Abstract
Purpose of review
To summarize key findings of the current literature on functional neuroimaging in migraine and to describe how these studies have changed our view of the disorder. Recent findings: Recent studies have started to investigate not only the global cerebral activation pattern during migraine attacks, but to address specific aspects of migraine attacks such as photophobia, osmophobia as well as pain perception with the aim of disentangling the underlying mechanisms. There is also more and more evidence that the migraine brain is abnormal even outside of attacks and that repeated attacks are leading to functional and structural alterations in the brain, which may in turn drive the transformation of migraine to its chronic form. Some new results are pinpointing towards a potential role of interesting new brain areas in migraine pathophysiology such as the temporal cortex or the basal ganglia.
Summary
Neuroimaging studies are beginning to shed light on the mechanisms underlying the development and evolution of migraine and its specific symptoms. Future studies have the potential to also improve our understanding of established and upcoming treatment approaches and to monitor treatment effects in an objective and non-invasive way.
Keywords: fMRI, MRS, voxel based morphometry, PET, migraine
1. Introduction
According to the World Health Organization (WHO) migraine affects about 15 percent of the population. It is the most common neurological disease and it is ranked 12th amongst women and 19th in the general population for the degree of handicap it causes. Although advances in migraine research have contributed to an improved understanding of the disease, the use of advanced MRI techniques has allowed for the investigation of migraine patients during the ictal and interictal period [1]. What has become apparent is that migraine is not simply a disease that relates to pain occurring intermittently or constantly, but a process that over time either affects the brain in a profound manner or acts on a predisposed brain (genetic) that may have an underlying difference in function or structure. In the past decade, a number of fundamental changes have been shown to occur in the brain of migraineurs including: (1) an abnormal function of key brain areas and networks and (2) changes brain gray and white matter structure and (3) changes in brain chemistry. The evaluation of brain targets of migraine-related therapeutic approaches including medications and neuromodulation is also feasible with modern imaging techniques. This review collates recent advances in brain imaging research in migraine that relate to these changes and provides an opinion on future research directions.
2. Migraine - An Under appreciated Disease of the Brain
We are just beginning to understand why some people suffer from migraine and others don’t. Genetics have shown to be involved in migraine and in particular glutamate pathways seem to be of importance [2], but in contrast to familial hemiplegic migraine, the amount of variance that genetics can explain is limited so far. Environmental factors are also clearly involved in precipitating migraine (e.g., weather [3, 4]; stress [5, 6]; physiological factors [7]). Whatever the case, brain systems are involved in the migraine attack. The neurobiological instigator/s of a migraine attack are unknown and may include alterations in altered traffic in trigeminal afferent systems as a result of instigating factors such as cytokines or mediated via endogenous brain systems themselves (e.g., cortical spreading depression) [8–10]. The spectrum of ictal and interictal phases is unclear because pro-dromal/pre-ictal symptoms and post-ictal symptoms as well as non-pain ictal symptoms (e.g., nausea) are part of changes that involve the brain directly. Cortical spreading depression may have more deleterious effects on brain function than that observed from subjective reports such as auras [11]. Thus the constellation of changes from autonomic (including changes in appetite, sleep propensity), to sensory (pain, vision, auditory, gustatory, olfactory), to cognitive (including language), all involve multiple brain systems. Some of these alterations may be adaptive. Over time, the repeated onslaught of migraine attacks includes a multitude of effects on brain systems that may result in maladaptive (neuroplastic) changes in brain structure and function promoting the transformation to chronic migraine. As such, changes are an inevitable consequence of each attack. However, such changes are most likely reversible and treatment is hence essential. Thus, migraine should be considered a “disease of the brain”.
3. Imaging the Ictal and Interictal States – Insights into altered Function
A number of recent studies have evaluated changes in migraine brain function in both the ictal and interictal states utilizing fMRI or PET in episodic and chronic migraine. We will discuss the pertinent findings separately for the brainstem, sub-cortical and cortical areas, but clearly the brain is an integrated system and more recent studies of brain networks in migraine are beginning to foster the understanding of functional connectivity in the migraine brain on a systems level [12]. In addition, new insights are being garnered from imaging studies on brain changes associated with migraine progression.
3.1. The Ictal State
Most of the studies in the ictal state have captured measures relating to spontaneous or triggered migraine attacks (and pain processing in particular) or applied evoked stimuli in order to exacerbate migraine symptoms (pain, olfactory, light).
3.1.1. Ictal Changes – Brainstem and Cerebellum
The early reports of brain activation in migraine implicated the brainstem in attack generation [13]. These PET studies were pivotal in bringing to our attention major changes in brain activity associated with migraine and opened a new era in the study of the disease [14, 15]. These studies focused the attention on brainstem regions that were abnormally active in the migraine state and in light of previous reports of migraines being generated by implantation of deep brain stimulation electrodes into the periaqueductal grey matter in the brainstem, they were interpreted as possible ‘migraine generator’ regions. Other studies showing increased iron accumulation in the periaqueductal gray (PAG) further supported the notion of a major contribution of alterations in brainstem function in migraine [14, 16]. Alterations in the PAG have also been documented using VBM (see below) adding further credence to the notion that such brainstem abnormalities may contribute to the migraine condition [17]. In an fMRI evaluation of migraine patients compared with healthy controls, visual stimulation induced changes in the red nucleus and substantia nigra [18]. However, these structures were also shown to be activated in other non-migraine pain studies [19]. In addition, PET studies of 5HT1A receptor availability (using [(18)F]MPPF PET tracer) during migraine showed an increased [(18)F]MPPF binding potential in an area referred to as the pontine raphe when comparing headache-free migraineurs and control subjects [20].
Newer PET studies in spontaneous migraine attacks again noted activation in the pons [21, 22]. Because of the limited spatial resolution of PET, it is difficult to determine exactly, which brainstem nuclei are corresponding to these activations, but one study focusing on the laterality by investigating patients with side-locked migraines reported the brainstem activation to occur ipsilaterally to the pain, suggesting that lateralization of the pain is a matter of lateralized brainstem dysfunction [23]. Subsequent fMRI studies showed changes in other brain-stem regions including midbrain regions such as the nucleus cuneiformis [24]. All these brainstem changes may reflect abnormal processing in modulatory brainstem circuits aiming at suppressing nociceptive drive within the trigeminovascular system. Another intriguing finding reported recently was the observation that characteristics of trigeminal transmission in the trigeminal nucleus caudalis may predict migraine attacks [25]. All these changes may reflect damage to modulatory systems that then cannot prevent nociceptive drive from trigeminovascular afferents.
3.1.2. Ictal Changes – Subcortical Regions
Attention on a potential involvement of subcortical grey matter in migraine focused on the thalamus and hypothalamus in the past. Regarding the hypothalamus, activation has been reported in one study during spontaneous migraine attacks [22], but this finding was not consistent in other reports [21]. This apparent inconsistency is not fully understood so far, but the time point of investigation during the migraine attack (early or late in the ictal state) may affect the degree of measurable hypothalamic activation as Denuelle studied patients relatively early after attack onset and the hypothalamus may have a particular role in the early phase of migraine attacks such as in the premonitory phase.
Regarding the role of the thalamus, more literature is available. The thalamus is now considered to be pivotal in the manifestation of extracephalic allodynia [26]. The posterior thalamus is activated in a rodent model of migraine (as measured electrophysiologically by increased responses to brush and pain) and there are increased responses in a similar thalamic area (pulvinar) in the ictal as compared to the interictal phase in migraine patients as measured using fMRI [27]. Even more intriguing is the observation that this same area may be involved in other manifestations of ictal hypersensitivity to sensory stimuli including photophobia: direct retino-thalamic projections to trigeminovascular neurons have been reported providing a mechanism by which a non-noxious stimulus may exacerbate headache (i.e., photophobia) [28]. Fiber tracking studies in humans have reported connections from the optic nerve to the posterior thalamus terminating in a similar region as reported in the animal study suggesting that this mechanism of headache exacerbation by sensory stimuli also exists in humans [29].
3.1.3. Ictal Changes – Cortical Regions
Cortical changes in the ictal period have been shown in regions normally associated with pain processing (cingulate cortex, insula, prefrontal cortex and other areas) [21] as well as in others not commonly reported. One such region is the temporal lobe [30]. Activation of this region in response to a noxious heat was shown to be exacerbated in the ictal vs. interictal state in migraineurs. In addition, the temporal pole showed stronger functional connectivity with several brain regions relative to controls, suggesting that TP hyperexcitability and abnormal connectivity may contribute to clinical abnormalities in migraine. These data are supported by prior PET data indicating activation of the temporal lobein ictal migraine (Figure 1) [21]. In addition to the temporal pole, occipital cortex responses have been investigated in a few studies [31, 32]. Low intensity photo stimulation activated the visual cortex during migraine attacks and after headache relief but not during the attack-free interval [32] indicating increased cortical excitability during the attack.
Figure 1.
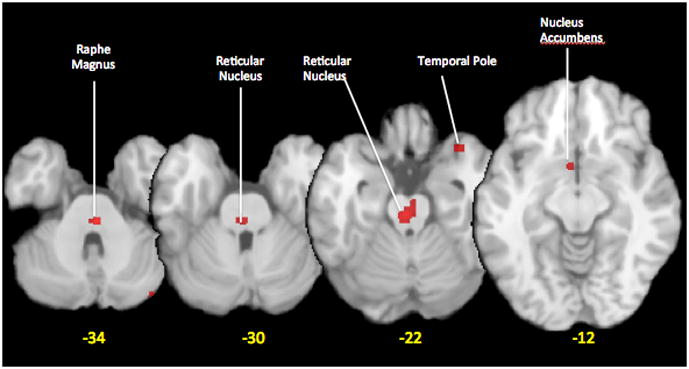
Metanalysis using activation likelihood estimation method [(ALE; GingerAle 2.1 software, Research Imaging Institute, San Antonio, Texas) [33] of brain activations during migraine attacks as measured in H215O-PET studies. The ALE map was thresholded at p<0.05 FDR corrected. Data were obtained from studies on spontaneous (Weiller 1995, spontaneous migraine [13]; Afridi et al., 2005, spontaneous migraine [17]; Denuelle 2007 spontaneous [22]) or evoked migraine (Afridi 2005, GTN triggered migraine [23]) were used.
3.2. The Interictal State
Some of the studies noted above also evaluated changes in the interictal state. There is no consensus regarding the definition of temporal boundaries of the interictal state and subclinical or functional changes may be taking place long after or long before an attack (see [34]). All things considered, distinguishing ictal and interictal phase may be arbitrary and may not really reflect the biology of the disorder. Possiblya dynamic process is ongoing and the ictal event is akin to a volcanic eruption, but the underlying flow of magma below the surface is continuing. Recent studies, both functional and morphometric (see section 3.3. below) gathered a substantial body of evidence suggesting that the interictal brain activity and metabolism is abnormal. One such study indicated a widespread increase of cerebral glucose metabolism in cerebral pain processing are as in migraineurs interictally [35]. This could be a non-specific finding in primary headache disorders, though, as very similar findings were reported in cluster headache patients out of attacks [36].
3.2.1. Interictal Changes – Brainstem
A few studies have examined interictal changes in the brainstem in response to experimental pain stimuli [24, 25]. In these studies, a nociceptive stimulus was used to activate the trigeminal pathways during an fMRI experiment. The nucleus cuneiformis (NCF) and pons are two regions, which have been suggested to correlate with activation to evoked stimuli in the interictal state that are also activated in the migraine state. It may depend on the nature and intensity of the stimulus since some stimuli (e.g., olfactory) do not produce any differences in the brainstem, but activation in the pons is observed in spontaneous migraine attacks, which may be part of the migraine attack [37].
3.2.2. Interictal Changes – Subcortical Regions
Prior studies have shown changes in the hypothalamus and thalamus in response to stimuli such as pain during the interictal period. Recently, we have reported changes in a number of sub-cortical regions including the basal ganglia [38]. These areas differed in their response to experimental pain in patients with high vs. low frequency episodic migraine. While pain imaging studies show consistent activation in acute and chronic pain in various parts of the basal ganglia [39], the latter study provided evidence for a differential processing in the basal ganglia of patients with high versus low migraine attack frequency.
3.2.3. Interictal Changes – Cortical Regions
Previous studies have reported evidence of increased cortical activity in response to sensory stimuli in migraineurs including olfactory hypersensitivity [40]. There was increased activation of the temporal pole in migraineurs in response to olfactory stimuli and increased activation was shown in a similar area when painful stimuli were applied in migraineurs interictally as compared to control subjects [30]. As noted above, the activated area in the temporal lobe was shown to have abnormal functional connectivity with the entire network of brain areas processing pain. Moreover, patients with mesial temporal lobe epilepsy often suffer from unilateral headaches with the side of the temporal lobe abnormality predicting the headache side [41]. All these observations argue in favor of a potential role of the temporal pole in migraine and headache pathophysiology, but more research is needed to better understand the exact relationship.
In another study, patients with migraine showed greater activation in the perigenual part of the anterior cingulate cortex at 51°C and reduced activation in the bilateral somatosensory cortex at 53°C compared to healthy controls [42] suggesting that differential modulatory influences may be at play at different stimulus intensities, but these results need to be confirmed by other studies first.
Repetitive painful stimulation within the territory of the trigeminal nerve over several days produced an abnormal plasticity in response to the stimulation in the cingulate and prefrontal cortex in migraineurs vs. healthy controls [43]. While responses to the painful stimulus increased over time in healthy volunteers, they were instead reduced in the group of migraineurs [43]. The authors interpreted this as a result of alterations in (pain)modulatory/inhibitory circuits, which may relate to the lack of habituation [44] and increased cortical excitability to stimuli previously reported in electrophysiological studies [45]. Imaging studies of interictal cortical excitability also proved a lack of habituation to light in migraineurs [46]. In a study of affective processing in migraine patients compared with healthy controls, pain-related adjectives vs. negative adjectives were used to generate mental images during an fMRI experiment. This resulted in increased activations in a number of cortical regions mediating the affective dimension of pain in migraineurs as compared to non-migraine subjects [47]. The authors concluded that migraine patients had an increased sensitivity to emotional inputs with strong recruitment of affective cortical areas.
4. Imaging Changes of Brain Structure – migraine-related gray matter changes
Ever since the landmark reports on alterations in gray matter in cluster headache [48] and chronic pain patients [49], evaluation of similar changes in migraine patients has shown a significant gray matter loss. Morphometric changes have been reviewed in detail elsewhere [50]. Abnormalities have been reported in multiple brain areas as evidenced by voxel based morphometry (VBM) and diffusion tensor imaging. It is an ongoing matter of debate whether such changes are cause or consequence of migraine, but the fact that at least in many VBM studies changes correlated with disease duration argues in favor of the latter. Research in osteoarthritis has suggested that grey matter changes observed with VBM are indeed reversible [51] and the same is most likely true for migraine also. The exact underlying mechanisms leading to alterations in grey matter density as evidenced by VBM remain to be elucidated. Such alterations may reflect alterations in dendritic complexity or changes in the numbers of synapses or simply in water content. White matter changes as measured using fractional anisotropy with diffusion tensor imaging are thought to reflect alterations in the integrity of connections/white matter tracts. Alterations in the thalamocortical tract are evident in migraineurs [52]. The importance of such measures is that they may allow a measure of the migraine state – in that changes may be an index of the disorder, its progression or an effective therapy.
4.1. Brainstem Changes
Although some suggestion of alterations in the brainstem gray matter volume were intuited by functional data, evidence for alterations in brainstem nuclei have only been reported by few of groups [17, 53].
4.2. Subcortical Changes
An increased caudate volume has recently been reported in high frequency vs. low frequency episodic migraineurs [38]. To date, we are unaware of any other subcortical regions showing changes in gray matter volume, but given the relative ease of the approach, we expect a slew of data in this area.
4.3. Cortical Changes
Cortical changes with gray matter loss as measured using VBM have been reported by a number of studies as indicated above [54]. Using another technique to investigate grey matter abnormalities, DaSilva and colleagues studied the cortical thickness and found thickening of the somatosensory cortex in migraineurs relative to healthy controls [55]. This may be due to increased afferent activity within the somatosensory system in migraine patients [56]. More recently, reports of decreased gray matter volume in episodic migraine (vs. control) in the following cortical regions were published: Superior temporal gyrus, inferior frontal gyrus, and precentralgyrus [57]. More changes were observed in chronic migraine patients (vs. controls) that included gray matter volumetric decreases in the anterior cingulate cortex, amygdala, parietal operculum, middle and inferior frontal gyrus, inferior frontal gyrus, and bilateral insula. A correlation with gray matter decreases and migraine frequency was shown in the anterior cingulate cortex. Such data has been replicated by other groups showing similar changes in the frontal lobes [53].
5. Imaging Chemical Changes
Magnetic Resonance Spectroscopy (MRS) allows to measure brain chemistry in single voxels (i.e., one brain area) or as a composite measure for the entire brain. Changes in excitatory or inhibitory amino acids, alterations in metabolites of energy function (mitochondria) and measures of metabolites that are indicators of neuronal integrity can be measured. A few reports have measured MRS changes in migraine patients. This technique offers the opportunity to not only investigate brain chemistry in the disease state, but to also monitor how treatments may alter, reverse or normalize the chemical milieu in the brain.
5.1. MRS and Migraine
In one of the first reports on MRS measures in patients with migraine with and without aura [58], differences in metabolites in the visual cortex between these two groups of patients were shown in response to a visual stimulation. Specifically, the data indicated a diminished mitochondrial functioning in the migraine with aura patients. Such differentiation is important since it indicates a deficient energy reserve and a potentially more severe disease state in patients who clinically manifest with aura (viz., increased burden of white matter lesion). A similar study involving visual stimulation and applying spectroscopic measures of visual cortex characteristics investigated subgroups of aura patients. Measures of lactate differentiated patients with visual aural from those who had aura plus other symptoms (e.g., paresthesia) [59] in that both groups had high lactate levels, but only those with aura-plus showed increased lactate levels with visual stimulation. A more recent study aimed to determine a ‘metabolic’ dose-response relationship between aura duration and severity metabolites as measured by phosphorus spectroscopy (31P-MRS) or proton spectroscopy (1H-MRS) [60]. The phosphocreatine/phosphate ratio decreased significantly in patients with increasing aura duration but they did not find any differences for 1H-MRS between patients and controls. The study provides important information on differences in patients with different aura phenotypes (e.g., motor vs. non-motor aura) in that patients with motor aura had lower phosphocreatine/phosphate ratios than patients with non-motor aura. No association of abnormal 31P-MRS levels and migrainous stroke was found in another study [61]. Other 31P-MRS studies have implicated alterations in magnesium ion levels that may contribute to cortical hyperexcitability [62]. We have reported abnormalities in glutamatergic metabolites in the anterior cingulate and insula in migraine patients vs. healthy controls [63]. In this report using a linear discriminant analysis (LDA), a clear separation between subject cohorts based on N-acetyl aspartyl glutamate (NAAG) and glutamine (Gln) in the ACC and insula could be observed. While few studies of altered brain chemistry have been performed, clearly this avenue of research should produce novel insights into alterations of brain chemistry that include energy function (mitochondrial), excitatory cortical transmission in migraine, and measures of neuronal integrity.
5.2. MRS and Familial Hemiplegic Migraine (FHM)
Familial Hemiplegic Migraine is a monogenic variant of migraine, characterized by motor deficits during the aura, often beginning in childhood [64]. The first reports of MRS measures in FHM Type 1evaluated metabolic alterations in the cerebellum (since about 20% of these patients have cerebellar signs and symptoms) [65]. Specifically N-acetyl aspartate (NAA) and glutamate (Glu) were significantly reduced while myo-inositol (mI) was increased in FHM patients compared with healthy controls. Toldo and colleagues report on multimodal measures (that included conventional MRI, MRS, and DWI) in a patient with a prolonged attack of FHM showing hypoperfusion and cortical swelling as well as a decrease in the N-acetylaspartate/creatine ratio. The MRI and SPECT abnormalities resolved during the interictal phase, no follow-up of MRS measures performed [66].
6. Personal View – The Future Potential of Imaging in Migraine
A summary of the current state of the field is provided in Table 1. Below we note some thoughts on how imaging may impact the future of understanding migraine and possible implications for treatments.
Table 1.
Summary of Brain Regions activated in the ictal and interictal state in fMRI or PET studies.
| REGION | ACTIVATION IN ICTAL STATE (spontaneous or triggered migraine attacks) | ACTIVATION IN INTERICTAL STATE | ACTIVATION IN ICTAL STAE (in response to experimental stimuli) | ACTIVATION IN INTERICTAL STATE (in response to experimental stimuli) | PUTATIVE ROLE IN MIGRAINE |
|---|---|---|---|---|---|
| BRAINSTEM AND CEREBELLUM | |||||
| PAG | fMRI photostimulation trigger attacks [18] | Descending modulation of nociceptive sensory transmission. | |||
| Red Nucleus | fMRI photostimulation-triggered attacks [18] | TBD | |||
| Substantia Nigra | fMRI photostimulation triggered attacks) [18] | TBD | |||
| Dorsal Pons | PET [9] PET GTN-triggered attacks [23] PET [17] PET [22] |
fMRI olfactory stimulation in attack vs. interictal phase [32] fMRI trigeminal stimulation with ammonia gas intranasally in attack vs. interictal phase [20] |
Asymmetry of brainstem dysfunction may play a role in lateralization of pain. | ||
| Locus coeruleus | PET [9] fMRI photostimulation-triggered attacks) [18] |
Modulation of cortical excitability. | |||
| Cuneiform Nucleus | fMRI Heat Stimuli (hypoactivation in migraineurs) [24] | Modulation of Sensory Transmission? | |||
| Cerebellum | PET [22] PET GTN-triggered attacks [23] |
TBD. | |||
| SUBCORTICAL REGIONS | |||||
| Thalamus | PET [17] | fMRI mechanical and thermal stimulation in migraineurs with allodynia [27] | Generation of extracephalic allodynia. | ||
| Basal Ganglia | PET GTN-triggered attacks [23] |
fMRI Thermal Stimuli in HF vs. LF migraine [38] | TBD | ||
| Hypothalamus | PET [22] | Generation of premonitory symptoms. | |||
| CORTICAL REGIONS | |||||
| Frontal Cortex | PET [22] PET [23] PET [17] |
fMRI trigeminal stimulation with ammonia gas intranasally in migraineurs vs. controls [37] | Modulation of pain perception through cognitive control. | ||
| Cingulate Cortex | PET(9)(Weiller et. al.). PET [22] PET GTN-triggered attacks [23] PET [17] |
fMRI trigeminal stimulation with ammonia gas intranasally in migraineurs vs. controls [43] fMRI thermal stimulation in migraineurs vs. healthy controls [42] |
Probably multiple mechanisms depending on exact location of ACC/PCC activation. | ||
| Temporal Cortex | PET [13] PET [17] |
PET migraineurs with permanent OHS vs. controls [40] | fMRI thermal painful stimulation in attack vs. interictal phase [30] fMRI olfactory stimulation in attack vs. interictal phase [32] |
fMRI thermal painful stimulation in migraineurs vs. controls [30] PET olfactory stimulation in migraineurs with OHS vs. controls [40] |
Processing of pain-related short-term memories; association with olfactory symptoms. |
| Parieto-occipital Junction | PET [13] | TBD. | |||
| Occipital Cortex | fMRI photostimulation-triggered attacks [18] | PET photostimulation [32] | PET olfactory stimulation in migraineurs with OHS vs. controls [40] PET luminous stimulation with and without concomitant trigeminal pain stimulation in migraineurs vs. controls [46] |
Generation of photophobia. | |
| Insula | PET GTN-triggered attacks [23] PET [17] |
fMRI olfactory stimulation in attack vs. interictal phase [32] | Migrainous pain processing. | ||
| Amygdala | fMRI olfactory stimulation in attack vs. interictal phase [32] | Processing of the negative affective component of migrainous pain. | |||
Abbreviations: OHS = olfactory hypersensitivity, TBD = to be determined; HF = high frequency; LF = low frequency.
6.1. Insights into the Neurobiology of Migraine
Imaging has allowed for new insights into the disease state and an unparalleled window into the brain and its activity that has already provided important insights into neural networks involved in migraine. The obvious ones relate to those around pain, photophobia or osmophobia. In addition alterations in modulatory systems have been reported. What is new is the ability to now study these systems in the context of networks without the need of specific stimuli: the study of resting state networks (RSN’s) may be useful to differentiate one state from another [67, 68]. Clearly an alteration in one region affects others and so even if the brunt of the disease affects thalamic relay stations or other specific areas, RSN’s may provide insights if not differentiate disease states or specific subtypes (e.g. genetic forms, episodic versus chronic migraine etc.). In addition, the evaluation of geno-phenotype interactions including imaging measures will also provide valuable insights into the neurobiology of the disease.
6.2. Imaging Drug Effects
We think that there area number of important issues, where imaging may have a role in improving our understanding of effects and complications of anti-migraine drugs:
The first is the still largely unexplored effect of medication overuse, especially with opioids in inducing a “drug resistance” in response to migraine preventives and the mechanism of medication overuse leading to migraine worsening and transformation. Little attention has been focused on imaging such changes in patients. A recent study used fMRI to evaluate brain changes in medication overuse headaches (MOH) [69]. In this report, patients with MOH were compared with healthy controls and reduced pain-related activity was reported in the primary somatosensory cortex, inferior parietal lobule, and supramarginalgyrus during medication withdrawal. These changes normalized 6 months after withdrawal suggesting that overuse-related abnormalities in pain processing are fully reversible, which is well in line with the clinical experience that a large proportion of patients improve after withdrawal of acute headache medications.
The second is to evaluate the effects of drugs on brain function. The issues relate to drug development (see below), but also understanding some clinical observations on drug effects in migraine patients. For example, triptans may be associated with allodynia upon administration [70]. A paper from a German group [71] evaluated the differences of sumatriptan vs. saline administration on subjective responses and also cortical processing using fMRI in a group of healthy volunteers. Normally non-painful soft brushing elicited ‘unpleasant’ effects during the sumatriptan condition correlating with activation of the anterior insula, lateral orbitofrontal, anterior cingulate cortex and medial thalamus (affective pain matrix) while activation in sensory systems (e.g., primary somatosensory cortex) was similar for the two conditions. The authors suggested that these changes may be a result of sensitization of nociceptors or low threshold unmyelinated afferents (see [72, 73]). In addition some drugs such as opioids may themselves result in significant functional and morphometric changes in the brain [74] that may in turn affect the disease or treatment responses.
The effects and role of dopamine in migraine has been the topic of a number of recent papers [75–78]. Evaluating the effects of dopaminergic modulation with approved drugs using imaging may provide additional insights to guide clinical trials (see drug development and biomarkers below).
6.3. Migraine transformation
As noted by others, migraine may be thought of as a chronic disease with episodic features [79]. The disease may increase in frequency (progression) or transform from an episodic to a chronic form (i.e. 15 or more days with headache per month). Few studies have evaluated functional and structural changes associated with disease progression or transformation. As discussed above, the study by Ferraro et al. provided some insight into potential mechanisms of medication overuse [69] and the above mentioned study by Adjeran et al. showed that repetitive trigeminal pain stimulation in migraineurs leads to an abnormal activation pattern in areas implicated in pain modulation [43], but other than that we are not aware of any recent functional imaging literature addressing the mechanisms of migraine progression and transformation. We believe that this should be an important future research focus.
6.4. Drug Development
Animal models have limited applicability if the model does only partly reflect key elements of the human condition. Just like in other neurological diseases affecting the CNS, this also applies to animal models of migraine, which are indeed largely models of trigeminal nociception or of cortical spreading depression and do probably not reflect the complexity of migraine. Since neural circuits may provide a ‘language of translation’ [80], it may now be possible to evaluate current and future models to determine if changes in neural circuits are equivalent across species. As such, this would provide a basis for improved drug development. In addition, the use of imaging in the early phases of drug trials can not only define drug targets (functionally), but may also reveal, which regions and mechanisms relate to potential side effects (e.g., medial thalamus in drowsiness [81, 82]). Furthermore, one may be able to reduce the necessary group sizes e.g. in early phase II trials when sensitive and objective imaging measures of drug effects are available. Evaluation of drugs at later stages of drug development may allow for defining how and whether drugs affect the disease course.
6.5. Migraine, Brain Maturation and Ageing
Little is known about migraine effects on the developing brain and vice versa. A number of research groups have begun to explore this in the context of understanding how brain maturation before and with puberty may differentially affect brain systems. Given that the disease affects individuals for a significant time, and for many starts in childhood or adolescence, potential insights into how neural circuits may change during this time may confer new insights into potential therapies or how current therapies may be optimized. In this context, it will also be important to better understand how migraine affects the ageing brain as some patients suffer well into their 60ies. Furthermore, there is also an obvious need to study the effects of sex hormones.
6.6. Understanding Brain Changes through Peri-ictal Changes – Opportunities to decrease the disease burden
Evaluation of the peri-ictal state in episodic migraine will offer opportunities to understand alterations in neural circuits that may then be targeted by preventive treatments including pharmacological, neuromodulatory and behavioral approaches. For those suffering from chronic migraine, the understanding of differences in brain systems between such patients and patients with episodic migraine should also provide insights on potential mechanisms that may be targeted therapeutically.
6.7. Biomarkers and Migraine
Perhaps the most exciting opportunity for a future contribution of imaging to migraine research and patient care is the possibility of providing a biomarker for the disease state [83–85]. Such efforts are ongoing in the pain field and may allow a specific diagnosis (including migraine state – where in the continuum progression or transformation) and the ability to objectively assess therapeutic efficacy.
7. Conclusions
Imaging has changed the way we understand migraine. It is in its relative infancy but as more researchers get involved in these exciting developments and the techniques become widely available, we expect to see more and more important contributions, which will change the way we understand and treat the disease.
Key points.
Migraine is a disease of the brain; repeated attacks result in functional and morphological changes
Imaging is providing new insights into brain function and structure that may provide objective markers of the disease
Imaging is adding insights into brain changes associated with progression; new insights into the processes of transformation from episodic to chronic migraine and drug resistance are now possible.
New approaches and targets for therapies can be evaluated through imaging in vivo
The efficacy of novel treatment approaches can be monitored in an objective way
Acknowledgments
The work was supported by grants from NIH (K24 NS064050 (NINDS) and R01 NS056195 (NINDS) to DB. We also gratefully acknowledge Michaela Andelova, MUDr. for help with Table 1.
Footnotes
Conflict of Interest: TS and DB have no conflicts to declare.
References (Annotated for those published between 2010 and 2011)
- 1.Cutrer FM, O’Donnell A, Sanchez del Rio M. Functional neuroimaging: enhanced understanding of migraine pathophysiology. Neurology. 2000;55(9 Suppl 2):S36–45. [PubMed] [Google Scholar]
- 2.Anttila V, Stefansson H, Kallela M, et al. Genome-wide association study of migraine implicates a common susceptibility variant on 8q22. 1. Nat Genet. 2010;42(10):869–73. doi: 10.1038/ng.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Villeneuve PJ, Szyszkowicz M, Stieb D, Bourque DA. Weather and emergency room visits for migraine headaches in Ottawa, Canada. Headache. 2006;46(1):64–72. doi: 10.1111/j.1526-4610.2006.00322.x. [DOI] [PubMed] [Google Scholar]
- 4.Vijayan N, Gould S, Watson C. Exposure to sun and precipitation of migraine. Headache. 1980;20(1):42–3. doi: 10.1111/j.1526-4610.1980.hed2001042.x. [DOI] [PubMed] [Google Scholar]
- 5.Robbins L. Precipitating factors in migraine: a retrospective review of 494 patients. Headache. 1994;34(4):214–6. doi: 10.1111/j.1526-4610.1994.hed3404214.x. [DOI] [PubMed] [Google Scholar]
- 6.Galiano L, Montiel I, Falip R, et al. [Stress as a precipitating factor in migraine] Rev Neurol. 1995;23(122):830–2. [PubMed] [Google Scholar]
- 7.MacGregor EA. Migraine headache in perimenopausal and menopausal women. Curr Pain Headache Rep. 2009;13(5):399–403. doi: 10.1007/s11916-009-0065-2. [DOI] [PubMed] [Google Scholar]
- 8.Dalkara T, Zervas NT, Moskowitz MA. From spreading depression to the trigeminovascular system. Neurol Sci. 2006;27 (Suppl 2):S86–90. doi: 10.1007/s10072-006-0577-z. [DOI] [PubMed] [Google Scholar]
- 9***.Zhang X, Levy D, Kainz V, et al. Activation of central trigeminovascular neurons by cortical spreading depression. Ann Neurol. 2011;69(5):855–65. doi: 10.1002/ana.22329. An important mechanistic paper. The authors demonstrate that cortical spreading depression (CSD) can activate central trigeminovascular neurons in the spinal trigeminal nucleus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ebersberger A, Schaible HG, Averbeck B, Richter F. Is there a correlation between spreading depression, neurogenic inflammation, and nociception that might cause migraine headache? Ann Neurol. 2001;49(1):7–13. [PubMed] [Google Scholar]
- 11.Dreier JP. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat Med. 2011;17(4):439–47. doi: 10.1038/nm.2333. [DOI] [PubMed] [Google Scholar]
- 12.Sprenger TSC, Valet M, Staehle K, Toelle TR, Foerschler, Zimmer C, Goadsby PJ. Abnormal Interictal Large-Scale Brain Network Connectivity in Episodic Migraine. Headache. 2010;50(71 suppl 1) [Google Scholar]
- 13.Weiller C, May A, Limmroth V, et al. Brain stem activation in spontaneous human migraine attacks. Nat Med. 1995;1(7):658–60. doi: 10.1038/nm0795-658. [DOI] [PubMed] [Google Scholar]
- 14.Welch KM, Nagesh V, Aurora SK, Gelman N. Periaqueductal gray matter dysfunction in migraine: cause or the burden of illness? Headache. 2001;41(7):629–37. doi: 10.1046/j.1526-4610.2001.041007629.x. [DOI] [PubMed] [Google Scholar]
- 15.Knight YE, Goadsby PJ. The periaqueductal grey matter modulates trigeminovascular input: a role in migraine? Neuroscience. 2001;106(4):793–800. doi: 10.1016/s0306-4522(01)00303-7. [DOI] [PubMed] [Google Scholar]
- 16.Kruit MC, Launer LJ, Overbosch J, et al. Iron accumulation in deep brain nuclei in migraine: a population-based magnetic resonance imaging study. Cephalalgia. 2009;29(3):351–9. doi: 10.1111/j.1468-2982.2008.01723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rocca MA, Ceccarelli A, Falini A, et al. Brain gray matter changes in migraine patients with T2-visible lesions: a 3-T MRI study. Stroke. 2006;37(7):1765–70. doi: 10.1161/01.STR.0000226589.00599.4d. [DOI] [PubMed] [Google Scholar]
- 18.Cao Y, Aurora SK, Nagesh V, et al. Functional MRI-BOLD of brainstem structures during visually triggered migraine. Neurology. 2002;59(1):72–8. doi: 10.1212/wnl.59.1.72. [DOI] [PubMed] [Google Scholar]
- 19.Dunckley P, Wise RG, Fairhurst M, et al. A comparison of visceral and somatic pain processing in the human brainstem using functional magnetic resonance imaging. J Neurosci. 2005;25(32):7333–41. doi: 10.1523/JNEUROSCI.1100-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Demarquay G, Lothe A, Royet JP, et al. Brainstem changes in 5-HT1A receptor availability during migraine attack. Cephalalgia. 2011;31(1):84–94. doi: 10.1177/0333102410385581. [DOI] [PubMed] [Google Scholar]
- 21.Afridi SK, Giffin NJ, Kaube H, et al. A positron emission tomographic study in spontaneous migraine. Arch Neurol. 2005;62(8):1270–5. doi: 10.1001/archneur.62.8.1270. [DOI] [PubMed] [Google Scholar]
- 22.Denuelle M, Fabre N, Payoux P, et al. Hypothalamic activation in spontaneous migraine attacks. Headache. 2007;47(10):1418–26. doi: 10.1111/j.1526-4610.2007.00776.x. [DOI] [PubMed] [Google Scholar]
- 23.Afridi SK, Matharu MS, Lee L, et al. A PET study exploring the laterality of brainstem activation in migraine using glyceryl trinitrate. Brain. 2005;128(Pt 4):932–9. doi: 10.1093/brain/awh416. [DOI] [PubMed] [Google Scholar]
- 24.Moulton EA, Burstein R, Tully S, et al. Interictal dysfunction of a brainstem descending modulatory center in migraine patients. PLoS One. 2008;3(11):e3799. doi: 10.1371/journal.pone.0003799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25**.Stankewitz A, Aderjan D, Eippert F, May A. Trigeminal nociceptive transmission in migraineurs predicts migraine attacks. J Neurosci. 2011;31(6):1937–43. doi: 10.1523/JNEUROSCI.4496-10.2011. A novel insight into potentilal migraine mecahnisms and process. The authors report on oscillating behaviors in the trigeminal ganglia that may be a key player in the generation of migraine headache. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burstein R, Cutrer MF, Yarnitsky D. The development of cutaneous allodynia during a migraine attack clinical evidence for the sequential recruitment of spinal and supraspinal nociceptive neurons in migraine. Brain. 2000;123 (Pt 8):1703–9. doi: 10.1093/brain/123.8.1703. [DOI] [PubMed] [Google Scholar]
- 27**.Burstein R, Jakubowski M, Garcia-Nicas E, et al. Thalamic sensitization transforms localized pain into widespread allodynia. Ann Neurol. 2010;68(1):81–91. doi: 10.1002/ana.21994. A translational paper in humans and animals that reports on how nociceptive information is transformed beyond the location of the migraine headahce (face, body and limbs) in an animla model of migraine and the human migraine attack providing a rational model for treatment evaluation in animals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28***.Noseda R, Kainz V, Jakubowski M, et al. A neural mechanism for exacerbation of headache by light. Nat Neurosci. 2010;13(2):239–45. doi: 10.1038/nn.2475. A major new avenue in migraine researchh on how light may exacerbate migraine through non-image-forming retinal pathway that modulates the activity of dura-sensitive thalamocortical neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maleki N, Becerra L, Upadhyay J, et al. Direct optic nerve pulvinar connections defined by diffusion MR tractography in humans: Implications for photophobia. Hum Brain Mapp. 2011 doi: 10.1002/hbm.21194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30*.Moulton EA, Becerra L, Maleki N, et al. Painful heat reveals hyperexcitability of the temporal pole in interictal and ictal migraine States. Cereb Cortex. 2011;21(2):435–48. doi: 10.1093/cercor/bhq109. The first study to clearly demonstrate that ictal and interictal pain produces significant functional changes in the temporal lobe that may provide a basis for some of the complex perceptive changes in migraine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bramanti P, Grugno R, Vitetta A, et al. Ictal and interictal hypoactivation of the occipital cortex in migraine with aura. A neuroimaging and electrophysiological study. Funct Neurol. 2005;20(4):169–71. [PubMed] [Google Scholar]
- 32**.Denuelle M, Boulloche N, Payoux P, et al. A PET study of photophobia during spontaneous migraine attacks. Neurology. 2011;76(3):213–8. doi: 10.1212/WNL.0b013e3182074a57. The first study to demonstrate that ictal photophobia is linked with a visual cortex hyperexcitability potentially implicating other mechanisms aside from the trigeminal system in photophobia. [DOI] [PubMed] [Google Scholar]
- 33.Eickhoff SB, Laird AR, Grefkes C, et al. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30(9):2907–26. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen WT, Lin YY, Fuh JL, et al. Sustained visual cortex hyperexcitability in migraine with persistent visual aura. Brain. 2011;134(Pt 8):2387–95. doi: 10.1093/brain/awr157. [DOI] [PubMed] [Google Scholar]
- 35.Lee JH, Durand R, Gradinaru V, et al. Global and local fMRI signals driven by neurons defined optogenetically by type and wiring. Nature. 2010;465(7299):788–92. doi: 10.1038/nature09108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sprenger T, Ruether KV, Boecker H, et al. Altered metabolism in frontal brain circuits in cluster headache. Cephalalgia. 2007;27(9):1033–42. doi: 10.1111/j.1468-2982.2007.01386.x. [DOI] [PubMed] [Google Scholar]
- 37*.Stankewitz A, May A. Increased limbic and brainstem activity during migraine attacks following olfactory stimulation. Neurology. 2011;77(5):476–82. doi: 10.1212/WNL.0b013e318227e4a8. Another example of sensitiization of neural circuits by sensory stimuli. [DOI] [PubMed] [Google Scholar]
- 38**.Maleki N, Becerra L, Nutile L, et al. Migraine attacks the Basal Ganglia. Mol Pain. 2011;7:71. doi: 10.1186/1744-8069-7-71. Detailed examination of alterations in basal ganglia in migraine as a consequnce of migriaine frequency implicating multisystem regional brain involvement in migraine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Borsook D, Upadhyay J, Chudler EH, Becerra L. A key role of the basal ganglia in pain and analgesia--insights gained through human functional imaging. Mol Pain. 2010;6:27. doi: 10.1186/1744-8069-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Demarquay G, Royet JP, Mick G, Ryvlin P. Olfactory hypersensitivity in migraineurs: a H(2)(15)O-PET study. Cephalalgia. 2008;28(10):1069–80. doi: 10.1111/j.1468-2982.2008.01672.x. [DOI] [PubMed] [Google Scholar]
- 41.Nunes JC, Zakon DB, Claudino LS, et al. Hippocampal sclerosis and ipsilateral headache among mesial temporal lobe epilepsy patients. Seizure. 2011;20(6):480–4. doi: 10.1016/j.seizure.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 42.Tessitore A, Russo A, Esposito F, et al. Interictal cortical reorganization in episodic migraine without aura: an event-related fMRI study during parametric trigeminal nociceptive stimulation. Neurol Sci. 2011;32 (Suppl 1):S165–7. doi: 10.1007/s10072-011-0537-0. [DOI] [PubMed] [Google Scholar]
- 43.Aderjan D, Stankewitz A, May A. Neuronal mechanisms during repetitive trigemino-nociceptive stimulation in migraine patients. Pain. 2010;151(1):97–103. doi: 10.1016/j.pain.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 44.Coppola G, Pierelli F, Schoenen J. Habituation and migraine. Neurobiol Learn Mem. 2009;92(2):249–59. doi: 10.1016/j.nlm.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 45.Coppola G, Pierelli F, Schoenen J. Is the cerebral cortex hyperexcitable or hyperresponsive in migraine? Cephalalgia. 2007;27(12):1427–39. doi: 10.1111/j.1468-2982.2007.01500.x. [DOI] [PubMed] [Google Scholar]
- 46**.Boulloche N, Denuelle M, Payoux P, et al. Photophobia in migraine: an interictal PET study of cortical hyperexcitability and its modulation by pain. J Neurol Neurosurg Psychiatry. 2010;81(9):978–84. doi: 10.1136/jnnp.2009.190223. Study showing that interical visual stimuli activate the occipital cortex in migraineurs, whereas the occipital cortex is not activated (as measured with PET) in control sugjects. Concomitant pain stimulation potentiated its activation in migraineurs and led to activation in controls also. [DOI] [PubMed] [Google Scholar]
- 47.Eck J, Richter M, Straube T, et al. Affective brain regions are activated during the processing of pain-related words in migraine patients. Pain. 2011;152(5):1104–13. doi: 10.1016/j.pain.2011.01.026. Increased activations in a number of cortical regions mediating the affective dimension of pain in migraineurs as compared to non-migraine subjects were shown. The study indicates that migraineurs possess an increased sensitivity to emotional inputs with strong recruitment of affective cortical areas. [DOI] [PubMed] [Google Scholar]
- 48.May A, Ashburner J, Buchel C, et al. Correlation between structural and functional changes in brain in an idiopathic headache syndrome. Nat Med. 1999;5(7):836–8. doi: 10.1038/10561. [DOI] [PubMed] [Google Scholar]
- 49.Apkarian AV, Sosa Y, Sonty S, et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004;24(46):10410–5. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.May A. New insights into headache: an update on functional and structural imaging findings. Nat Rev Neurol. 2009;5(4):199–209. doi: 10.1038/nrneurol.2009.28. [DOI] [PubMed] [Google Scholar]
- 51.Rodriguez-Raecke R, Niemeier A, Ihle K, et al. Brain gray matter decrease in chronic pain is the consequence and not the cause of pain. J Neurosci. 2009;29(44):13746–50. doi: 10.1523/JNEUROSCI.3687-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DaSilva AF, Granziera C, Tuch DS, et al. Interictal alterations of the trigeminal somatosensory pathway and periaqueductal gray matter in migraine. Neuroreport. 2007;18(4):301–5. doi: 10.1097/WNR.0b013e32801776bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schmitz N, Admiraal-Behloul F, Arkink EB, et al. Attack frequency and disease duration as indicators for brain damage in migraine. Headache. 2008;48(7):1044–55. doi: 10.1111/j.1526-4610.2008.01133.x. [DOI] [PubMed] [Google Scholar]
- 54.Rocca MA, Ceccarelli A, Falini A, et al. Diffusion tensor magnetic resonance imaging at 3. 0 tesla shows subtle cerebral grey matter abnormalities in patients with migraine. J Neurol Neurosurg Psychiatry. 2006;77(5):686–9. doi: 10.1136/jnnp.2005.080002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.DaSilva AF, Granziera C, Snyder J, Hadjikhani N. Thickening in the somatosensory cortex of patients with migraine. Neurology. 2007;69(21):1990–5. doi: 10.1212/01.wnl.0000291618.32247.2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hadjikhani N. Relevance of cortical thickness in migraine sufferers. Expert Rev Neurother. 2008;8(3):327–9. doi: 10.1586/14737175.8.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Valfre W, Rainero I, Bergui M, Pinessi L. Voxel-based morphometry reveals gray matter abnormalities in migraine. Headache. 2008;48(1):109–17. doi: 10.1111/j.1526-4610.2007.00723.x. [DOI] [PubMed] [Google Scholar]
- 58.Sarchielli P, Tarducci R, Presciutti O, et al. Functional 1H-MRS findings in migraine patients with and without aura assessed interictally. Neuroimage. 2005;24(4):1025–31. doi: 10.1016/j.neuroimage.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 59.Sandor PS, Dydak U, Schoenen J, et al. MR-spectroscopic imaging during visual stimulation in subgroups of migraine with aura. Cephalalgia. 2005;25(7):507–18. doi: 10.1111/j.1468-2982.2005.00900.x. [DOI] [PubMed] [Google Scholar]
- 60.Schulz UG, Blamire AM, Corkill RG, et al. Association between cortical metabolite levels and clinical manifestations of migrainous aura: an MR-spectroscopy study. Brain. 2007;130(Pt 12):3102–10. doi: 10.1093/brain/awm165. [DOI] [PubMed] [Google Scholar]
- 61.Schulz UG, Blamire AM, Davies P, et al. Normal cortical energy metabolism in migrainous stroke: A 31P-MR spectroscopy study. Stroke. 2009;40(12):3740–4. doi: 10.1161/STROKEAHA.109.558163. [DOI] [PubMed] [Google Scholar]
- 62.Boska MD, Welch KM, Barker PB, et al. Contrasts in cortical magnesium, phospholipid and energy metabolism between migraine syndromes. Neurology. 2002;58(8):1227–33. doi: 10.1212/wnl.58.8.1227. [DOI] [PubMed] [Google Scholar]
- 63.Prescot A, Becerra L, Pendse G, et al. Excitatory neurotransmitters in brain regions in interictal migraine patients. Mol Pain. 2009;5:34. doi: 10.1186/1744-8069-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barrett CF, van den Maagdenberg AM, Frants RR, Ferrari MD. Familial hemiplegic migraine. Adv Genet. 2008;63:57–83. doi: 10.1016/S0065-2660(08)01003-1. [DOI] [PubMed] [Google Scholar]
- 65.Dichgans M, Herzog J, Freilinger T, et al. 1H-MRS alterations in the cerebellum of patients with familial hemiplegic migraine type 1. Neurology. 2005;64(4):608–13. doi: 10.1212/01.WNL.0000151855.98318.50. [DOI] [PubMed] [Google Scholar]
- 66.Toldo I, Cecchin D, Sartori S, et al. Multimodal neuroimaging in a child with sporadic hemiplegic migraine: a contribution to understanding pathogenesis. Cephalalgia. 2011;31(6):751–6. doi: 10.1177/0333102410392068. [DOI] [PubMed] [Google Scholar]
- 67.van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol. 2010;20(8):519–34. doi: 10.1016/j.euroneuro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 68.Rosazza C, Minati L. Resting-state brain networks: literature review and clinical applications. Neurol Sci. 2011 doi: 10.1007/s10072-011-0636-y. [DOI] [PubMed] [Google Scholar]
- 69**.Ferraro S, Grazzi L, Mandelli ML, et al. Pain Processing in Medication Overuse Headache: A Functional Magnetic Resonance Imaging (fMRI) Study. Pain Med. 2011 doi: 10.1111/j.1526-4637.2011.01183.x. Authors report a reduction of experimental pain-induced activations in several brain areas including the primary somatosensory cortex in patients with medication overuse headache (MOH) during medication withdrawal as compared to controls. These abnormalities resolved 6 months after withdrawal indicating a reversible reorganization of pain-processing in MOH. [DOI] [PubMed] [Google Scholar]
- 70.Linde M, Elam M, Lundblad L, et al. Sumatriptan (5-HT1B/1D-agonist) causes a transient allodynia. Cephalalgia. 2004;24(12):1057–66. doi: 10.1111/j.1468-2982.2004.00782.x. [DOI] [PubMed] [Google Scholar]
- 71.Kramer HH, Lundblad L, Birklein F, et al. Activation of the cortical pain network by soft tactile stimulation after injection of sumatriptan. Pain. 2007;133(1–3):72–8. doi: 10.1016/j.pain.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 72.Bjornsdotter M, Loken L, Olausson H, et al. Somatotopic organization of gentle touch processing in the posterior insular cortex. J Neurosci. 2009;29(29):9314–20. doi: 10.1523/JNEUROSCI.0400-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Loken LS, Wessberg J, Morrison I, et al. Coding of pleasant touch by unmyelinated afferents in humans. Nat Neurosci. 2009;12(5):547–8. doi: 10.1038/nn.2312. [DOI] [PubMed] [Google Scholar]
- 74.Upadhyay J, Maleki N, Potter J, et al. Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain. 2010;133(Pt 7):2098–114. doi: 10.1093/brain/awq138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dusitanond P, Young WB. Neuroleptics and migraine. Cent Nerv Syst Agents Med Chem. 2009;9(1):63–70. doi: 10.2174/187152409787601888. [DOI] [PubMed] [Google Scholar]
- 76.Garcia-Martin E, Martinez C, Serrador M, et al. Dopamine receptor 3 (DRD3) polymorphism and risk for migraine. Eur J Neurol. 2010;17(9):1220–3. doi: 10.1111/j.1468-1331.2010.02988.x. [DOI] [PubMed] [Google Scholar]
- 77.Charbit AR, Akerman S, Goadsby PJ. Dopamine: what’s new in migraine? Curr Opin Neurol. 2010;23(3):275–81. doi: 10.1097/WCO.0b013e3283378d5c. [DOI] [PubMed] [Google Scholar]
- 78.Rollene NL, Khan Z, Schroeder DR, et al. Migraines and ovarian hyperstimulation syndrome: a dopamine connection. Fertil Steril. 2011;95(1):417–9. doi: 10.1016/j.fertnstert.2010.08.041. [DOI] [PubMed] [Google Scholar]
- 79.Bigal ME, Lipton RB. Clinical course in migraine: conceptualizing migraine transformation. Neurology. 2008;71(11):848–55. doi: 10.1212/01.wnl.0000325565.63526.d2. [DOI] [PubMed] [Google Scholar]
- 80.Borsook D, Becerra L, Hargreaves R. A role for fMRI in optimizing CNS drug development. Nat Rev Drug Discov. 2006;5(5):411–24. doi: 10.1038/nrd2027. [DOI] [PubMed] [Google Scholar]
- 81.Becerra L, Harter K, Gonzalez RG, Borsook D. Functional magnetic resonance imaging measures of the effects of morphine on central nervous system circuitry in opioid-naive healthy volunteers. Anesth Analg. 2006;103(1):208–16. doi: 10.1213/01.ane.0000221457.71536.e0. table of contents. [DOI] [PubMed] [Google Scholar]
- 82.Dang-Vu TT, Schabus M, Desseilles M, et al. Functional neuroimaging insights into the physiology of human sleep. Sleep. 2010;33(12):1589–603. doi: 10.1093/sleep/33.12.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Borsook D, Becerra L, Hargreaves R. Biomarkers for chronic pain and analgesia. Part 1: the need, reality, challenges, and solutions. Discov Med. 2011;11(58):197–207. [PubMed] [Google Scholar]
- 84.Borsook D, Becerra L. CNS animal fMRI in pain and analgesia. Neurosci Biobehav Rev. 2011;35(5):1125–43. doi: 10.1016/j.neubiorev.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Borsook D, Hargreaves R, Becerra L. Can Functional Magnetic Resonance Imaging Improve Success Rates in CNS Drug Discovery? Expert Opin Drug Discov. 2011;6(6):597–617. doi: 10.1517/17460441.2011.584529. [DOI] [PMC free article] [PubMed] [Google Scholar]


