic actions on the aged brain after ischemic injury, the development and use of a model that replicates the clinical findings is an essential component for progress in the field.
Acknowledgments
The authors thank Genentech for their kind gift of tPA (OR-208707) to perform these studies and Karen Martin, PhD of the WVU Miscroscopic Imaging Facility (P20 RR016440) for assistance with microscopy. We also thank the National Institutes of Health for support of this project through the National Institute of Neurological Diseases and Stroke (RO1NS061954 to J.D.H.).
Disclosure Summary: The authors do not have any actual or potential conflicts of interest to disclose that could have inappropriately influenced this work.
Footnotes
- E2
- Estradiol
- ER
- estrogen receptor
- GFAP
- glial fibrillary acidic protein
- HRT
- hormone replacement therapy
- Iba-1
- ionic calcium binding protein
- MCA
- middle cerebral artery
- MCAO
- occlusion of the right middle cerebral artery
- mNSS
- modified Neurological Severity Score
- OVX
- ovariectomized
- tPA
- tissue plasminogen activator
- TTC
- 2,3,5-triphenyltetrazolium chloride
- WHI
- Women's Health Initiative.
References
- 1. Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, Baird A, Kotchen T, Curb JD, Black H, Rossouw JE, Aragaki A, Safford M, Stein E, Laowattana S, Mysiw WJ. 2003. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women's Health Initiative: a randomized trial. JAMA 289:2673–2684 [DOI] [PubMed] [Google Scholar]
- 2. Bath PM, Gray LJ. 2005. Association between hormone replacement therapy and subsequent stroke: a meta-analysis. BMJ 330:342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sare GM, Gray LJ, Bath PM. 2008. Association between hormone replacement therapy and subsequent arterial and venous vascular events: a meta-analysis. Eur Heart J 29:2031–2041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Simpkins JW, Rajakumar G, Zhang YQ, Simpkins CE, Greenwald D, Yu CJ, Bodor N, Day AL. 1997. Estrogens may reduce mortality and ischemic damage caused by middle cerebral artery occlusion in the female rat. J Neurosurg 87:724–730 [DOI] [PubMed] [Google Scholar]
- 5. Dubal DB, Kashon ML, Pettigrew LC, Ren JM, Finklestein SP, Rau SW, Wise PM. 1998. Estradiol protects against ischemic injury. J Cereb Blood Flow Metab 18:1253–1258 [DOI] [PubMed] [Google Scholar]
- 6. Toung TJ, Traystman RJ, Hurn PD. 1998. Estrogen-mediated neuroprotection after experimental stroke in male rats. Stroke 29:1666–1670 [DOI] [PubMed] [Google Scholar]
- 7. Culmsee C, Vedder H, Ravati A, Junker V, Otto D, Ahlemeyer B, Krieg JC, Krieglstein J. 1999. Neuroprotection by estrogens in a mouse model of focal cerebral ischemia and in cultured neurons: evidence for a receptor-independent antioxidative mechanism. J Cereb Blood Flow Metab 19:1263–1269 [DOI] [PubMed] [Google Scholar]
- 8. Merchenthaler I, Dellovade TL, Shughrue PJ. 2003. Neuroprotection by estrogen in animal models of global and focal ischemia. Ann NY Acad Sci 1007:89–100 [DOI] [PubMed] [Google Scholar]
- 9. Rau SW, Dubal DB, Böttner M, Gerhold LM, Wise PM. 2003. Estradiol attenuates programmed cell death after stroke-like injury. J Neurosci 23:11420–11426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hoffman GE, Merchenthaler I, Zup SL. 2006. Neuroprotection by ovarian hormones in animal models of neurological disease. Endocrine 29:217–231 [DOI] [PubMed] [Google Scholar]
- 11. Connell BJ, Crosby KM, Richard MJ, Mayne MB, Saleh TM. 2007. Estrogen-mediated neuroprotection in the cortex may require NMDA receptor activation. Neuroscience 146:160–169 [DOI] [PubMed] [Google Scholar]
- 12. Lebesgue D, Chevaleyre V, Zukin RS, Etgen AM. 2009. Estradiol rescues neurons from global ischemia-induced cell death: multiple cellular pathways of neuroprotection. Steroids 74:555–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rosen CL, Dinapoli VA, Nagamine T, Crocco T. 2005. Influence of age on stroke outcome following transient focal ischemia. J Neurosurg 103:687–694 [DOI] [PubMed] [Google Scholar]
- 14. Dinapoli VA, Rosen CL, Nagamine T, Crocco T. 2006. Selective MCA occlusion: a precise embolic stroke model. J Neurosci Methods 154:233–238 [DOI] [PubMed] [Google Scholar]
- 15. DiNapoli VA, Huber JD, Houser K, Li X, Rosen CL. 2008. Early disruptions of the blood-brain barrier may contribute to exacerbated neuronal damage and prolonged functional recovery following stroke in aged rats. Neurobiol Aging 29:753–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li Y, Chen J, Chen XG, Wang L, Gautam SC, Xu YX, Katakowski M, Zhang LJ, Lu M, Janakiraman N, Chopp M. 2002. Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology 59:514–523 [DOI] [PubMed] [Google Scholar]
- 17. Yang Y, Shuaib A, Li Q. 1998. Quantification of infarct size on focal cerebral ischemia model of rats using a simple and economical method. J Neurosci Methods 84:9–16 [DOI] [PubMed] [Google Scholar]
- 18. Selvamani A, Sohrabji F. 2010. Reproductive age modulates the impact of focal ischemia on the forebrain as well as the effects of estrogen treatment in female rats. Neurobiol Aging 31:1618–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barreto G, Veiga S, Azcoitia I, Garcia-Segura LM, Garcia-Ovejero D. 2007. Testosterone decreases reactive astroglia and reactive microglia after brain injury in male rats: role of its metabolites, oestradiol and dihydrotestosterone. Eur J Neurosci 25:3039–3046 [DOI] [PubMed] [Google Scholar]
- 20. Cordeau P, Jr, Lalancette-Hébert M, Weng YC, Kriz J. 2008. Live imaging of neuroinflammation reveals sex and estrogen effects on astrocyte response to ischemic injury. Stroke 39:935–942 [DOI] [PubMed] [Google Scholar]
- 21. Barreto G, Santos-Galindo M, Diz-Chaves Y, Pernía O, Carrero P, Azcoitia I, Garcia-Segura LM. 2009. Selective estrogen receptor modulators decrease reactive astrogliosis in the injured brain: effects of aging and prolonged depletion of ovarian hormones. Endocrinology 150:5010–5015 [DOI] [PubMed] [Google Scholar]
- 22. Pekny M, Eliasson C, Siushansian R, Ding M, Dixon SJ, Pekna M, Wilson JX, Hamberger A. 1999. The impact of genetic removal of GFAP and/or vimentin on glutamine levels and transport of glucose and ascorbate in astrocytes. Neurochem Res 24:1357–1362 [DOI] [PubMed] [Google Scholar]
- 23. Dubal DB, Rau SW, Shughrue PJ, Zhu H, Yu J, Cashion AB, Suzuki S, Gerhold LM, Bottner MB, Dubal SB, Merchanthaler I, Kindy MS, Wise PM. 2006. Differential modulation of estrogen receptors (ERs) in ischemic brain injury: a role for ERα in estradiol-mediated protection against delayed cell death. Endocrinology 147:3076–3084 [DOI] [PubMed] [Google Scholar]
- 24. Sampei K, Goto S, Alkayed NJ, Crain BJ, Korach KS, Traystman RJ, Demas GE, Nelson RJ, Hurn PD. 2000. Stroke in estrogen receptor-alpha-deficient mice. Stroke 31:738–743; discussion 744 [DOI] [PubMed] [Google Scholar]
- 25. Dubal DB, Zhu H, Yu J, Rau SW, Shughrue PJ, Merchenthaler I, Kindy MS, Wise PM. 2001. Estrogen receptor α, not β, is a critical link in estradiol-mediated protection against brain injury. Proc Natl Acad Sci USA 98:1952–1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wise PM, Dubal DB, Rau SW, Brown CM, Suzuki S. 2005. Are estrogens protective or risk factors in brain injury and neurodegeneration? Reevaluation after the Women's Health Initiative. Endocr Rev 26:308–312 [DOI] [PubMed] [Google Scholar]
- 27. Suzuki S, Brown CM, Dela Cruz CD, Yang E, Bridwell DA, Wise PM. 2007. Timing of estrogen therapy after ovariectomy dictates the efficacy of its neuroprotective and antiinflammatory actions. Proc Natl Acad Sci USA 104:6013–6018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rocca WA, Grossardt BR, Shuster LT. 2010. Oophorectomy, reproductive senescence, estrogen, and cognitive aging: the timing hypothesis. Neurodegener Dis 7:163–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Popa-Wagner A, Badan I, Vintilescu R, Walker L, Kessler C. 2006. Premature cellular proliferation following cortical infarct in aged rats. Rom J Morphol Embryol 47:215–228 [PubMed] [Google Scholar]
- 30. Balaban B, Tok F, Yavuz F, Yaar E, Alaca R. 2011. Early rehabilitation outcome in patients with middle cerebral artery stroke. Neurosci Lett 498:204–207 [DOI] [PubMed] [Google Scholar]
- 31. Lukanova A, Lundin E, Zeleniuch-Jacquotte A, Muti P, Mure A, Rinaldi S, Dossus L, Micheli A, Arslan A, Lenner P, Shore RE, Krogh V, Koenig KL, Riboli E, Berrino F, Hallmans G, Stattin P, Toniolo P, Kaaks R. 2004. Body mass index, circulating levels of sex-steroid hormones, IGF-I and IGF-binding protein-3: a cross-sectional study in healthy women. Eur J Endocrinol 150:161–171 [DOI] [PubMed] [Google Scholar]
- 32. McCullough LD, Blizzard K, Simpson ER, Oz OK, Hurn PD. 2003. Aromatase cytochrome P450 and extragonadal estrogen play a role in ischemic neuroprotection. J Neurosci 23:8701–8705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Peterson RS, Lee DW, Fernando G, Schlinger BA. 2004. Radial glia express aromatase in the injured zebra finch brain. J Comp Neurol 475:261–269 [DOI] [PubMed] [Google Scholar]
- 34. Strom JO, Theodorsson A, Theodorrsson E. 2011. Mechanism of estrogens' dose-dependent neuroprotective and neurodamaging effects in experimental models of cerebral ischemia. Int J Mol Sci 12:1533–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]