Abstract
The inflammasome-forming NLRs are well characterized members of a protein complex mediating the activation of caspase-1 and the cleavage of pro-IL-1β and pro-IL-18 into their active, secreted forms. New data suggest that components of the inflammasome cascade may have roles in influencing inflammasome-independent pathways of cytokine production. These influences on other immune cytokine pathways are complemented by data suggesting that non-inflammasome cytokines can influence the activation of the inflammasome, either directly or by influencing transcription of inflammasome components. The crosstalk between these cytokine cascades may lead to increased abilities for the cell to respond to diverse pathogen threats.
Keywords: Inflammasome, IL-1β, NLR, ASC
Introduction
IL-1β and IL-18 are potent pro-inflammatory cytokines that promote a variety of innate immune processes associated with infection, inflammation, and autoimmunity [1]. A fine balance is required to ensure host defense against viral and bacterial pathogens without tissue damage caused by an over-abundant inflammatory response. Perhaps this is why mammals have developed a two-step system for the regulation of these cytokines. IL-1β and IL-18 are produced as cytosolic precursors and, unlike most secreted proteins, they typically require secondary proteolytic cleavage induced by the inflammasome for activation and secretion. The inflammasome is composed of either a nucleotide-binding domain leucine-rich repeat (NLR) protein such as NLRP1, NLRP3, NLRC4, or NLRC5, the HIN-200 domain-containing protein Aim2, or the cytosolic RNA helicase RIG-I; tethered by the adaptor molecule ASC/Pycard to caspase-1, which provides the enzymatic activity of the complex [1,2]. Assembly of the inflammasome and enzymatic processing of IL-1β and IL-18 is activated by a wide range of stimuli, including bacteria, viruses, and danger signals (DAMPs), such as ATP.
Despite the clear role of the inflammasome in IL-1β and IL-18 processing, evidence is emerging to suggest that the function of the inflammasome and its constituents extends beyond these cytokines. For example, Asc, Caspase-1, and Nlrp3-deficient mice each are resistant to septic shock, whereas IL-1β/IL-18 double knockout mice are susceptible [3,4]. Likewise, clearance of flagellated bacteria is dependent on NLRC4 and caspase-1, but is independent of IL-1β and IL-18 [5]. A variety of effector mechanisms for caspase-1 have been proposed that could explain these IL-1β/IL-18- independent activities [6]. The best characterized of these is the ability of caspase-1 to promote pyroptosis independent of other inflammasome components [7]. Additionally, there are several cellular processes and signaling pathways that the inflammasome NLRs and ASC have recently been shown to regulate independent of caspase-1 activation. In the first part of this review, we discuss the emerging role of the inflammasome components in the regulation of non-inflammasome cytokines. Evidence is also emerging to suggest that non-inflammasome cytokines are involved in the regulation of the inflammasome, and this is discussed in the later part of the review. The crosstalk between the inflammasome and non-inflammasome cytokines is likely to provide expanded control of inflammatory processes that have biological consequences for the response to viral and bacterial pathogens and in autoimmunity.
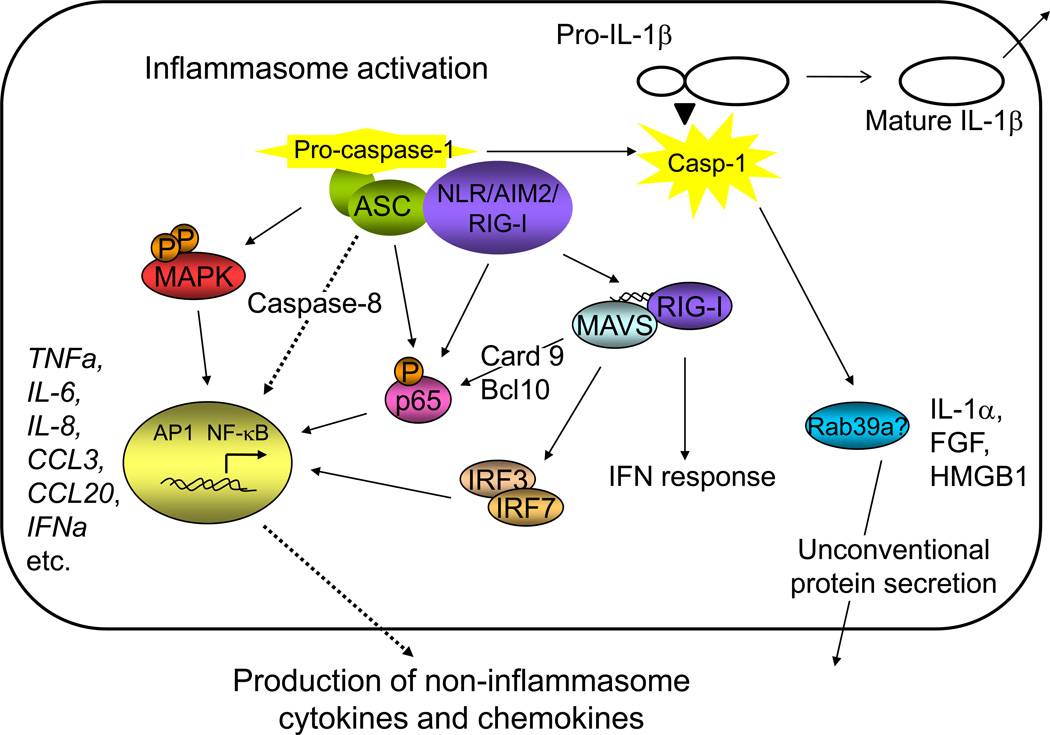
Influence of inflammasome components on the activation of non-Inflammasome cytokines and cytokine signaling pathways (Figure 1)
Figure 1. Influence of inflammasome components on the activation of non-Inflammasome cytokines and cytokine signaling pathways.
Recent evidence suggests that ASC has an inflammasome-independent role in the activation of MAP kinase and NF-κB signaling, which leads to the transcriptional activation of a panel of cytokines and chemokines. Inflammasome NLRs, including NLRP3 and NLRC5, and the inflammasome viral DNA sensors AIM2 and RIG-I have also been shown to influence the activation of NF-κB and subsequent activation of cytokine transcription. Additional evidence suggests that AIM2 may directly affect the induction of IFN-α by viruses through interaction with the RIG-I/MAVS complex. Recent evidence also suggests that caspase-1 may influence the production of additional cytokines that are processed through the unconventional secretory pathway.
The emerging role for ASC in the regulation of non-inflammasome cytokines and chemokines through NF-κB and MAP kinase signaling
Recent evidence suggests that ASC has a role in several inflammatory processes independent of its role in caspase-1 activation and IL-1β/IL-18 processing. As an example, ASC is required for a caspase-1-independent form of cell death in response to several pathogens [8–10]. Asc−/− mice are less susceptible to Mycobacterium tuberculosis, yet have unimpaired production of IL-1β[11,12]. Furthermore, ASC, but not other inflammasome components, is required for antigen-induced murine arthritis [13,14] and humoral immunity following vaccination [15]. In the latter study, changes in the activation of Mip1β and Mip2 were observed, suggesting that the role of ASC in the activation of cytokines and chemokines may extend beyond IL-1β and IL-18. A more recent study demonstrated that the inflammasome is activated in mice following administration of a high fat diet and that levels of Tnfa and Mcp1 in livers from Asc−/− mice are reduced following administration of insulin, providing further evidence for a role for Asc extends beyond the processing of IL-1β and IL-18 [16].
Recent studies have revealed a possible mechanism for ASC-dependent activation of non-inflammasome cytokines and chemokines. ASC is required for the activation of an array of inflammatory cytokines in monocytic cell lines following infection with Porphyromonas gingivalis, including TNF-α, IL-6, IL-8 and IL-10 [17]. The activation of these cytokines was independent of IL-1R signaling and caspase-1 activation, but correlated with the activation of NF-κB. NF-κB activation by ASC has also been verified in 293T cells by genetic reconstitution [18–20], while the role of ASC in NF-κB activation in mouse macrophages is less clear. A recent study has expanded the list of ASC-dependent cytokines in human and mouse macrophages to include a panel of chemokines whose expression is controlled by the MAP kinases ERK and JNK [21]. MAP kinase activity was shown to be regulated by the dual-specificity phosphatase DUSP10/MKP5 and be independent of the inflammasome. ASC has also recently been shown to activate AP1 signaling in a reconstituted cell system and to transcriptionally induce TNF, IL-6, IL-8 and CXCL2. Activation of AP1 required MAP kinase activation and caspase-8 signaling, but was independent of caspase-1 [22]. Collectively, these studies suggest that ASC may mediate the activation NF-κB, MAP kinase, and caspase-8 signaling pathways to transcriptionally activate non-inflammasome cytokines and chemokines.
Potential roles for the inflammasome NLRs, RIG-I and Aim2 in the regulation of non-inflammasome cytokines and chemokines
There is evidence to indicate that the inflammasome NLRs may have functions independent of the inflammasome as well. In a renal ischemia-reperfusion injury model, the absence of Nlrp3, but not Asc, Il1β or Il18, was sufficient to protect mice [23]. Many of the noninflammasome NLRs (e.g., NOD1, NOD2 and NLRP12) have defined roles in the NF-κB and MAP kinase pathways [24,25]; however, it is unknown whether the inflammasome NLRs have similar effects. The possible exception is NLRC5, an additional member of the NLR protein family that has recently been identified as a potential inflammasome protein [26,27]. NLRC5 binds to IKKα and IKKβ and blocks signaling dependent on NF-κB, including the production of TNF-α and IL-6 [28]. This role of NLRC5 in influencing NF-κB signaling is controversial and has been confirmed in some studies [29] but not others [27,30,31]. A mechanism for NLRC5 in influencing interferon responses has also been suggested. NLRC5 binds RIG-I and MDA5 to inhibit type-I interferon responses, analogous to the role of the non-inflammasome protein NLRX1 [25,28]. Other studies have indicated that overexpression of NLRC5 can lead to the induction of IFN-γ-dependent signaling pathways, including STAT1 phosphorylation, and that knockdown of NLRC5 can abrogate Sendai virus and poly (I:C)-induced type-I interferon responses [30,31]. Once again, these results are confounded by conflicting results from other studies [27,29]. The use of different cell types in these studies make it difficult to directly compare results; however, it is possible that this novel NLR protein plays an important role in the regulation of both inflammasome and non-inflammasome cytokines and may provide insight into the mechanisms by which these pathways intersect. The identification of specific NLRC5-dependent ligands will likely aid in elucidating its role.
Additional studies show cross-regulation between inflammasome activation and non-inflammasome cytokines in the viral response. Cytosolic viral RNA is known to activate a complex containing RIG-I and MAVS that induces both IRF-mediated transcription of type I interferon and NF-κB-mediated transcription of IL-6 and TNFα. Recently, RIG-I was also shown to bind ASC in a caspase-1-activating inflammasome that processes IL-1β [32]. Likewise, the viral DNA inflammasome sensor Aim2 has been suggested in some studies [33,34], but not others [35,36] to have a secondary role in the regulation of NF-κB and the transcription of IFN-β. Thus, RIG-I and Aim2 may both serve dual roles as viral inflammasome sensors and activators of non-inflammasome cytokines.
Role for the inflammasome in the secretion of leaderless proteins other than IL-1β and IL-18
Most secreted proteins contain signal peptides that mediate targeting to the endoplasmic reticulum in the classical secretory pathway. The proteins are then transported through the Golgi apparatus, where Golgi-derived secretory vesicles fuse with the plasma membrane for release into the extracellular space. More than 20 proteins in addition to IL-1β and IL-18 are known to be released by unconventional protein secretion independently of the ER and Golgi. The list includes signaling molecules involved in inflammatory, cell survival, and repair responses, such as HMGB1, IL-1α, galectins 1 and 3, and FGF2. Though these proteins are not thought to be direct substrates for caspase-1 cleavage, recent studies suggest that caspase-1 is required for their secretion [37]. Nlrp3 and Asc also have a demonstrated role in the LPS-driven release of IL-1α [38,39] and HMGB1 [3]. Several additional non-cytokine substrates for caspase-1 have been identified including caspase-7 [40,41], and it is possible that one of these substrates may mediate the processing of these leaderless cytokines. Alternatively, several trafficking proteins have been identified as caspase-1 substrates, including Rac2, Rab GDI, Rho RDI beta, and RAB7 [40]. A recent study has also identified Rab39a as a caspase-1 binding partner that is involved in the secretion of IL-1β [42], and it is possible that Rab39a or one of these other trafficking proteins may regulate the secretion of non-inflammasome cytokines by the unconventional secretory pathway.
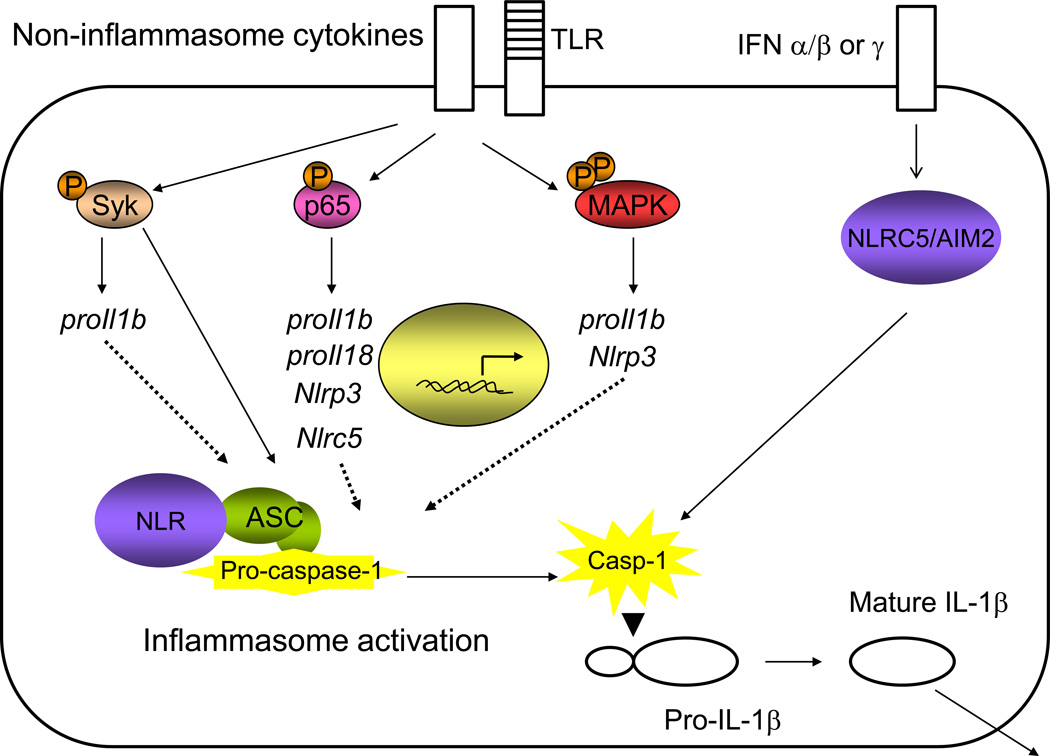
Influence of non-inflammasome cytokines and cytokine signaling pathways on the inflammasome (Figure 2)
Figure 2. Influence of non-inflammasome cytokines and cytokine signaling pathways on the inflammasome.
Many immune pathways downstream of non-inflammasome cytokines influence inflammasome activation, either by transcriptional upregulation of inflammasome components or direct interaction with the inflammasome. While the understanding of the transcriptional control of the NLR sensors is not well understood, it has been demonstrated that NF-κB signaling, Src family kinases, Syk kinase, MAP kinase, and interferon signaling are involved in NLR transcription. Syk kinase and molecules in the interferon and NF-κB signaling cascades can also interact with inflammasome components to influence inflammasome activation.
Effects of TNF-α, MAP kinase and NF-κB on the inflammasome
The canonical model of inflammasome activation involves “Signal 1”, transcriptional upregulation of pro-IL1b and pro-IL18 often induced by TLR stimulation, followed by “Signal 2”, caspase-1-mediated cleavage of pro-IL-1β and pro-IL-18 into their mature forms (reviewed in[1]). Early data indicated that TNF-α could induce IL-1β secretion [43]. More recently it has been shown that TNF-α, and to a lesser extent IL-1α and IL-1β itself, could induce caspase-1 activation and IL-1β secretion [44]. These data indicate that other cytokines may be able to substitute for a TLR-mediated stimulus to induce “Signal 1”. This TNF-α-mediated caspase-1 activation was shown to require translation and NF-κB activation. However, unlike LPS pretreatment, TNF-α pretreatment resulted in sustained ATP-dependent IL-1β secretion by the NLRP3 inflammasome, implying that inflammasome induction by cytokines may amplify an inflammatory response and differ in the quality of inflammasome activation. Additionally, the use of pharmacological inhibitors has indicated that TLR-mediated signaling through TAK1 may influence the activity of the inflammasome in a transcription-independent manner [45].
It is likely that additional cytokines will be shown to induce signal 1 in inflammasome activation. The human monocyte-derived cell line THP-1 is routinely used in studies of the inflammasome following PMA maturation, which leads to a strong increase in the transcription of pro-IL1b [46], suggesting that MAP kinase activation may lead to signal 1. Other studies have indicated that NF-κB stimulation via RANKL or stimulation via PMA or IFN-γ cannot lead to signal 1 in mouse macrophages [44], meaning that this may be an instance of differential control of the inflammasome in different cell types.
In addition, early studies have shown that the expression of NLRP3 is induced by TNF-α in human monocytes, indicating another way in which cytokines can amplify inflammasome activation [47]. Like the expression of pro-Il1b, the expression of Nlrp3 has also been found to be dependent on NF-κB activation [48]. In fact, the expression of Nlrp3 was influenced by many of the same stimuli that induce the expression of pro-IL1b, indicating that these stimuli may be leading to more amplification than previously appreciated [49]. Influenza virus infection has also been shown to induce Nlrp3 transcription in mouse airway epithelial cells and total lung homogenates [50]. Analysis of the NLRP3 promoter revealed the presence of Sp1, c-Myb, AP-1, and c-Ets sites, indicating that the regulation of this sensor is likely complex [51]. NLRC5 has also been shown to be transcriptionally regulated by NF-κB [28]. Further, Syk has been shown to enhance inflammasome activation by binding Asc following phosphorylation by Lyn in response to malarial hemozoin [52] or by influencing pro-IL1b transcription in response to Candida albicans [53]. The transcriptional regulation of inflammasome components likely represents an area of convergence of many proinflammatory pathways.
Effects of interferon on the inflammasome
Increasing evidence suggests that interferon signaling can also influence inflammasome activation. Type-I interferon levels can enhance Aim2 protein levels, while deficiency in the interferon receptor or STING signaling pathways reduces the activity of this inflammasome [34,35,46,54]. Other studies have indicated that IFN-β’s role in enhancing the activity of the Aim2 inflammasome is not dependent on changes in Aim2 transcription and depends on influencing the accessibility of Aim2 ligands [36]. In addition, transcription of the novel inflammasome member NLRC5 has been shown to be regulated by IFN-γ and IFN-γ-dependent infection and its promoter contains two predicted STAT binding sites [29,30,55]. Another study suggested that NLRC5 is transcriptionally regulated by two stimuli of type-I interferon, poly (I:C) and Sendai virus [31]. It will be interesting to determine whether NLRC5 senses pathogens that are also strong inducers of interferon.
An intriguing study also highlights the potential for direct interaction between the interferon and inflammasome pathways. Pretreatment with IFN-β was shown to inhibit the activation of caspase-1 downstream of stimulation with NLRP1 and NLRP3 agonists and exacerbate infection with the NLRP3-dependent pathogen Candida albicans [56]. These data are particularly interesting as they provide an explanation for the immunosuppression observed following viral infection. In addition, this study showed that monocytes from patients undergoing IFN-β treatment produced reduced levels of IL-1β, providing a mechanism for the utility of IFN-β as an MS treatment [56]. IFN-γ has also been shown to enhance the ability of macrophages to produce IL-1β, although the mechanism is unknown [57].
Conclusions
Our original understanding of the inflammasome was as a platform for the activation of caspase-1 and cleavage of the pro-forms of IL-1β and IL-18 into active cytokines. Emerging data indicates that the components of the inflammasome cascade can have roles in pathways independent of their roles in the inflammasome. New data is highlighting potential roles for other inflammatory cytokines in influencing inflammasome activation via transcription and mechanisms other than transcription as well. While the study of pro-inflammatory cytokines has been bifurcated into examination of either the interferon pathway or inflammasome-dependent IL-1β secretion, it is now evident that substantial crosstalk exists between these pathways. Likewise, the members of the NLR family were originally thought of as either inflammasome-forming NLRs or non-inflammasome NLRs. While it is still true that the primary function of the inflammasome NLRs is the formation of the inflammasome complex for IL-1β and IL-18 secretion, many of these NLRs serve functions similar to those described for non-inflammasome NLRs. In fact, these proteins may be functioning as parts of different multicomponent signalosomes depending on cell type and other NLR proteins present and may not be restricted to acting in only one of these signalosomes. This type of response may allow multiple signals from diverse pathogen-sensing pathways to be integrated and allow the cell to generate an appropriate response. It is likely that the data presented in this review are only the first observations of this sort of crosstalk between inflammasome components and other cytokine signaling pathways and that many more will be described in the future.
Highlights.
The IL-1β/IL-18-processing inflammasome is composed of an NLR sensor, Aim2 or RIG-I; the adaptor ASC/Pycard; and caspase-1.
Recent evidence suggests that many of these inflammasome components have secondary roles in the transcriptional induction of non-inflammasome cytokines through the activation of NF-κB and MAP kinase signaling pathways.
Caspase-1 also is proposed to have a role in the processing of growth factors through the unconventional secretory pathway.
A variety of studies also suggest that non-inflammasome cytokines may influence the activation of the inflammasome through transcriptional induction of inflammasome components and direct interaction with inflammasome components.
The cross-regulation between these inflammatory cytokine cascades may lead to increased abilities for the cell to respond appropriately to diverse pathogen threats.
Acknowledgements
This work was supported by NIH grants 5-U19-AI1077437-02 and 2-R37-AI029564-16A2.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare no conflicts of interest.
References and Recommended Reading
* Of special interest
** Of outstanding interest
- 1.Davis BK, Wen H, Ting JP. The Inflammasome NLRs in Immunity, Inflammation, and Associated Diseases. Annu Rev Immunol. 2011;29:707–735. doi: 10.1146/annurev-immunol-031210-101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poeck H, Ruland J. From virus to inflammation: Mechanisms of RIG-I-induced IL-1beta production. European journal of cell biology. 2011 doi: 10.1016/j.ejcb.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 3. Lamkanfi M, Sarkar A, Vande Walle L, Vitari AC, Amer AO, Wewers MD, Tracey KJ, Kanneganti TD, Dixit VM. Inflammasome-dependent release of the alarmin HMGB1 in endotoxemia. Journal of immunology. 2010;185:4385–4392. doi: 10.4049/jimmunol.1000803.This study shows that mice deficient in the genes for the inflammasome components Asc Caspase-1, and Nlrp3 succeptibility to LPS-induced septic shock, whereas IL-1β/IL-18 double knockout mice remain susceptible. This provides direct evidence that the NLRP3 inflammasome has roles that extend beyond the processing of IL-1β and IL18. HMGB1 was shown to have a critical role in the IL-1β/IL-18-independent endotoxemia.
- 4. Sarkar A, Hall MW, Exline M, Hart J, Knatz N, Gatson NT, Wewers MD. Caspase-1 regulates Escherichia coli sepsis and splenic B cell apoptosis independently of interleukin-1beta and interleukin-18. American journal of respiratory and critical care medicine. 2006;174:1003–1010. doi: 10.1164/rccm.200604-546OC. This study shows that the Nlrc4 inflammasome mediates the clearance of flagellated bacteria, whereas IL-1β and IL-18 are not sufficient. This provides additional evidence of a role for caspase-1 that is independent of IL-1β and IL-18, this time in the context of the Nlrc4 inflammasome. Pyroptosis is identified as a mechanism for caspase-1-mediated bacterial clearance.
- 5.Miao EA, Leaf IA, Treuting PM, Mao DP, Dors M, Sarkar A, Warren SE, Wewers MD, Aderem A. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nature immunology. 2010;11:1136–1142. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamkanfi M. Emerging inflammasome effector mechanisms. Nature reviews. Immunology. 2011;11:213–220. doi: 10.1038/nri2936. [DOI] [PubMed] [Google Scholar]
- 7. Broz P, von Moltke J, Jones JW, Vance RE, Monack DM. Differential requirement for Caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing. Cell host & microbe. 2010;8:471–483. doi: 10.1016/j.chom.2010.11.007. These authors demonstrate that NLRP3 and NLRC4 can both respond to Salmonella typhimurium to activate an inflammasome for IL-1β processing along with caspase 1 and ASC. A separate inflammasome complex that does not include ASC was involved in pyroptosis in response to NLRC4-dependentSalmonellarecognition.
- 8.Willingham SB, Bergstralh DT, O'Connor W, Morrison AC, Taxman DJ, Duncan JA, Barnoy S, Venkatesan MM, Flavell RA, Deshmukh M, et al. Microbial pathogen-induced necrotic cell death mediated by the inflammasome components CIAS1/cryopyrin/NLRP3 and ASC. Cell Host Microbe. 2007;2:147–159. doi: 10.1016/j.chom.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duncan JA, Gao X, Huang MT, O'Connor BP, Thomas CE, Willingham SB, Bergstralh DT, Jarvis GA, Sparling PF, Ting JP. Neisseria gonorrhoeae activates the proteinase cathepsin B to mediate the signaling activities of the NLRP3 and ASC-containing inflammasome. J Immunol. 2009;182:6460–6469. doi: 10.4049/jimmunol.0802696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang MT, Taxman DJ, Holley-Guthrie EA, Moore CB, Willingham SB, Madden V, Parsons RK, Featherstone GL, Arnold RR, O'Connor BP, et al. Critical role of apoptotic speck protein containing a caspase recruitment domain (ASC) and NLRP3 in causing necrosis and ASC speck formation induced by Porphyromonas gingivalis in human cells. J Immunol. 2009;182:2395–2404. doi: 10.4049/jimmunol.0800909. [DOI] [PubMed] [Google Scholar]
- 11.McElvania Tekippe E, Allen IC, Hulseberg PD, Sullivan JT, McCann JR, Sandor M, Braunstein M, Ting JP. Granuloma formation and host defense in chronic Mycobacterium tuberculosis infection requires PYCARD/ASC but not NLRP3 or caspase-1. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012320. e12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mayer-Barber KD, Barber DL, Shenderov K, White SD, Wilson MS, Cheever A, Kugler D, Hieny S, Caspar P, Nunez G, et al. Caspase-1 independent IL-1beta production is critical for host resistance to mycobacterium tuberculosis and does not require TLR signaling in vivo. Journal of immunology. 2010;184:3326–3330. doi: 10.4049/jimmunol.0904189. These two studies show that ASC has an inflammasome-independent role in the host resistance to Mycobacterium tuberculosis. The role for Nlrp3 differed in the two studies, however, both studies agreed that caspase-1 is dispensable.
- 13.Kolly L, Karababa M, Joosten LA, Narayan S, Salvi R, Petrilli V, Tschopp J, van den Berg WB, So AK, Busso N. Inflammatory role of ASC in antigen-induced arthritis is independent of caspase-1, NALP-3, and IPAF. Journal of immunology. 2009;183:4003–4012. doi: 10.4049/jimmunol.0802173. [DOI] [PubMed] [Google Scholar]
- 14. Ippagunta SK, Brand DD, Luo J, Boyd KL, Calabrese C, Stienstra R, Van de Veerdonk FL, Netea MG, Joosten LA, Lamkanfi M, et al. Inflammasome-independent role of apoptosis-associated speck-like protein containing a CARD (ASC) in T cell priming is critical for collagen-induced arthritis. The Journal of biological chemistry. 2010;285:12454–12462. doi: 10.1074/jbc.M109.093252.These two studies show an inflammasome-independent role for ASC in antigen-induced arthritis.
- 15. Ellebedy AH, Lupfer C, Ghoneim HE, DeBeauchamp J, Kanneganti TD, Webby RJ. Inflammasome-independent role of the apoptosis-associated speck-like protein containing CARD (ASC) in the adjuvant effect of MF59. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:2927–2932. doi: 10.1073/pnas.1012455108. This study demonstrates an inflammasome-independent role for ASC in humoral immunity following vaccination. ASC−/−mice has decreased levels of Mip1b and Mip2, suggesting a possible link between ASC and chemokine induction.
- 16.Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, Brickey WJ, Ting JP. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12:408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taxman DJ, Zhang J, Champagne C, Bergstralh DT, Iocca HA, Lich JD, Ting JP. Cutting edge: ASC mediates the induction of multiple cytokines by Porphyromonas gingivalis via caspase-1-dependent and -independent pathways. Journal of immunology. 2006;177:4252–4256. doi: 10.4049/jimmunol.177.7.4252. [DOI] [PubMed] [Google Scholar]
- 18.Manji GA, Wang L, Geddes BJ, Brown M, Merriam S, Al-Garawi A, Mak S, Lora JM, Briskin M, Jurman M, et al. PYPAF1, a PYRIN-containing Apaf1-like protein that assembles with ASC and regulates activation of NF-kappa B. The Journal of biological chemistry. 2002;277:11570–11575. doi: 10.1074/jbc.M112208200. [DOI] [PubMed] [Google Scholar]
- 19.Masumoto J, Dowds TA, Schaner P, Chen FF, Ogura Y, Li M, Zhu L, Katsuyama T, Sagara J, Taniguchi S, et al. ASC is an activating adaptor for NF-kappa B and caspase-8-dependent apoptosis. Biochemical and biophysical research communications. 2003;303:69–73. doi: 10.1016/s0006-291x(03)00309-7. [DOI] [PubMed] [Google Scholar]
- 20.Hasegawa M, Imamura R, Kinoshita T, Matsumoto N, Masumoto J, Inohara N, Suda T. ASC-mediated NF-kappaB activation leading to interleukin-8 production requires caspase-8 and is inhibited by CLARP. The Journal of biological chemistry. 2005;280:15122–15130. doi: 10.1074/jbc.M412284200. [DOI] [PubMed] [Google Scholar]
- 21. Taxman DJ, Holley-Guthrie EA, Huang MT, Moore CB, Bergstralh DT, Allen IC, Lei Y, Gris D, Ting JP. The NLR adaptor ASC/pycard regulates DUSP10, MAP kinase (MAPK) and chemokine induction independent of the inflammasome. J Biol Chem. 2011 doi: 10.1074/jbc.M111.221077. This study identifies a panel of cytokines and chemokines that are reduced in ASC-deficient cells, providing evidence for a broader role for ASC in the transcriptional regulation of non-inflammasome cytokines and chemokines. ASC-dependent MAP kinase activation is described as a mechanism for inflammasome-independent cytokine transcription. MAP kinase activation is also shown to be regulated by the dual specificity phosphatase DUSP10.
- 22. Hasegawa M, Imamura R, Motani K, Nishiuchi T, Matsumoto N, Kinoshita T, Suda T. Mechanism and repertoire of ASC-mediated gene expression. Journal of immunology. 2009;182:7655–7662. doi: 10.4049/jimmunol.0800448. Using a reconstituted cell system, this study identifies AP1 as a transcription factor that mediates ASC-dependent IL-8 promoter activation. AP-1 activation required MAP kinase activation and caspase-8, but was independent of caspase-1 activity. Several additional ASC-dependent genes were identified by microarray analysis.
- 23.Shigeoka AA, Mueller JL, Kambo A, Mathison JC, King AJ, Hall WF, Correia Jda S, Ulevitch RJ, Hoffman HM, McKay DB. An inflammasome-independent role for epithelial-expressed Nlrp3 in renal ischemia-reperfusion injury. Journal of immunology. 2010;185:6277–6285. doi: 10.4049/jimmunol.1002330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanneganti TD, Lamkanfi M, Nunez G. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007;27:549–559. doi: 10.1016/j.immuni.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Ting JP, Duncan JA, Lei Y. How the noninflammasome NLRs function in the innate immune system. Science. 2010;327:286–290. doi: 10.1126/science.1184004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis BK, Roberts RA, Huang MT, Willingham SB, Conti BJ, Brickey WJ, Barker BR, Kwan M, Taxman DJ, Accavitti-Loper MA, et al. Cutting edge: NLRC5-dependent activation of the inflammasome. J Immunol. 2011;186:1333–1337. doi: 10.4049/jimmunol.1003111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kumar H, Pandey S, Zou J, Kumagai Y, Takahashi K, Akira S, Kawai T. NLRC5 deficiency does not influence cytokine induction by virus and bacteria infections. Journal of immunology. 2011;186:994–1000. doi: 10.4049/jimmunol.1002094. These two studies indicate that the NLR protein NLRC5 plays a role in inflammasome formation. NLRC5 may function in a complex with NLRP3 and modulate the activity of the NLRP3 inflammasome. As NLRC5 is also involved in interferon responses, formation of the NLRC5/NLRP3 inflammasome complex may allow for more fine tuning of the inflammatory response.
- 28.Cui J, Zhu L, Xia X, Wang HY, Legras X, Hong J, Ji J, Shen P, Zheng S, Chen ZJ, et al. NLRC5 negatively regulates the NF-kappaB and type I interferon signaling pathways. Cell. 2010;141:483–496. doi: 10.1016/j.cell.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benko S, Magalhaes JG, Philpott DJ, Girardin SE. NLRC5 limits the activation of inflammatory pathways. Journal of immunology. 2010;185:1681–1691. doi: 10.4049/jimmunol.0903900. [DOI] [PubMed] [Google Scholar]
- 30.Kuenzel S, Till A, Winkler M, Hasler R, Lipinski S, Jung S, Grotzinger J, Fickenscher H, Schreiber S, Rosenstiel P. The nucleotide-binding oligomerization domain-like receptor NLRC5 is involved in IFN-dependent antiviral immune responses. Journal of immunology. 2010;184:1990–2000. doi: 10.4049/jimmunol.0900557. [DOI] [PubMed] [Google Scholar]
- 31.Neerincx A, Lautz K, Menning M, Kremmer E, Zigrino P, Hosel M, Buning H, Schwarzenbacher R, Kufer TA. A role for the human nucleotide-binding domain, leucine-rich repeat-containing family member NLRC5 in antiviral responses. The Journal of biological chemistry. 2010;285:26223–26232. doi: 10.1074/jbc.M110.109736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Poeck H, Bscheider M, Gross O, Finger K, Roth S, Rebsamen M, Hannesschlager N, Schlee M, Rothenfusser S, Barchet W, et al. Recognition of RNA virus by RIG-I results in activation of CARD9 and inflammasome signaling for interleukin 1 beta production. Nature immunology. 2010;11:63–69. doi: 10.1038/ni.1824. This study indicated that the RNA helicase RIG-I can induce inflammasome activation in addition to its other well-known roles. RIG-I was shown to form a complex with MAVS, CARD9 and Bcl-10 to activate NF-κB and proil1b transcription. RIG-I was also in a second complex with ASC but not MAVS, CARD9, or NLRP3, to mediate inflammasome activation directly. The related RNA helicase MDA5 was also able to influence NLRP3-dependent inflammasome activation.
- 33.Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rathinam VA, Jiang Z, Waggoner SN, Sharma S, Cole LE, Waggoner L, Vanaja SK, Monks BG, Ganesan S, Latz E, et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol. 2010;11:395–402. doi: 10.1038/ni.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones JW, Kayagaki N, Broz P, Henry T, Newton K, O'Rourke K, Chan S, Dong J, Qu Y, Roose-Girma M, et al. Absent in melanoma 2 is required for innate immune recognition of Francisella tularensis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:9771–9776. doi: 10.1073/pnas.1003738107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernandes-Alnemri T, Yu JW, Juliana C, Solorzano L, Kang S, Wu J, Datta P, McCormick M, Huang L, McDermott E, et al. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat Immunol. 2010;11:385–393. doi: 10.1038/ni.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Keller M, Ruegg A, Werner S, Beer HD. Active caspase-1 is a regulator of unconventional protein secretion. Cell. 2008;132:818–831. doi: 10.1016/j.cell.2007.12.040. This study recognizes caspase-1 as a regulator of leaderless proteins that undergo ER/Golgi-independent secretion, including IL-1α and FGF-2. Regulation is not through direct enzymatic cleavage by caspase-1, but does involve a direct interaction.
- 38.Kuida K, Lippke JA, Ku G, Harding MW, Livingston DJ, Su MS, Flavell RA. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 39.Sutterwala FS, Ogura Y, Szczepanik M, Lara-Tejero M, Lichtenberger GS, Grant EP, Bertin J, Coyle AJ, Galan JE, Askenase PW, et al. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24:317–327. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 40.Shao W, Yeretssian G, Doiron K, Hussain SN, Saleh M. The caspase-1 digestome identifies the glycolysis pathway as a target during infection and septic shock. The Journal of biological chemistry. 2007;282:36321–36329. doi: 10.1074/jbc.M708182200. [DOI] [PubMed] [Google Scholar]
- 41.Lamkanfi M, Kanneganti TD, Van Damme P, Vanden Berghe T, Vanoverberghe I, Vandekerckhove J, Vandenabeele P, Gevaert K, Nunez G. Targeted peptidecentric proteomics reveals caspase-7 as a substrate of the caspase-1 inflammasomes. Molecular & cellular proteomics : MCP. 2008;7:2350–2363. doi: 10.1074/mcp.M800132-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Becker CE, Creagh EM, O'Neill LA. Rab39a binds caspase-1 and is required for caspase-1-dependent interleukin-1beta secretion. The Journal of biological chemistry. 2009;284:34531–34537. doi: 10.1074/jbc.M109.046102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dinarello CA, Cannon JG, Wolff SM, Bernheim HA, Beutler B, Cerami A, Figari IS, Palladino MA, Jr, O'Connor JV. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. The Journal of experimental medicine. 1986;163:1433–1450. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Franchi L, Eigenbrod T, Nunez G. Cutting edge: TNF-alpha mediates sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation. Journal of immunology. 2009;183:792–796. doi: 10.4049/jimmunol.0900173. These authors show that TNF-α can induce "signal 1" for inflammasome activation. Interestingly, this TNF-α-mediated induction of proil1b transcription had different kinetics than LPS-mediated proil1b transcription, providing a potential for fine tuning of inflammasome activation.
- 45.Gong YN, Wang X, Wang J, Yang Z, Li S, Yang J, Liu L, Lei X, Shao F. Chemical probing reveals insights into the signaling mechanism of inflammasome activation. Cell research. 2010;20:1289–1305. doi: 10.1038/cr.2010.135. [DOI] [PubMed] [Google Scholar]
- 46.Burckstummer T, Baumann C, Bluml S, Dixit E, Durnberger G, Jahn H, Planyavsky M, Bilban M, Colinge J, Bennett KL, et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nature immunology. 2009;10:266–272. doi: 10.1038/ni.1702. [DOI] [PubMed] [Google Scholar]
- 47.O'Connor W, Jr, Harton JA, Zhu X, Linhoff MW, Ting JP. Cutting edge: CIAS1/cryopyrin/PYPAF1/NALP3/CATERPILLER 1.1 is an inducible inflammatory mediator with NF-kappa B suppressive properties. Journal of immunology. 2003;171:6329–6333. doi: 10.4049/jimmunol.171.12.6329. [DOI] [PubMed] [Google Scholar]
- 48.Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Guarda G, Zenger M, Yazdi AS, Schroder K, Ferrero I, Menu P, Tardivel A, Mattmann C, Tschopp J. Differential expression of NLRP3 among hematopoietic cells. Journal of immunology. 2011;186:2529–2534. doi: 10.4049/jimmunol.1002720. These authors generated a mouse in which Nlrp3 was replaced by egfp to allow examination of regulatory elements driving Nlrp3 transcription. They showed that NLRP3 is inducible in macrophages following treatment with inflammatory stimuli in the draining lymph nodes in vivo. These mice will be a useful reagent for examination ofNlrp3transcriptional regulation.
- 50.Allen IC, Scull MA, Moore CB, Holl EK, McElvania-TeKippe E, Taxman DJ, Guthrie EH, Pickles RJ, Ting JP. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009;30:556–565. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anderson JP, Mueller JL, Misaghi A, Anderson S, Sivagnanam M, Kolodner RD, Hoffman HM. Initial description of the human NLRP3 promoter. Genes and immunity. 2008;9:721–726. doi: 10.1038/gene.2008.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shio MT, Eisenbarth SC, Savaria M, Vinet AF, Bellemare MJ, Harder KW, Sutterwala FS, Bohle DS, Descoteaux A, Flavell RA, et al. Malarial hemozoin activates the NLRP3 inflammasome through Lyn and Syk kinases. PLoS pathogens. 2009;5 doi: 10.1371/journal.ppat.1000559. e1000559. In this study, the authors show that malarial hemozoin is recognized by the NLRP3 inflammasome. This recognition induces phosphorylation of Syk by the Src family kinase Lyn followed by the formation of a complex between phosphorylated Syk and NLRP3 and ASC.
- 53. Gross O, Poeck H, Bscheider M, Dostert C, Hannesschlager N, Endres S, Hartmann G, Tardivel A, Schweighoffer E, Tybulewicz V, et al. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459:433–436. doi: 10.1038/nature07965. These authors demonstrated that Syk controls inflammasome activation directly as well as at the level of transcription to induce antifungal immune responses. Syk forms a complex with CARD9, similar to the RIG-I/CARD9 interaction shown in (36), to induce NF-κ-dependent proil1b transcription, and is also directly influences NLRP3-dependent inflammasome activation, potentially in similar manner to (52). These data indicate that the inflammasome has a crucial role in immune responses to fungal infection.
- 54.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meissner TB, Li A, Biswas A, Lee KH, Liu YJ, Bayir E, Iliopoulos D, van den Elsen PJ, Kobayashi KS. NLR family member NLRC5 is a transcriptional regulator of MHC class I genes. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:13794–13799. doi: 10.1073/pnas.1008684107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Guarda G, Braun M, Staehli F, Tardivel A, Mattmann C, Forster I, Farlik M, Decker T, Du Pasquier RA, Romero P, et al. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity. 2011;34:213–223. doi: 10.1016/j.immuni.2011.02.006. This study was the first to show that type I interferon can modulate inflammasome activation directly, as well as acting at the level of transcription. Type I interferon production was also able to reduce IL-1β production in response to infection with Candida albicans in a mouse model, as well as increasing fungal susceptibility, potentially providing a mechanism for immunosuppression following viral infections. Further, this study showed that monocytes from IFN-β treated MS patients, but not healthy controls, produced reduced levels of IL-1β, demonstrating a mechanism for the utility of IFN-β treatment in M.S.
- 57.Netea MG, Nold-Petry CA, Nold MF, Joosten LA, Opitz B, van der Meer JH, van de Veerdonk FL, Ferwerda G, Heinhuis B, Devesa I, et al. Differential requirement for the activation of the inflammasome for processing and release of IL-1beta in monocytes and macrophages. Blood. 2009;113:2324–2335. doi: 10.1182/blood-2008-03-146720. [DOI] [PMC free article] [PubMed] [Google Scholar]