Abstract
This paper reports the identification, expression, binding kinetics, and functional studies of two novel type III lamprey GnRH receptors (lGnRH-R-2 and lGnRH-R-3) in the sea lamprey, a basal vertebrate. These novel GnRH receptors share the structural features and amino acid motifs common to other known gnathostome GnRH receptors. The ligand specificity and activation of intracellular signaling studies showed ligands lGnRH-II and -III induced an inositol phosphate (IP) response at lGnRH-R-2 and lGnRH-R-3, whereas the ligand lGnRH-I did not stimulate an IP response. lGnRH-II was a more potent activator of lGnRH-R-3 than lGnRH-III. Stimulation of lGnRH-R-2 and lGnRH-R-3 testing all three lGnRH ligands did not elicit a cAMP response. lGnRH-R-2 has a higher binding affinity in response to lGnRH-III than lGnRH-II, whereas lGnRH-R-3 has a higher binding affinity in response to lGnRH-II than IGnRH-III. lGnRH-R-2 precursor transcript was detected in a wide variety of tissues including the pituitary whereas lGnRH-R-3 precursor transcript was not as widely expressed and primarily expressed in the brain and eye of male and female lampreys. From our phylogenetic analysis, we propose that lGnRH-R-1 evolved from a common ancestor of all vertebrate GnRH receptors and lGnRH-R-2 and lGnRH-R-3 likely occurred due to a gene duplication within the lamprey lineage. In summary, we propose from our findings of receptor subtypes in the sea lamprey that the evolutionary recruitment of specific pituitary GnRH receptor subtypes for particular physiological functions seen in later evolved vertebrates was an ancestral character that first arose in a basal vertebrate.
GnRH action is mediated through high-affinity binding with the GnRH receptor (GnRH-R), a rhodopsin-like seven-transmembrane G protein-coupled receptor. Pituitary GnRH receptors are thought to signal primarily through Gαq/11, resulting in the stimulation of the inositol phosphate (IP) second messenger system; however, Gαs activation and cAMP signaling have been reported as well (1–5). Since the first successful cloning of a GnRH receptor transcript from the mouse (6), more than 83 GnRH receptor cDNA have been cloned (7–9). In an earlier study, an identified lamprey (l) GnRH-R-1 was suggested to be an ancestral GnRH receptor because it did not group with any of the clusters of vertebrate GnRH receptors (8). lGnRH-R-1 retains the conserved structural features and amino acid motifs of other known GnRH-R and includes a lengthy C-terminal tail (8). lGnRH-R-1 activated IP on stimulation with lGnRH-I or -III (8, 10). The lamprey GnRH receptor also stimulated the cAMP signaling system in a dose-dependent manner, which, through mutagenesis studies, was shown to depend on the presence of the C-terminal tail (10). In these same studies, the C-terminal tail was shown to be required for rapid ligand-dependent internalization, binding affinity, and, to some degree, cell surface expression. Finally, pharmacological profiling, in conjunction with these and previous efficacy data, confirmed that the lamprey GnRH receptor was lamprey GnRH-III selective, and the ability to couple to Gαs is conferred by the first 40 amino acids of the C-terminal tail.
The study of GnRH receptors in basal and later evolved vertebrates can provide insight into the molecular mechanisms of signaling of this receptor family. Numerous full-length GnRH receptor sequences have been identified, with more than one receptor isoform identified within a single species. In most vertebrates there are usually two to three forms of GnRH receptors present (11), although there are fewer GnRH receptor genes in mammals compared with protochordates, fish, and amphibians (12). To date, there is only a partial understanding of the physiological significance of each receptor type with regard to the spatial expression of GnRH receptors because more than one receptor type can be expressed in the same tissue. Lampreys along with hagfish are the only living representatives of the agnathans, the most ancient class of vertebrates, whose lineage dates back over 550 million years (13). Lampreys, which express three hypothalamic forms of GnRH, lamprey GnRH-I, -II, and -III are important to our understanding of the reproductive endocrinology of the first vertebrates and are likely to have retained key characteristics of the ancestral GnRH and GnRH receptor from which modern GnRH isoforms and GnRH receptors arose as reviewed elsewhere (13). Lamprey GnRH-I differs at four positions compared with lamprey GnRH-II and -III with nonconservative substitutions, whereas lamprey GnRH-II and type 2 GnRH differ from each other by only one conservative substitution. As an agnathan, one of the two most basal vertebrates, the sea lamprey has become a model system for analysis of the evolution of the neuroendocrine regulation of reproduction (13). In this study, we have identified two additional novel GnRH receptors in the lamprey and have performed expression, phylogenetic analysis, and pharmacological and functional studies, elucidating molecules from a basal vertebrate that provide insight into the molecular evolution of this receptor family.
Materials and Methods
Animals
Adult male and female lampreys (Petromyzon marinus) were collected from the Cocheco River fish ladder in Dover, New Hampshire and transferred to the University of New Hampshire (UNH) Anadromous Fish and Aquatic Invertebrate Research Laboratory in Durham, New Hampshire. The lampreys were maintained in accordance with the UNH animal care guidelines. The animals were killed and the dissected tissues were immediately snap frozen in liquid nitrogen and stored at −80 C, or the dissected tissues were immediately placed in RNAlater (Ambion, Austin, TX) and stored at −20 C.
Receptor sequence identification and peptides
Partial sequences of lGnRH-R-2 and lGnRH-R-3 (NCBI accession nos. DQ915102 and DQ915103, respectively) were first obtained from the sea lamprey genome sequencing project trace files available from the Genome Institute at Washington University (St. Louis, MO). Later, full-length sequences encoding for lGnRH-R-2 and lGnRH-R-3 were submitted to NCBI by T. Ikemoto and M.K. Park (NCBI accession nos. EF166082 and EF166083 respectively). Subsequently, lGnRH-R-2 and lGnRH-R-3 cDNA clones were obtained from the current study (NCBI accession nos. HM641828 and HM641829, respectively); each deduced sequence differs by one amino acid (402 and 225, respectively). GnRH peptides used in this study were lGnRH-I (pEHYSLEWKPG-NH2), lGnRH-II (pEHWSHGWFPG-NH2), lGnRH-III (pEHWSHDWKPG-NH2), and chicken (c)GnRH-II (pEHWSHGWYPG-NH2) (American Peptide Co., Sunnyvale, CA).
Cloning of lGnRH-R-2 and lGnRH-R-3 cDNA
All tissues used were homogenized with the aid of a Tissue Lyser II (QIAGEN, Valencia, CA). RNeasy Lipid Tissue Kit (QIAGEN) was used according to the manufacturer's instructions to isolate total RNA, except for the cloning of lGnRH-R-2 where TRI-Reagent (Molecular Research Center, Inc., Cincinnati, OH) was used according to the manufacturer's instructions.
For cloning of lGnRH-R-2 cDNA, total RNA was extracted from three pooled pituitary glands of adult female lampreys. cDNA synthesis was performed with 2.3 μg of the pooled total RNA using the Superscript III cDNA synthesis system (Invitrogen, Carlsbad, CA), with the application of 3′-rapid amplification of cDNA ends primer A (CLONTECH Laboratories, Inc., Mountain View, CA). Oligonucleotide primers (Integrated DNA Technologies, Coralville, IA) were designed. The synthesized cDNA was used as a template for PCR amplification of lGnRH-R-2 mRNA using 10 μm primers lGnRH-R-2-full-forward (5′-CACCGGT[A]CCATG[G]CTTCATCATCGCCCACGTGGAC-3′) and lGnRH-R-2-full-reverse (5′-TATTATACCGGTCTATCCCTGGGTGGCATGAGGATC-3′). Phusion High-Fidelity DNA polymerase (New England Biolabs, Ipswich, MA) was used with 2% DMSO in a total reaction volume. The approximately 1400-bp product was excised and purified using QIAEX II gel extraction kit (QIAGEN). This purified PCR product was used as a template for a subsequent amplification, and the PCR product was purified and used for subsequent ligation reactions with pcDNA3.1D/V5-His-TOPO (Invitrogen). After transformation and preparing plasmid DNA, the presence of inserts of the expected size was verified by KpnI and AgeI-restriction digest and positives sequenced [Seqwright Laboratories (Houston, TX) or UNH Hubbard Center for Genome Studies (Durham, NH)].
For cloning of lGnRH-R-3 cDNA, each pituitary, brain and testis total RNA was individually extracted from male lampreys. cDNA synthesis was performed with 2.0 μg of the total RNA of each tissue using the Superscript III cDNA synthesis system (Invitrogen), and 3′-GSP primer (5′-ATCCTTGCTCCTCGTCCCGTCATTGA-3′). The synthesized cDNA from each tissue was pooled and used as a template for PCR amplification of lGnRH-R-3 mRNA using primers lGnRH-R-3-full-forward (5′-CACCGGT[A]CCATG[G]CGTTACTAGCGCACGCGTGTAAC-3′) and lGnRH-R-3-full-reverse (5′-TATTATACCGGTTCATTGAACGGGGGGGCACCCCTG-3′). Phusion High-Fidelity DNA polymerase (New England Biolabs) was used with 3% DMSO in a total reaction volume. The purified approximately 1300-bp product was used for subsequent ligation reactions, processed similar to the final steps of the lGnRH-R-2 cloning.
Tissue distribution of lGnRH-R-2 and lGnRH-R-3
Total RNA was extracted from various tissues (100–300 mg total in pool) dissected from three male and three female lampreys, using the RNeasy Lipid Tissue Kit (QIAGEN). RT-PCR was performed using the AccessQuick RT-PCR System (Promega Corp., Madison, WI.) according to manufacturer's instruction with 0.4 μg of deoxyribonuclease I-treated RNA per reaction. Primers GnRHR2F4 (5′-AAGCTGGTGGCCATGTACTC-3′; 439–458 in cDNA) and GnRHR2R2 (5′-CCAGTGGCCTCGTACAAAAT-3′; 1293–1274) were used for lGnRH-R-2 amplification whereas GnRHR3F4 (5′-CTCTTCACCCTCCTCGTGAT-3′; 388–407) and GnRHR3R3 (5′-GAG TCC AGC AGA CGA GGA AC-3′; 976–957) were used for lGnRH-R-3 amplification. Primers for elongation factor 1α (EF1A) amplification are EF1A open reading frame-F1 (5′-CCTCCATCCATCATGGGCAAGGAAAAG-3′) and EF1A R (5′-ACCGGCCTCAAACTCACCTA-3′).
Cell culture and gene transfection
COS-7 cells were maintained in DMEM (high glucose, without sodium pyruvate; Invitrogen) containing a final concentration of 10% fetal bovine serum and incubated in a humidified atmosphere at 37 C in 5% CO2. COS-7 cells transiently transfected by the application of Lipofectamine were used for subsequent IP and cAMP assays as previously described (8, 10). COS-7 cells transiently transfected by the application of electroporation were used for subsequent whole-cell binding assays as previously described (14).
Iodination of cGnRH-II
cGnRH-II was iodinated using a modification of the Chloramine-T method as previously described (15). The iodinated peptide was then purified by adaptation of the methodology previously described (16).
Whole-cell radioligand binding assay
Radioligand binding assays were performed in COS-7 cells transiently transfected with lGnRH-R-1, lGnRH-R-2, and lGnRH-R-3 expression constructs. The lGnRH-R-1 expression construct used was described previously (8, 10). With the exception of [125I]cGnRH-II used as the radiolabeled ligand, the binding assays were performed as described previously (14). All assays were performed four times in triplicate. The IC50 values derived in these experiments are the concentration of the competing ligand required to displace 50% of the specific radioligand binding. In all results presented, IC50 values derived from competitive binding assays are used as a measure of relative apparent binding affinity of each ligand to make a comparison between the three receptors (lGnRH-R-1, lGnRH-R-2, and lGnRH-R-3).
IP assay
Total IP accumulation in response to lGnRH-I, -II, and -III stimulation was performed as previously described (8) with the exception of the incubation time being 48-h after transfection before a 24-h treatment to 2 μCi/ml myo[2-3H]inositol (GE Healthcare, Lawrence, MA). This method was initially adapted from Refs. 17–19). Cells transfected with empty vector were used as negative controls whereas cells transfected with lGnRH-R-1 were used as positive controls. Each receptor was tested independently with all three ligands in triplicate in two or three experiments.
cAMP assay
Measurement of cAMP accumulation was performed as previously described (10). Cells transfected with empty vector were used as negative controls whereas cells transfected with lGnRH-R-1 were used as positive controls. All treatments were performed in triplicate and in three different trials.
Phylogenetic analysis
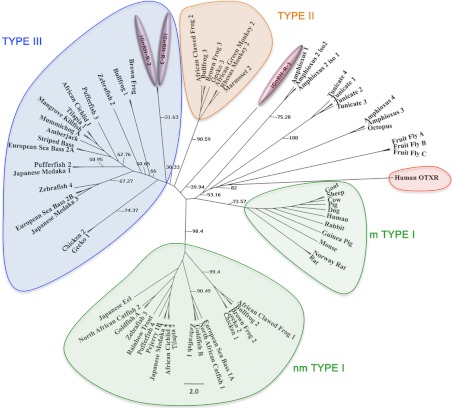
Sixty-eight published GnRH receptor sequences were retrieved from the NCBI database including representative sequences from invertebrate and vertebrate sequences (accession numbers are in the legend of Fig. 5). The human oxytocin receptor was used as an outgroup. Full amino acid sequences were aligned using the MAFFT (20) sequence alignment method in conjunction with Seaview version 4.2 (21). The aligned sequences were analyzed using PAUP* version 4.0 β 10 (22). The JTT, amino acid rate matrix (23), was used to run a 1000 bootstrap analysis in GARLI (24). To complete the bootstrap analyses with GARLI, we used Grid computing (25) through the Lattice Project (26), which includes clusters and desktops in one encompassing system (27). A 50% majority rule consensus tree was then generated from the results in PAUP* and finished in FigTree version 1.3.1 (http://tree.bio.ed.ac.uk/software/figtree/).
Fig. 5.
The molecular phylogenetic analysis was constructed using maximum-likelihood method using 68 deduced amino acid sequences of the vertebrate and invertebrate GnRH receptors. Numbers on the branches indicate bootstrap probabilities following 1000 replications in constructing the tree. The DDBJ/ MBL/ GenBank accession numbers of amino acid sequences used for the phylogenetic analysis are as follows: African cichlid 1, Haplochromis burtoni (AY705931.1); African cichlid, H. burtoni (AY028476.1); African green monkey, Cercopithecus aethiops (AF353988.1); amberjack, Seriola dumerili (AJ130876.1); African clawed frog 1, Xenopus laevis (AF172330.1); African clawed frog 2, X. laevis (AF257320.1); Amphioxus 1, Branchiostoma floridae (EU433377.1); Amphioxus 2*, B. floridae (EU433378.1); Amphioxus 3, B. floridae (EU433380.1); Amphioxus 4, B. floridae (FJ426561.1); brown frog 1, Rana dybowskii (AF236879.2); brown frog 2, R. dybowskii (AF236877.2); brown frog 3, R. dybowskii (AF236878.1); bullfrog 1, R. catesbeiana (AF144063.1); bullfrog 2, R. catesbeiana (AF153913.1); bullfrog 3, R. catesbeiana (AF224277.1); chicken 1, Gallus gallus (AJ304414.1); chicken 2, G. gallus (AY895154.1); cow, Bos Taurus (U00934.1); dog, Canis lupus familiaris (AF206513.1); European sea bass 1A, Dicentrarchus labrax (AJ606683.2), European sea bass 2A, D. labrax (AJ419594.1); European sea bass 2B, D. labrax (AJ606686.2); fruit fly C, Drosophila melanogaster (AE014134.5); fruit fly B, D. melanogaster (AE014134.5); fruit fly A, D. melanogaster (AE014134.5); gecko 1/IIIl, Eublepharis macularius (DQ269481.1); gecko 2, E. macularius (AB109032.1); gecko 3, E. macularius (DQ269482.1); goat, Capra hircus (EF150356.1); goldfish B, Carassius auratus (AF121846.1); goldfish A, C. auratus (AF121845.1); guniea pig, Cavia porcellus (AF426176.3); human, Homo sapiens (NM_000406.2); Japanese eel, Anguilla japonica (AB041327.1); Japanese medaka 1, Oryzais latipes (AB057677.1); Japanese medaka 2, O. latipes (AB057676.1); Japanese medaka 3, O. latipes (AB083364.1); mangrove kill fish, Kryptolebias marmoratus (DQ996268.2); marmoset 2, Callithrix jacchus (AF368286.1); mouse, Mus musculus (L01119.1); mummichog, Fundulus heteroclitus (AB426466.1); North African catfish 1, Clarias gariepinus (X97497.2); North African catfish 2, C. gariepinus (AF329894.1); Norway rat, Rattus norvegicus (NM_031038.3); octopus, Octopus vulgaris (AB185200.1); Pejerrey 1B, Odontesthes bonariensis (DQ875596.1); pig, Sus scrofa (L29342.1); pufferfish, Tetraodon nigroviridis (AB212821.1); pufferfish, T. nigroviridis (AB212816.1); pufferfish, T. nigroviridis (AB212819.1); pufferfish, T. nigroviridis (AB212825.1); rabbit, Oryctolagus cuniculus (AY781779.1); rainbow trout, Oncorhyncus mykiss (AJ272116.1); rhesus monkey 2, Mucaca mulatta (AF353987.1); rat, Rattus sp (AF353987.1); sea lamprey 1, Petromyzon marinus (AF439802.1); sea lamprey 2, P. marinus (DQ915103); sea lamprey 3, P. marinus (DQ915102); sheep, Ovis aries (X72088.1); striped bass, Morone saxatilis (AF218841.1); tunicate 1, Ciona intestinalis (AY742888.1); tunicate 2, C. intestinalis (AY742889.1); tunicate 3 C. intestinalis (AY742890.1); tunicate 4, C. intestinalis (AY742891.1); tilapia type 1, Oreochromis niloticus (AB111356.2); tilapia type 2, O. niloticus (AB111357.2); zebrafish 1, D. rerio (NM_001144980.1); zebrafish 2, Danio rerio (NM_001144979.1); zebrafish 3, D. rerio (NM_001177450.1); zebrafish 4, D. rerio (NM_001098193.1); oxytocin receptors: human oxytocin R, human oxytocin receptor (NM_000916.3) was used as the outgroup. Fruitfly adipokinetic hormone receptors were used because they are the closest insect relatives to GnRH-R. *, A second C-terminal tail truncated sequence for this GnRH receptor subtype was included in the analysis. (EU433379.1).
Results
Cloning and gene structure of two novel lGnRH-R-2 and lGnRH-R-3 cDNA
A PCR product was cloned from a cDNA pool derived from three individual female lamprey pituitary glands, and the derived sequence was termed lGnRH-R-2 (Fig. 1). Similarly, a PCR cDNA product was cloned and sequenced from a cDNA pool derived from the pituitary gland, brain, and testis of two individual male lampreys and termed lGnRH-R-3 (Fig. 1). Expression constructs containing the entire coding sequence of each receptor construct were prepared and used in subsequent in vitro assays.
Fig. 1.
Alignment of human, mouse, African green monkey, bullfrog, leopard gecko, chicken, zebrafish, and lamprey GnRH receptor sequences. The sequences (see Fig. 5, accession details) were aligned with ClustalW2 (28), and an image was generated by editing and annotating, the style based on the output initially produced with BOXSHADE 3.21. Ubiquitously conserved amino acid residues are shown with dark background shading, and amino acids with conserved substitutions are shown with light shading Semiconserved substitutions are shown with a dot on the consensus line. Bold horizontal lines indicate predicted TMD (TMD 1–7). GnRH receptor subtypes are labeled with ordinal numbers according to the published terminology (7, 14, 47, 50) and grouped together. Residues, microdomains, and motifs discussed in the text are bracketed above and below the alignments.
Gene structure of lGnRH-R-2 and lGnRH-R-3
Bioinformatic comparisons of the cDNA sequences confirmed that lGnRH-R-2 and lGnRH-R-3 consists of 466 and 451 amino acids, respectively, and are predicted to have three coding exons. This is based on: 1) the identification of two complete exons for both receptors (the presumed last two for lGnRH-R-2 and first two for lGnRH-R-3) in a repeat-masked version (v 6.0) of the current lamprey genome assembly (http://petromyzon.msu.edu/drupal6/) via queries employing BLASTN (29–31) with the cDNA sequences; and 2) the similar exon/intron organization apparent among the three lamprey GnRH receptors, with all three exons identified in the genome for lGnRH-R-1. The predicted exons for lGnRH-R-2 lie within bases 1–594, 595–798, and 799-1401 of the cDNA sequence, whereas the predicted exons for lGnRH-R-3 lie within the bases 1–645, 646–865, and 866-1356 of the cDNA sequence. The amino-terminal extracellular domain is coded by exon 1 in both lGnRH-R-2 and lGnRH-R-3. Exon 1 in both lamprey GnRH receptors additionally codes for transmembrane domain (TMD) 1, intracellular loop (ICL) 1, TMD2, extracellular loop (ECL) 1, TMD3, ICL2 and part of TMD4. Exon 2 in both lamprey GnRH receptors codes for the remainder of TMD4, ECL2, TMD5, and part of ICL3, whereas exon 3 codes for the remainder of ICL3, TMD6, ECL3, TMD7, and the C-terminal cytoplasmic domain.
The amino acid sequences of lGnRH-R-2 and lGnRH-R-3 were compared by global alignment (32) and local alignment (29, 30) using GnRH receptor subtypes (Table 1). The amino acid sequence identity of lGnRH-R-2 and lGnRH-R-3 is 54% in a local alignment, whereas it is at 41% in a global alignment. Compared with lGnRH-R-1, lGnRH-R-2 has a higher local and similar global amino acid sequence identity (52% vs. 38%) compared with that of lGnRH-R-3 (47% vs. 38%) (Table 1). The highest homology in global alignments to lGnRH-R-2 was with zebrafish 2 and chicken III GnRH-R (42%), whereas in local alignments the highest homology was with zebrafish 2 GnRH receptor only (56%). The highest homology in global alignments to lGnRH-R-3 was with chicken III GnRH-R (46%), whereas in local alignments the highest homology was with leopard gecko 1 GnRH-R (Table 1).
Table 1.
Comparison of global and local amino acid sequences identity of GnRH-R subtypes
| GnRH receptor subtype | Identity (%) with lGnRH-R-2 (global/local) | Identity (%) with lGnRH-R-3 (global/local) |
|---|---|---|
| Lamprey 1 | 38/52 | 38/47 |
| Lamprey 2 | 100/100 | 41/54 |
| Lamprey 3 | 41/54 | 100/100 |
| Zebrafish 1 | 36/46 | 39/48 |
| Zebrafish 2 | 42/56 | 42/56 |
| Zebrafish 3 | 36/51 | 37/49 |
| Zebrafish 4 | 39/51 | 42/54 |
| Bullfrog 1 | 39/52 | 44/50 |
| Bullfrog 2 | 35/50 | 40/49 |
| Bullfrog 3 | 40/52 | 43/50 |
| Leopard gecko 1 | 41/52 | 45/57 |
| Leopard gecko 2 | 37/50 | 38/49 |
| Leopard gecko 3 | 40/53 | 43/52 |
| Chicken I | 39/54 | 38/46 |
| Chicken III | 42/52 | 46/52 |
| African green monkey 2 | 38/48 | 40/53 |
| Human 1a | 37/40 | 40/46 |
The C-terminal portions with no correspondence were not considered in the global alignment with the tailless human receptor.
Comparisons of functionally important residues and microdomains were performed. lGnRH-R-2 and lGnRH-R-3, like lGnRH-R-1, have aspartic acid in both loci of the helix2/helix7 microdomain (Fig. 1, 4a and 4b), which is an arrangement unique to nonmammalian GnRH receptors (33, 34). Ligand binding residues were compared between the three lamprey GnRH receptor subtypes. The aspartic acid residue in TMD2 (34)(Fig. 1, feature 1) is conserved in all three receptors. Whereas the ligand binding residue asparagine that is also present in TMD2 (35) is substituted with a histidine in lGnRH-R-1, although it is conserved in lGnRH-R-2 and lGnRH-R-3 (Fig. 1, feature 2). Similarly, the lysine residue in TMD3 (36) is substituted with arginine residue in lGnRH-R-1, although it is conserved in lGnRH-R-2 and lGnRH-R-3 (Fig. 1, feature 3). The glutamate residue in ECL3 (37) is not conserved in lGnRH-R-1, whereas it is conserved in lGnRH-R-2 and lGnRH-R-3 (Fig. 1, feature 12).
Fig. 4.
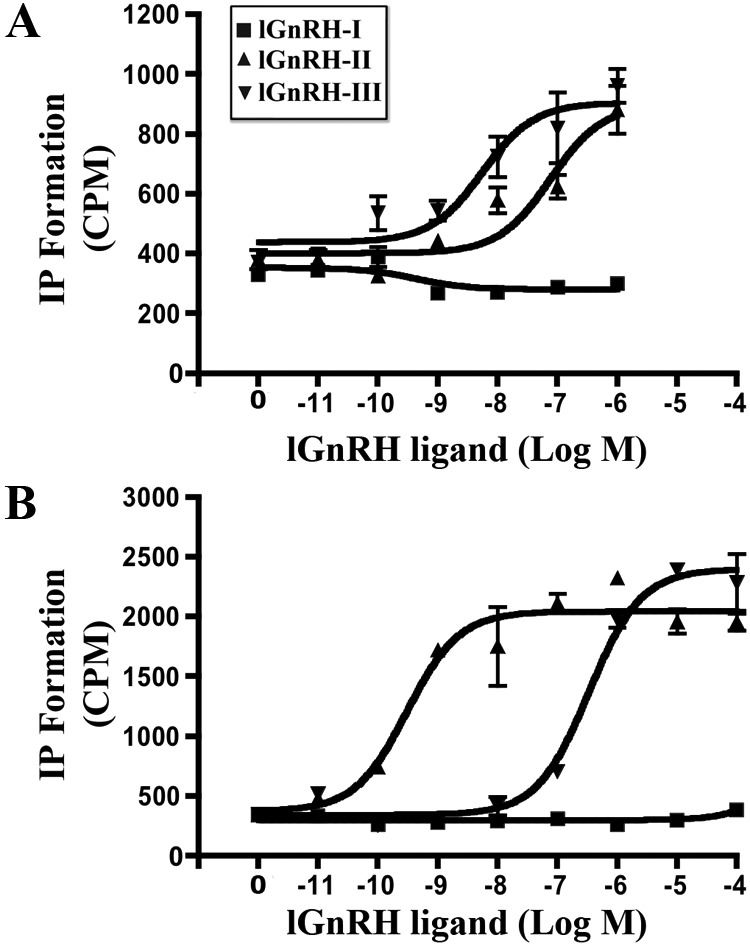
IP production of lGnRH-R-2 and lGnRH-R-3 in response to (10−11 m to 10−4m) lGnRH-I (■), (10−11m to 10−4m) lGnRH-II (▴), and (10−11m to 10−4 m) GnRH-III (▾) in COS-7 cells transiently transfected with lGnRH-R-2 (A) and lGnRH-R-3 (B) expression constructs. The data are from two to three independent experiments performed in triplicate.
Important Gαq/11 coupling motifs were compared between the lGnRH receptors. The arginine cage motif (38) is DRHSAI, DRHAAV, DRYSAV in lGnRH-R-1, lGnRH-R-2, and lGnRH-R-3, respectively (Fig. 1, feature 11). The alanine in ICL3 (39) is conserved in all three identified lGnRH receptors (Fig. 1, feature 8). Lysine residue (1) and the motif DRS (40, 41) identified as Gαq/11-coupling residues were also compared and found to be L and DRH in lGnRH-R-1 and lGnRH-R-2 whereas it was found to be L and DRY in lGnRH-R-3 (Fig. 1, feature 7 and 6). The leucine residue (Leu 237) in IL3 of the mouse GnRH receptor previously identified as important for Gαq/11 (42) was compared and is shown to be substituted with an isoleucine residue in all three lamprey GnRH receptors (Fig. 1, feature 10).
The HFRK motif identified as necessary for cAMP signaling in the bullfrog (4) is substituted with a HVRR motif within a 40-amino acid region of lGnRH-R-1 (Fig. 1, feature 5) (10). The motifs identified in lGnRH-R-2 and lGnRH-R-3 within this same region are PLGP and QWDG, respectively. The amino acid residues that were previously identified in the mouse GnRH-R, and found to be involved in Gαs coupling (K71KLSR75; L58; L80) (43) correspond to residues (TKSH; C; I), (RRSH; W; L), and (RGSH; R; L) in lGnRH-R-1, lGnRH-R-2, and lGnRH-R-3, respectively (Fig. 1, feature 9).
Differential tissue distribution of lGnRH-R-2 and lGnRH-R-3 mRNA expression in male and female lampreys
Amplification of lGnRH-R-2 cDNA in male lampreys was detected in muscle, liver, skin, thyroid, intestine, eye, brain, and pituitary tissues; however, no cDNA detection was observed in the testes, heart, and kidney tissues (Fig. 2A). This was similar to the expression pattern observed in female lampreys except for detection of lGnRH-R-2 cDNA in the kidney of the female lamprey. In direct contrast, lGnRH-R-3 mRNA expression (Fig. 2B) was not as extensive as lGnRH-R-2 mRNA expression (Fig. 2A). Of all the tissue tested, amplification of lGnRH-R-3 cDNA was only detected in the intestine, eye, and brain of male lamprey (Fig. 2B). In female lampreys lGnRH-R-3 cDNA was also detected in the intestine, eye, and brain in addition to expression detected in the ovary and skin (Fig. 2B). Elongation factor 1α was amplified in all tissue cDNA tested (Fig. 2C).
Fig. 2.
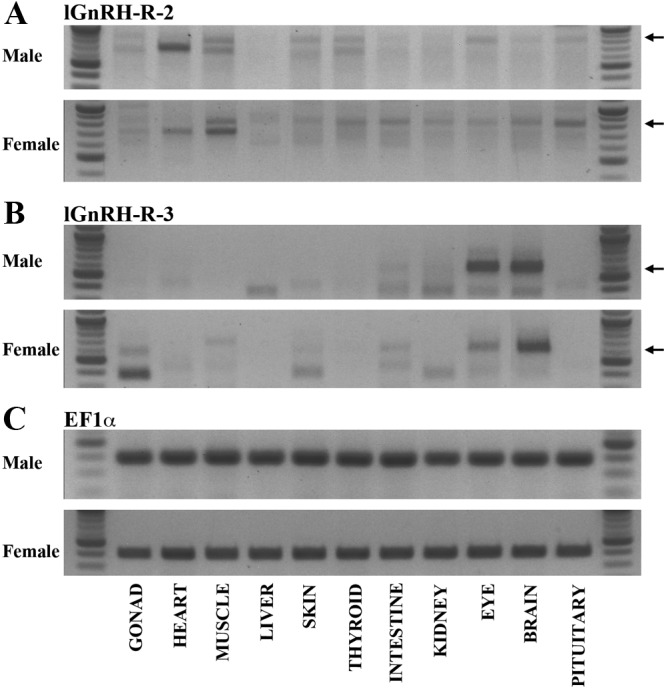
Tissue distribution of lGnRH-R-2 (A) and lGnRH-R-3 (B) mRNA expression in male and female lampreys. Arrows indicate the corresponding products. RNA integrity and cDNA production were verified by amplification of elongation factor 1α (C). The expected DNA fragment sizes were 855 and 589, respectively. Each amplified fragment of lGnRH-R-2 and -3 spans all three exons.
lGnRH-II and lGnRH-III bind to both novel lGnRH-R-2 and lGnRH-R-3
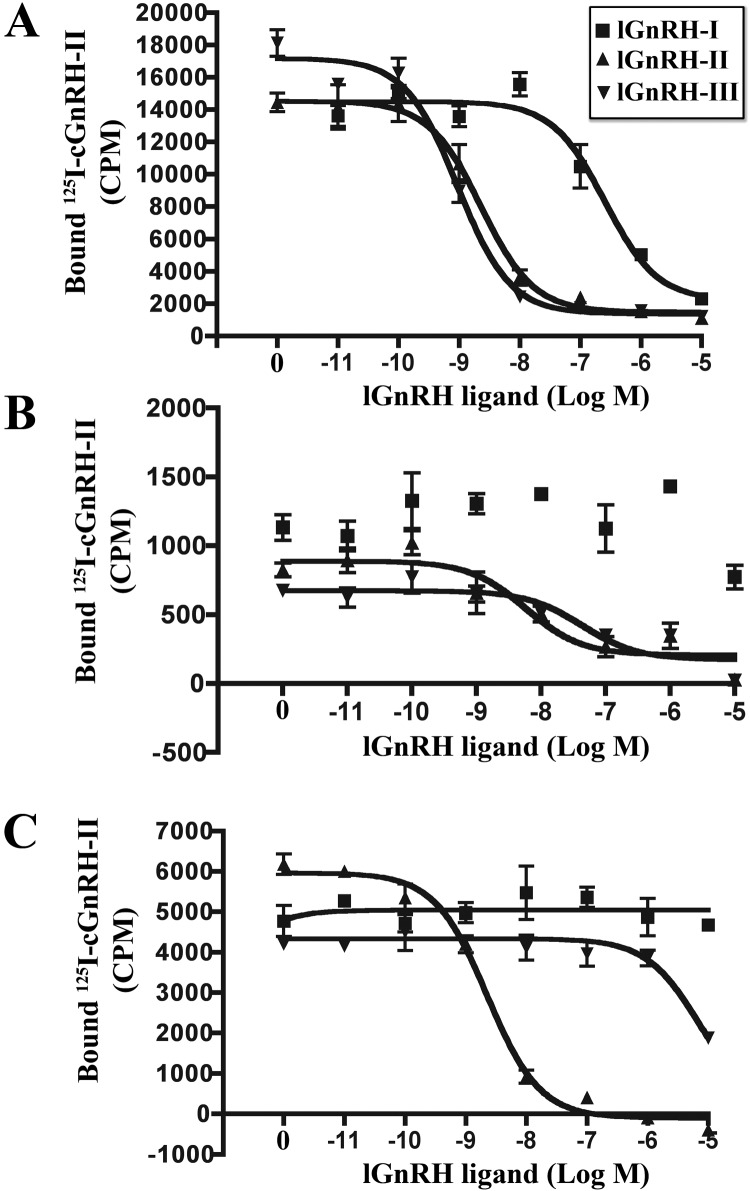
COS-7 cells transiently transfected with lGnRH-R-1, lGnRH-R-2, and lGnRH-R-3 expression constructs bound lGnRH-II and lGnRH-III and showed displacement of [125I]cGnRH-II with both lGnRH-II and lGnRH-III (Fig. 3, A–C) in a concentration-dependent fashion. Our findings show that lGnRH-I only bound to lGnRH-R-1 (Fig. 3A) but not to lGnRH-R-2 (Fig. 3B) or lGnRH-R-3 (Fig. 3C). The binding affinities of lGnRH-R-1 were IC50: 152 nm (lGnRH-I) and IC50: 0.9 nm (lGnRH-III) (Table 2 and Fig. 3A). These binding affinities were similar to those previously reported to lGnRH-R-1 with GnRH-I (IC50, 118 nm) and GnRH-III (IC50, 0.708 nm) (10). lGnRH-II (IC50, 1.32 nm) and lGnRH-III (0.9 nm) exhibited similar binding affinities for lGnRH-R-1, both of which were 137-fold higher than that of lGnRH-I (IC50, 152 nm) (Table 2) (Fig. 3A). lGnRH-III (IC50, 29.17) exhibited a higher binding affinity to lGnRH-R-2 than lGnRH-II (IC50, 181 nm) (Fig. 3B and Table 2). lGnRH-II (IC50, 1.45) exhibited a higher binding affinity to lGnRH-R-3 than lGnRH-III (IC50, 3491 nm) (Fig. 3C). The binding affinity for lGnRH-III was 6-fold higher than lGnRH-II; in contrast, the binding affinity for lGnRH II was 2400-fold higher than lGnRH-III. The binding affinity and displacement of [125I]cGnRH-II were tested with cGnRH-II resulting in IC50 values of 0.7 nm, 307 nm, and 1.47 nm observed for lGnRH-R-1, -R-2, and -R-3, respectively (Table 2). The maximal binding measured (counts per minute) demonstrated that lGnRH-R-3 is expressed 3-fold more than that of lGnRH-R-2, whereas lGnRH-R-1 is 6-fold more than lGnRH-R-3, leaving lGnRH-R-2 18-fold less than that of lGnRH-R-1 (Fig. 3, A–C). The results indicate a relatively low expression of lGnRH-R-2 in COS-7 cells.
Fig. 3.
Ligand binding of lGnRH-R-1, lGnRH-R-2, and lGnRH-R-3. Competitive displacement of [125H]cGnRH-II with serial dilutions (10−11m to 10−5m) of lGnRH-I (■), (10−11m to 10−5M) lGnRH-II (▴) and (10−11m to 10−5m) lGnRH-III (▾) in COS-7 cells transiently transfected with lGnRH-R-1 (A), lGnRH-R-2 (B), and lGnRH-R-3 (C) expression constructs. The data presented are from four independent experiments each performed in triplicate. Nonspecific binding, determined in nontransfected cells, was subtracted from maximal counts per minute.
Table 2.
Receptor binding and peptide-stimulated IP accumulation in response to GnRH analogs in COS-7 cells expressing lGnRH-R-1, lGnRH-R-2, and lGnRH-R-3
| Ligand | Ligand binding IC50 (nm)a |
IP accumulation EC50 (nm)b |
||||
|---|---|---|---|---|---|---|
| lGnRH-R-1 | lGnRH-R-2 | lGnRH-R-3 | lGnRH-R-1 | lGnRH-R-2 | lGnRH-R-3 | |
| lGnRH-I | 152 ± 35.58c | ND | ND | ND | ND | |
| lGnRH-II | 1.32 ± 0.43c | 181 ± 15c | 1.45 ± 0.44c | 29.1 ± 23.59d | 5.52 ± 0.24e | |
| lGnRH-III | 0.9 ± 0.06c | 29.17 ± 11.67c | 3491 ± 1213.5c | 5.49 ± 0.32d | 283 ± 148d | |
| cGnRH-II | 0.70 ± 0.06c | 307 ± 264.5c | 1.47 ± 0.27c | |||
All experiments were performed on up to four separate experiments in triplicate.
IC50 values (relative binding affinities) for GnRH analogs;
EC50 values for agonist activity of GnRH analogs;
Data are mean ± se. of four experiments;
Data are mean ± se. of three experiments;
Data are mean ± se. of two experiments. ND, Non-detected.
Both novel lGnRH-R-2 and lGnRH-R-3 signal through the IP pathway on lGnRH-II and lGnRH-III stimulation
lGnRH-II and lGnRH-III stimulated IP production in lGnRH-R-2 and lGnRH-R-3 transfected COS-7 cells (Fig. 4, A and B). Neither of the receptors showed an accumulation of IP with lGnRH-I (Fig. 4, A and B). lGnRH-III was more potent in stimulation of IP production at lGnRH-R-2 than lGnRH-II (EC50, 5.49 vs. 29.1 nm) (Table 2). In contrast, lGnRH-II was more potent in stimulation of IP production at lGnRH-R-3 than lGnRH-III (EC50, 5.52 vs. 283 nm). In lGnRH-R-2 transfected cells, the potency of lGnRH-III stimulation was 5-fold higher than with lGnRH-II. lGnRH-II was 51-fold more potent than lGnRH-III for production of IP via activation of lGnRH-R-3 (Table 2).
Neither of the two novel lGnRH-R-2 and lGnRH-R-3 activates the cAMP pathway with all endogenous lGnRH ligands
No detectable responses of cAMP with lGnRH-R-2 and lGnRH-R-3 were observed in transiently transfected COS-7 cells in stimulation with lGnRH-I, lGnRH-II, or lGnRH-III at increasing concentrations (data not shown).
Phylogenetic analysis
A consensus phylogenetic tree based on the alignment of the entire protein sequence is presented (Fig. 5). Vertebrate and invertebrate GnRH receptors separate into four clades as previously described (7, 44, 45). The novel lGnRH-R-2 and lGnRH-R-3 separates into the type III clade, whereas lGnRH-R-1 does not separate into any of the clades, although it appears to be more closely related to the type II clade of GnRH receptors.
Discussion
Two full-length cDNA encoding novel GnRH receptors, lGnRH-R-2 and lGnRH-R-3, have been identified and classified as type III receptors. Analysis of the encoded amino acid sequences showed conservation of the characteristic motifs of GnRH receptors and high overall similarity to previously identified GnRH receptors. The two lamprey GnRH receptors were shown to be functional through stimulation of IP in transiently transfected COS-7 cells when treated with the ligands, lGnRH-II and -III. Stimulation of lGnRH-R-2 and lGnRH-R-3 with increasing doses of each of the three GnRH ligands did not elicit a cAMP response, supporting evidence that a key motif (HVRR-like) in the C-terminal tail is required for cAMP activation. Lamprey GnRH-R-3 precursor transcript was primarily expressed in the brain and eye of male and female lampreys. Lamprey GnRH-R-2 precursor transcript was more widely expressed in various tissues compared with that of lGnRH-R-3. In summary, the GnRH systems in the lamprey, like other species, exhibit species-specific recruitment of receptor subtypes and ligands within different tissues, and there are also differences in the spatiotemporal expression patterns of GnRH receptor subtypes.
Of the numerous available forms of GnRH receptor nomenclature that have been proposed by several investigators, in this paper we adopt the phylogenetic classification in which three distinct classes of GnRH receptors from the vertebrate lineage grouped into separate clusters: type I (mammalian and nonmammalian), type II, and type III GnRH receptors (7). From our phylogenetic analysis using full-length sequences, we propose that lGnRH-R-1 evolved from a common ancestor of all vertebrate GnRH receptors and lamprey GnRH-R-2 and lGnRH-R-3 likely occurred due to a gene duplication within the lamprey lineage. These novel GnRH receptors share the structural features and amino acid motifs common to other known gnathostome type II/III GnRH receptors. In an earlier study, lGnRH-R-1 was classified with the type II GnRH receptors; however, it must be noted that only the TMD of the proteins were used in the phylogenetic analysis (44). Within our phylogenetic tree, lGnRH-R-1 does not group with any of the clades; however, it is more closely related to the type II GnRH receptor clade than any other clade identified within the phylogenetic tree. lGnRH-R-2 and lGnRH-R-3 are neatly clustered among the type III GnRH receptors and are therefore both termed “type III” GnRH receptors. Vertebrate type I GnRH receptors are represented in teleosts, amphibians, reptiles, and avian species and in mammals. The type II receptors include receptors from amphibians, reptiles, and mammals; however, type II receptors are inactivated in most mammals (46). The type II GnRH receptors are more closely related to type III GnRH receptors than to type I receptors, and it has been suggested that these two GnRH receptor subtypes may have arisen from genome duplication in an ancestral gene in the extinct ancestral vertebrates (7). Unlike the type II GnRH receptors, the type III GnRH receptors include sequences from teleost fish, amphibians, reptiles, and avian species but do not occur in mammals. Our new findings show that an ancestral extant vertebrate, the lamprey, has two type III GnRH receptors and an ancestral type GnRH receptor that is more closely related to the type II receptors. These data provide evidence that type II and type III GnRH receptors arose from an ancestral gene in early vertebrate evolution.
In an attempt to attribute potential functions of lGnRH-R-2 and lGnRH-R-3, tissue distribution of the mRNA expression was performed in tissues inclusive of the nervous system and peripheral tissues. Comparisons of the expression patterns of the two novel lamprey GnRH receptors identified in this study with the previously characterized expression pattern of lGnRH-R-1 (8) demonstrates that lGnRH-R-1 is less ubiquitously expressed within the male and female lampreys than lGnRH-R-2, although, lGnRH-R-1 mRNA expression was located in both the pituitary and male gonads (8). lGnRH-R-2 mRNA expression was also more diverse than lGnRH-R-3 mRNA expression. It is expressed in neuroendocrine, but not in gonadal tissues of both males and females. In contrast, lGnRH-R-3 is not expressed in pituitary tissue of either males or females and is only expressed in the gonadal tissues of females. Therefore, the different expression patterns of lGnRH-R-1, -2, and -3 suggest differential regulation within male and female pituitary and gonadal tissues in sea lampreys.
Of the three lGnRH receptors identified in the lamprey, lGnRH-R-1 and lGnRH-R-2 are expressed in the pituitary gland, whereas lGnRH-R-2 and lGnRH-R-3 are expressed in the brain of both adult male and female lampreys. Attributing physiological significance to each receptor type by the spatial expression of GnRH receptors is further complicated because several studies have shown that more than one receptor type can be expressed in the same tissue. For example, in the zebrafish, the anatomical distribution of the four GnRH receptors (type III and type I) is widespread in the brain, eye, and gonads and additionally, all four of the GnRH receptors are expressed in the pituitary (45). The sea bass possesses five isoforms of GnRH receptor (type III and type I), and all but one are expressed in the pituitary (47). The current study suggests that in the sea lamprey either lGnRH-R-1 or lGnRH-R-2 may play a role in transcriptional regulation of the gonadotropin expression in the pituitary gland. Therefore, GnRH systems in the lamprey, like other species, exhibit species-specific recruitment of receptor subtypes and ligands within different tissues, and there is also plasticity in the spatiotemporal expression patterns of GnRH receptor subtypes (47–51). The predominance of one receptor subtype compared with the others in a particular tissue in lamprey was not investigated and should be the focus of future investigations.
The occurrence of three forms of GnRH peptide hormones in the lamprey suggested the possibility that the endogenous receptors may respond in a differential manner to specific ligands. Activation of the three lamprey GnRH receptor isoforms by the three naturally occurring endogenous ligands was investigated in this study in an attempt to further classify the receptors in terms of physiological functionality, because deciphering ligand-binding interactions is important for understanding receptor function, indicative of receptor activation (7). Previous studies examining the binding affinity of lGnRH-R-1 were performed (10); however, at the time of the study, the lGnRH-II ligand had not been isolated. A later study showed that lGnRH-II did induce an IP response (52), although no indication of binding affinity or maximal binding was investigated. The analyses performed in this study comparing the pharmacological profiles of each lGnRH receptor demonstrate that the lamprey receptors have varying responses in relation to the endogenous GnRH peptides in the lamprey. lGnRH-I binds to lGnRH-R-1 but not to lGnRH-R-2 or -3. However this response in binding affinity of lGnRH-R-1 is 137-fold lower than the binding affinity to lGnRH-II and lGnRH-III. This was in clear contrast to what was observed for lGnRH-R-2 and lGnRH-R-3. lGnRH-III exhibited a 6-fold higher binding affinity to lGnRH-R-2 compared with lGnRH-II, whereas lGnRH-II exhibited a 2400-fold higher binding affinity to lGnRH-R-3 than lGnRH-III. The promiscuity of receptor subtype activation in vitro by different endogenous GnRH ligand isoforms is seen in teleost fish (47, 49), amphibians (50, 51), and reptilian species, suggesting a complex interplay between ligands and receptors. The findings in this study support the evidence of the promiscuity of receptor subtype activation by different GnRH ligands.
Most nonmammalian GnRH peptides are at least 1 order of magnitude less active than mammalian GnRH at mammalian type I GnRH receptors because they lack a charged residue in position 8 of the decapeptide (53). Nonmammalian GnRH receptors are less selective for mammalian GnRH compared with nonmammalian GnRH (49, 54, 55). It is now firmly established that GnRH-II (relative to GnRH-I) has a higher binding affinity for all nonmammalian vertebrate receptors irrespective of their classification based on structural similarities (56). For example, both type I goldfish GnRH receptors have a higher ligand selectivity for GnRH-II, whereas in the catfish one of the two type I GnRH receptors has a higher ligand binding selectivity for the endogenous GnRH-II than for GnRH-I; however, both receptors also had a higher ligand-binding selectivity for GnRH-II compared with the other analogs (55). The results of the current study show that both lGnRH-R-1 and lGnRH-R-3 have a higher ligand selectivity to lGnRH-II, whereas lGnRH-R-2 has a higher ligand selectivity to lGnRH-III than lGnRH-II.
A comparison of the major ligand binding sites identified in mammalian GnRH receptors shows that Asp98 (57) is conserved in lGnRH-R-1, lGnRH-R-2, and lGnRH-R-3, whereas lysine in TMD3 (36) and Asn102 (35) are both conserved in lGnRH-R-2 and lGnRH-R-3, but not in lGnRH-R-1, although the lysine in TMD3 is conservatively substituted with an arginine residue in lGnRH-R-1, preserving agonist binding to the receptor. These receptor residues are believed to interact with the N- and C-terminal residues of the GnRH ligands (35, 36, 57). Previous studies show that GnRH receptors may be configured such that the spatial arrangement of the receptor binding sites accommodates binding of configured and nonconfigured ligands, supported by the presence of aspartic acid in both loci of the functional helix2/helix7 microdomain (58). Aspartic acid is conserved in all lGnRH receptors studied; however, only binding to lGnRH-R-1 was observed with lGnRH-I.
An earlier truncation study performed with the lGnRH-R-1 showed that within the first 40 amino acids of the C-terminal tail of lGnRH-R-1 lies the residues necessary for Gαs coupling (10). The HFRK motif identified as necessary for cAMP signaling in the bullfrog (4) is substituted with a HVRR motif within this 40-amino acid region of lGnRH-R-1 and was previously proposed to be responsible for Gαs coupling (10). The PLGP and QWDG motifs in lGnRH-R-2 and lGnRH-R-3, respectively, which lie within the same region, may therefore explain the lack of cAMP response observed in these lamprey GnRH receptors. This strengthens the previous implication of the HVRR motif of lGnRH-R-1 being responsible for cAMP response. There is also a clear differential activation of signal transduction cascades of the lamprey GnRH receptors. Although lGnRH-R-1, lGnRH-R-2, and lGnRH-R-3 are able to stimulate IP accumulation, only lGnRH-R-1 is able to stimulate production of cAMP. This plasticity in activation of second messenger cascades through GnRH receptors is also observed in the amphioxus and the zebrafish (44, 45).
Understanding the in vitro expression, potency of IP accumulation and binding affinity of these novel lamprey receptors provides an in-depth understanding of the GnRH systems in an ancestral extant vertebrate. lGnRH-R-1 is the only receptor in lampreys in which lGnRH-I can bind and transduce both IP and cAMP signal transduction, likely due to the HFRK motif in the C-terminal end (10). lGnRH-R-2 has a higher binding affinity and IP potency in response to lGnRH-III than lGnRH-II, whereas lGnRH-R-3 has a higher binding affinity and IP potency in response to lGnRH-II than IGnRH-III. lGnRH-R-1 and lGnRH-R-2 are both present in the pituitary gland of the adult sea lamprey; in addition, lGnRH-R-1 is present in the male gonads, and lGnRH-R-3 is present in the female gonads. Although in vitro ligand selectivity does not necessarily correlate with in vivo functional significance (14), lGnRH-I, -II, and -III may all be physiological regulators of the pituitary gland in the sea lamprey; however, the cognate receptor for lGnRH-I remains lGnRH-R-1 exclusively. lGnRH-R-1 may transduce both lGnRH-III and lGnRH-II signal in the male gonads, and lGnRH-R-3 may transduce both lGnRH-II and lGnRH-III signal in female gonads. Further studies into the predominance in receptor isoform expression in specific tissues and the changes in expression due to age, reproductive status, and sex of the sea lamprey will provide key information on the functional capacity of each receptor subtype. We propose that an ancestral type II-like receptor, lGnRH-R-1, evolved from a common ancestor of all vertebrate GnRH receptors, and the two type III receptors, lGnRH-R-2 and lGnRH-R-3, likely arose by gene duplication and diverged from lGnRH-R-1 during the evolution of the lamprey lineage. In summary, we further propose from our findings of receptor subtypes in the sea lamprey that the evolutionary recruitment of specific GnRH receptor subtypes for particular physiological functions seen in later evolved vertebrates was an ancestral character that first arose in a basal vertebrate.
Acknowledgments
We thank the following University of New Hampshire personnel from the Center for Comparative and Molecular Biology: Dr. Mihael Freamat, Scott Morin, Takayoshi Kosugi, Samantha Murphy, Jason Dahlstrom, Kari Britt, Kristen Gazda, Dana Daukss, Jeff Neale, and Lisa Merrill for their excellent assistance and/or excellent advice.
This work was supported by National Science Foundation Grant 0849569 (to S.A.S.) and by NIH Grant 5421RR024477-02 (to S.A.S.). Partial funding was provided by the New Hampshire Agricultural Experiment Station. This is Scientific Contribution No. 2472.
The sequences reported in this paper have been deposited in the DNA Data Bank of Japan/European Molecular Biology Laboratory/GenBank database: accession numbers are HM641828 and HM641829 for lGnRH-R-2 and lGnRH-R-3, respectively.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ECL
- Extracellular loop
- EF1A
- elongation factor 1α
- GnRH-R
- GnRH receptor
- ICL
- intracellular loop
- IP
- inositol phosphate
- TMD
- transmembrane domain.
References
- 1. Arora KK, Sakai A, Catt KJ. 1995. Effects of second intracellular loop mutations on signal transduction and internalization of the gonadotropin-releasing hormone receptor. J Biol Chem 270:22820–22826 [DOI] [PubMed] [Google Scholar]
- 2. Grosse R, Schmid A, Schöneberg T, Herrlich A, Muhn P, Schultz G, Gudermann T. 2000. Gonadotropin-releasing hormone receptor initiates multiple signaling pathways by exclusively coupling to G(q/11) proteins. J Biol Chem 275:9193–9200 [DOI] [PubMed] [Google Scholar]
- 3. Liu F, Usui I, Evans LG, Austin DA, Mellon PL, Olefsky JM, Webster NJ. 2002. Involvement of both G(q/11) and G(s) proteins in gonadotropin-releasing hormone receptor-mediated signaling in L β T2 cells. J Biol Chem 277:32099–32108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Oh DY, Song JA, Moon JS, Moon MJ, Kim JI, Kim K, Kwon HB, Seong JY. 2005. Membrane-proximal region of the carboxyl terminus of the gonadotropin-releasing hormone receptor (GnRHR) confers differential signal transduction between mammalian and nonmammalian GnRHRs. Mol Endocrinol 19:722–731 [DOI] [PubMed] [Google Scholar]
- 5. Stanislaus D, Ponder S, Ji TH, Conn PM. 1998. Gonadotropin-releasing hormone receptor couples to multiple G proteins in rat gonadotrophs and in GGH3 cells: evidence from palmitoylation and overexpression of G proteins. Biol Reprod 59:579–586 [DOI] [PubMed] [Google Scholar]
- 6. Tsutsumi M, Zhou W, Millar RP, Mellon PL, Roberts JL, Flanagan CA, Dong K, Gillo B, Sealfon SC. 1992. Cloning and functional expression of a mouse gonadotropin-releasing hormone receptor. Mol Endocrinol 6:1163–1169 [DOI] [PubMed] [Google Scholar]
- 7. Millar RP, Lu ZL, Pawson AJ, Flanagan CA, Morgan K, Maudsley SR. 2004. Gonadotropin-releasing hormone receptors. Endocr Rev 25:235–275 [DOI] [PubMed] [Google Scholar]
- 8. Silver MR, Nucci NV, Root AR, Reed KL, Sower SA. 2005. Cloning and characterization of a functional type II gonadotropin-releasing hormone receptor with a lengthy carboxy-terminal tail from an ancestral vertebrate, the sea lamprey. Endocrinology 146:3351–3361 [DOI] [PubMed] [Google Scholar]
- 9. Sower S, Nucci NV, Silver MR. 2004. Gonadotropin-releasing hormone, family of. In: Martini L, ed. Encyclopedia of endocrine diseases. Amsterdam; Boston: Elsevier, Inc.; 306–316 [Google Scholar]
- 10. Silver MR, Sower SA. 2006. Functional characterization and kinetic studies of an ancestral lamprey GnRH-III selective type II GnRH receptor from the sea lamprey, Petromyzon marinus. J Mol Endocrinol 36:601–610 [DOI] [PubMed] [Google Scholar]
- 11. Millar RP. 2005. GnRHs and GnRH receptors. Anim Reprod Sci 88:5–28 [DOI] [PubMed] [Google Scholar]
- 12. Morgan K, Millar RP. 2004. Evolution of GnRH ligand precursors and GnRH receptors in protochordate and vertebrate species. Gen Comp Endocrinol 139:191–197 [DOI] [PubMed] [Google Scholar]
- 13. Sower SA, Freamat M, Kavanaugh SI. 2009. The origins of the vertebrate hypothalamic-pituitary-gonadal (HPG) and hypothalamic-pituitary-thyroid (HPT) endocrine systems: new insights from lampreys. Gen Comp Endocrinol 161:20–29 [DOI] [PubMed] [Google Scholar]
- 14. Joseph NT, Morgan K, Sellar R, McBride D, Millar RP, Dunn IC. 2009. The chicken type III GnRH receptor homologue is predominantly expressed in the pituitary, and exhibits similar ligand selectivity to the type I receptor. J Endocrinol 202:179–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sower SA, Balz E, Aquilina-Beck A, Kavanaugh SI. 2011. Seasonal changes of brain GnRH-I, -II, and -III during the final reproductive period in adult male and female sea lamprey. Gen Comp Endocrinol 170:276–282 [DOI] [PubMed] [Google Scholar]
- 16. Stopa EG, Sower SA, Svendsen CN, King JC. 1988. Polygenic expression of gonadotropin-releasing hormone (GnRH) in human? Peptides 9:419–423 [DOI] [PubMed] [Google Scholar]
- 17. Berg KA, Clarke WP, Chen Y, Ebersole BJ, McKay RD, Maayani S. 1994. 5-Hydroxytryptamine type 2A receptors regulate cyclic AMP accumulation in a neuronal cell line by protein kinase C-dependent and calcium/calmodulin-dependent mechanisms. Mol Pharmacol 45:826–836 [PubMed] [Google Scholar]
- 18. Ikemoto T, Park MK. 2003. Identification and characterization of the reptilian GnRH-II gene in the leopard gecko, Eublepharis macularius, and its evolutionary considerations. Gene 316:157–165 [DOI] [PubMed] [Google Scholar]
- 19. Okubo K, Nagata S, Ko R, Kataoka H, Yoshiura Y, Mitani H, Kondo M, Naruse K, Shima A, Aida K. 2001. Identification and characterization of two distinct GnRH receptor subtypes in a teleost, the medaka Oryzias latipes. Endocrinology 142:4729–4739 [DOI] [PubMed] [Google Scholar]
- 20. Katoh K, Misawa K, Kuma K, Miyata T. 2002. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res 30:3059–3066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Galtier N, Gouy M, Gautier C. 1996. SEAVIEW and PHYLO_WIN: two graphic tools for sequence alignment and molecular phylogeny. Comput Appl Biosci 12:543–548 [DOI] [PubMed] [Google Scholar]
- 22. Swofford DL. 2003. PAUP*: Phylogenetic analysis using parsimony (and other methods). In 4.0 Beta edition. Sunderland, MA: Sinauer Associates [Google Scholar]
- 23. Jones DT, Taylor WR, Thornton JM. 1992. The rapid generation of mutation data matrices from protein sequences. Comput Appl Biosci 8:275–282 [DOI] [PubMed] [Google Scholar]
- 24. Zwickl DJ. 2006. GARLI (genetic algorithm for rapid likelihood inference). In: Genetic algorithm approaches for the phylogenetic analysis of large biological sequence datasets under the maximum likelihood criterion. Austin, TX: University of Texas, Austin [Google Scholar]
- 25. Cummings M, Huskamp JC. 2005. Grid computing. Educause Rev 40:116–117 [Google Scholar]
- 26. Bazinet AL, Cummings MP. 2009. The Lattice Project: A grid research and production environment combining multiple grid computing models. In: Weber MHW, eds. Distributed and grid computing: Science made transparent for everyone. Principles, applications and supporting communities. Tectum Publishing House, Marburg; 2–13 [Google Scholar]
- 27. Myers DS, Bazinet AL, Cummings MP. 2008. Expanding the reach of grid computing: combining Globus- and BOINC-based systems. In: Talbi E-G, Zomaya A, eds. Grids for bioinformatics and computational biology, Wiley book series on parallel and distributed computing. John Wiley & Sons, New York; 71–85 [Google Scholar]
- 28. Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948 [DOI] [PubMed] [Google Scholar]
- 29. Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol 215:403–410 [DOI] [PubMed] [Google Scholar]
- 30. Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. States D, Gish W, Altschul SF. 1991. Improved sensitivity of nucleic acid database searches using application-specific scoring matrices. Methods 3:66–77 [Google Scholar]
- 32. Myers EW, Miller W. 1988. Optimal alignments in linear space. Comput Appl Biosci 4:11–17 [DOI] [PubMed] [Google Scholar]
- 33. Flanagan CA, Zhou W, Chi L, Yuen T, Rodic V, Robertson D, Johnson M, Holland P, Millar RP, Weinstein H, Mitchell R, Sealfon SC. 1999. The functional microdomain in transmembrane helices 2 and 7 regulates expression, activation, and coupling pathways of the gonadotropin-releasing hormone receptor. J Biol Chem 274:28880–28886 [DOI] [PubMed] [Google Scholar]
- 34. Zhou W, Flanagan C, Ballesteros JA, Konvicka K, Davidson JS, Weinstein H, Millar RP, Sealfon SC. 1994. A reciprocal mutation supports helix 2 and helix 7 proximity in the gonadotropin-releasing hormone receptor. Mol Pharmacol 45:165–170 [PubMed] [Google Scholar]
- 35. Davidson JS, McArdle CA, Davies P, Elario R, Flanagan CA, Millar RP. 1996. Asn102 of the gonadotropin-releasing hormone receptor is a critical determinant of potency for agonists containing C-terminal glycinamide. J Biol Chem 271:15510–15514 [DOI] [PubMed] [Google Scholar]
- 36. Zhou W, Rodic V, Kitanovic S, Flanagan CA, Chi L, Weinstein H, Maayani S, Millar RP, Sealfon SC. 1995. A locus of the gonadotropin-releasing hormone receptor that differentiates agonist and antagonist binding sites. J Biol Chem 270:18853–18857 [DOI] [PubMed] [Google Scholar]
- 37. Flanagan CA, Becker II, Davidson JS, Wakefield IK, Zhou W, Sealfon SC, Millar RP. 1994. Glutamate 301 of the mouse gonadotropin-releasing hormone receptor confers specificity for arginine 8 of mammalian gonadotropin-releasing hormone. J Biol Chem 269:22636–22641 [PubMed] [Google Scholar]
- 38. Ballesteros J, Kitanovic S, Guarnieri F, Davies P, Fromme BJ, Konvicka K, Chi L, Millar RP, Davidson JS, Weinstein H, Sealfon SC. 1998. Functional microdomains in G-protein-coupled receptors. The conserved arginine-cage motif in the gonadotropin-releasing hormone receptor. J Biol Chem 273:10445–10453 [DOI] [PubMed] [Google Scholar]
- 39. Myburgh DB, Millar RP, Hapgood JP. 1998. Alanine-261 in intracellular loop III of the human gonadotropin-releasing hormone receptor is crucial for G-protein coupling and receptor internalization. Biochem J 331:893–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Arora KK, Cheng Z, Catt KJ. 1997. Mutations of the conserved DRS motif in the second intracellular loop of the gonadotropin-releasing hormone receptor affect expression, activation, and internalization. Mol Endocrinol 11:1203–1212 [DOI] [PubMed] [Google Scholar]
- 41. Kitanovic S, Yuen T, Flanagan CA, Ebersole BJ, Sealfon SC. 2001. Insertional mutagenesis of the arginine cage domain of the gonadotropin-releasing hormone receptor. Mol Endocrinol 15:390–397 [DOI] [PubMed] [Google Scholar]
- 42. Chung HO, Yang Q, Catt KJ, Arora KK. 1999. Expression and function of the gonadotropin-releasing hormone receptor are dependent on a conserved apolar amino acid in the third intracellular loop. J Biol Chem 274:35756–35762 [DOI] [PubMed] [Google Scholar]
- 43. Arora KK, Krsmanovic LZ, Mores N, O'Farrell H, Catt KJ. 1998. Mediation of cyclic AMP signaling by the first intracellular loop of the gonadotropin-releasing hormone receptor. J Biol Chem 273:25581–25586 [DOI] [PubMed] [Google Scholar]
- 44. Tello JA, Sherwood NM. 2009. Amphioxus: beginning of vertebrate and end of invertebrate type GnRH receptor lineage. Endocrinology 150:2847–2856 [DOI] [PubMed] [Google Scholar]
- 45. Tello JA, Wu S, Rivier JE, Sherwood NM. 2008. Four functional GnRH receptors in zebrafish: analysis of structure, signaling, synteny and phylogeny. Integr Comp Biol 48:570–587 [DOI] [PubMed] [Google Scholar]
- 46. Stewart AJ, Katz AA, Millar RP, Morgan K. 2009. Retention and silencing of prepro-GnRH-II and type II GnRH receptor genes in mammals. Neuroendocrinology 90:416–432 [DOI] [PubMed] [Google Scholar]
- 47. Moncaut N, Somoza G, Power DM, Canário AV. 2005. Five gonadotrophin-releasing hormone receptors in a teleost fish: isolation, tissue distribution and phylogenetic relationships. J Mol Endocrinol 34:767–779 [DOI] [PubMed] [Google Scholar]
- 48. Ikemoto T, Park MK. 2007. Comparative analysis of the pituitary and ovarian GnRH systems in the leopard gecko: signaling crosstalk between multiple receptor subtypes in ovarian follicles. J Mol Endocrinol 38:289–304 [DOI] [PubMed] [Google Scholar]
- 49. Illing N, Troskie BE, Nahorniak CS, Hapgood JP, Peter RE, Millar RP. 1999. Two gonadotropin-releasing hormone receptor subtypes with distinct ligand selectivity and differential distribution in brain and pituitary in the goldfish (Carassius auratus). Proc Natl Acad Sci USA 96:2526–2531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Troskie BE, Hapgood JP, Millar RP, Illing N. 2000. Complementary deoxyribonucleic acid cloning, gene expression, and ligand selectivity of a novel gonadotropin-releasing hormone receptor expressed in the pituitary and midbrain of Xenopus laevis. Endocrinology 141:1764–1771 [DOI] [PubMed] [Google Scholar]
- 51. Wang L, Bogerd J, Choi HS, Seong JY, Soh JM, Chun SY, Blomenröhr M, Troskie BE, Millar RP, Yu WH, McCann SM, Kwon HB. 2001. Three distinct types of GnRH receptor characterized in the bullfrog. Proc Natl Acad Sci USA 98:361–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kavanaugh SI, Nozaki M, Sower SA. 2008. Origins of gonadotropin-releasing hormone (GnRH) in vertebrates: identification of a novel GnRH in a basal vertebrate, the sea lamprey. Endocrinology 149:3860–3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Millar RP, Flanagan CA, Milton RC, King JA. 1989. Chimeric analogues of vertebrate gonadotropin-releasing hormones comprising substitutions of the variant amino acids in positions 5, 7, and 8. Characterization of requirements for receptor binding and gonadotropin release in mammalian and avian pituitary gonadotropes. J Biol Chem 264:21007–21013 [PubMed] [Google Scholar]
- 54. Barran PE, Roeske RW, Pawson AJ, Sellar R, Bowers MT, Morgan K, Lu ZL, Tsuda M, Kusakabe T, Millar RP. 2005. Evolution of constrained gonadotropin-releasing hormone ligand conformation and receptor selectivity. J Biol Chem 280:38569–38575 [DOI] [PubMed] [Google Scholar]
- 55. Bogerd J, Diepenbroek WB, Hund E, van Oosterhout F, Teves AC, Leurs R, Blomenröhr M. 2002. Two gonadotropin-releasing hormone receptors in the African catfish: no differences in ligand selectivity, but differences in tissue distribution. Endocrinology 143:4673–4682 [DOI] [PubMed] [Google Scholar]
- 56. Pfleger KD, Bogerd J, Millar RP. 2002. Conformational constraint of mammalian, chicken, and salmon GnRHs, but not GnRH II, enhances binding at mammalian and nonmammalian receptors: evidence for preconfiguration of GnRH II. Mol Endocrinol 16:2155–2162 [DOI] [PubMed] [Google Scholar]
- 57. Flanagan CA, Rodic V, Konvicka K, Yuen T, Chi L, Rivier JE, Millar RP, Weinstein H, Sealfon SC. 2000. Multiple interactions of the Asp(2.61(98)) side chain of the gonadotropin-releasing hormone receptor contribute differentially to ligand interaction. Biochemistry 39:8133–8141 [DOI] [PubMed] [Google Scholar]
- 58. Sun YM, Flanagan CA, Illing N, Ott TR, Sellar R, Fromme BJ, Hapgood J, Sharp P, Sealfon SC, Millar RP. 2001. A chicken gonadotropin-releasing hormone receptor that confers agonist activity to mammalian antagonists. Identification of D-Lys(6) in the ligand and extracellular loop two of the receptor as determinants. J Biol Chem 276:7754–7761 [DOI] [PubMed] [Google Scholar]