Abstract
Glucagon like peptide-1 (GLP-1) and GLP-2 are hormones secreted by intestinal L cells that stimulate glucose-dependent insulin secretion and regulate intestinal growth, respectively. Mice with deletion of the glucagon receptor (Gcgr) have high levels of circulating GLP-1 and GLP-2. We sought to determine whether the increased level of the glucagon-like peptides is due to L cell hyperplasia. We found, first, that high levels of the glucagon-like peptides increase L cell number but does not affect the number of other intestinal epithelial cell types. Second, a large proportion of ileal L cells of Gcgr−/− mice coexpressed glucose-dependent insulinotropic peptide (GIP). Cells coexpressing GIP and GLP-1 are termed LK cells. Third, the augmentation in L cell number was due to a higher rate of proliferation of L cell progenitors rather than to the entrance of mature L cells into the cell cycle. Fourth, a high concentration of the glucagon-like peptides in the circulation augmented the mRNA levels of transcription factors expressed by late but not early enteroendocrine progenitors. Fifth, the administration of exendin 9–39, a GLP-1 receptor antagonist, resulted in a decrease in the rate of L cell precursor proliferation. Finally, we determined that L cells do not express the GLP-1 receptor, suggesting that the effect of GLP-1 is mediated by paracrine and/or neuronal signals. Our results suggest that GLP-1 plays an important role in the regulation of L cell number.
The cells of the intestinal epithelium are generated by common precursors located in the base of the crypts (1–4). These precursors produce the absorptive cells (columnar cells or enterocytes) and secretory cells (Paneth, goblet, and enteroendocrine cells) (5). Enteroendocrine cells are scattered throughout the mucosa, comprising approximately 1% of the cells lining the intestinal lumen (6). There are over 15 different types of enteroendocrine cells, which form the largest population of hormone-producing cells in the body (6).
The L cells, located mainly in the mucosa of ileum and colon, are the main source of glucagon-like peptide-1 (GLP-1). GLP-1 is an enteric hormone encoded by a single glucagon gene (7–9). The precursor peptide, proglucagon, undergoes tissue-specific posttranslational processing. In the α-cells of the pancreas, proglucagon is processed to glucagon (10). In intestine, proglucagon generates not only GLP-1 but also GLP-2, a hormone that induces epithelial cell proliferation and regeneration (2, 11, 12), oxyntomodulin, and glicentin. Although GLP-2 and glucagon show some sequence homology to GLP-1, these peptides exhibit no interactions with the GLP-1 receptor (GLP-1r) at physiologically relevant concentrations (13–15). Two of the enteroendocrine hormones, GLP-1 and glucose-dependent insulinotropic peptide (GIP), produced by L cells and the K cells of the duodenum, respectively, are termed incretins because they are secreted after a meal, stimulating glucose-dependent insulin secretion (8).
The identity of the signals regulating the number of each cell type within the various intestinal cell lineages is not known. In this study, we sought to determine whether L cell number is regulated by GLP-1. This question was examined in mice lacking the glucagon receptor (Gcgr), which were reported to have high circulating levels of GLP-1 and GLP-2 (16, 17). We found that high levels of the glucagon-like peptides increase the number of L cells that coexpress GIP (LK cells) but do not affect the number of other intestinal epithelial cell types and that the administration of exendin 9–39 (Ex 9–39), a GLP-1r antagonist, resulted in a decrease in the rate of L cell precursor proliferation. Our results suggest that GLP-1 plays an important role in the regulation of LK cell number.
Materials and Methods
Animals
The generation of Gcgr knockout mice has been previously described (16). Gcgr+/+ and Gcgr−/− in a C57BL6/J background were derived from heterozygote crossings (16). Three- to 4-month-old mice were used in all experiments. Animals were fed ad libitum with free access to water and maintained in a murine hepatitis virus-free barrier facility on a 12-h light, 12-h dark cycle. Two-month-old male CD-1 mice were purchased from Charles River (Wilmington, MA). All animal protocols were approved by the Institutional Animal Care and Use Committee.
Preparation of tissue sections
Mice (not fasted) were perfused through the heart with 4% paraformaldehyde (PF). Samples of 3 cm duodenum (proximal to the pylorus), 8 cm ileum (proximal to the cecum), and 4 cm colon were collected and cleaned of mesenteric fat, gently flushed with PBS, postfixed for 1 h in 4% PF, cryopreserved in a 30% sucrose solution, and embedded in Shandon M1 matrix (Thermo Scientific, Pittsburgh, PA), and 20-μm frozen sections were obtained using a cryostat microtome (Leica Jung 3050S).
Immunocytochemistry
Sections were processed for immunostaining as previously described (18). Primary antisera raised in different hosts were used to localize two antibodies in the same tissue section. The bound antibodies were visualized with corresponding anti-primary host X secondary antibody linked to molecules that fluoresce at different wavelengths, respectively. Source and dilutions of antibodies used in this study are described in Supplemental Table 1 (published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org).
Antibody to the GLP-1r
Antisera to the GLP-1r was a generous gift from Dr. Habener (Harvard Medical School, Boston, MA). This antibody has been previously characterized (19, 20). In addition, we tested the specificity of the GLP-1r antibody using antisera that was immunoneutralized with a specific blocking peptide. The 18-amino-acid peptide, previously described by Heller et al. (20), was synthesized and more than 90% HPLC purified by Biomatik (Cambridge, Ontario, Canada). The sequence from N terminus to C terminus was as follows: TVSLSETVQKWREYRHQC. The peptide was modified by conjugation to keyhole limpet hemocyanin through free-SH Cys. The GLP-1r antibody was mixed with a 5-fold (by weight) excess of blocking peptide in a small volume (500 μl) of PBS, incubated in a rotator overnight at 4 C, and centrifuged at 10,000 rpm for 10 min, and the supernatant was used for immunostaining.
Visualization of goblet cells
Cells were visualized using the periodic-acid Schiff (PAS) staining kit (Polysciences, Warrington, PA) as indicated by the provider. Paneth cells are also PAS+, but they are located in the base of the crypt and were not counted.
Cell proliferation
We could not employ the commonly used pulse/chase methodology in which bromodeoxyuridine (BrdU) or [3H]thymidine is administered once to mice, and the fate of labeled cells is then followed. Rapidly proliferating cells divide the label between daughter cells, consequently diluting the label (21). Although early work detected enteroendocrine cells containing [3H]thymidine in the nucleus several days after a pulse with the isotope (2), it is likely that the cells containing the isotope withdrew from the cell cycle just after the injection of the isotope, preventing its dilution. The number of these cells is so small that it precludes an analysis of the rate of proliferation using a classical pulse/chase scheme.
In our studies, BrdU was added to drinking water (80 mg/100 ml) for 6 d, and the mice were then euthanized. Tissue sections were processed for visualization of BrdU and GLP-1 as described previously (18). The number of GLP-1+BrdU+ over the total number of GLP-1+ cells was scored. Results in the experimental group were normalized to the value obtained in controls. At least 300 GLP-1+ cells were scored per mouse (three mice per line). Alternatively, proliferating cells were identified by immunostaining with antisera to Ki67, a marker of proliferating cells.
Determination of apoptosis
Sections were processed for visualization of active caspase 3, according to manufacturer's protocol (Chemicon, Temecula, CA). The number of caspase 3+ cells was determined in 10 random cross-sections of the colon from three mice per strain. The number of caspase 3+ cells over total number of crypts was determined.
Isolation of intestinal cells
Mice were anesthetized, and a 10-cm segment of ileum (proximal to the cecum), a 3-cm segment of the duodenum (proximal to the pylorus), and 4 cm of colon (next to the cecum) were used for isolation of epithelial cells following a previously described technique (22) with minor modifications. Epithelial cell suspensions were collected in nuclease-free tubes filled with 1 ml PBS and centrifuged at 1000 rpm for 2 min at 4 C. The pellet was collected for RNA isolation.
Confocal microscopy
Confocal images were obtained using a Radiance 2000 confocal microscope (Bio-Rad, Hercules, CA) attached to a Zeiss Axioskop microscope (Carl Zeiss Inc., Thornwood, NY) on every 10th section. Images at 1260 × 1260 pixels were obtained and processed using Adobe Photoshop version 6.0 (Adobe Systems, Mountain View, CA).
Morphometry
At least 10 cross-sections (one from every 10 sections) were chosen to determine numbers of immunostained cells per villus or crypt, n = 3 per group. Only correctly oriented villi/crypt were examined, i.e. those in which it was possible to visually follow without interruption the continuation of a crypt into the villi. To determine the number of different intestinal epithelial cells, a minimum of 200 villi and crypts were scored per mouse per line.
To determine the surface area of villi, tissue samples measuring 1.0 cm were taken from identical anatomical positions of the ileum of 4-month-old male Gcgr+/+ and Gcgr−/− mice, four mice per line. Tissues were fixed, sectioned (20 μm) in a cryostat microtome, and stained with hematoxylin. For each tissue sample, microscopic measurements were determined in a blinded fashion from a minimum of 25 well-oriented villi from at least five nonconsecutive cross-sections per animal. Villus plus crypt surface area was measured using ImageJ (imagej.nih.gov/ij), and the data, in arbitrary units of numeric value, are graphically represented as mean ± sem.
Quantification of the length of the transit amplifying (TA) region
The TA region was identified by Ki67 staining and was measured in cross-sections of colonic crypts using Image J. For each mouse, an average of 25 well-oriented crypts were scored, and a ratio of the length of the region containing Ki67+ nuclei over the total crypt length was determined (n = 3 mice per strain).
Exendin 4 administration
CD-1 mice were injected with exendin 4 (10 nmol/kg body weight) (23) (Bachem, Torrance, CA) dissolved in PBS plus 1% BSA twice a day for 2 wk or vehicle (PBS plus 1% BSA).
Dipeptidyl-peptidase 4 (DPP4) inhibitor administration
CD-1 mice were fed a regular diet (rodent chow no. 5015; Research Diets, New Brunswick, NJ) or a diet containing the sitagliptin analog MK0626 [rodent chow no. 5015 (Purina) plus 4 g/kg MK0626; Merck, Rathway, NJ) for 2 months. This treatment has been shown to completely inhibit plasma DDP4 levels in control mice 1 wk after the initiation of treatment (24, 25) (Merck, unpublished results). This diet did not induce differences in weight or in fasting and fed blood glucose levels.
Ex 9–39 administration
The GLP-1r antagonist Ex 9–39 was purchased from California Peptide Research Inc. (Napa, CA). All experiments were performed using the same batch of Ex 9–39. The stock solution (1 μg/μl) was diluted in PBS containing 1% BSA to achieve the final concentrations employed for injection.
Intraperitoneal glucose tolerance tests
Intraperitoneal glucose tolerance tests were performed in mice after an overnight fast (16–18 h). Fasting blood glucose levels were measured. Then, Ex 9–39 (5 μg) or saline (control) was administered ip 20 min before glucose loading (1.5 mg glucose/g body weight). Blood was drawn from the tail vein at 0, 30, 60, 90, and 120 min after glucose administration, and blood glucose levels were measured by the glucose oxidase method using a Precision Xtra glucometer (Abbott, Alameda, CA).
Mouse genotyping
Primer sequences and PCR protocol for Gcgr mice were as previously described (16). Because Gcgr−/− mice develop islet cell hyperplasia (16, 18), samples of pancreas were taken from each mouse to confirm the islet phenotype.
RNA isolation and quantitative real-time PCR
Intestinal cells were isolated, and RNA isolation and quantitative real-time PCR were performed as described previously (26) and in Supplemental Data 1. The level of the specific gene (X) transcript was normalized to the expression of TATA box binding protein (TBP) or 18S rRNA as endogenous control. For data analysis, the ΔΔCt method was used; for each gene, fold changes were calculated as the difference in gene expression between Gcgr+/+, Gcgr−/−, and Ex 9–39-injected Gcgr−/− mice. All reactions were run in triplicate, and each gene expression assay was repeated at least three times.
Measurement of GIP and GLP-2 levels in plasma
Circulating levels of GIP were determined by ELISA (Millipore, St. Charles, MO) that measures both intact GIP (1–42) and its major metabolite GIP (3–42) in mouse plasma following manufacturer's instructions. Total plasma GLP-2 levels were assessed using a mouse GLP-2 assay kit (YK142 mouse GLP-2 EIA; Yanaihara Institute Inc., Shizuoka, Japan/Biovendor LLC, Candler, NC). This assay has a high specificity to mouse GLP-2.
Cytospin preparation and measurement of cell size
Mice were killed, and intestinal cell suspensions prepared from ileum and colon were subjected to cytocentrifugation on pretreated glass slides for 5 min at 1000 rpm (Cytospin 4; Shandon). The cytospin preparations were fixed for 10 min in a solution of 4% PF and processed for immunostaining. To measure the size of proliferating cells, slides were immunostained for visualization of Ki67 in the nuclei and β-catenin in the cell membrane. Surface area of cells was measured using Image J, and the results are expressed in arbitrary units.
Statistical analysis
Values indicate the mean ± sem. For comparison between two groups, unpaired two-tailed Student's t test was used. A P value < 0.05 was considered significant. Because of the small sample size, no mathematical correction was made for multiple comparisons (27).
Results
Specific increase in the number of cells expressing GLP-1
To ascertain whether the rise in circulating levels of the glucagon-like peptides in Gcgr−/− mice was correlated with an increase in the number of GLP-1+ cells, the number of L cells in the ileum of Gcgr−/− and Gcgr+/+ mice was determined.
Morphometric analysis of sections immunostained for visualization of GLP-1 showed that the average number of L cells per villi plus crypts was higher in Gcgr−/− than in Gcgr+/+ mice (Fig. 1, A–C). Because it has been reported that some L cells coexpress GIP (28, 29), we sought to determine whether the lower small intestine contained cells coexpressing GLP-1 and GIP (termed LK cells) (28, 29) and whether their relative number increased in Gcgr−/− mice. We found that the ileum of the Gcgr−/− mice contained LK cells (Fig. 1D). Morphometric analysis indicated that the number of LK cells per villi plus crypts increased approximately 4-fold in the ileum of Gcgr−/− compared with Gcgr+/+ mice (Fig. 1E). This increase cannot be accounted for by differences in villi size, because the size of the villi was similar in Gcgr+/+ (Fig. 1F) and Gcgr−/− (Fig. 1G) mice. This result was confirmed by morphometric analysis of the surface area of villi (Fig. 1H). Moreover, the percentage of LK cells over total number of L cells scored was significantly higher in the ileum of Gcgr−/− than of Gcgr+/+ mice (Fig. 1I). In contrast to ileum, the number of LK cells in duodenum was similar in Gcgr−/− and Gcgr+/+ mice (Fig. 1J).
Fig. 1.
Increase in L cell number in ileum. A and B, Photomicrographs illustrate cells immunostained for GLP-1 in ileum of Gcgr+/+ (A) and Gcgr−/− (B) mice. Scale bar, 20 μm. C, Histogram documents that the number of L cells per villus and crypt is higher in Gcgr−/− than in Gcgr+/+ mice; n = 6 mice per line, at least 300 villi, and crypts per mouse were scored. **, P < 0.005. D, Immunohistochemical localization of GLP-1 (red) and GIP (green) in a LK cell (yellow) located in the ileum of Gcgr−/− mice. Scale bar, 15 μm. E, The number of cells coexpressing GLP-1 and GIP (LK cells) per villus plus crypt of ileum is higher in Gcgr−/− than in Gcgr+/+ mice; n = 4 mice per line. *, P < 0.05. F and G, Representative sections document that the thickness of the intestinal wall and villi length were similar in Gcgr+/+ (F) and Gcgr−/− (G) mice. H, Villi in ileum of Gcgr+/+ and Gcgr−/− have similar surface area; n = 4 mice per line, and at least 100 villi were scored per animal. I, Graph illustrates the specific increase in the ratio of LK cells over total number of L cells scored in ileum of Gcgr−/− and Gcgr+/+ mice. *, P < 0.05; n = 4 per line. J, Duodenum of Gcgr−/− and Gcgr+/+ mice have similar number of cells coexpressing GLP-1 and GIP (LK cells); n = 4 mice per line. K, Photomicrograph illustrates two GLP-1+ (cytoplasm, red) and Pdx-1+ (nucleus, green) cells in the ileum of Gcgr−/− mice. Scale bar, 15 μm. For all measurement of cell numbers, at least 300 villi plus crypt were scored.
Because Pdx-1, a transcription factor (TF) involved in endocrine differentiation in pancreas and intestine (30, 31), is required for GIP, but not for GLP-1, expression (32, 33), we sought to determine whether the GLP-1+ cells of the ileum expressed Pdx-1. Immunostaining for GLP-1 and Pdx-1 revealed the presence of doubly labeled cells (Fig. 1K). These observations strongly suggest that the GLP-1+Pdx-1+ cells also expressed GIP. This possibility is supported by the presence of GLP-1+GIP+ cells (Fig. 1D) and by the report that cells that coexpress GIP and GLP-1 are Pax6 and Pdx-1 positive, whereas cells expressing only GLP-1 are Pax6 positive but do not express Pdx-1 (32). Taken together, these findings indicate that ablation of Gcgr results in an increase in the number of LK cells in the ileum but not in duodenum.
Next, we tested whether ablation of Gcgr affected the number of other intestinal epithelial cells. Immunohistochemical and morphometric analysis indicated that the number of mucin-secreting goblet cells (nonendocrine secretory lineage) (Fig. 2, A–C), and of cells expressing serotonin (Fig. 2, D–F) and cholecystokinin (CCK) (Fig. 2, G–I) (endocrine lineage), was similar in the ileum of Gcgr+/+ and Gcgr−/− mice, indicating that deletion of Gcgr affects only L cell number.
Fig. 2.
The number of other intestinal epithelial cells is not affected. The number of goblet (A–C), serotonin (D–F), and CCK (G–I) cells in the ileum is similar in Gcgr+/+ and Gcgr−/− mice. At least 200 villi per mouse for each cell type, three mice per line, were examined. The three cell types are illustrated in the respective photomicrographs. Photomicrographs A, D, and G correspond to Gcgr+/+ mice; photomicrographs B, E, and H correspond to Gcgr−/− mice. Scale bars, 50 μm (A and B) and 20 μm (D, E, G, and H). Goblet cells were identified using the PAS technique, whereas serotonin and CCK cells were identified by immunocytochemistry.
Ablation of Gcgr resulted in an elongation of the gut. Determination of the length of the intestine from duodenum to rectum in fresh (not perfused) tissues indicated that the intestine of Gcgr−/− was 20% longer than that of Gcgr+/+ mice (Fig. 3A). The augmentation in gut length could be due to the action of GLP-2, which induces intestinal hypertrophy (34). In agreement with recently published observations (17), we found that the circulating level of GLP-2 was significantly higher in Gcgr−/− than in Gcgr+/+ mice (Fig. 3B).
Fig. 3.
A, Increased gut length in Gcgr−/− compared with Gcgr+/+. B, Plasma level of GLP-2 was higher in Gcgr−/− than in Gcgr+/+ mice. C, Effect of DPP4 inhibition in CD-1 mice. Sections of ileum of CD-1 mice were stained for visualization of GLP-1, and the number of L cells per surface area of villi was determined; n = 4 mice per treatment, at least 300 villi, and crypts per mouse were scored per antibody staining. CD, Control diet; ED, experimental diet. D–F, Apoptosis; D and E, caspase3+ cells in ileum (D) and colon (E) of Gcgr−/− mice. Note cluster of stained cells in villus of ileum. Scale bar, 30 μm. F, Deletion of Gcgr inhibited apoptosis of intestinal epithelial cells. The number of apoptotic cells (caspase 3+), was lower in Gcgr−/− than in Gcgr+/+ mice (200 crypts scored per mouse and three mice examined per strain). G, Mature L cells are quiescent. Photomicrograph illustrates a section of ileal crypts of Gcgr−/− mice processed for visualization of GLP-1 (red) and Ki67 (green). Note that the GLP-1+ cell (arrowhead) does not contain Ki67. Scale bar, 20 μm. This cell is shown in the inset at higher magnification. **, P < 0.005 and ***, P < 0.001.
Glucagon-like peptides affect L cell number of control CD-1 mice
Then we tested whether the effect of the glucagon-like peptides on L cell number was restricted to the Gcgr−/− mice. The administration of the GLP-1r agonist exendin-4 to CD-1 mice did not affect L cell number, the size of the crypt plus villi, or the length of the TA region (not shown). In contrast, size of the villi plus crypt compartment increased in mice fed with a diet containing an inhibitor to DPP4 (Supplemental Table 2), the enzyme responsible for degradation of GLP-1, GLP-2, GIP, and other molecules (35). For this reason, L cell number per villi plus crypt was normalized to the corresponding surface area. Mice fed the experimental diet showed a significant increase in the number of L cells (per surface area of villus plus crypt) in the distal ileum (Fig. 3C) and a 25% increase in the length of the TA region (not shown). In contrast to L cells, the number of CCK cells was similar in both groups of mice in the distal ileum (not shown).
Decreased number of apoptotic cells in intestinal mucosa of Gcgr−/− mice
To determine whether the increase in L cell number in Gcgr−/− mice is due to a reduction in the rate of programmed cell death, we measured the rate of apoptosis in Gcgr+/+ and Gcgr−/− mice. Apoptotic cells, identified by the expression of active caspase-3, were more easily quantified in colon than in ileum. In ileum, positive cells cluster at the tip of the villi and are visible only when the apex is in the plane of section (Fig. 3D), making them difficult to quantify. The colon mucosa lacks villi, and apoptotic cells are distributed throughout the surface of the epithelium. The number of apoptotic cells per crypt, identified in cross-sections of the colon of Gcgr−/− (Fig. 3E) and Gcgr+/+ (not shown), was lower in Gcgr−/− than in Gcgr+/+ mice (Fig. 3F). This decrease in the rate of cell death could be due to the high circulating levels of GLP-1 and GLP-2, because both of these glucagon-like peptides have been shown to have antiapoptotic effects (36, 37).
Deletion of Gcgr increases proliferation of L precursor cells
The increase in L cell number could be due not only to a decrease in apoptosis but also to an increase in L cell proliferation. Because differentiated enteroendocrine cells are quiescent (5), the increased number of L cells in Gcgr −/− mice could be due either to the reentry of the cells to the cell cycle and/or to increased replication of L cell precursors. Double-label immunohistochemical visualization of GLP-1 and Ki67 in tissue sections of the ileum of Gcgr−/− and Gcgr+/+ mice confirmed that L cells did not express the proliferation marker (Fig. 3G). This finding demonstrates that mutation of Gcgr does not induce differentiated L cells to reenter the cell cycle.
Then, we sought to determine whether the rise in the number of L cells in Gcgr−/− mice was due to the increased proliferation of progenitors responsible for the L cell lineage. The protocol used was designed to increase the number of labeled differentiated cells. Gcgr−/− and Gcgr+/+ mice were administered BrdU in the drinking water for 6 d and the ileum examined with histological techniques at the end of that period. Although the turnover time of mucosal cells is believed to be 3–4 d (2, 38), the presence of a large number of GLP-1+Brdu− cells after the 6-d exposure to BrdU indicates that they have a much longer cycle. GLP-1+Brdu− were termed old L cells because these cells became quiescent before the initiation of the treatment with the thymidine analog, whereas cells generated during the administration of BrdU contained GLP-1 and BrdU and were termed new L cells (Fig. 4, A and B). Progenitor cells are believed to cycle every 12 h (39, 40), and it is likely that several generations of precursors were labeled before they began to differentiate. Thus, the long-term labeling protocol facilitated the replication analysis because it amplified the number of BrdU+ L cells accumulated during the period of exposure to BrdU. Precursors with a low rate of proliferation produced fewer GLP-1+BrdU+ daughter cells than more actively dividing progenitors (Fig. 4, A and B).
Fig. 4.
Increased L cell precursor proliferation. A and B, Predictions from analysis of rate of precursor cell proliferation after a long-term exposure to BrdU. Precursors that differentiated before the BrdU administration lack BrdU (BrdU−GLP-1+, old L cells). In model A, a precursor cell (BrdU−GLP-1−) divides only once during the exposure to BrdU, and the two daughter cells (BrdU+GLP-1−) become quiescent and initiate GLP-1 expression (BrdU+GLP-1+). In B, precursor cells undergo two cycles of cell division, generating four daughter cells before becoming quiescent and differentiating into L cells. The ratio of (new) BrdU+GLP-1+ cells generated during the period of exposure to BrdU to the total number of L cells scored (GLP1+ BrdU− plus GLP-1+ BrdU+) is higher in B than in A. C, Photomicrograph illustrates a new L cell [GLP-1+ (red) BrdU+ (green)] in Gcgr−/− mice generated from proliferating progenitors. This cell is shown in higher magnification in the inset. D, The number of GLP-1+BrdU+ cells per total number of GLP-1+ cells was determined in ileum of Gcgr+/+, Gcgr−/−, and Gcgr−/− mice injected with Ex 9–39; n = 3 mice per group. Results from Gcgr−/− mice were normalized to the value obtained for the Gcgr+/+ line. Note the increase in the number of new L cells in Gcgr−/− mice and its decrease in Gcgr−/− mice that received Ex 9–39; n = 3 mice per group. **, P < 0.01; *, P < 0.05.
Comparison of the number of GLP-1+BrdU+ cells (Fig. 4C) that were generated during the period of BrdU labeling revealed that the ratio of GLP-1+ to BrdU+ vs. the total number of GLP-1+ L cells was 75% higher in Gcgr−/− mice than in Gcgr+/+ mice (Fig. 4D). The fact that the number of other enteroendocrine cells was similar in Gcgr−/− and Gcgr+/+ mice suggests that deletion of Gcgr specifically increased the rate of proliferation of L cell progenitors.
The increase in progenitor cell proliferation in Gcgr−/− mice was correlated with an elongation of the TA region, where progenitor cells are located. We examined colon because it provides a highly purified preparation of crypts for cytospin (see below). Cross-sections of similar regions of colon from Gcgr+/+ and Gcgr−/− mice were processed for visualization of Ki67 (Fig. 5, A and B), and the length of the TA region was measured. This analysis revealed that the ratio of the length of the proliferative compartment to the length of the crypt was higher in Gcgr−/− mice than in Gcgr+/+ littermates (Fig. 5D), supporting the finding that deletion of Gcgr induces precursor cell proliferation. The increase in length of the TA region in colonic crypts was not due to hyperplasia of the precursor cells in Gcgr−/− mice because Ki67+ precursor cells of Gcgr+/+ (Fig. 5E) and Gcgr−/− (Fig. 5F) mice, examined in cytospin preparations, were of similar size (Gcgr+/+, 5.49 ± 0.1; Gcgr−/−, 5.7 ± 0.1, arbitrary units, 100 cells measured per line). In agreement with the morphometric data, PCR analysis indicated that the mRNA levels of Ki67 were higher in Gcgr−/− than in Gcgr+/+ mice (Fig. 5G).
Fig. 5.
Increased length of TA region. A–C, Comparison of the length of the TA region in colonic crypts of Gcgr+/+ (A), Gcgr−/− (B), and Gcgr−/− mice plus Ex 9–39 (C) immunostained for Ki67. Note that proliferating cells occupy the lower half of the crypt in A and the majority of the crypt in B. The length of the TA region decreased in Gcgr−/− mice injected with Ex 9–39 (C). Scale bar, 30 μm. D, Morphometric analysis confirms the augmentation in the size of the TA region in Gcgr−/− mice and the inhibitory effect of the GLP-1r antagonist on the length of the TA region; n = 3 mice per line, and at least 25 correctly oriented colonic crypts were measured. *, P < 0.05; **, P < 0.005. E and F, Cell size was determined in cytospin preparations of isolated mucosa from Gcgr+/+ (E) and Gcgr−/− (F) immunostained for Ki67 (green, nuclei) and β-catenin (red, cell membrane). Scale bar, 25 μm. G, Real-time PCR analysis indicates an increase in Ki67 mRNA level in Gcgr−/− when compared with Gcgr+/+ mice and a decrease in Gcgr−/− mice that received Ex 9–39. Results are expressed as fold increase over levels found in Gcgr+/+ mice; n = 4. ***, P < 0.001.
Rate of L cell precursor proliferation is inhibited by a GLP-1r antagonist
To determine whether GLP-1 mediates the increase in L cell number, the activity of the incretin was inhibited by Ex 9–39, a specific GLP-1r antagonist (13, 41). To choose a concentration of Ex 9–39 for our studies, we first used osmotic minipumps (Alzet, Cupertino, CA) that released 150 pm/kg/min for 14 d (42). In agreement with reports by others (43), mice receiving continuous administration of Ex 9–39 had approximately 2-fold higher fasting blood glucose levels than control mice. However, histological analysis of pancreas indicated the presence of apoptotic nuclei (Supplemental Fig. 1A), disrupted islet structure, and the existence of many empty spaces within the islet (Supplemental Fig. 1B), probably due to cell death, indicating that this dose was deleterious to Gcgr−/− mice.
To avoid the toxic effect of the continuous infusion of the antagonist, we used Gcgr−/− mice that received once-daily ip injection of either saline or Ex 9–39 (50 nmol/kg in saline) over 2 wk (six mice per treatment). This dose of Ex 9–39 has been shown by others to dysregulate glucose homeostasis (44). The effect of Ex 9–39 on glucose tolerance, determined by ip glucose tolerance test, lasted for approximately 4 h and did not induce apoptosis (Supplemental Fig. 1C) or visibly altered islet histology (Supplemental Fig. 1D).
Analysis of the rate of L cell precursor proliferation indicated that the number of new L cells decreased after inhibition of GLP-1r signaling (Fig. 4D). Because a decrease in the rate of replication should be correlated with a reduction in the length of the proliferative zone, we examined whether the administration of Ex 9–39 to Gcgr−/− mice affected the length of the TA region. The length of the TA region in colon, which was larger in Gcgr−/− than in Gcgr+/+ mice, decreased significantly after administration of Ex 9–39 (Fig. 5, C and D). Moreover, the antagonist also decreased the level of Ki67 mRNA (Fig. 5G). These observations demonstrate that the increase in L cell number was partially blocked by the GLP-1r antagonist. In contrast, Ex 9–39 had no effect on the length of the TA region in control CD-1 mice (Supplemental Fig. 2, A–C), suggesting the presence of a compensatory effect by other molecules on the regulation of proliferation of crypt progenitor cells. As previously reported for C57BL6/J mice (42), our results indicate that Ex 9–39 did not affect random or fasting glucose levels of CD-1 mice.
L cells do not express the GLP-1r
To ascertain whether GLP-1 has either a direct or an indirect effect on intestinal progenitor cells, we sought to determine whether GLP-1-positive cells expressed the GLP-1r. Immunohistochemical analysis of ileum of Gcgr−/− mice indicated that the GLP-1r was not expressed by L cells (Fig. 6, A and B) or by cells in the TA region (M.H.K. and G.T., unpublished observations). Similar results were obtained in Gcgr+/+ mice (results not shown). As expected, the antibody stained pancreatic islets (Fig. 6C). Immunoneutralization of the antibody eliminated staining from pancreatic islets (Fig. 6D), documenting the specificity of the antibody.
Fig. 6.
L cells do not express the GLP-1r. Photomicrograph illustrates a section of ileum of Gcgr−/− mice processed for visualization of GLP-1 (A) and GLP-1r (B). Each color is illustrated in a separate panel. Note that the GLP-1+ cell in A does not express the GLP-1r in B; the position of the GLP-1+ cells is illustrated in B with an arrowhead. Scale bar, 20 μm. C, Photomicrograph illustrates a pancreatic islet of wild-type mouse immunostained for GLP-1r as a positive control. D, Pancreatic islet incubated with GLP-1r antibody immunoabsorbed with a specific GLP-1r blocking peptide lacks immunostaining. Scale bars, 20 μm. The circle of bars indicates the location of an islet.
Ablation of Gcgr affects the level of expression of TF expressed by late but not early progenitor cells
The increase in the number of L cell progenitors in Gcgr−/− mice suggested that the level of expression of TF involved in the differentiation of these precursors would increase. To test this possibility, the mRNA levels of several regulatory factors postulated to direct the early and late differentiation of enteroendocrine cell types were determined in isolated intestinal epithelial cells of ileum of Gcgr−/− and Gcgr+/+ mice. This approach avoids the inclusion of nonepithelial cell layers in the expression profiling.
The TF Ngn3 (neurogenin3) drives precursor cells toward an enteroendocrine fate (45, 46), whereas NeuroD, which acts downstream of Ngn3, has been reported to be required for the differentiation of secretin and CCK cells (47). Pax6 is required for activation of glucagon and GLP-1 expression in pancreatic α-cells and L cells, respectively (48). Nkx2.2 is essential for the differentiation of several enteroendocrine populations such as the CCK, GIP, somatostatin, GLP-1, and gastrin cells (49). The differentiation of L and somatostatin cells is also dependent on the TF Foxa1 and Foxa 2 (50), whereas GIP expression is activated by both Pax6 and Pdx-1 (31, 33, 51).
Real-time PCR analysis revealed that the level of Ngn3 and of Foxa1/2 (forkhead box A) mRNA was similar in Gcgr+/+ and Gcgr −/− mice (Fig. 7A). In contrast, NeuroD (neurogenic differentiation 1), Nkx2.2 (NK2 transcription factor related, locus 2 transcript variant 2), and Pax6 (paired box gene 6) mRNA levels were higher in Gcgr−/− than in Gcgr+/+ mice. Interestingly, mRNA levels of Pdx-1 (pancreatic and duodenal homeobox 1) increased over 3-fold in ileum of mutant mice (Fig. 7A), in agreement with the augmentation in the number of GLP-1+ cells coexpressing GIP in ileum in that line. However, ablation of Gcgr did not affect Pdx-1 mRNA levels in duodenum, which were similar in Gcgr−/− and Gcgr+/+ mice (not shown).
Fig. 7.

Effect of ablation of Gcgr on expression of transcription factors. A, Real-time PCR analysis of TF expression in isolated intestinal cells of ileum of Gcgr+/+, Gcgr−/−, and Gcgr−/− mice that received Ex 9–39. Fold change in Gcgr−/− and Gcgr−/− plus Ex 9–39 was normalized to values of Gcgr+/+. Note that the level of expression of Ngn3 mRNA was similar in Gcgr+/+ and Gcgr−/− mice, whereas mRNA coding for NeuroD, Nkx2.2, Pdx-1, and Pax6 increased in the mutant strain and diminished after Ex 9–39 treatment; n = 3 mice per line. *, P < 0.05. B, Real-time PCR analysis indicates that the level of preproglucagon (PPG), PC3/1, and DPP4 mRNA was similar in Gcgr+/+ and Gcgr−/− mice. Results are the average from three independent experiments. C and D, The level of GIP mRNA measured by real-time PCR (C) and of GIP level in the circulation (D) was similar in Gcgr+/+ and Gcgr−/− mice. Three mice per line were examined.
The administration of Ex 9–39 affected the level of expression of TF involved in the commitment of progenitors to the LK cell lineage. Real-time PCR analysis revealed a significant reduction in the level of Pdx-1, NeuroD, Nkx2.2, and Pax6 mRNA in Gcgr−/− that received Ex 9–39 compared with levels present in Gcgr−/− mice injected with saline (Fig. 7A). Ex 9–39 also decreased the mRNA level of Pax6 and NeuroD expression in wild-type mice (Supplemental Fig. 2D). These observations support the involvement of GLP-1 in the regulation of expression of these genes. In contrast, the level of Foxa1/2 mRNA was not affected by the GLP-1r antagonist.
Deletion of Gcgr does not affect GLP-1 mRNA or GIP levels
We then asked whether the increased number of L cells contributed to the augmentation in the GLP-1 levels in the circulation. Real-time PCR analysis revealed the presence of similar levels of preproglucagon mRNA in cells of the intestinal mucosa of ileum of Gcgr+/+ and Gcgr−/− mice (Fig. 7B). Others have also reported no change in intestinal preproglucagon (PPG) mRNA content in mice with chemical ablation of Gcgr and high circulating levels of GLP- 1 (52). Ileum of Gcgr+/+ and Gcgr−/− mice had similar levels of proprotein convertase subtilisin/kexin type 1 (Pcsk3/1) mRNA, the enzyme responsible for the processing of proglucagon into GLP-1 and GLP-2, and of DPP4 mRNA (Fig. 7B). Levels of GIP mRNA in ileum (Fig. 7C) and of GIP in plasma, tested in fasted and fed mice by ELISA (Fig. 7D), were also similar in Gcgr−/− and Gcgr+/+ mice.
Discussion
In this study, we demonstrate that high levels of glucagon-like peptides in the circulation result in an augmentation in the number of L cells in ileum and that this increase is not due to proliferation of mature L cells because these cells remain quiescent. Rather, progenitor cells generate more L cells, presumably because the crypts contain more precursors committed to an L cell lineage that divide and/or have an increased rate of proliferation. This observation and the fact that the mutation does not affect the number of goblet cells, CCK, or serotonin cells suggest that commitment to different endocrine fates is established at the progenitor level. Our studies also suggest that L and LK cells of the small intestine share a common origin and that high levels of the glucagon-like peptides preferentially affect LK precursors (Fig. 8A). Thus, although in Gcgr+/+ mice, the progeny of L cell precursors comprises 80% GLP-1+ cells and 20% GLP-1+GIP+ cells, proliferating precursors of Gcgr−/− mice generate 55% GLP-1+ and 45% GLP-1+GIP+ cells.
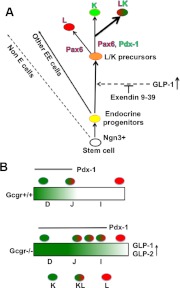
Fig. 8.
A, Proposed model for enteroendocrine cell differentiation in mouse ileum. Stem cells generate endocrine and nonendocrine lineages. Ngn3 induces endocrine differentiation in progenitors. A subset of progenitors are activated by GLP-1 to increase expression of TF, such as NeuroD, Nkx2.2, Pdx-1, and Pax6, that are involved in differentiation of these progenitors to the LK endocrine fate. Our results indicate that Ex 9–39 inhibits the involvement of GLP-1 in this process. Progenitors that will differentiate into L cells express Pax6, which activates the glucagon gene. Precursors that will generate LK and K cells activate Pax6 plus Pdx-1 and Pdx-1 expression, respectively. B, Extension of Pdx-1 regional expression results in increased LK cell number. This model illustrates that in Gcgr+/+, the caudal boundary of Pdx-1 expression terminates in the anterior gastrointestinal tract. In Gcgr−/−, high levels of the glucagon-like peptides enhance Pdx-1 expression and extend its caudal boundary up to the ileum, leading to an increased number of LK cells in the caudal gastrointestinal tract. D, Duodenum; I, ileum; J, jejunum. Bar indicates the rostrocaudal gradient of Pdx-1 expression; E, endocrine; EE, enteroendocrine.
Increased levels of the glucagon-like peptides did not affect the level of Ngn3 mRNA, indicating that the rate of proliferation of endocrine cell precursors in the initial stages of differentiation was not altered. This observation demonstrates that commitment to the different lineages does not occur at this early stage (Fig. 8A). In contrast, the levels of NeuroD, Nkx2.2, Pdx-1, and Pax6 mRNA were higher in Gcgr−/− than in littermate controls, in agreement with their involvement in L cell differentiation. Although the ablation of each of those transcription factors affects a variety of enteroendocrine cell types (53), the fact that their increase in level of expression is correlated only with an augmentation in L cell number is consistent with a model of enteroendocrine cell differentiation in which the number of the various cell types is differentially regulated.
The observation that the increased number of L cells in Gcgr−/− mice is not correlated with a rise in the levels of mRNA for the corresponding hormones suggest that the level of preproglucagon (PPG) mRNA in the new L cells is extremely low, failing to change the overall message levels in the tissue. If the new L cells are immature, it is likely that they do not secrete GLP-1 or GLP-2. If so, the high level of circulating glucagon-like peptides in Gcgr−/− mice is probably due to the increased secretory activity of the preexisting L cells. However, the mRNA level of PC3/1 and DPP4 were also similar in Gcgr+/+ and Gcgr−/− mice, indicating that the augmented secretory activity of the old L cells was not correlated with increased synthesis of preproglucagon or decreased degradation of the mature incretin. These observations are puzzling and indicate that additional studies are required to identify the mechanisms underlying the increased levels of the glucagon-like peptides in the circulation. These considerations also imply that increased glucagon-like peptide levels induced proliferation of a subset of enteroendocrine precursor cells (the new L cells) but that a different set of signals is required to carry these cells to full maturity.
Our findings support the possibility that L cell progenitor proliferation is activated by GLP-1 because the effect of the incretin is down-regulated by Ex 9–39. However, the administration of Ex 9–39 to control CD-1 mice did not affect the length of the TA region, suggesting the presence of compensatory signals. In support of this proposition, mice lacking the GLP-1r show an enhancement of GIP action that compensates the GLP-1 deficiency (44). It is possible that differences in the relation between GLP-1 and GIP levels such as those present in Gcgr−/− and wild-type mice, may affect the degree of participation of these hormones in the regulation of the rate of replication of L precursor cells. Although Ex 9–39 did not affect length of the TA region in control mice, it decreased the level of expression of NeuroD and Pax6 mRNA, suggesting that the inhibition of GLP-1r activity reduced the proportion of precursor cells that differentiate into mature L cells.
The specificity of Ex 9–39 is concentration dependent, because it does not bind to receptors for other members of the glucagon-like family of peptides at low concentrations (54–56), such as that used in this study, but binds to the GIP and GLP-2 receptor when used at micromolar concentrations in vitro (57–59). Therefore, it is likely that the GLP-1r antagonist did not bind to GIP or GLP-2 receptor in Gcgr−/− mice. Although the plasma half-life of Ex 9–39 is approximately 33 min (60), and as reported here, its action on glucose tolerance lasted for approximately 4 h, the length of time it affects the intestinal progenitor cells is unknown. Because L cells do not express GLP-1r, the action of the antagonist is likely to be indirect and to be relayed by paracrine or neural signals that regulate L cell function (61–64). GLP-1r is expressed by neurons of the myenteric plexus (64), and GLP-1 may regulate the activity of muscarinic receptors located in the intestinal crypt (65) and the rate of precursor cell replication. This possibility is supported by observations indicating an important role of released acetylcholine in the control of stem cell niche proliferation (66). These considerations indicate that the effect of Ex 9–39 cannot be estimated only by the length of time it affects β-cell function but also by its indirect action on the different cell types that mediate its effect on L cells. Our current results indicate that although the protocol of Ex 9–39 administration used in this study does not provide a constant inhibition of GLP-1r activity, which has deleterious consequences for pancreatic cells of Gcgr−/− mice (Supplemental Fig. 1), this dose resulted in decreased proliferation of a subpopulation of L cell precursors.
The intestine of Gcgr−/− mice is longer than in the Gcgr+/+ line. This difference is presumably due to a higher rate of crypt neogenesis and crypt bifurcation, which are the mechanisms believed to be responsible for intestinal growth (66, 67). The increase in the length of the intestine may be mediated by GLP-2, which has been proposed to induce intestinal hypertrophy (34). The fact that the surface area of the villi was similar in Gcgr−/− and Gcgr+/+ mice suggests that, in Gcgr−/− mice, GLP-2 affects the length of the ileum but not its thickness. The effect of GLP-2 on intestinal cell proliferation is mediated by the Wnt/β-catenin pathway (68), a signaling system that is also involved in the activation of preproglucagon gene expression (69), raising the possibility that GLP-1 and GLP- 2 may act in synergy to activate the rate of proliferation/differentiation of L precursor cells. This possibility is supported by our findings that L cell number increase in CD-1 mice treated with a DPP4 inhibitor but remained unchanged after the administration of Exendin 4. Additional studies are required to precisely define the combined effect of the glucagon-like peptides in the regulation of intestinal L cell number.
It is of interest that the signals mediating the activation of L cell precursor proliferation and differentiation affect precursors of the ileum but not of duodenum, indicating regional differences in the response of progenitor cells to the glucagon-like peptides. This difference is likely to be determined by distinct molecular determinants expressed in these two regions. For instance, Gata4 and Pdx-1 are two TF that are highly expressed in the rostral but not in caudal regions of the gut (31, 70), and it is likely that other still unknown factors establish the molecular phenotype of caudal regions and that combinatorial activities of these factors determine the response of the different segments of the gut to extrinsic signals.
A GLP-1r agonist is reported to increase Pdx-1 levels in pancreas (71), suggesting that the incretin is likely to activate Pdx-1 expression in L cell precursors of the lower intestinal tract. Our findings indicate that high levels of GLP-1 in Gcgr−/− mice extend the normal caudal boundary of Pdx-1 expression from the duodenum (31) to the ileum. Presumably, Pdx-1 expression in the ileum is responsible for the induction of GIP in LK cells (Fig. 8B).
In summary, we demonstrate the presence of specific progenitors for the L cell lineage in the intestinal crypts and that GLP-1 is involved in the regulation of the rate of proliferation of these precursor cells. These observations have important implications for designing therapies for metabolic diseases. For instance, it is known that GLP-1 increased after bariatric surgery (72, 73) and that this elevation is correlated with increased number in LK cells (74). The identification of the signals that regulate the proliferation and maturation of the L cell precursor population could provide important insights on the mechanisms responsible for the resolution of diabetes after surgery.
Supplementary Material
Acknowledgments
This work was supported by Grant R21 HL091344 from the National Institutes of Health (NIH), Merck & Co., and the Juvenile Diabetes Research Foundation.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BrdU
- Bromodeoxyuridine
- CCK
- cholecystokinin
- DPP4
- dipeptidyl-peptidase 4
- Ex 9–39
- exendin 9–39
- Foxa
- forkhead box A
- GIP
- glucose-dependent insulinotropic peptide
- GLP-1
- glucagon-like peptide-1
- GLP-1r
- GLP-1 receptor
- NeuroD
- neurogenic diffrentiation 1
- Ngn3
- neurogenin3
- Nkx2.2
- NK2 transcription factor related, lodus 2 transcript variant 2
- PAS
- periodic-acid Schiff
- Pdx-1
- pancreatic and duodenal homeobox 1
- PF
- paraformaldehyde
- TA
- transit amplifying
- TF
- transcription factor.
References
- 1. Booth C, Potten CS. 2000. Gut instincts: thoughts on intestinal epithelial stem cells. J Clin Invest 105:1493–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cheng H, Leblond CP. 1974. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. III. Entero-endocrine cells. Am J Anat 141:503–519 [DOI] [PubMed] [Google Scholar]
- 3. Haegebarth A, Clevers H. 2009. Wnt signaling, lgr5, and stem cells in the intestine and skin. Am J Pathol 174:715–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. 2009. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459:262–265 [DOI] [PubMed] [Google Scholar]
- 5. Cheng H, Leblond CP. 1974. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian theory of the origin of the four epithelial cell types. Am J Anat 141:537–561 [DOI] [PubMed] [Google Scholar]
- 6. Schonhoff SE, Giel-Moloney M, Leiter AB. 2004. Development and differentiation of gut endocrine cells. Endocrinology 145:2639–2644 [DOI] [PubMed] [Google Scholar]
- 7. Kieffer TJ, Habener JF. 1999. The glucagon-like peptides. Endocrine Reviews 20:876–913 [DOI] [PubMed] [Google Scholar]
- 8. Drucker DJ. 2006. The biology of incretin hormones. Cell Metab 3:153–165 [DOI] [PubMed] [Google Scholar]
- 9. Holst JJ. 1997. Enteroglucagon. Annu Rev Physiol 59:257–271 [DOI] [PubMed] [Google Scholar]
- 10. Furuta M, Yano H, Zhou A, Rouillé Y, Holst JJ, Carroll R, Ravazzola M, Orci L, Furuta H, Steiner DF. 1997. Defective prohormone processing and altered pancreatic islet morphology in mice lacking active SPC2. Proc Natl Acad Sci USA 94:6646–6651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Crosnier C, Stamataki D, Lewis J. 2006. Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nat Rev Genet 7:349–359 [DOI] [PubMed] [Google Scholar]
- 12. Drucker DJ. 2005. Biologic actions and therapeutic potential of the proglucagon-derived peptides. Nat Clin Pract Endocrinol Metab 1:22–31 [DOI] [PubMed] [Google Scholar]
- 13. Göke R, Fehmann HC, Linn T, Schmidt H, Krause M, Eng J, Göke B. 1993. Exendin-4 is a high potency agonist and truncated exendin-(9–39)-amide an antagonist at the glucagon-like peptide 1-(7–36)-amide receptor of insulin-secreting β-cells. J Biol Chem 268:19650–19655 [PubMed] [Google Scholar]
- 14. Drucker DJ. 2002. Gut adaptation and the glucagon-like peptides. Gut 50:428–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thorens B. 1992. Expression cloning of the pancreatic β cell receptor for the gluco-incretin hormone glucagon-like peptide 1. Proc Natl Acad Sci USA 89:8641–8645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gelling RW, Du XQ, Dichmann DS, Romer J, Huang H, Cui L, Obici S, Tang B, Holst JJ, Fledelius C, Johansen PB, Rossetti L, Jelicks LA, Serup P, Nishimura E, Charron MJ. 2003. Lower blood glucose, hyperglucagonemia, and pancreatic α-cell hyperplasia in glucagon receptor knockout mice. Proc Natl Acad Sci USA 100:1438–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ali S, Lamont BJ, Charron MJ, Drucker DJ. 2011. Dual elimination of the glucagon and GLP-1 receptors in mice reveals plasticity in the incretin axis. J Clin Invest 121:1917–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vuguin PM, Kedees MH, Cui L, Guz Y, Gelling RW, Nejathaim M, Charron MJ, Teitelman G. 2006. Ablation of the glucagon receptor gene increases fetal lethality and produces alterations in islet development and maturation. Endocrinology 147:3995–4006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tornehave D, Kristensen P, Rømer J, Knudsen LB, Heller RS. 2008. Expression of the GLP-1 receptor in mouse, rat, and human pancreas. J Histochem Cytochem 56:841–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heller RS, Kieffer TJ, Habener JF. 1997. Insulinotropic glucagon-like peptide I receptor expression in glucagon-producing α-cells of the rat endocrine pancreas. Diabetes 46:785–791 [DOI] [PubMed] [Google Scholar]
- 21. Escobar M, Nicolas P, Sangar F, Laurent-Chabalier S, Clair P, Joubert D, Jay P, Legraverend C. 2011. Intestinal epithelial stem cells do not protect their genome by asymmetric chromosome segregation. Nat Commun 2:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weiser MM, Ryzowicz S, Soroka CJ, Albini B. 1987. In vitro translation of rat intestinal RNA prepared from isolated villus and crypt cells and from the epithelium-denuded intestine. Synthesis of intestinal basement membrane. Trans Assoc Am Physicians 100:316–328 [PubMed] [Google Scholar]
- 23. Hadjiyanni I, Baggio LL, Poussier P, Drucker DJ. 2008. Exendin-4 modulates diabetes onset in nonobese diabetic mice. Endocrinology 149:1338–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mu J, Woods J, Zhou YP, Roy RS, Li Z, Zycband E, Feng Y, Zhu L, Li C, Howard AD, Moller DE, Thornberry NA, Zhang BB. 2006. Chronic inhibition of dipeptidyl peptidase-4 with a sitagliptin analog preserves pancreatic β-cell mass and function in a rodent model of type 2 diabetes. Diabetes 55:1695–1704 [DOI] [PubMed] [Google Scholar]
- 25. Kim SJ, Nian C, Doudet DJ, McIntosh CH. 2008. Inhibition of dipeptidyl peptidase IV with sitagliptin (MK0431) prolongs islet graft survival in streptozotocin-induced diabetic mice. Diabetes 57:1331–1339 [DOI] [PubMed] [Google Scholar]
- 26. Kedees MH, Grigoryan M, Guz Y, Teitelman G. 2009. Differential expression of glucagon and glucagon-like peptide 1 receptors in mouse pancreatic α and β cells in two models of α cell hyperplasia. Mol Cell Endocrinol 311:69–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rothman KJ. 1990. Statistics in nonrandomized studies. Epidemiology 1:417–418 [DOI] [PubMed] [Google Scholar]
- 28. Mortensen K, Christensen LL, Holst JJ, Orskov C. 2003. GLP-1 and GIP are colocalized in a subset of endocrine cells in the small intestine. Regul Pept 114:189–196 [DOI] [PubMed] [Google Scholar]
- 29. Theodorakis MJ, Carlson O, Michopoulos S, Doyle ME, Juhaszova M, Petraki K, Egan JM. 2006. Human duodenal enteroendocrine cells: source of both incretin peptides, GLP-1 and GIP. Am J Physiol Endocrinol Metab 290:E550–E559 [DOI] [PubMed] [Google Scholar]
- 30. Ahlgren U, Jonsson J, Edlund H. 1996. Arrested development of the pancreas in IPF1/Pdx-1 deficient mice reveals that the pancreatic mesenchyme develops independently of the pancreatic epithelium. Development 122:1409–1416 [DOI] [PubMed] [Google Scholar]
- 31. Offield MF, Jetton TL, Labosky PA, Ray M, Stein RW, Magnuson MA, Hogan BL, Wright CV. 1996. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development 122:983–995 [DOI] [PubMed] [Google Scholar]
- 32. Fujita Y, Chui JW, King DS, Zhang T, Seufert J, Pownall S, Cheung AT, Kieffer TJ. 2008. Pax6 and Pdx1 are required for production of glucose-dependent insulinotropic polypeptide in proglucagon-expressing L cells. Am J Physiol Endocrinol Metab 295:E648–E657 [DOI] [PubMed] [Google Scholar]
- 33. Jepeal LI, Fujitani Y, Boylan MO, Wilson CN, Wright CV, Wolfe MM. 2005. Cell-specific expression of glucose-dependent-insulinotropic polypeptide is regulated by the transcription factor PDX-1. Endocrinology 146:383–391 [DOI] [PubMed] [Google Scholar]
- 34. Drucker DJ, Erlich P, Asa SL, Brubaker PL. 1996. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc Natl Acad Sci USA 93:7911–7916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Deacon CF, Knudsen LB, Madsen K, Wiberg FC, Jacobsen O, Holst JJ. 1998. Dipeptidyl peptidase IV resistant analogues of glucagon-like peptide-1 which have extended metabolic stability and improved biological activity. Diabetologia 41:271–278 [DOI] [PubMed] [Google Scholar]
- 36. Drucker DJ. 2003. Glucagon-like peptides: regulators of cell proliferation, differentiation, and apoptosis. Mol Endocrinol 17:161–171 [DOI] [PubMed] [Google Scholar]
- 37. Rowland KJ, Brubaker PL. 2008. Life in the crypt: a role for glucagon-like peptide-2? Mol Cell Endocrinol 288:63–70 [DOI] [PubMed] [Google Scholar]
- 38. Aiken KD, Roth KA. 1992. Temporal differentiation and migration of substance P, serotonin, and secretin immunoreactive enteroendocrine cells in the mouse proximal small intestine. Dev Dyn 194:303–310 [DOI] [PubMed] [Google Scholar]
- 39. Barker N, van de Wetering M, Clevers H. 2008. The intestinal stem cell. Genes Dev 22:1856–1864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. van der Flier LG, Clevers H. 2009. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol 71:241–260 [DOI] [PubMed] [Google Scholar]
- 41. Drucker DJ. 2007. The role of gut hormones in glucose homeostasis. J Clin Invest 117:24–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. De León DD, Li C, Delson MI, Matschinsky FM, Stanley CA, Stoffers DA. 2008. Exendin-(9–39) corrects fasting hypoglycemia in SUR-1−/− mice by lowering cAMP in pancreatic β-cells and inhibiting insulin secretion. J Biol Chem 283:25786–25793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. De León DD, Deng S, Madani R, Ahima RS, Drucker DJ, Stoffers DA. 2003. Role of endogenous glucagon-like peptide-1 in islet regeneration after partial pancreatectomy. Diabetes 52:365–371 [DOI] [PubMed] [Google Scholar]
- 44. Baggio L, Kieffer TJ, Drucker DJ. 2000. Glucagon-like peptide-1, but not glucose-dependent insulinotropic peptide, regulates fasting glycemia and nonenteral glucose clearance in mice. Endocrinology 141:3703–3709 [DOI] [PubMed] [Google Scholar]
- 45. Jenny M, Uhl C, Roche C, Duluc I, Guillermin V, Guillemot F, Jensen J, Kedinger M, Gradwohl G. 2002. Neurogenin3 is differentially required for endocrine cell fate specification in the intestinal and gastric epithelium. EMBO J 21:6338–6347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lee CS, Perreault N, Brestelli JE, Kaestner KH. 2002. Neurogenin 3 is essential for the proper specification of gastric enteroendocrine cells and the maintenance of gastric epithelial cell identity. Genes Dev 16:1488–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Naya FJ, Huang HP, Qiu Y, Mutoh H, DeMayo FJ, Leiter AB, Tsai MJ. 1997. Diabetes, defective pancreatic morphogenesis, and abnormal enteroendocrine differentiation in β2/neuroD-deficient mice. Genes Dev 11:2323–2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Trinh DK, Zhang K, Hossain M, Brubaker PL, Drucker DJ. 2003. Pax-6 activates endogenous proglucagon gene expression in the rodent gastrointestinal epithelium. Diabetes 52:425–433 [DOI] [PubMed] [Google Scholar]
- 49. Desai S, Loomis Z, Pugh-Bernard A, Schrunk J, Doyle MJ, Minic A, McCoy E, Sussel L. 2008. Nkx2.2 regulates cell fate choice in the enteroendocrine cell lineages of the intestine. Dev Biol 313:58–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ye DZ, Kaestner KH. 2009. Foxa1 and Foxa2 control the differentiation of goblet and enteroendocrine L- and D-cells in mice. Gastroenterology 137:2052–2062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chen C, Fang R, Davis C, Maravelias C, Sibley E. 2009. Pdx1 inactivation restricted to the intestinal epithelium in mice alters duodenal gene expression in enterocytes and enteroendocrine cells. Am J Physiol Gastrointest Liver Physiol 297:G1126–G1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sloop KW, Cao JX, Siesky AM, Zhang HY, Bodenmiller DM, Cox AL, Jacobs SJ, Moyers JS, Owens RA, Showalter AD, Brenner MB, Raap A, Gromada J, Berridge BR, Monteith DK, Porksen N, McKay RA, Monia BP, Bhanot S, Watts LM, Michael MD. 2004. Hepatic and glucagon-like peptide-1-mediated reversal of diabetes by glucagon receptor antisense oligonucleotide inhibitors. J Clin Invest 113:1571–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lee CS, Kaestner KH. 2004. Clinical endocrinology and metabolism. Development of gut endocrine cells. Best Pract Res Clin Endocrinol Metab 18:453–462 [DOI] [PubMed] [Google Scholar]
- 54. Schirra J, Sturm K, Leicht P, Arnold R, Göke B, Katschinski M. 1998. Exendin(9–39)amide is an antagonist of glucagon-like peptide-1(7–36)amide in humans. J Clin Invest 101:1421–1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kolligs F, Fehmann HC, Göke R, Göke B. 1995. Reduction of the incretin effect in rats by the glucagon-like peptide 1 receptor antagonist exendin (9–39) amide. Diabetes 44:16–19 [DOI] [PubMed] [Google Scholar]
- 56. Lovshin J, Estall J, Yusta B, Brown TJ, Drucker DJ. 2001. Glucagon-like peptide (GLP)-2 action in the murine central nervous system is enhanced by elimination of GLP-1 receptor signaling. J Biol Chem 276:21489–21499 [DOI] [PubMed] [Google Scholar]
- 57. Gremlich S, Porret A, Hani EH, Cherif D, Vionnet N, Froguel P, Thorens B. 1995. Cloning, functional expression, and chromosomal localization of the human pancreatic islet glucose-dependent insulinotropic polypeptide receptor. Diabetes 44:1202–1208 [DOI] [PubMed] [Google Scholar]
- 58. Wheeler MB, Gelling RW, McIntosh CH, Georgiou J, Brown JC, Pederson RA. 1995. Functional expression of the rat pancreatic islet glucose-dependent insulinotropic polypeptide receptor: ligand binding and intracellular signaling properties. Endocrinology 136:4629–4639 [DOI] [PubMed] [Google Scholar]
- 59. Tang-Christensen M, Larsen PJ, Thulesen J, Rømer J, Vrang N. 2000. The proglucagon-derived peptide, glucagon-like peptide-2, is a neurotransmitter involved in the regulation of food intake. Nat Med 6:802–807 [DOI] [PubMed] [Google Scholar]
- 60. Edwards CM, Todd JF, Mahmoudi M, Wang Z, Wang RM, Ghatei MA, Bloom SR. 1999. Glucagon-like peptide 1 has a physiological role in the control of postprandial glucose in humans: studies with the antagonist exendin 9–39. Diabetes 48:86–93 [DOI] [PubMed] [Google Scholar]
- 61. Lim GE, Brubaker PP. 2006. Glucagon-like peptide 1 secretion by the L-cell. The view from within. Diabetes 55:S70–S77 [Google Scholar]
- 62. Amato A, Cinci L, Rotondo A, Serio R, Faussone-Pellegrini MS, Vannucchi MG, Mulè F. 2010. Peripheral motor action of glucagon-like peptide-1 through enteric neuronal receptors. Neurogastroenterol Motil 22:664-e203 [DOI] [PubMed] [Google Scholar]
- 63. Amato A, Rotondo A, Cinci L, Baldassano S, Vannucchi MG, Mulè F. 2010. Role of cholinergic neurons in the motor effects of glucagon-like peptide-2 in mouse colon. Am J Physiol Gastrointest Liver Physiol 299:G1038–G1044 [DOI] [PubMed] [Google Scholar]
- 64. Körner M, Stöckli M, Waser B, Reubi JC. 2007. GLP-1 receptor expression in human tumors and human normal tissues: potential for in vivo targeting. J Nucl Med 48:736–743 [DOI] [PubMed] [Google Scholar]
- 65. Lundgren O, Jodal M, Jansson M, Ryberg AT, Svensson L. 2011. Intestinal epithelial stem/progenitor cells are controlled by mucosal afferent nerves. PLoS One 6:e16295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bjerknes M, Cheng H. 2005. Gastrointestinal stem cells. II. Intestinal stem cells. Am J Physiol Gastrointest Liver Physiol 289:G381–G387 [DOI] [PubMed] [Google Scholar]
- 67. Totafurno J, Bjerknes M, Cheng H. 1987. The crypt cycle. Crypt and villus production in the adult intestinal epithelium. Biophys J 52:279–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dubé PE, Rowland KJ, Brubaker PL. 2008. Glucagon-like peptide-2 activates β-catenin signaling in the mouse intestinal crypt: role of insulin-like growth factor-I. Endocrinology 149:291–301 [DOI] [PubMed] [Google Scholar]
- 69. Daoudi M, Hennuyer N, Borland MG, Touche V, Duhem C, Gross B, Caiazzo R, Kerr-Conte J, Pattou F, Peters JM, Staels B, Lestavel S. 2011. PPARβ/δ activation induces enteroendocrine L cell GLP-1 production. Gastroenterology 140:1564–1574 [DOI] [PubMed] [Google Scholar]
- 70. Bosse T, Piaseckyj CM, Burghard E, Fialkovich JJ, Rajagopal S, Pu WT, Krasinski SD. 2006. Gata4 is essential for the maintenance of jejunal-ileal identities in the adult mouse small intestine. Mol Cell Biol 26:9060–9070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Stoffers DA, Kieffer TJ, Hussain MA, Drucker DJ, Bonner-Weir S, Habener JF, Egan JM. 2000. Insulinotropic glucagon-like peptide 1 agonists stimulate expression of homeodomain protein IDX-1 and increase islet size in mouse pancreas. Diabetes 49:741–748 [DOI] [PubMed] [Google Scholar]
- 72. Näslund E, Backman L, Holst JJ, Theodorsson E, Hellström PM. 1998. Importance of small bowel peptides for the improved glucose metabolism 20 years after jejunoileal bypass for obesity. Obes Surg 8:253–260 [DOI] [PubMed] [Google Scholar]
- 73. Laferrère B. 2009. Effect of gastric bypass surgery on the incretins. Diabetes Metab 35:513–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Speck M, Cho YM, Asadi A, Rubino F, Kieffer TJ. 2011. Duodenal-jejunal bypass protects GK rats from β-cell loss and aggravation of hyperglycemia and increases enteroendocrine cells coexpressing GIP and GLP-1. American journal of physiology. Endocrinology and metabolism 300:E923–932 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.