Abstract
Glycosaminoglycans (GAG) have diverse functions that regulate macromolecular assembly in the extracellular matrix. During pregnancy, the rigid cervix transforms to a pliable structure to allow birth. Quantitative assessment of cervical GAG is a prerequisite to identify GAG functions in term and preterm birth. In the current study, total GAG levels increased at term, yet the abundance, chain length, and sulfation levels of sulfated GAG remained constant. The increase in total GAG resulted exclusively from an increase in hyaluronan (HA). HA can form large structures that promote increased viscosity, hydration, and matrix disorganization as well as small structures that have roles in inflammation. HA levels increased from 19% of total GAG in early pregnancy to 71% at term. Activity of the HA-metabolizing enzyme, hyaluronidase, increased in labor, resulting in metabolism of large to small HA. Similar to mice, HA transitions from high to low molecular weight in term human cervix. Mouse preterm models were also characterized by an increase in HA resulting from differential expression of the HA synthase (Has) genes, with increased Has1 in preterm in contrast to Has2 induction at term. The Has2 gene but not Has1 is regulated in part by estrogen. These studies identify a shift in sulfated GAG dominance in the early pregnant cervix to HA dominance in term and preterm ripening. Increased HA synthesis along with hyaluronidase-induced changes in HA size in mice and women suggest diverse contributions of HA to macromolecular changes in the extracellular matrix, resulting in loss of tensile strength during parturition.
Understanding the molecular mechanisms of cervical remodeling is critical for development of therapies to prevent preterm birth (PTB). PTB accounts for 12.7% of all births in the United States and is a primary cause of infant morbidity and mortality in the first year of life and the root of lifelong health problems in survivors (1, 2). The cervix, a collagen-rich connective tissue, must remain closed during pregnancy, yet simultaneously undergo a progressive remodeling to prepare for birth. This remodeling can be loosely divided into four overlapping phases: 1) Softening: the slow progressive phase, beginning in early pregnancy when progesterone levels are high and estrogen levels are relatively low (3); 2) Ripening: the accelerated phase at the end of pregnancy when progesterone action is blunted and estrogen levels are high; 3) Dilation: with onset of uterine contractions, there is a maximal loss of tensile strength in the cervix, resulting in cervical opening to allow passage of a term fetus; 4) Postpartum repair: after parturition the cervix undergoes a recovery phase dominated by proinflammatory and immunosuppressive immune cells that likely facilitate tissue repair (4, 5).
Studies suggest that mechanisms of cervical remodeling are well conserved between human and mouse (6). In contrast, the mechanism of premature ripening differs from physiological ripening. Premature ripening induced by infection or a progesterone receptor antagonist occurs by mechanisms distinct from each other. These mechanisms also differ from term, physiological ripening as determined by differences in gene expression, immune cell populations, and collagen structure (7–10).
The cervical extracellular matrix (ECM) consists of numerous structural components including fibrillar collagens, elastin, matricellular proteins, proteoglycans, and glycosaminoglycans (GAG). Alterations in the structure and composition of the ECM accompany all phases of physiological cervical remodeling (6). Early in pregnancy there is a reduction in collagen cross-links and matricellular proteins. These alterations in the ECM likely contribute to the initial decline in cervical tensile strength during cervical softening (3, 11). Late in gestation, changes in collagen fibril structure and packing correlate with changes in GAG composition. These changes are hypothesized to contribute to the maximal loss of tensile strength of the term cervix and the ability of the cervix to dilate upon generation of coordinated uterine contractions (6, 12–14).
Five linear, complex, polydisperse GAG are made in mammalian systems. They include hyaluronan (HA), chondroitin sulfate (CS), dermatan sulfate (DS), heparan sulfate (HS), and keratan sulfate (KS). These highly hydrophilic macromolecules maintain tissue hydration and compressive strength. With the exception of HA, GAG chains are sulfated and covalently attached to a core protein; the ensuing macromolecules are termed “proteoglycans” (15). The structural and functional diversity of GAG is regulated by the type of GAG, size of the GAG chains, composition, and degree of sulfation, as well as the ability to bind collagen and other proteins including enzymes and growth factors (16–18). Given their multifactorial capabilities in other biological systems (16, 19–22), these macromolecules are proposed to play important and diverse roles in ECM remodeling of cervix during pregnancy as well as postpartum.
HA has previously been reported to play a role in cervical remodeling. During ripening, increased HA content results from increased expression of HA synthase 2 (Has2) mRNA in mice and women (13, 23). HA transitions in size from a predominantly high molecular weight HA before birth to an increase in low molecular weight HA during labor or postpartum. Large molecular weight (MW) HA can provide increased tissue viscoelasticity, whereas low MW HA has proinflammatory roles (23–25). The breakdown of HA from large to small MW is regulated by the enzyme hyaluronidase. Hyaluronidase activity has not been reported in the pregnant cervix; however, studies in rat and women report the efficacy of exogenous hyaluronidase application to the cervix in promotion of cervical dilation and decreasing the duration of labor (26, 27).
In addition to HA, sulfated GAG (sGAG) in numerous proteoglycans are expressed in the pregnant cervix. Transcripts encoding the core protein of several proteoglycans are unchanged in the human and murine cervix through pregnancy and parturition (11, 28). However, changes in abundance of the core protein or changes in the GAG chain (type, sulfation, and chain length) have not been well studied during pregnancy. Based on their roles in other biological systems, modulation of the GAG chain can affect collagen fibril packing and tissue viscoelasticity. For example, changes in sulfation pattern or chain length have been suggested to affect GAG binding to other molecules (29, 30). Given the potential importance of GAG composition to the mechanical properties of the cervix, in the current study we sought to quantify changes in GAG composition, size, and sulfation through normal pregnancy as well as in infection-mediated and non-infection-mediated preterm birth models.
Materials and Methods
Mice
Mice were housed under a 12-h light, 12-h dark photoperiod at 22 C and were of mixed strain (C57B6/129Sv). Mice in these studies were 2–6 months old and nulliparous. Female mice were housed with males from 0800 h to 1600 h and then checked for vaginal plugs. The day of plug formation was counted as d 0, and birth occurred on d 19. Most samples were collected at midday except for d 18 samples, which were collected in the evening of d 18. All studies were conducted in accordance with the standards of humane animal care as described in the NIH Guide for the Care and Use of Laboratory Animals. The research protocols were approved by the institutional animal care and research advisory committee.
Treatment of animals
Tissue collection
Cervices were isolated by dissection at the uterocervical junction, and all vaginal tissue was removed. For histological and immunohistochemical analysis, cervices were fixed in 4% paraformaldehyde (Sigma Aldrich, St. Louis, MO) and paraffin embedded.
Infection-induced preterm model
Preterm labor was induced in gestation d 15 mice as previously described (8). Lipopolysaccharide (LPS, 150 μg; Sigma) or sterile water (sham) was injected between two gestational sacs in the left uterine horn. The animals were euthanized after 6 h for cervical tissue collection. Performance of surgery under sterile conditions as described was found to be critical to prevent global activation of proinflammatory responses in the sham surgery controls. Under these conditions, PTB occurred on average 7–9 h after LPS injection but not sham surgery. To ensure initiation of PTB at a similar time point in gestation as the RU486 model, LPS was administered early on gestation d 15 (∼ 0700 h) and cervices were collected at midday on d 15.
Noninfection PTB model
RU486 (M 8046, Sigma), was used to simulate premature progesterone withdrawal. A dose of 0.5 mg/200 μl glyceryl trioleate was injected sc late on the night of gestation d 14, and cervical tissue was collected 12 h later on gestation d 15 because this dose will cause delivery 13–16 h after injection (31). As a 12-h vehicle control, 50 μl of ethanol in 150 μl of glyceryl trioleate were administered sc.
Tamoxifen treatment
Mice were anesthetized using Avertin. Tamoxifen pellets (5 mg pellet, 21-d release, Innovative Research of America, Sarasota, FL) were inserted sc in gestation d 15 mice, and the incision in the upper back was closed with sterile wound clips. Animals were killed on gestation d 18.
Ovariectomized nonpregnant model
Six to 8-wk old female mice were anesthetized with Avertin. Hair was shaved from an area on the lower back, and a single horizontal cut was made. The peritoneum above the right and left ovary was punctured and ovaries were removed. Steroid pellets (Innovative Research of America) were inserted in the upper back as described above: 17β-estradiol (E2) pellet (0.05 mg 30-d release) and/or progesterone (P4) pellet (0.05 mg 30-d release). The incisions were closed with wound clips. Cervical tissue was collected after 10 d. RU486 (0.5 mg) was injected 12 h before tissue collection. Each group (sham, E2, E2+P4, or E2+P4+RU486) included four to five mice.
Human samples
Human cervical tissues from nonpregnant women were obtained at the time of hysterectomy for benign gynecological indications with no cervical pathology. Cervical tissues and mucus from pregnant women were obtained at the time of cesarean hysterectomy for indications that did not involve cervical pathology using a protocol approved by the Institutional Review Board at the University of Texas Southwestern Medical Center. Cervical mucus was collected with an endobrush. Tissues and mucus were stored at −80 C until use. Although there was no histological evidence of infection in the not in labor (NIL) group, there was evidence of infection in some but not all of the in labor (IL) samples. Cervical Tissue. Nonpregnant cervical stromal-enriched tissue (n = 4) were from premenopausal women. Pregnant cervical stromal-enriched tissues were used from term NIL (n = 5) and term IL (n = 5). Cervical Mucus. Cervical mucus was collected from gestation wk 35–39, NIL (n = 6), and 40–42 wk, IL (n = 2).
Fluorophore-assisted carbohydrate electrophoresis (FACE)
Cervices were collected and wet and dry weight was taken before and after lyophilization, respectively. Tissues were then digested in 100 mm ammonium acetate with 0.0005% phenol red (pH 7.0) containing 0.25 mg/ml proteinase-K (Roche, Indianapolis, IN) for 4 h at 60 C. Proteinase K was inactivated by boiling and undigested tissues were pelleted by centrifugation. An aliquot of 12.5 μl supernatant equal to 0.125 mg dry weight of digested cervix was processed for FACE analysis as described elsewhere with minor modifications (32, 33).
HA and CS/DS disaccharides were generated by digestion with Hyaluronidase SD (10 mU) at 37 C for 1 h followed by Chondroitinase ABC (10 mU) for 2 h. HS was broken down into disaccharides by digestion with a mixture of heparitinase and heparitinase 1 and 2 (2.5mU per enzyme) with 100 mm calcium acetate at 37 C for 3 h. KS disaccharides were generated by Keratanase II (5 mU) and/or endo-β-galactosidases (5 mU) incubation for 3 h at 37 C. Corneas were used to monitor effectiveness of KS digestion. All of the GAG digesting enzymes were obtained from the Associates of Cape Cod (East Falmouth, MA). Digests of HA, CS/DS, or KS disaccharides were fluorescently labeled with 2-Aminoacridone (Invitrogen, Carlsbad, CA). Samples were incubated overnight at 37 C with 5 μl of 2.5 m NaCNBH4. 2-Aminoacridone and NaCNBH4 were added simultaneously to HS digests. After overnight incubation at 37 C, 2.5 μl of glycerol were added, and 5-μl samples were run on gels that were prepared as described elsewhere (32). Gels were imaged using a Fuji FLA-5000 phosphoimager. GAG levels were quantified by signal intensity using Fujifilm Multiguage 3.0 software. Standards containing known amounts of CS/DS disaccharides and HA were used to estimate individual GAG expression. To estimate the average chain length of CS/DS, the total amount of the CS/DS disaccharides were divided by the total amount of disaccharides at the nonreducing termini of the GAG chains that are calculated from chondroitinase ABC and mercuric ion-treated samples as previously described (32, 34).
HA MW gels
HA MW of mouse cervical tissues and human mucus were determined as described previously with some modifications (23). An aliquot equal to 0.4 mg dry weight of Proteinase-K-digested tissue or 1 mg of human mucus was treated with 3 μl of deoxyribonuclease (Ambion, Austin, TX) and 3 μl of ribonuclease (Roche) for 5 h at 37 C. Samples were boiled to inactivate enzymes, and GAG were precipitated in 80% ethanol at −20 C overnight. After centrifugation, pellets were resuspended in 15 μl of Tris-Na Acetate-EDTA (pH.7.9) and 3 μl loading buffer (0.2% Bromophenol Blue, 1 ml TAE buffer, and 8.5ml glycerol). Samples were run on a 1% agarose gel (Seakem HGT; Cambrex, Rockland, ME) made in TAE buffer. The gel was prerun for approximately 1 h at 100 V before loading samples and HA size standards (Hyalose, Oklahoma City, OK). After electrophoresis at 100 V, the gel was equilibrated in 30% ethanol for 30 min, followed by incubation in Stains-All solution 2.5 mg/ml (Sigma) overnight in the dark. Gel was destained in water until bands were visualized before scanning.
Hyaluronidase enzyme activity measurement by fluorescence resonance energy transfer (FRET)
Hyaluronidase enzyme activity was measured using a modified FRET-HA assay (35). The substrate, FRET-HA, was made by covalently conjugating fluorescent donor and acceptor molecules to the carboxylic acid functional groups of HA (sodium hyaluronate, Thermo Fisher Scientific, Pittsburgh, PA). HA was dissolved to 1.25 mg/ml in dH2O. The HA solution was diluted 1:2 in dimethylsulfoxide, and fluorescein amine and rhodamine B amine (both predissolved as dimethylsulfoxide stock solutions) were simultaneously added to final concentrations of 0.42 mg/ml for each of the fluorophores. Acetaldehyde and cyclohexyl isocyanide were added to 0.04% (vol/vol), and the reaction was allowed to proceed for 48 h at 25 C. Afterward, the solution was diluted 1:14 in ethanol/guanidine HCl (50 μl of 3 m guanidine HCl per 900 μl of 100% ethanol), and the HA was precipitated overnight at −20 C. The precipitate was then dissolved in 1 ml of dH2O, followed by extensive dialysis against dH2O. Purity of the dual-labeled HA conjugate was ascertained by applying the sample to a Sephadex G-75 size exclusion column.
Cervical tissue was homogenized in PBS (pH 6.0) with protease inhibitor cocktail (Thermo Fisher Scientific). Samples were centrifuged for 10 min at 16,000 × g. Protein concentration of supernatants was determined by the BCA protein assay Kit (Pierce Chemical Co., Rockford, IL). Protein (25 μg) and 10 μl of FRET-HA substrate were mixed in PBS (pH 6.0) making up a 250 μl mixture. Similarly, varying concentrations of bovine testes hyaluronidase (0–2000 mU/ml) and FRET-HA were prepared to generate a standard curve. Fluorescence was measured in a Tecan Safire 2 microplate reader and analyzed by Magellan software (Tecan, Männedorf Switzerland).
RNA isolation and quantitative real-time PCR
Total RNA was extracted using RNA Stat-60 (Tel-Test, Friendswood, TX) and treated with deoxyribonuclease I (DNA-Free; Ambion). cDNA synthesis was performed using 2 μg total RNA in a 100-μl volume (TaqMan cDNA synthesis kit; Applied Biosystems, Foster City, CA). Quantitative real-time PCR (qPCR) was performed using SYBR Green and a PRISM7900HT Sequence Detection System (Applied Biosystems, Foster City, CA). Aliquots (20 ng) of cDNA were used for each reaction and were run in triplicate. Each gene was normalized to the expression of the housekeeping genes cyclophilin or 36B4 (51). In the case of mouse studies, expression was calculated relative to gestation d 15 cervical tissues and in the human cervical tissue studies relative to nonpregnant tissue as the external calibrator in the 2-ΔΔCt method, as described in User Bulletin No. 2 (Applied Biosystems). Primer sequences used in the qPCR are provided in Supplemental Table 1 published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org).
Statistical analysis
Data were analyzed using one-way ANOVA with pair wise multiple comparisons performed with Dunnett's test for data normally distributed. Data are displayed as the mean ± sem. Values of P ≤ 0.05 are considered statistically significant.
Results
Dynamic changes in GAG composition during normal pregnancy are driven by a progressive increase in HA
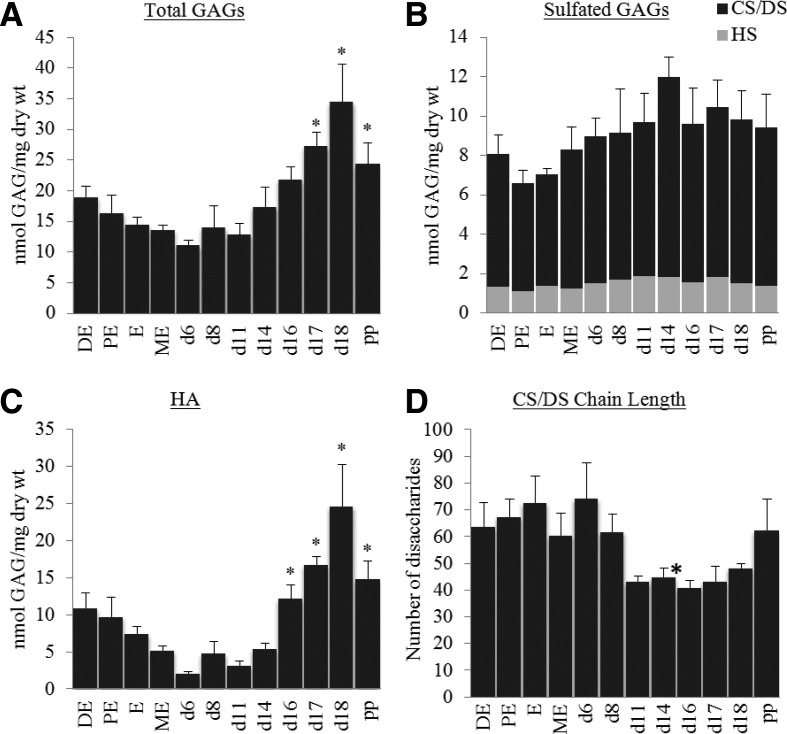
FACE analysis was used to quantify HA, CS/DS, HS, and KS. With the exception of KS, all other GAG were present in the nonpregnant and pregnant mouse cervix. Although there were modest changes in GAG over the nonpregnant estrous cycle, these changes were not significant. Compared with gestation d 6 the total amount of GAG increased toward the end of pregnancy and remained elevated up to 1 d postpartum (Fig. 1A, P > 0.05). Although CS/DS and HS levels remained constant, HA levels increased significantly at term and postpartum (Fig. 1, B and C; P > 0.05). Maximal levels of HA on gestation d 18 were almost 12-fold greater than gestation d 6. The chain length of CS/DS hybrid chains was estimated over the course of pregnancy (Fig. 1D). Although there was a trend for shorter chains toward the end of gestation, statistical significance was not achieved for most time points. Digestion of cervical extracts with keratanases (keratanase II and endo-β-galactosidase) did not yield disaccharides, suggesting little to no expression of KS in mouse cervix (data not shown). Cornea tissue was used as a positive control as this tissue is rich in KS-GAG.
Fig. 1.
Quantification of GAG content and chain length in nonpregnant and pregnant mice. Cervices were collected from cycled nonpregnant (DE, diestrus; PE, proestrus; E, estrus; and ME, metestrus), pregnancy d 6–d 18 and postpartum 1 d (pp) and analyzed by FACE. A, Total GAG expression (sum of HA, CS/ DS, and HS). B, Total sGAG (CS/DS and HS). C, Total HA. D, Estimation of chain length. CS/DS chain length ranges from 40–75 disaccharides. *, P < 0.05 when compared with pregnant d 6 cervix (n = 4–6 samples per time point).
In the cervix, CS/DS was sulfated predominantly at the 4-position (ΔDi-4s) with very low expression of ΔDi-6s or ΔDi-4s, 6s (Supplemental Fig 1, left panel). Heparin sulfate GAG in the cervix are comprised of disaccharides without sulfation as well as mono and multiple sulfated disaccharides (Supplemental Fig 1, right panel).
As illustrated in Fig. 2, there is a dynamic shift in the relative proportion of sulfated) vs. non-sGAG from nonpregnant to early pregnancy. In the nonpregnant estrous cervix, non-sGAG and sGAG were nearly equal. On gestation d 6, sGAG made up nearly 81% of total GAGs as HA levels were quite low. Subsequently, from early pregnancy to late pregnancy there was both a 3-fold increase in the total amount of GAG as well as a shift in the proportion of sGAG vs. non-sGAG. This shift results from the marked increase in HA, whereas sGAG remain relatively constant. In the postpartum period, whereas total GAG and HA levels begin to decline, HA still accounts for the majority of cervical GAG.
Fig. 2.
Dynamic changes in mouse cervical GAG composition in nonpregnant estrus, gestation d 6, gestation d 18, and postpartum samples. Most notable is the predominance of CS/DS GAG in early pregnancy (67%) as opposed to HA predominance (71%) in late pregnancy. Four to six samples were evaluated for each time point. NP, Not pregnant.
Hyaluronidase enzyme activity and HA size during late pregnancy in mouse
HA functions are dependent in part on its size. We have previously reported a shift from high-MW HA during cervical ripening (d 18) to low-MW HA postpartum (23). To better define the regulation of this shift, activity of the HA-metabolizing enzyme, hyaluronidase, was assayed by a FRET assay. Relative to gestation d 15, hyaluronidase activity begins to increase in labor and is significantly higher shortly postpartum (pp 4 h) in mouse cervical extracts (Fig. 3A, P > 0.05). As shown in Fig. 3B, HA breakdown to low MW HA at 4 h postpartum correlates with the rise in hyaluronidase activity.
Fig. 3.
Hyaluronidase activity is increased IL and early postpartum in mouse cervix (panel A, n = 4 per time point; *, P < 0.05 when compared with the d 15 group). Consistent with the timing of increased hyaluronidase activity, lower MW HA is increased postpartum as shown in panel B. Cervical tissues were collected from pregnancy d 16–d 18 and postpartum 4–24 h (pp). Representative gel from one of three experiments.
Human cervical HA and hyaluronidase expression
In the mouse cervix we observed a decline in HA size, which correlates to an increase in hyaluronidase activity. To determine whether similar mechanisms are in place in the human cervix, HA size and expression of the HA-metabolizing enzyme HYAL2 were evaluated in human cervical mucus or tissue collected from nonpregnant or pregnant women at or near term.
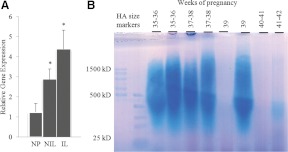
Although there are five hyaluronidase genes, Hyal1 and Hyal2 are the predominant isoforms expressed in somatic cells (36). We have previously reported expression of HYAL1 in human cervical tissue with no change in expression from women at term but not in labor compared with cervices from term pregnancies in labor (14). In the current study we observed increased HYAL2 mRNA expression in term pregnant vs. nonpregnant cervical tissue. Similar to HYAL1 expression, there was no significant difference in HYAL2 expression in term human cervix that was NIL compared with IL tissues (Fig. 4A). Because HA is secreted into the cervical mucus, which can be obtained noninvasively from women, HA MW was evaluated in cervical mucus obtained from eight women ranging from gestation wk 35 to wk 42 (Fig. 4B). In most samples, HA was abundant and ranged in size from high to low MW. It is interesting to note that in two samples collected from women in labor at 40–42 wk, HA levels were reduced and what remained was low MW HA.
Fig. 4.
Human cervical tissue HYAL2 mRNA expression is increased at term. *, P < 0.05 when compared with the NP (n = 4–5 samples). NP, NIL, and IL represent nonpregnant, term not in labor, and term in labor (panel A). Assessment of HA size distribution in cervical mucus from women at gestation wk 35–39. Lanes 1 and 2 contain HA MW standards. Lanes 3–8 evaluate samples from women at 35–39 wk gestation whose cervix is not dilated, and lanes 9 and 10 contain samples from women at 40–42 wk gestation with dilation more than or equal to 2 cm (panel B).
HA, but not sGAG, is elevated in cervix of PTB models
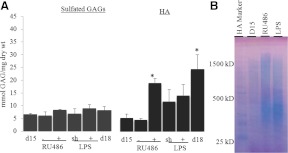
The observation that cervical GAG composition dynamically changes during pregnancy, along with previous work suggesting that premature ripening can occur by multiple mechanisms (8), led us to evaluate GAG composition in PTB. FACE analysis was evaluated in mice treated ± RU486 (noninfection model), or ± LPS (infection model) and compared with normal untreated controls at gestation d 15 and d 18. Similar to term ripening, there was no statistically significant change in levels of sGAG (CS/DS and HS) between normal pregnancy (d 15 and d 18) and preterm models (Fig. 5A). In contrast to sGAG, HA was significantly elevated in RU486-treated cervices compared with both gestation d 15 control and untreated d 15 samples (Fig. 5A, P > 0.05). HA levels were increased with LPS treatment, but there was a similar increase in the LPS control animals undergoing sham surgery. These increases were not significantly greater than untreated d 15. The percentage of HA shifted from approximately 44% of total GAG in d 15 control to 70% in RU486-injected cervices, whereas HA expression of LPS sham and LPS-injected cervices were approximately 62% of the total GAG. Assessment of HA MW indicated the HA population in RU486- and LPS-treated mice was a mix of both high and low MW HA (Fig. 5B).
Fig. 5.
HA, but not sGAG, is elevated in premature cervical ripening. Cervices were collected on the 15th day of pregnancy from untreated (d 15), RU486 vehicle (−), RU486 (0.5 mg) (+), and LPS sham surgery (sh) or LPS-treated mice (+) and from gestation d 18 (d 18). Samples were analyzed by FACE. *, P < 0.05 when compared with d 15 untreated cervix; n = 3–4 (panel A). Assessment of HA size distribution in mouse cervical tissue of preterm models. HA Marker, HA MW standards, d 15, gestation d 15; RU-d 15, RU486-treated; and LPS-d 15, LPS-treated. Gel represents one of three experiments performed (panel B).
Immunofluorescence of cervical HA: localization and expression
Immunofluorescence staining for HA was performed to evaluate localization and pattern of HA in both PTB models. On gestation d 15, low HA expression is observed in the matrix surrounding the stroma with no staining in the pericellular matrix surrounding the epithelia. As previously reported, during ripening on gestation d 18, HA expression is increased in the stroma as well as in the pericellular matrix surrounding the epithelia (14). In gestation d 15 cervices from mice treated with RU486 or LPS, there was increased expression of HA in the stroma compared with untreated d 15. The level of stromal HA in both preterm models appeared to be reduced when compared with term ripening, and epithelial HA expression was negligible (Fig. 6).
Fig. 6.
Cellular localization of cervical HA in preterm models is confined to the stroma in contrast to term pregnancy (d 18) where HA is localized to both epithelial and stromal ECM. Green, HA; blue, nucleus; S, stroma; E, epithelia.
Differential gene expression of HA synthesis in term vs. preterm ripening
The enzyme responsible for HA synthesis, Has, is encoded by three genes, Has1, Has2, and Has3. Transcripts encoding Has2 are up-regulated during term ripening but not in infection-mediated or RU486-induced premature ripening (8). In the current study we evaluated expression of all three HA synthases by qPCR. In contrast to term, increased HA synthesis in cervices of both PTB models appears to arise from increased mRNA expression of Has1 (Fig. 7; P > 0.05). Has3 expression remains low in both term and preterm cervices. Transcripts encoding the HA-metabolizing enzyme, Hyal1, were expressed at similar levels in PTB models compared with untreated control d 15. Although the 2-fold increase in Hyal2 expression in term and PTB models did not achieve statistical significance, Hyal2 tended to increase at term and after treatment with RU486 or LPS. Nonetheless, the magnitude of this increase in hyaluronidase gene expression paled relative to the striking increases in HA synthases (Fig. 7).
Fig. 7.
Gene expression of HA synthases (Has1, Has2, and Has3) and hyaluronidases (Hyal1 and Hyal2). The study groups are d 15, d 15RU486 control or treatment (−, +), d 15 LPS sham surgery or treatment (sh, +), and d 18. Gene expression is normalized to d 15 untreated of each group. *, P < 0.05 when compared with d 15 untreated cervix (n = 5–6/group). RU486 and LPS groups were tested separately with d 15 untreated group using one-way ANOVA.
Has2 expression is regulated, in part, by estrogen
Increased Has2 expression at term suggests a potential regulation by steroid hormones of pregnancy. Because estrogen levels normally increase at the end of pregnancy, two approaches were taken to assess regulation by estrogens. Administration of the estrogen receptor α antagonist, tamoxifen, on d 15 resulted in reduced Has2, but not Has1, Has3, Hyal1, or Hyal2, gene expression (Fig. 8A; P > 0.05). Consistent with reduced Has2 transcripts, HA content was reduced 50% with tamoxifen treatment (Fig. 8B; P > 0.05). The specificity of this effect was confirmed with the known estrogen-responsive gene, trefoil factor 1 (Tff1). Furthermore, administration of estradiol to nonpregnant ovariectomized mice increased Has2 gene expression and HA content, with no significant effect on the other synthases or hyaluronidases (Fig. 8, C and D; P > 0.05). Taken together, these results suggest that estrogen-induced activation of ERα plays an important role in up-regulation of cervical HA at term. Interestingly, progesterone treatment resulted in abrogation of E2-induced increases in HA content, suggesting that decreased progesterone responsiveness at term, either through decreased progesterone secretion from the corpus luteum (37), increased metabolism (31, 38, 39), or alterations in progesterone receptors (40, 41), may amplify estrogen-induced increases in cervical HA at term. Administration of RU486 along with E2 + P4 reduced the level of Has2 induction yet increased Has1 synthesis (Fig. 8D; P > 0.05). Increased Has1 expression in the RU486-treated ovariectomy model is consistent with the results in the RU486-induced PTB model.
Fig. 8.
Cervical Has2 expression is regulated in part by estrogen. A, Gene expression of HA synthases (Has1, Has2, Has3) and hyaluronidases (Hyal1 and Hyal2) in cervical tissues (d 18) from mice treated with (+) or without (−) tamoxifen. The estrogen-regulated gene trefoil factor 1 (Tff1) was used to assess responsiveness of tissue to tamoxifen treatment. Data represent mean ± sem of four animals per group. B, Cervical HA content in gestation d 18 mice treated with (+) or without (−) tamoxifen; n = 3–4 (P < 0.05). C, Gene expression of Has1, Has2, Has3, Hyal1, Hyal2, and Tff1 in cervical tissues from ovariectomized (ovx) mice treated with sham pellets (Sham), E2, E2+P4, or E2+P4+RU486. D, Cervical HA content in ovariectomized (ovx) mice treated with E2 (0.05 mg pellet per mouse) or E2 and P4 (0.05 mg pellet per mouse) for 10 d. RU486 (500 μg/mouse) was injected 12 h before tissue collection. Data represent mean ± sem of four animals per group. *, P < 0.05 compared with control cervix or ovx sham in tamoxifen and ovx study, respectively. Tmx, Tamoxifen.
Discussion
The current study provides a comprehensive analysis of cervical GAG over the course of term pregnancy as well as in two mouse models of PTB. GAG play numerous functions in the ECM to regulate mechanical properties of a tissue, cell proliferation, cell adhesion, growth factor signaling, immune cell function, and collagen structure. Although these functional attributes of GAG are postulated to be important to the cervical remodeling process, identification of specific GAG functions is hampered by our lack of understanding of GAG composition and structure. Published GAG studies using human cervical tissue are numerous, but variations in sample size, pregnancy time point that tissue/mucus was collected, and regional variations of sample within the cervix likely contribute to the lack of a consistent findings from study to study (13, 27, 42–44). The current study uses a mouse model allowing for the collection of well-defined time points in gestation and analysis of the entire tissue, thus preventing regional variations. We also apply a sensitive, quantitative assay to assess GAG. These studies have led to two novel insights. First, cervical GAG composition and abundance change dramatically during pregnancy and parturition. The ratio between the non-sGAG (HA) and sGAG (CS, DS, and HS) is altered as HA progressively increases through normal pregnancy and labor. Second, although similar changes in GAG composition occur in term and preterm cervical ripening, the regulation of these processes appears to differ between term and PTB. This conclusion is supported by the differential induction of Has2 vs. Has1 genes in term and preterm models, respectively, as well as the evidence that Has2 is regulated, in part, by estrogens in contrast to Has1 expression.
The total amount of GAG as measured by FACE increased through pregnancy with highest levels recorded at term in the mouse cervix. This is consistent with our previous estimate of total GAG in mouse cervix by hexuronic acid assay (45). The increase in total GAG results solely from increased HA synthesis toward the end of pregnancy. A steady level of sGAG (CS/DS and HS) was maintained in pregnant and nonpregnant cervices and sulfation at the fourth position of N-acetyl-galactosamine was the most abundant form of sGAG at all time points. KS GAG chains were not detectable in the mouse cervix although transcripts encoding the KS containing proteoglycan, fibromodulin are expressed throughout pregnancy. The sensitivity of FACE to detect picomolar quantities of GAG and our ability to detect KS GAG in the cornea, but not the cervix, suggest that cervical fibromodulin may lack KS GAG chains. Further studies are required to confirm this observation as well as determine how fibromodulin functions are affected by the absence of KS chains.
Numerous proteoglycans are expressed in the cervix including members of the small leucine-rich proteoglycan family (e.g. decorin, biglycan, fibromodulin, osteomodulin, and asporin) as well as the large proteoglycan, versican. Although total sGAG levels were unchanged, we must consider the possibility that selective loss of GAG chains on some proteoglycans, along with an increase in sGAG chains on other proteoglycans, could account for no net change in sGAG. Purification of individual proteoglycans by anion exchange chromatography followed by FACE analysis to quantify GAG chain length and composition is required to better elucidate potential alterations in proteoglycan function that arise from regulated changes in GAG composition and length. It is possible that changes in degree of sulfation and GAG chain length of a particular proteoglycan could alter the binding to other extracellular components including collagen and HA, which in turn would modulate cervical ECM structure and strength during remodeling. In support of this hypothesis, recent studies report that the binding of proteoglycan to LDL is increased by elongated GAG chains while inhibited by higher sulfation (46).
Increased HA synthesis during term cervical ripening coincides with the maximal increase in tissue compliance as previously described and is a conserved element of ripening in numerous species (13, 14, 38, 47, 48). The importance of HA to this process is further emphasized in the current study by the observation that the increase in total GAG during mouse pregnancy results solely from the increase in HA in both term and premature cervical ripening. Furthermore, the transition from cervical ripening to labor is accompanied by a shift from predominantly high-MW HA to low-MW HA in both women and mice. The MW shift correlates with an increase in hyaluronidase activity and/or transcripts encoding Hyal2. We have previously suggested that HA may have multiple functions in the cervix based on size. High-MW HA has the potential to promote increased tissue viscoelasticity during cervical ripening. The smaller HA at the time of labor promotes a loss of structural integrity as well as activates inflammatory cell cascades important in tissue repair and matrix cleanup (23). Importantly, we also report a rise in hyaluronidase activity during labor. HA breakdown by hyaluronidases may have importance in cervical function during labor as suggested by studies in both rat and humans, in which cervical application of hyaluronidase accelerates cervical ripening and reduces the length of labor (27). This finding emphasizes the need for further studies to understand the regulation of hyaluronidase synthesis as well as determine whether the small-MW HA produced as a result of hyaluronidase action has specific functions in the latter stages of labor or postpartum.
Similar to term ripening, HA levels were significantly increased in noninfection PTB and although not significant, there was a trend for increased HA content with infection-induced PTB. The level of expression was lower than that of gestation d 18 and was confined to the stromal matrix in contrast to both the pericellular matrix surrounding the cervical epithelial cells and the stroma as seen in the term cervix. Recent studies provide evidence that the mechanisms of premature cervical ripening occur by distinct mechanisms dependent on etiology (8). Consistent with differing mechanisms in term and preterm, Has1 gene expression was up-regulated in the two PTB models but was not induced by estradiol whereas the Has2 gene appears to be regulated by estradiol in term ripening (Figs. 7 and 8). Further studies are required to understand the mechanism by which RU486 induces Has1 expression.
Elevations in HA synthesis are reported to arise in response to both sterile and nonsterile injury (49), similar to our findings with intrauterine injection of sterile water. Further studies are required to distinguish the observed increase in HA content with sterile injury (sham surgery, Fig. 5) compared with the distinct LPS-induced increase in HA that is accompanied by an increase in Has1 gene expression (Fig. 7) and increased expression of proinflammatory genes (8). The importance of HA to preterm cervical ripening in women is suggested by previous studies that report an increase in high-MW HA in human cervical mucus taken from women with threatened PTB (50).
In closing, these studies provide new insights into hormonally regulated changes in cervical GAG over the course of pregnancy and parturition. The significance of HA synthesis and breakdown in term and preterm cervical remodeling is highlighted in this study. Estrogens, in part, induce Has2 gene expression but not Has1. Further studies are required to determine whether the actions of estrogens are by direct binding of estrogen receptors to response elements on the Has2 gene. Finally, although global changes in sGAG levels, the degree of sulfation, and chain length appear constant through mouse pregnancy, future studies to purify individual proteoglycans followed by GAG chain analysis are necessary before the importance of proteoglycans and sGAG chains to cervical remodeling can be ruled out. The contribution of GAG to modulation of ECM function, strength, and structure is well established in numerous biological systems. Further studies to understand GAG function in the process of cervical remodeling will likely provide novel insights into the development of clinical tools to detect impending PTB and therapies to prevent PTB.
Supplementary Material
Acknowledgments
We thank the Human Tissue and Biological Core Laboratory (University of Texas Southwestern Medical Center) for providing human cervical tissue and mucus samples We thank Drs. Mark Lehrman and Ningguo Gao (Department of Pharmacology, UT Southwestern Medical Center) for assistance with FACE gel preparation techniques. We also thank Dr. Meredith Akins (Department of OB/GYN, UT Southwestern Medical Center) for critical reading of the manuscript.
This work was supported by The National Institutes of Health Grant 5-PO1-HD011149.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CS
- Chondroitin sulfate
- DS
- dermatan sulfate
- E2
- 17β-estradiol
- ECM
- extracellular matrix
- FACE
- fluorophore-assisted carbohydrate electrophoresis
- FRET
- fluorescence resonance energy transfer
- GAG
- glycosaminoglycan
- HA
- hyaluronan
- Has
- HA synthase
- HS
- heparan sulfate
- IL
- in labor
- KS
- keratan sulfate
- LPS
- lipopolysaccharide
- MW
- molecular weight
- NIL
- not in labor
- P4
- progesterone
- PTB
- preterm birth
- qPCR
- quantitative real-time PCR
- sGAG
- sulfated GAG.
References
- 1. Martin JA. 2011. Preterm births - United States, 2007. MMWR Surveill Summ 60(Suppl):78–79 [PubMed] [Google Scholar]
- 2. Cunningham F, Leveno K, Bloom S, Hauth J, Rouse D, Spong C. 2010. Williams obstetrics. 23rd ed New York: McGraw-Hill Professional [Google Scholar]
- 3. Read CP, Word RA, Ruscheinsky MA, Timmons BC, Mahendroo MS. 2007. Cervical remodeling during pregnancy and parturition: molecular characterization of the softening phase in mice. Reproduction 134:327–340 [DOI] [PubMed] [Google Scholar]
- 4. Timmons BC, Fairhurst AM, Mahendroo MS. 2009. Temporal changes in myeloid cells in the cervix during pregnancy and parturition. J Immunol 182:2700–2707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Timmons BC, Mahendroo MS. 2006. Timing of neutrophil activation and expression of proinflammatory markers do not support a role for neutrophils in cervical ripening in the mouse. Biol Reprod 74:236–245 [DOI] [PubMed] [Google Scholar]
- 6. Timmons B, Akins M, Mahendroo M. 2010. Cervical remodeling during pregnancy and parturition. Trends Endocrinol Metab 21:353–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gonzalez JM, Xu H, Chai J, Ofori E, Elovitz MA. 2009. Preterm and term cervical ripening in CD1 Mice (Mus musculus): similar or divergent molecular mechanisms? Biol Reprod 81:1226–1232 [DOI] [PubMed] [Google Scholar]
- 8. Holt R, Timmons BC, Akgul Y, Akins ML, Mahendroo M. 2011. The molecular mechanisms of cervical ripening differ between term and preterm birth. Endocrinology 152:1036–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hirsch E, Filipovich Y, Mahendroo M. 2006. Signaling via the type I IL-1 and TNF receptors is necessary for bacterially induced preterm labor in a murine model. Am J Obstet Gynecol 194:1334–1340 [DOI] [PubMed] [Google Scholar]
- 10. Akins ML, Luby-Phelps K, Mahendroo M. 2010. Second harmonic generation imaging as a potential tool for staging pregnancy and predicting preterm birth. J Biomed Opt 15:026020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Akins ML, Luby-Phelps K, Bank RA, Mahendroo M. 2011. Cervical softening during pregnancy: regulated changes in collagen cross-linking and composition of matricellular proteins in the mouse. Biol Reprod 84:1053–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Myers KM, Paskaleva AP, House M, Socrate S. 2008. Mechanical and biochemical properties of human cervical tissue. Acta Biomater 4:104–116 [DOI] [PubMed] [Google Scholar]
- 13. Osmers R, Rath W, Pflanz MA, Kuhn W, Stuhlsatz HW, Szeverényi M. 1993. Glycosaminoglycans in cervical connective tissue during pregnancy and parturition. Obstet Gynecol 81:88–92 [PubMed] [Google Scholar]
- 14. Straach KJ, Shelton JM, Richardson JA, Hascall VC, Mahendroo MS. 2005. Regulation of hyaluronan expression during cervical ripening. Glycobiology 15:55–65 [DOI] [PubMed] [Google Scholar]
- 15. Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME. 2009. Essentials of glycobiology. 2nd ed Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; [PubMed] [Google Scholar]
- 16. Fuster MM, Esko JD. 2005. The sweet and sour of cancer: glycans as novel therapeutic targets. Nat Rev Cancer 5:526–542 [DOI] [PubMed] [Google Scholar]
- 17. Jiang D, Liang J, Noble PW. 2011. Hyaluronan as an immune regulator in human diseases. Physiol Rev 91:221–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Raman R, Sasisekharan V, Sasisekharan R. 2005. Structural insights into biological roles of protein-glycosaminoglycan interactions. Chem Biol 12:267–277 [DOI] [PubMed] [Google Scholar]
- 19. Wight TN, Kinsella MG, Qwarnström EE. 1992. The role of proteoglycans in cell adhesion, migration and proliferation. Curr Opin Cell Biol 4:793–801 [DOI] [PubMed] [Google Scholar]
- 20. Karangelis D, Asimakopoulou A, Kanakis I, Tagarakis GI, Koufakis T, Triposkiadis F, Tsilimingas N, Karamanos NK. 2011. Monitoring serum chondroitin sulfate levels in patients submitted to coronary artery bypass surgery. Biomed Chromatogr 25:748–750 [DOI] [PubMed] [Google Scholar]
- 21. Pickford CE, Holley RJ, Rushton G, Stavridis MP, Ward CM, Merry CL. 2011. Specific glycosaminoglycans modulate neural specification of mouse embryonic stem cells. Stem Cells 29:629–640 [DOI] [PubMed] [Google Scholar]
- 22. Lin R, Rosahl TW, Whiting PJ, Fawcett JW, Kwok JC. 2011. 6-Sulphated chondroitins have a positive influence on axonal regeneration. PLoS One 6:e21499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ruscheinsky M, De la Motte C, Mahendroo M. 2008. Hyaluronan and its binding proteins during cervical ripening and parturition: dynamic changes in size, distribution and temporal sequence. Matrix Biol 27:487–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liang J, Jiang D, Jung Y, Xie T, Ingram J, Church T, Degan S, Leonard M, Kraft M, Noble PW. 2011. Role of hyaluronan and hyaluronan-binding proteins in human asthma. J Allergy Clin Immunol 128:403–411.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang TL, Hsu HC, Yang KC, Yao CH, Lin FH. 2010. Effect of different molecular weight hyaluronans on osteoarthritis-related protein production in fibroblast-like synoviocytes from patients with tibia plateau fracture. J Trauma 68:146–152 [DOI] [PubMed] [Google Scholar]
- 26. Byers BD, Bytautiene E, Costantine MM, Buhimschi CS, Buhimschi I, Saade GR, Goharkhay N. 2010. Hyaluronidase modifies the biomechanical properties of the rat cervix and shortens the duration of labor independent of myometrial contractility. Am J Obstet Gynecol 203:596.e1–5 [DOI] [PubMed] [Google Scholar]
- 27. Spallicci MD, Chiea MA, Singer JM, Albuquerque PB, Bittar RE, Zugaib M. 2007. Use of hyaluronidase for cervical ripening: a randomized trial. Eur J Obstet Gynecol Reprod Biol 130:46–50 [DOI] [PubMed] [Google Scholar]
- 28. Westergren-Thorsson G, Norman M, Björnsson S, Endrésen U, Stjernholm Y, Ekman G, Malmström A. 1998. Differential expressions of mRNA for proteoglycans, collagens and transforming growth factor-β in the human cervix during pregnancy and involution. Biochim Biophys Acta 1406:203–213 [DOI] [PubMed] [Google Scholar]
- 29. Kuschert GS, Coulin F, Power CA, Proudfoot AE, Hubbard RE, Hoogewerf AJ, Wells TN. 1999. Glycosaminoglycans interact selectively with chemokines and modulate receptor binding and cellular responses. Biochemistry 38:12959–12968 [DOI] [PubMed] [Google Scholar]
- 30. O'Keeffe D, Olson ST, Gasiunas N, Gallagher J, Baglin TP, Huntington JA. 2004. The heparin binding properties of heparin cofactor II suggest an antithrombin-like activation mechanism. J Biol Chem 279:50267–50273 [DOI] [PubMed] [Google Scholar]
- 31. Mahendroo MS, Cala KM, Russell DW. 1996. 5α-Reduced androgens play a key role in murine parturition. Mol Endocrinol 10:380–392 [DOI] [PubMed] [Google Scholar]
- 32. Calabro A, Hascall VC, Midura RJ. 2000. Adaptation of FACE methodology for microanalysis of total hyaluronan and chondroitin sulfate composition from cartilage. Glycobiology 10:283–293 [DOI] [PubMed] [Google Scholar]
- 33. Rosa RG, Akgul Y, Joazeiro PP, Mahendroo M. 2012. Changes of large molecular weight hyaluronan and versican in the mouse pubic symphysis through pregnancy. Biol Reprod 86:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Calabro A, Midura R, Wang A, West L, Plaas A, Hascall VC. 2001. Fluorophore-assisted carbohydrate electrophoresis (FACE) of glycosaminoglycans. Osteoarthritis Cartilage 9(Suppl A):S16–S22 [DOI] [PubMed] [Google Scholar]
- 35. Zhang LS, Mummert ME. 2008. Development of a fluorescent substrate to measure hyaluronidase activity. Anal Biochem 379:80–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Girish KS, Kemparaju K. 2007. The magic glue hyaluronan and its eraser hyaluronidase: a biological overview. Life Sci 80:1921–1943 [DOI] [PubMed] [Google Scholar]
- 37. Virgo BB, Bellward GD. 1974. Serum progesterone levels in the pregnant and postpartum laboratory mouse. Endocrinology 95:1486–1490 [DOI] [PubMed] [Google Scholar]
- 38. Mahendroo MS, Porter A, Russell DW, Word RA. 1999. The parturition defect in steroid 5α-reductase type 1 knockout mice is due to impaired cervical ripening. Mol Endocrinol 13:981–992 [DOI] [PubMed] [Google Scholar]
- 39. Ishida M, Choi JH, Hirabayashi K, Matsuwaki T, Suzuki M, Yamanouchi K, Horai R, Sudo K, Iwakura Y, Nishihara M. 2007. Reproductive phenotypes in mice with targeted disruption of the 20α-hydroxysteroid dehydrogenase gene. J Reprod Dev 53:499–508 [DOI] [PubMed] [Google Scholar]
- 40. Fang X, Wong S, Mitchell BF. 2002. Messenger RNA for progesterone receptor isoforms in the late-gestation rat uterus. Am J Physiol Endocrinol Metab 283:E1167–E1172 [DOI] [PubMed] [Google Scholar]
- 41. Condon JC, Jeyasuria P, Faust JM, Wilson JW, Mendelson CR. 2003. A decline in the levels of progesterone receptor coactivators in the pregnant uterus at term may antagonize progesterone receptor function and contribute to the initiation of parturition. Proc Natl Acad Sci USA 100:9518–9523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kitamura K, Ito A, Mori Y, Hirakawa S. 1979. Changes in the human uterine cervical collagenase with special reference to cervical ripening. Biochem Med 22:332–338 [DOI] [PubMed] [Google Scholar]
- 43. Fischer DC, Henning A, Winkler M, Rath W, Haubeck HD, Greiling H. 1996. Evidence for the presence of a large keratan sulphate proteoglycan in the human uterine cervix. Biochem J 320( Pt 2):393–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. El Maradny E, Kanayama N, Kobayashi H, Hossain B, Khatun S, Liping S, Kobayashi T, Terao T. 1997. The role of hyaluronic acid as a mediator and regulator of cervical ripening. Hum Reprod 12:1080–1088 [DOI] [PubMed] [Google Scholar]
- 45. Xu X, Akgul Y, Mahendroo M, Jerschow A. 2010. Ex vivo assessment of mouse cervical remodeling through pregnancy via 23Na MRS. NMR Biomed 23:907–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Little PJ, Ballinger ML, Osman N. 2007. Vascular wall proteoglycan synthesis and structure as a target for the prevention of atherosclerosis. Vasc Health Risk Manag 3:117–124 [PMC free article] [PubMed] [Google Scholar]
- 47. Golichowski AM, King SR, Mascaro K. 1980. Pregnancy-related changes in rat cervical glycosaminoglycans. Biochem J 192:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Uldbjerg N, Ekman G, Malmström A, Olsson K, Ulmsten U. 1983. Ripening of the human uterine cervix related to changes in collagen, glycosaminoglycans, and collagenolytic activity. Am J Obstet Gynecol 147:662–666 [DOI] [PubMed] [Google Scholar]
- 49. Taylor KR, Yamasaki K, Radek KA, Di Nardo A, Goodarzi H, Golenbock D, Beutler B, Gallo RL. 2007. Recognition of hyaluronan released in sterile injury involves a unique receptor complex dependent on Toll-like receptor 4, CD44, and MD-2. J Biol Chem 282:18265–18275 [DOI] [PubMed] [Google Scholar]
- 50. Obara M, Hirano H, Ogawa M, Tsubaki H, Hosoya N, Yoshida Y, Miyauchi S, Tanaka T. 2001. Changes in molecular weight of hyaluronan and hyaluronidase activity in uterine cervical mucus in cervical ripening. Acta Obstet Gynecol Scand 80:492–496 [PubMed] [Google Scholar]
- 51. Laborda J. 1991. 36B4 cDNA used as an estradiol-independent mRNA control is the cDNA for human acidic ribosomal phosphoprotein PO. Nucleic Acids Res 19 (14):3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.