Abstract
Previously we have shown that a reduction in γ-amino butyric acid (GABA) inhibition is critical for the mechanism initiating puberty onset because chronic infusion of the GABAA receptor antagonist, bicuculline, significantly increased GnRH release and accelerated the timing of menarche and first ovulation in female rhesus monkeys. Because previous studies in our laboratory indicate that in prepubertal female monkeys, kisspeptin release in the medial basal hypothalamus is low, whereas kisspeptin-10 can stimulate GnRH release, we hypothesized that a low level of kisspeptin release prior to puberty onset is due to tonic GABA inhibition. To test this hypothesis we examined the effects of bicuculline infusion on kisspeptin release using a microdialysis method. We found that bicuculline at 1 μm dramatically stimulates kisspeptin release in the medial basal hypothalamus of prepubertal monkeys but had little effect on kisspeptin release in midpubertal monkeys. We further examined whether bicuculline-induced GnRH release is blocked by the presence of the kisspeptin antagonist, peptide 234. We found that inhibition of kisspeptin signaling blocked the bicuculline-induced stimulation of GnRH release, suggesting that kisspeptin neurons may relay inhibitory GABA signals to GnRH neurons. This implies that a reduction in tonic GABA inhibition of GnRH release is, at least in part, mediated through kisspeptin neurons.
It is well established that puberty is triggered with the elevation of pulsatile GnRH release into the portal-pituitary circulation (1, 2), although the neurobiological mechanisms leading to puberty onset remain elusive. In primates, an unambiguous gonadal steroid independent neural inhibition suppresses the release of gonadotropins (and presumably GnRH) during the prepubertal/juvenile period. Previously we have proposed that γ-amino butyric acid (GABA) is a neuronal substrate responsible for suppression of GnRH release before puberty onset. This is based on several findings. First, we have found through direct measurements from the stalk-median eminence (S-ME) that GABA levels are high when GnRH release is low in prepubertal monkeys whereas after the onset of puberty, when GnRH release is elevated, GABA levels are lower (3). Second, infusion of the GABAA receptor antagonist, bicuculline, into the S-ME stimulates GnRH release to a much greater extent in prepubertal than in pubertal monkeys, whereas infusion of GABA is effective in suppressing GnRH release in only pubertal monkeys, presumably because of the reduction in tonic GABA inhibition at the onset of puberty (3). Lastly, chronic infusion of bicuculline to the S-ME of juvenile female primates leads to precocious puberty and first ovulation (4).
Recently kisspeptin (KP) and its receptor, G protein-coupled receptor 54 (GPR54), have been implicated in the mechanism underlying puberty onset. In humans, mutations of the GPR54 gene are related to atypical pubertal timing or the absence of puberty (5, 6). Furthermore, mice lacking the GPR54 gene have immature gonads and fail to progress through puberty (7), and an absence of reproductive development is found in mice lacking the KiSS-1 gene (8, 9). Because mouse GnRH neurons express GPR54 (10), it is possible that increased GnRH release is a consequence of a pubertal increase in KP release. In fact, we found that developmental changes in hypothalamic KP release and the KP response to ovariectomy virtually mirror those of GnRH through pubertal development in female primates (11–13).
Important questions consequently arise. Does the pubertal reduction in GABA in the S-ME allow stimulation of KP release? If so, does KP, released as a consequence of reduced GABA tone, subsequently stimulate GnRH release? In the present study, we addressed these questions. We found the following: 1) the inhibition of GABA transmission by bicuculline-stimulated KP release in the medial basal hypothalamus (MBH) of female rhesus monkeys before, but not after, puberty onset and 2) the inhibition of KP signaling by the KP receptor antagonist, peptide 234, blocked the bicuculline-induced stimulation of GnRH release in prepubertal monkeys.
Materials and Methods
Animals
Six prepubertal (14.7 ± 0.3 months of age) and three midpubertal (31.0 ± 0.7 months of age) female rhesus monkeys (Macaca mulatta) were used in this study. All animals were born and raised at the Wisconsin National Primate Research Center and housed in pairs (cages 172 × 86 × 86 cm) in a room with a lighting schedule of 12 h of light and 12 h of dark, at a controlled temperature (22 C). They were fed a standard diet of Teklad Primate Chow (Harlan, Madison, WI) twice per day and water was available ad libitum. Fresh fruit or other enrichment was also provided on a daily basis. To accurately define the pubertal stage, all animals were examined daily for sex-skin development and menstrual status and received weekly blood sampling for circulating hormone measurements (14). The protocol was approved by the Animal Care and Use Committee; University of Wisconsin-Madison, and all experiments were conducted under the National Institutes of Health and U.S. Department of Agriculture guidelines.
Microdialysis experiments
To determine the effect of bicuculline infusion on KP release before and after the onset of puberty and GnRH release before puberty, two experiments were conducted using an in vivo microdialysis method, previously described (12, 13). Microdialysis probe tip locations are described in Fig. 1. Dialysates were collected at 10-min intervals from the S-ME/MBH for up to 12 h for each session. A minimum of 4 wk separated sampling sessions for each animal. In the first experiment, 10-min infusions (challenges) of either bicuculline (10−6 m; Sigma-Aldrich, St. Louis, MO) or vehicle [central nervous system perifusion fluid: 147 mm NaCl, 2.7 mm KCl, 1.2 mm CaCl2, 0.85 mm MgCl2, and bacitracin (4 U/ml)] commenced after 4 h of baseline dialysate collection. To eliminate potential interference of peptide release by the probe insertion procedure, dialysate samples collected during the first 2 h were eliminated and samples collected during the subsequent 2 h period were used to establish an experimental baseline of peptide release. Challenges were separated by 2 h and the order of the challenge condition was randomized for each experiment. In the second experiment, 10-min bicuculline or vehicle challenges commenced after 4 h of baseline dialysate collection. However, during some challenges in this experiment, the KP receptor antagonist (peptide 234, 10−8 m) was infused for a 30-min period, starting 10 min before and lasting 10 min after the bicuculline or vehicle challenges. In the first experiment, four prepubertal and three midpubertal animals were used with one to two challenges per animal for each condition (bicuculline and vehicle). In the second experiment, three prepubertal animals, including one animal from experiment 1, were used with one to two challenges per animal for each condition (bicuculline, bicuculline with peptide 234, and peptide 234 alone).
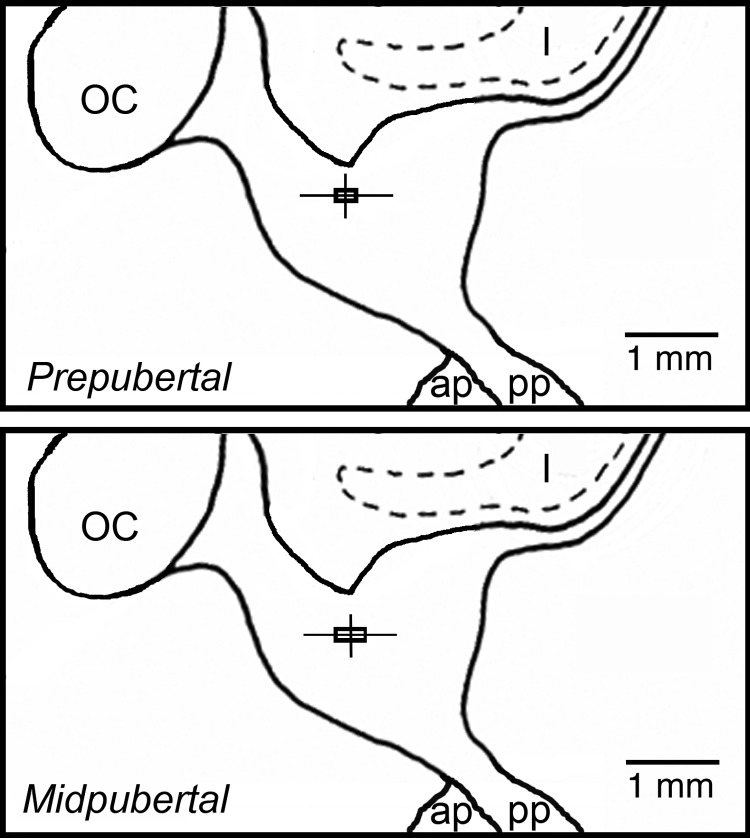
Fig. 1.
Midsagittal diagrams of the hypothalamus depicting microdialysis probe tip placements for prepubertal (top panel) and midpubertal (bottom panel) animals. The vertical and horizontal lines indicate the dorsal-ventral and anterior-posterior ranges of the probe tip locations. The intersections of these lines represent the mean probe tip placement; the boxes delineate ±sem. The lateral deviations from midline position (mean ± sem) were 0.27 ± 0.07 mm (range 0–0.75 mm) for prepubertal animal experiments (n = 12) and 0.33 ± 0.08 mm (range 0–0.5 mm) for midpubertal animals (n = 6). OC, Optic chiasm; I, infundibular recess of the third ventricle; ap, anterior pituitary; pp, posterior pituitary.
Radioimmunoassays
For each time point, 20 μl of sample was assayed. For kisspeptin, GQ2 antiserum was used as previously described (11, 15). Assay sensitivity was 0.05 pg/tube (or 2.5 pg/ml). Intra- and interassay coefficients of variation were 10.1 and 14.3%, respectively. For GnRH, R42 antiserum was used as previously described (16). Assay sensitivity was 0.02 pg/tube (or 1 pg/ml). Intra- and interassay coefficients of variation were 8.1 and 11.3%, respectively. Peptide levels in dialysates were expressed in picograms per milliliter every 10 minutes.
Statistical analyses
The release of both KP and GnRH fluctuates considerably. To discriminate a response to bicuculline among spontaneous peptide pulses, we calculated an average baseline release (per 10 min) from peptide levels in dialysates across a successive 50-min period (five 10 min samples collected 60 min before initiation of the first challenge). This corresponded to the period −110 min to −60 min with time 0 being the time that the first challenge was initiated. All data below assay sensitivity were adjusted to respective assay sensitivity levels. Using this value, we calculated peptide release from each 10-min sample as a percent of the baseline. We then calculated the mean (±sem) for each time point in all groups. The effects of a challenge were examined by one-way ANOVA followed by Bonferroni post hoc analyses. Comparisons between two conditions (e.g. bicuculline vs. vehicle infusion) were made by two-way ANOVA followed by Bonferroni post hoc analyses. We used GraphPad Prism software (San Diego, CA). Differences were considered significant at P < 0.05.
Results
Effects of bicuculline on kisspeptin release
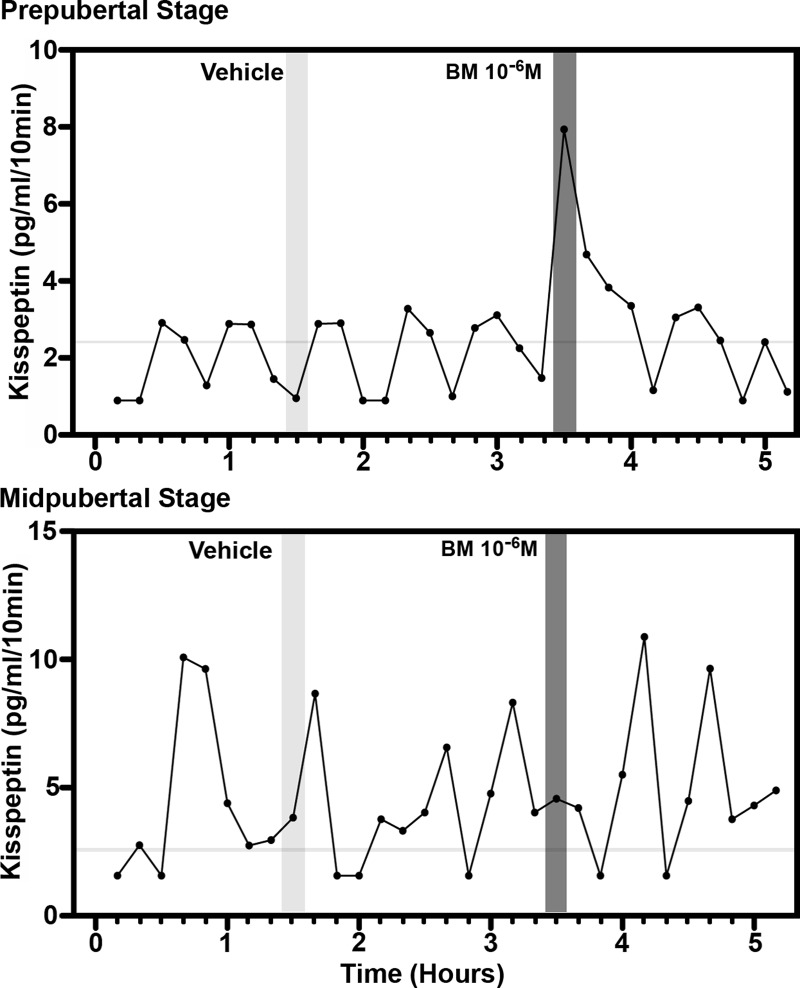
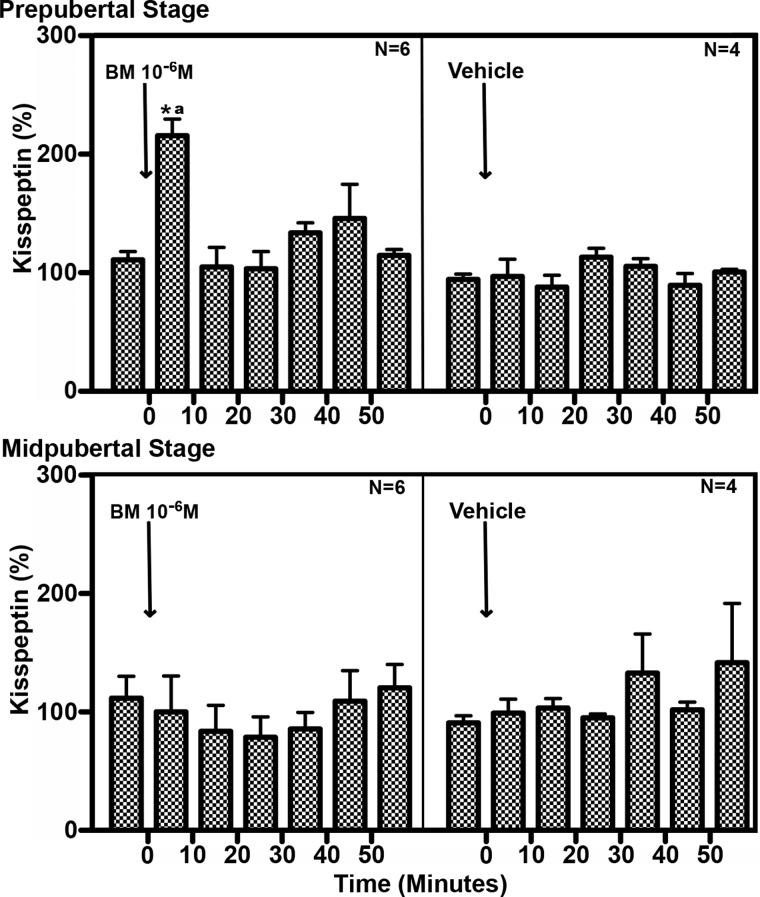
Infusion of the GABAA receptor antagonist, bicuculline (10−6 m), induced a rapid, transient stimulatory effect on KP release. This effect was found in animals at the prepubertal, but not pubertal, stage (Fig. 2). In prepubertal animals, bicuculline stimulated KP release during the 10-min exposure, and elevated KP release returned to baseline levels after cessation of the infusion (Fig. 3). Mean (±sem) release rose to 215 ± 14.1% of baseline (ANOVA: P = 0.006, post hoc: P < 0.05, n = 6; Fig. 3). This suggests the antagonist is transiently interfering with a tonic GABA-mediated inhibitory mechanism over KP release in prepubertal animals. In contrast, bicuculline (10−6 m) had no apparent effect on KP release in midpubertal animals. Vehicle infusion had no effects on KP release in prepubertal or midpubertal animals; consequently, the rise of KP release during bicuculline infusion was significant compared with the time period of vehicle infusion in prepubertal animals (two way ANOVA time vs. treatment interaction: P = 0.001, post hoc: P < 0.001; Fig. 3). These findings with KP were parallel to our previous findings showing that a similar bicuculline infusion strongly stimulated GnRH release in prepubertal female rhesus monkeys.
Fig. 2.
Representative cases for the effect of direct infusion of the GABAA receptor antagonist, bicuculline (BM; 10−6 m), on kisspeptin release in the S-ME/MBH of prepubertal (top panel) and midpubertal (bottom panel) animals. Shaded vertical bars represent the 10-min period of either vehicle or bicuculline infusions. Bicuculline infusion stimulated kisspeptin release in prepubertal, but not midpubertal, animals. Horizontal gray bars indicate the kisspeptin RIA sensitivity.
Fig. 3.
Statistical analyses of the effect of direct infusion of the GABAA receptor antagonist, bicuculline (BM; 10−6 m), or vehicle on kisspeptin release in the S-ME/MBH of prepubertal (top panel) and midpubertal (bottom panel) animals. After bicuculline infusion, kisspeptin release elevated significantly above average baseline kisspeptin release (as described in Materials and Methods) only in prepubertal animals. Vehicle infusion had no effect on kisspeptin release. *, One-way ANOVA (P = 0.06), Bonferroni post hoc comparing 0–10 min average to all other time points (P < 0.05); a, two-way ANOVA (P = 0.001 (interaction), Bonferroni post hoc comparing 0- to 10-min time points for each treatment (P < 0.001).
Blockade of the bicuculline-induced GnRH release by a kisspeptin receptor antagonist
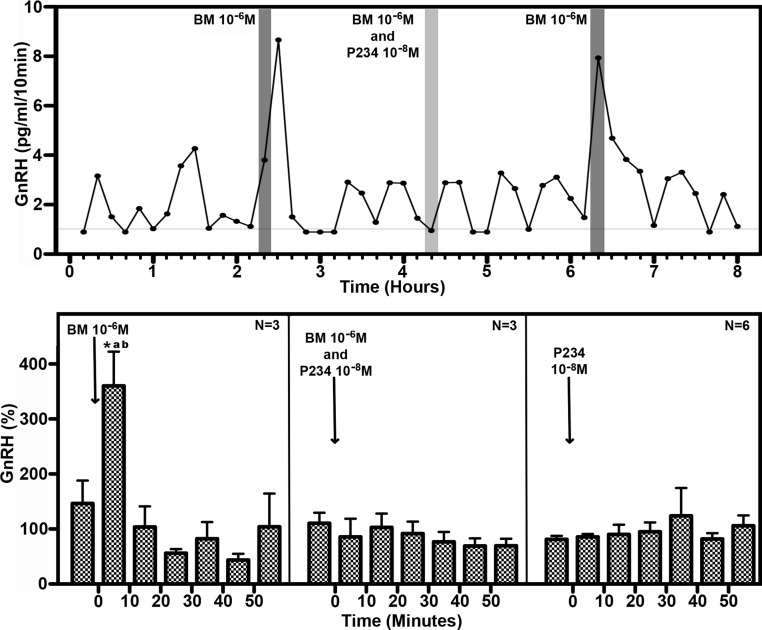
Our laboratory previously reported that GnRH release in prepubertal female rhesus monkeys is suppressed by a tonic inhibitory signal through a GABAA receptor-mediated mechanism. Consistent with that observation using a push-pull perfusion method, in the present study, bicuculline stimulated GnRH release in prepubertal animals with mean (±sem) release rising to 360 ± 62.1% of baseline (ANOVA: P = 0.001, post hoc: P < 0.05, n = 3; Fig. 4). Importantly, the pattern and time course of the GnRH response to bicuculline challenge were similar to those observed for the response of KP (Fig. 4). We found that infusion of the KP receptor antagonist (peptide 234, 10−8 m) during bicuculline (10−6 m) exposure clearly blocked the stimulatory effect of bicuculline on GnRH release. Infusion of peptide 234 alone had no effect on GnRH release. GnRH release was significantly stimulated by bicuculline infusion alone (two way ANOVA interaction: P < 0.0001) compared with infusions of bicuculline and peptide 234 simultaneously (post hoc: P < 0.001) and peptide 234 alone (post hoc: P < 0.001). Collectively the results of these experiments suggest that before puberty onset, GnRH release is inhibited by tonic GABA input through KP neurons.
Fig. 4.
Top panel, Representative case for the effect of direct infusion of the GABAA receptor antagonist, bicuculline (BM; 10−6 m), on GnRH release in the S-ME/MBH of prepubertal animals in the absence (dark shaded vertical bars) or presence (light shaded vertical bar) of the kisspeptin receptor antagonist, peptide 234 (P234; 10−8 m). P234 infusions were 30 min long from 10 min before starting bicuculline or vehicle infusion until 10 min after completing those infusions. Bicuculline stimulated GnRH release in prepubertal animals, whereas the bicuculline-induced GnRH release was blocked when P234 was present. Horizontal gray bar indicates the GnRH RIA sensitivity. Bottom panel, Statistical analyses of the effects of bicuculline, P234, or bicuculline with P234 on GnRH release in the S-ME/MBH of prepubertal animals. After bicuculline infusion, GnRH release elevated significantly above average baseline GnRH release (as described in Materials and Methods) only when P234 was not present. P234 alone had no effect on GnRH release. *, One-way ANOVA (P = 0.001), Bonferroni post hoc comparing 0- to 10-min average to all other time points (P < 0.05); a and b, two-way ANOVA [P < 0.0001 (interaction)], Bonferroni post hoc comparing 0- to 10-min time points between bicuculline alone vs. bicuculline with P234 (P < 0.001) (a) and bicuculline alone vs. P234 alone (b).
Discussion
In the present study, we found that infusion of the GABAA receptor antagonist, bicuculline, stimulated release of KP and GnRH and presence of the KP receptor antagonist, peptide 234, during bicuculline infusion blocked the bicuculline-induced GnRH release.
Release of GnRH at a sufficient level with defined pulsatile patterns is critical to the onset of puberty and reproductive function in general. After heightened gonadotropin release (and presumably GnRH release) at the neonatal period, gonadotropin levels are kept low until puberty onset in primates. This period is termed the juvenile hiatus of gonadotropin release and is not due to circulating gonadal steroids (17, 18). Rather, tonic central inhibition is responsible as gonadal dysgensis in human patients and gonadectomized monkeys undergo a similar suppressed gonadotropin release during the juvenile period (17, 19, 20). As a neural substrate for this tonic central inhibition, our laboratory previously has provided evidence for the role of GABA neurons. In fact, a reduction in tonic GABA inhibition over GnRH release was sufficient to induce puberty onset (3, 4). However, the precise mechanism for GABA control over GnRH release was not defined. Specifically, the question of whether GABA inhibition was directly to GnRH neurons or indirectly through interneurons, such as kisspeptin neurons, was not addressed.
In the present study, we found that the GABAA receptor antagonist, bicuculline, caused a rapid, transient increase of KP release in the S-ME/MBH. This effect was clear in prepubertal, but not midpubertal, females, which is strikingly similar to our earlier observations on the effect of bicuculline on GnRH release across the transition to puberty (2). It is likely that blockade of GABA neurotransmission is more effective in altering peptide release in prepubertal compared with midpubertal females due to the differences in tonic hypothalamic GABA release between these two stages. Specifically our previous findings show that hypothalamic GABA tone is significantly higher in prepubertal compared with midpubertal females and infusion of GABA was more effective at suppressing GnRH release in midpubertal compared with prepubertal animals (3), suggesting that the decrease in GABA tone across puberty is at least partly responsible for increased GnRH release at puberty onset. The current study verified that bicuculline infusion to the S-ME/MBH induces a rapid increase of GnRH release in prepubertal female monkeys and that this bicuculline-induced increase in GnRH release is sensitive to the presence of the KP receptor antagonist, peptide 234. Collectively the results of this study have defined KP neurons as a part of the neuronal network mediating GABAergic control of GnRH release before puberty in non-human primates.
If tonic GABA inhibition over GnRH neurons is mediated through KP neurons, it is expected that KP release would exhibit a developmental pattern that parallels GnRH developmental release patterns. Recent studies in our laboratory confirm that expectation. Specifically we found that mean KP release increased through pubertal development with timing similar to developmental increases in mean GnRH release (11, 13). In addition, the maturation of pulsatile KP release patterns and amplitude was similar to the developmental changes our laboratory previously observed for GnRH release (13, 21). Based on these observations, we have proposed that after puberty onset, the developmental increase in GnRH release is facilitated by KP release.
It should be noted that a complex set of neuronal inputs other than kisspeptin neurons are known to alter GnRH release in the control of reproductive function (22). For example, Plant and colleagues (23, 24) have proposed that neuropeptide Y neurons are responsible for the juvenile hiatus in LH secretion in male monkeys, although there is a subtle sex difference (18) in the intensity of central inhibition (i.e. complete suppression in males vs. incomplete in females). Perhaps that sex difference is attributable to central inhibition mediated by GABA neurons in females vs. neuropeptide Y neurons in males, although this needs confirmation. In addition, other known modifiers of GnRH release, including dynorphin, neurokinin B, leptin, and the excitatory neurotransmitter glutamate, may have a role in GnRH control before or after puberty. Although we found that a reduction in GABA tone in the MBH precedes a relative increase in glutamate tone (25) during the transition to puberty, the interactions of other neuropeptides and neurotransmitters in the control of GnRH release remains largely underinvestigated, particularly in primates. Nonetheless, it appears that developmental changes in KP are closely tied to pubertal changes of GnRH release in female primates, and now we believe that these two are coordinately regulated by the tonic GABA-inhibitory signal present during female prepubertal development. However, the question of what triggers the pubertal decrease in GABA tone remains unclear.
Recently we found that female rhesus monkeys that eat a high-calorie diet during prepubertal development reach menarche (puberty onset) at a younger age with coordinate increases in body growth rates and circulating leptin levels (26). Intriguingly, a recent study found that leptin action through leptin receptor (LepR) in the GABA neuronal network participates in the control of energy homeostasis in mice (27). It is tempting to speculate that this metabolic signal helps define pubertal timing through inhibitory inputs to GnRH neurons. In fact, recent findings in our laboratory indicate that LepR-expressing GABA neurons are concentrated in the premammallary nucleus (Terasawa E., unpublished observation), a region in which lesions in prepubertal female monkeys induces precocious puberty and precocious LH elevation (28, 29). Although whether a similar neurocircuitory of the LepR expressing GABA network is responsible for leptin action in primates and how the GABA network with LepR interacts with GnRH neurons needs to be defined, evidence is already mounting to suggest that hypothalamic GABA neurons with LepR may have a role in initiating puberty.
In conclusion, in the present study, we have found that interference with tonic GABA inhibition in the prepubertal female primate hypothalamus causes a rapid, transient increase in KP release. The same treatment had no effect in pubertal monkeys, when tonic GABA inhibition has presumably decreased. Moreover, the tonic inhibition by GABA transmission over KP neurons is, at least in part, responsible for low levels of GnRH release before puberty onset. Future work will aim to define factors that modify inhibitory GABA tone in the prepubertal female primate hypothalamus.
Acknowledgments
We express our sincere appreciation to Dr. Robert P. Millar (University of Edinburgh, Edinburgh, UK), Dr. Stephen R. Bloom (Imperial College, London, UK), and Dr. Terry Nett (Colorado State University, Fort Collins, Colorado) for their generous gifts of peptide 234, GQ2, and R-42, respectively. Without these research tools, we would not have been able to conduct this study.
This work was supported by National Institutes of Health Grants R01HD11355 and R01HD15433 and made possible to perform by National Institutes of Health support Grant P51OD011106 (to the Wisconsin National Primate Research Center).
Disclosure Summary: The authors have no conflicts to disclose.
Footnotes
- GABA
- γ-Amino butyric acid
- GPR54
- G protein-coupled receptor 54
- KP
- kisspeptin
- LepR
- leptin receptor
- MBH
- medial basal hypothalamus
- S-ME
- stalk-median eminence.
References
- 1. Wildt L, Marshall G, Knobil E. 1980. Experimental induction of puberty in the infantile female rhesus monkey. Science 207:1373–1375 [DOI] [PubMed] [Google Scholar]
- 2. Watanabe G, Terasawa E. 1989. In vivo release of luteinizing hormone releasing hormone increases with puberty in the female rhesus monkey. Endocrinology 125:92–99 [DOI] [PubMed] [Google Scholar]
- 3. Mitsushima D, Hei DL, Terasawa E. 1994. γ-Aminobutyric acid is an inhibitory neurotransmitter restricting the release of luteinizing hormone-releasing hormone before the onset of puberty. Proc Natl Acad Sci USA 91:395–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Keen KL, Burich AJ, Mitsushima D, Kasuya E, Terasawa E. 1999. Effects of pulsatile infusion of the GABAA receptor blocker bicuculline on the onset of puberty in female rhesus monkeys. Endocrinology 140:5257–5266 [DOI] [PubMed] [Google Scholar]
- 5. Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zhan D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. 2003. The GPR54 gene as a regulator of puberty. N Engl J Med 349:1614–1627 [DOI] [PubMed] [Google Scholar]
- 6. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. 2003. Hypogonadotropic hypogonadism due to loss of function of the kiss1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 100:10972–10976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Funes S, Hedrick JA, Vassileva G, Markowitz L, Abbondanzo S, Golovko A, Yang S, Monsma FJ, Gustafson EL. 2003. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun 312:1357–1363 [DOI] [PubMed] [Google Scholar]
- 8. d'Angelmont de Tassigny X, Fagg LA, Dixon JP, Day K, Leitch HG, Hendrick AG, Zhan D, Franceschini I, Caraty A, Carlton MB, Aparicio SA, Colledge WH. 2007. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci USA 104:10714–10719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lapatto R, Pallais JC, Zhang D, Chan YM, Mahan A, Cerrato F, Le WW, Hoffman GE, Seminara SB. 2007. Kiss1−/− mice exhibit more variable hypogonadism that Gpr54−/− mice. Endocrinology 148:4927–4936 [DOI] [PubMed] [Google Scholar]
- 10. Herbison AE, de Tassigny X, Doran J, Colledge WH. 2010. Distribution and postnatal development of Gpr54 gene expression in mouse brain and gonadotropin-releasing hormone neurons. Endocrinology 151:312–321 [DOI] [PubMed] [Google Scholar]
- 11. Keen KL, Wegner FH, Bloom SR, Ghatei MA, Terasawa E. 2008. An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology 149:4151–4157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guerriero KA, Keen KL, Millar RP, Terasawa E. 2012. Developmental changes in GnRH release in response to kisspeptin agonist and antagonist in female rhesus monkeys (Macaca mulatta): implication for the mechanism of puberty. Endocrinology 153:825–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guerriero KA, Keen KL, Terasawa E. 2012. Developmental increase in kisspeptin-54 release in vivo is independent of the pubertal increase in estradiol in female rhesus monkeys (Macaca mulatta). Endocrinology 153:1887–1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Terasawa E, Nass TE, Yeoman RR, Loose MD, Schultz NJ. 1983. Hypothalamic control of puberty in female rhesus macaque. In: Norman RL, ed. Neuroendocrine aspects of reproduction. New York: Academic Press; 149–182 [Google Scholar]
- 15. Dhillo WS, Chaudhri OB, Thompson EL, Murphy KG, Patterson M, Ramachandran R, Nijher GK, Amber V, Kokkinos A, Donaldson M, Ghatei MA, Bloom SR. 2007. Kisspeptin-54 stimulates gonadotropin release most potently during the preovulatory phase of the menstrual cycle in women. J Clin Endocrinol Metab 92:3958–3966 [DOI] [PubMed] [Google Scholar]
- 16. Gearing M, Terasawa E. 1988. Luteinizing hormone releasing hormone (LHRH) neuroterminals mapped using the push-pull perfusion method in the rhesus monkey. Brain Res Bull 21:117–121 [DOI] [PubMed] [Google Scholar]
- 17. Plant TM. 1980. The effects of neonatal orchidectomy on the developmental pattern of gonadotropin secretion in the male rhesus monkey (Macaca mulatta). Endocrinology 106:1451–1454 [DOI] [PubMed] [Google Scholar]
- 18. Plant TM, Witchel SF. 2006. Puberty in nonhuman primates and primates. In: Neill J, ed. The physiology of reproduction. 3rd ed Vol 2 San Diego: Academic Press; 2177–2230 [Google Scholar]
- 19. Conte FA, Grumbach MM, Kaplan SL, Reiter EO. 1980. Correlation of luteinizing hormone-releasing factor-induced luteinizing hormone and follicle-stimulating hormone release from infancy to 19 years with the changing pattern of gonadotropin secretion in agonadal patients: relation to the restraint of puberty. J Clin Endocrinol Metab 50:163–168 [DOI] [PubMed] [Google Scholar]
- 20. Terasawa E, Nass TE, Yeoman RR, Loose MD, Schultz NJ. 1983. Hypothalamic control of puberty in the female rhesus macaque. In: Norman RL, ed. Neuroendocrine aspects of reproduction. New York: Academic Press; 149–182 [Google Scholar]
- 21. Watanabe G, Terasawa E. 1989. In vivo release of luteinizing hormone releasing hormone increases with puberty in the female rhesus monkey. Endocrinology 1989 125:92–99 [DOI] [PubMed] [Google Scholar]
- 22. Terasawa E, Kurian JR. 2012. Neuroendocrine mechanism of puberty. In: Fink G, Pfaff DW, Levine JE, eds. Handbook of neuroendocrinology. London: Elsevier; 433–484 [Google Scholar]
- 23. Plant TM, Shahab M. 2002. Neuroendocrine mechanisms that delay and initiate puberty in higher primates. Physiol Behav 77:717–722 [DOI] [PubMed] [Google Scholar]
- 24. El Majdoubi M, Sahu A, Ramaswamy S, Plant TM. 2000. Neuropeptide Y: a hypothalamic brake restraining the onset of puberty in primates. Proc Natl Acad Sci USA 97:6179–6184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Terasawa E, Luchansky LL, Kasuya E, Nyberg CL. 1999. An increase in glutamate release follows a decrease in γ aminobutyric acid and the pubertal increase in luteinizing hormone releasing hormone release in the female rhesus monkeys. J Neuroendocrinol 11:275–282 [DOI] [PubMed] [Google Scholar]
- 26. Terasawa E, Kurian JR, Keen KL, Shiel NA, Colman RJ, Capuano SV. 2012. Body weight impact on puberty: effects of high-calorie diet on puberty onset in female rhesus monkeys. Endocrinology 153:1696–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vong L, Ye C, Yang Z, Choi B, Chua S, Jr, Lowell BB. 2011. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron 71:142–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Terasawa E, Bridson WE, Nass TE, Noonan JJ, Dierschke DJ. 1984. Developmental changes in the luteinizing hormone secretory pattern in peripubertal female rhesus monkeys: comparisons between gonadally intact and ovariectomized animals. Endocrinology 115:2233–2240 [DOI] [PubMed] [Google Scholar]
- 29. Schultz NJ, Terasawa E. 1988. Posterior hypothalamic lesions advance the time of the pubertal changes in luteinizing hormone release in ovariectomized female rhesus monkeys. Endocrinology 123:445–455 [DOI] [PubMed] [Google Scholar]