Abstract
Using functional optical imaging in vivo, we demonstrate that the γ mushroom body (MB) neurons of Drosophila melanogaster respond with axonal calcium influx when odors or electric shock stimuli are presented to the fly. Pairing of odor and electric shock stimuli in a single training trial or multiple, massed training trials failed to modify the odor-evoked calcium signal when flies were tested at several different times after training. In contrast, animals that received multiple but spaced odor–shock pairings exhibited a robust increase in calcium influx into the MB axons when tested between 18 and 48 h after training. This time window for the γ neuron memory trace is displaced relative to the modifications that occur between 9 and 24 h after training in the α branch of the α/β MB neurons. The α/β and the γ neuron long-term memory traces were both blocked by expressing a repressor of the transcription factor cAMP response element-binding protein or a calcium/calmodulin-dependent kinase II hairpin RNA. These results demonstrate that behavioral long-term olfactory memory is encoded as modifications of calcium influx into distinct MB neurons during overlapping but different windows of time after training.
Introduction
Drosophila exposed to odors paired with electric shock learn this association and display their memory as a selective avoidance of the odor paired with the negative reinforcer. The strength and duration of the memory is dependent on the training protocol. For instance, a single cycle of conditioning generates robust short-term memory that decays over the period of ∼1 d (Tully et al., 1994; Beck et al., 2000; Pascual and Préat, 2001). Multiple cycle conditioning with no rest between each cycle (massed conditioning) generates robust initial memory that decays over a few days, whereas introducing a rest between cycles (spaced conditioning) generates memory that persists for 4–7 d and is dependent on normal protein synthesis and cAMP response element-binding protein (Creb) activity at the time of conditioning (Tully et al., 1994; Perazzona et al., 2004; Yu et al., 2006). The different temporal forms of memory generated by different conditioning protocols are mechanistically distinct and must therefore generate distinct cellular memory traces that underlie the conditioned behavior.
Functional optical imaging has provided important insights into the neuroanatomy of cellular memory traces in the Drosophila brain that involve an increase or decrease of calcium influx into specific neurons, or increased synaptic transmission, in response to the conditioned odor after training (Yu et al., 2004, 2005, 2006; Liu and Davis, 2009). The memory traces observed are strong correlates of behavioral memory: they are generated only by conditioning protocols that produce behavioral performance gains, and insults that disrupt behavioral memory also disrupt the memory traces. These traces include a short-lived memory trace in the antennal lobe (Yu et al., 2004), a middle-term and branch-specific memory trace in the dorsal paired medial (DPM) neurons (Yu et al., 2005), and a long-term memory trace that forms in the α branch of the α/β mushroom body (MB) neurons (Yu et al., 2006).
Many studies have identified the MBs as crucial for olfactory associative learning and memory in insects (for review, see Davis, 2005), yet the α/β MB neurons comprise only one of three distinct classes of MB intrinsic neurons (α/β, α′/β′, and γ) whose axons extend to form five lobes of neuropil (α, β, α′, β′, and γ). The functional diversity of the different MB neurons has long been recognized based on differential expression of gene and protein markers (Yang et al., 1995; Crittenden et al., 1998) and behavioral transgenic rescue experiments that target expression of rescuing transgenes in specific neuronal subsets (Zars et al., 2000; McGuire et al., 2003). Here, we describe the properties of a recently discovered memory trace that forms in the γ neurons. This memory trace is similar to the previously described α/β MB memory trace in that it is detected as increased calcium influx into the MB axons and forms only after multiple, spaced conditioning trials. However, it is distinct with respect to its onset and persistence, forming by 18 h after conditioning and being detectable up to 48 h. Both memory traces mechanistically use Drosophila Creb and calcium-calmodulin dependent protein kinase II (CaMKII).
Materials and Methods
Transgenic animals and fly culture.
Flies were cultured on standard medium at room temperature and transferred overnight to a 25°C incubator before training. Flies carrying Uas transgenes containing the Drosophila Creb2-b repressor coding region [Uas–dCreb2-b (Yu et al., 2006)], CaMKII hairpin RNA [Uas–CaMKIIhpn (Ashraf et al., 2006)], or G-CaMP [Uas–G-CaMP (Wang et al., 2003; Colbran and Brown, 2004)] were used along with the 1471–Gal4 (expression pattern described by Isabel et al., 2004) and the c739–Gal4 driver (c739–Gal4 flies were from K. Kaiser, Division of Molecular Genetics, University of Glasgow, Scotland). The w(CS10) flies (CS flies carrying the w1118 mutation) served as a wild-type control in certain experiments.
Behavioral assays.
Except for the data presented in Figure 5G–I, olfactory learning was assayed using a modified olfactory classical conditioning procedure that allows the performance gains occurring from the pairing of an odor with electric shock to be measured relative to naive flies (Yu et al., 2006). A mixed population of male and female flies was exposed to two odors in succession, one odor [the conditioned stimulus (CS+)] paired with electric shock pulses (unconditioned stimulus), followed by a counter odor (the CS−) without electric shock during the training phase. After training, the flies were incubated at 25°C for the indicated times (3, 9, 18, 24, 48, or 96 h) before testing. For testing in the T-maze, separate groups of animals were tested during each time point with each group only being tested once. During testing, the flies were presented with both odors in a T-maze, and their avoidance of the CS+ was quantified. A corresponding naive control for each trained group underwent all of the manipulations as the trained flies except that they were not administered odor or electric shock. The performance indices (PIs) were then calculated for both the naive and trained group, and the ΔPI was obtained by subtracting the naive score from the score of the corresponding trained group. In all cases, only experiments in which the naive flies exhibited naive performance scores that were not significantly different from zero (t test) were used. This modification allowed us to make meaningful comparisons among groups because performance indices were normalized to naive performance (Yu et al., 2006). In addition, the assay allowed us to obtain an index of the performance gains attributable to conditioning with each specific odor as the CS+, so that these gains could be compared with the results obtained after optically imaging individual flies. Data for Figure 5G were obtained using the more traditional assay (Tully and Quinn, 1985) in which two groups of flies are trained to two different odors as the CS+ [3-octanol (Oct) and benzaldehyde (Ben), for instance], and their performance scores were subsequently averaged. For assaying long-term memory, multiple spaced training trials were performed, which are required to induce a longer-lasting memory (Tully et al., 1994). We used 5× spaced training to generate long-term memory, which persists for >4 d. Spaced training was performed with an interval of 15 min between each training cycle, whereas massed training was performed with no intertrial interval. Data from behavioral and imaging experiments were subjected to nonparametric statistical tests. The Wilcoxon test was used for evaluating significance from zero. A Kruskal–Wallis H statistic was computed when comparing different groups, followed by pairwise comparisons using Mann–Whitney.
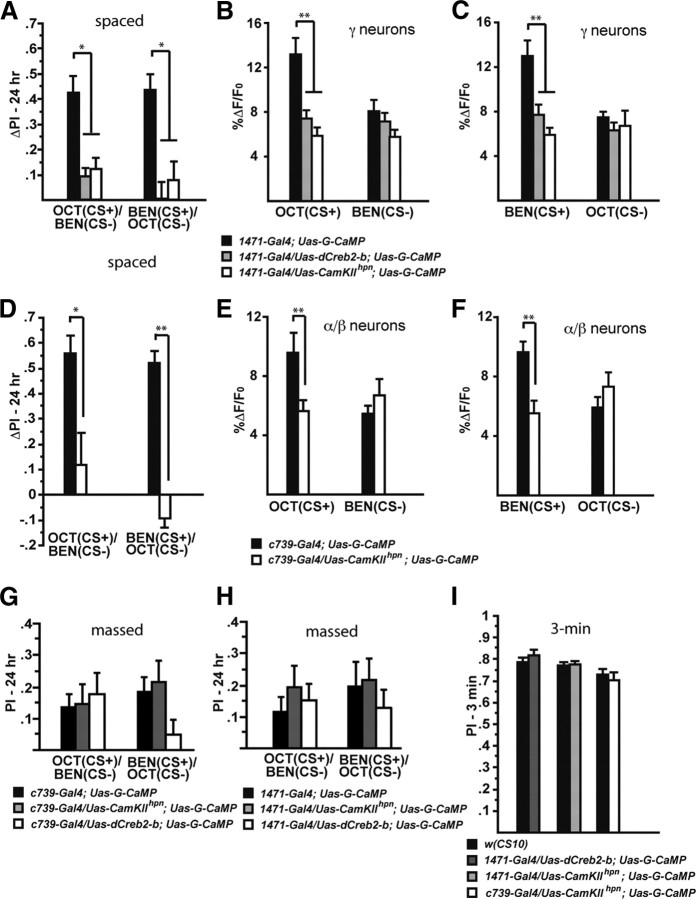
Figure 5.
The long-term memory trace in the axons of γ MB neurons is disrupted by expression of a Creb repressor and a CaMKII hairpin RNA. A, Expression of dCreb2-b or CaMKII hairpin RNA in neurons defined by 1471–Gal4 impairs long-term behavioral memory after spaced forward conditioning. Flies carrying Uas–dCreb2-b, Uas–G-CaMP, and 1471–Gal4 showed significantly reduced performance scores for both Oct (CS+) and Ben (CS+) compared with control flies carrying only Uas–G-CaMP and 1471–Gal4 (Mann–Whitney pairwise comparisons, p ≤ 0.0209). Similarly, flies carrying Uas–CaMKIIhpn, Uas–G-CaMP, and 1471–Gal4 showed significantly reduced performance scores for both odorants as the CS+ compared with the control flies (Mann–Whitney pairwise comparisons, p ≤ 0.0047; n = 12 for all groups). Error bars are the SEM. *p < 0.05. B, There was a significant difference in the measured calcium trace (%ΔF/F0) in response to Oct (CS+) 24 h after spaced forward conditioning between flies carrying only the expressed reporter (1471–Gal4; Uas–G-CaMP) and flies that carried the expressed reporter along with Uas–dCreb2-b or Uas–CaMKIIhpn (Kruskal–Wallis statistic of 16.401, p = 0.0003; Mann–Whitney pairwise comparisons, p ≤ 0.0057). There was no significant difference in response to the CS− among the genotypes (Kruskal–Wallis statistic of 2.856, p = 0.2398; Mann–Whitney pairwise comparisons, p ≥ 0.1391; n = 12–20 for all groups). Error bars are the SEM. **p < 0.01. C, There was a significant difference in the measured calcium trace (%ΔF/F0) in response to the CS+ (Ben) 24 h after spaced forward conditioning between flies carrying only the expressed reporter (1471–Gal4; Uas–G-CaMP) and flies that carried the expressed reporter along with Uas–dCreb2-b or Uas–CaMKIIhpn (Kruskal–Wallis statistic of 15.252, p = 0.0005; Mann–Whitney pairwise comparisons, p ≤ 0.0082). There was no significant difference in response to the CS− among the genotypes (Kruskal–Wallis statistic of 2.279, p = 0.3199; Mann–Whitney pairwise comparisons, p ≥ 0.1872; n = 10–12 for all groups). Error bars are the SEM. **p < 0.01. D, Expression of CaMKII hairpin RNA in neurons defined by c739–Gal4 results in the abolishment of long-term behavioral memory after spaced forward conditioning. Flies carrying Uas–CaMKIIhpn, Uas–G-CaMP, and c739–Gal4 showed significantly reduced performance scores for both Oct (CS+) and Ben (CS+) compared with control flies that carry only Uas–G-CaMP and c739–Gal4 (Mann–Whitney pairwise comparisons, p ≤ 0.0105; n = 6–12 for all groups). Error bars are the SEM. *p < 0.05, **p < 0.01. E, There was a significant difference in the measured calcium trace (%ΔF/F0) in response to the CS+ (Oct) 24 h after spaced forward conditioning between flies carrying only the expressed reporter (c739–Gal4; Uas–G-CaMP) and flies that carried the expressed reporter along with Uas–CaMKIIhpn (Mann–Whitney test, p = 0.0003). There was no significant difference in response to the CS− among the genotypes (Mann–Whitney test, p = 0.1914; n = 10–12 for all groups). Error bars are the SEM. **p < 0.01. F, There was a significant difference in the measured calcium trace (%ΔF/F0) in response to the CS+ (Ben) 24 h after spaced forward conditioning between flies carrying only the expressed reporter (c739–Gal4; Uas–G-CaMP) and flies that carried the expressed reporter along with Uas–CaMKIIhpn (Mann–Whitney test, p = 0.021). There was no significant difference in response to the CS− among the genotypes (Mann–Whitney test, p = 0.7416; n = 9–10 for all groups). Error bars are the SEM. **p < 0.01. G, Expression of dCreb2-b or CaMKII hairpin RNA in neurons defined by c739–Gal4 did not significantly impair 24 h behavioral memory after massed forward conditioning (Kruskal–Wallis statistic of 0.389 for Oct as the CS+ and 3.922 for Ben as the CS+; both <5.99). Flies carrying Uas–CaMKIIhpn, Uas–G-CaMP, and c739–Gal4 showed performance scores that were indistinguishable from control flies carrying only Uas–G-CaMP and c739–Gal4 for both odorants as the CS+ (Mann–Whitney pairwise comparisons, p ≥ 0.6033). Similarly, flies carrying Uas–dCreb2-b, Uas–G-CaMP, and c739–Gal4 showed performance scores for both Oct (CS+) and Ben (CS+) that were not significantly different from control flies (Mann–Whitney pairwise comparisons, p ≥ 0.0764; n = 12–18 for all groups). Error bars are the SEM. H, Expression of dCreb2-b or CaMKII hairpin RNA in neurons defined by 1471–Gal4 did not significantly impair 24 h behavioral memory after massed forward conditioning (Kruskal–Wallis statistic of 0.902 for Oct as the CS+ and 1.257 for Ben as the CS+; both <5.99). Flies carrying Uas–CaMKIIhpn, Uas–G-CaMP, and 1471–Gal4 showed performance scores that were indistinguishable from control flies carrying only Uas–G-CaMP and 1471–Gal4 for both odorants as the CS+ (Mann–Whitney pairwise comparisons, p ≥ 0.3865). Similarly, flies carrying Uas–dCreb2-b, Uas–G-CaMP, and 1471–Gal4 showed performance scores for both CS+ odors that were not significantly different from control flies (Mann–Whitney pairwise comparisons, p ≥ 0.2727; n = 12–18 for all groups). Error bars are the SEM. I, Three minute performance scores after 1× forward conditioning of flies expressing the dCreb2-b repressor or CaMKII hairpin RNA in combination with G-CaMP using 1471–Gal4 and c739–Gal4 were not significantly different from wild-type flies [w(CS10)] (Mann–Whitney pairwise comparisons, p ≥ 0.3367; n = 6–12 for all groups). Error bars are the SEM.
Functional cellular imaging.
We performed functional imaging procedures according to previously described protocols (Yu et al., 2004, 2005, 2006). Flies containing both a Gal4 driver and Uas–G-CaMP with or without an additional transgene of interest (Uas–dCreb2-b or Uas–CaMKIIhpn) were separated before behavioral testing from the remainder of the trained flies. The bulk of the trained flies were tested for behavioral memory as described above. Those removed for functional imaging were mounted in pipette tips, and their exposed heads were secured to the tip opening with silicon cement. To expose the brain, a small region of cuticle was removed from the top of the head capsule, and the exposed area was covered with a piece of plastic wrap. Confocal imaging was performed by mounting the flies under the 20× objective of a Leica TCS confocal microscope and imaged with a 488 nm excitation line. The emitted light was collected from 520 ± 15 nm. Two criteria were used to ensure that the same volume of the γ MB lobe was imaged between flies. First, the complete mediolateral extent of the γ lobe needed to be visible in the baseline image for functional imaging to continue. If not, the fly was discarded and another was prepared. Second, this volume was scanned in the z-plane to find the most intense focal plane, which we believe occurred when the focus was centered on the midpoint of the γ lobe in the dorsoventral axis. Odorants were spread on a small piece of filter paper inside a syringe barrel that was placed in line with the pressurized air flowing at a rate of 100 ml/min. Concentrated odorants were diluted in mineral oil. Odorant delivery was accomplished using a three-way Teflon valve under the control of a programmable timer, such that fresh air could be delivered to the animals for a determined period with an instantaneous switch to odor-laced air without altering the overall flow rate. Electric shock pulses were applied to the fly's abdomen. A total of 12 pulses of electric shock at 90 V were delivered with each shock lasting 1.25 s. Conditioned flies were collected after training and tested at 3, 9, 18, 24, and 48 h after training and tested for calcium influx into the MB axons when the CS+ and CS− odors were delivered at 5 min intervals.
Data analysis.
Images were acquired at approximately five frames per second at a resolution of 256 × 256 pixels, followed by image data analysis as described previously (Yu et al., 2004, 2005, 2006). Regions of interest were circumscribed, and a pseudocolor image of the %ΔF/F0 ratio was produced. The value F0 was calculated for each pixel within the region of interest as the fluorescence before odor application as averaged over five successive frames. The value ΔF was calculated for each pixel within the region of interest as the difference between the maximum average intensity during the 3 s odor application for five successive frames and F0.
Results
The γ MB neurons respond to odor and shock stimuli with axonal calcium influx
Previously, we showed that the axons of the α/β MB neurons responded with calcium influx after odor delivery or exposure to 90 V shock pulses to the fly (Yu et al., 2006). A memory trace forms in these neurons between 3 and 9 h after spaced conditioning and exhibits axon branch specificity, forming only in the α axon branch of the α/β MB neurons. In addition, we showed that the memory trace is dependent on normal protein synthesis at the time of conditioning and the activity of the transcription factor Creb. In this study, we first tested whether γ MB axons would respond similarly to odors delivered to the antennae and electric shock pulses delivered to the fly's abdomen. Flies carrying the calcium reporter transgene Uas–G-CaMP expressed by 1471–Gal4 were prepared for in vivo functional imaging of the brain by mounting them stably under a laser-scanning confocal microscope to detect basal fluorescence and the resulting change in fluorescence after the application of odor or electric shock. The Gal4 driver 1471–Gal4 is an enhancer detector element that drives expression specifically in the γ MB neurons (Isabel et al., 2004; Aso et al., 2009). The cell bodies of the γ MB neurons reside in the dorsal and posterior cellular cortex. In contrast to the branched α/β and α′/β′ neurons, each γ neuron possesses an unbranched axon (but see Discussion) that projects to the anterior face of the brain as a bundled peduncle with the axons of other MB neurons. The axons of the γ MB neurons turn medially in the anterior brain to form the γ lobe, which is oriented mediolaterally in the head capsule and horizontally relative to the long axis of the fly. We collected imaging data across time at the depth of the Drosophila brain at which the γ neurons are visible to visualize calcium influx into the axons of the γ MB neurons (Fig. 1A,B).
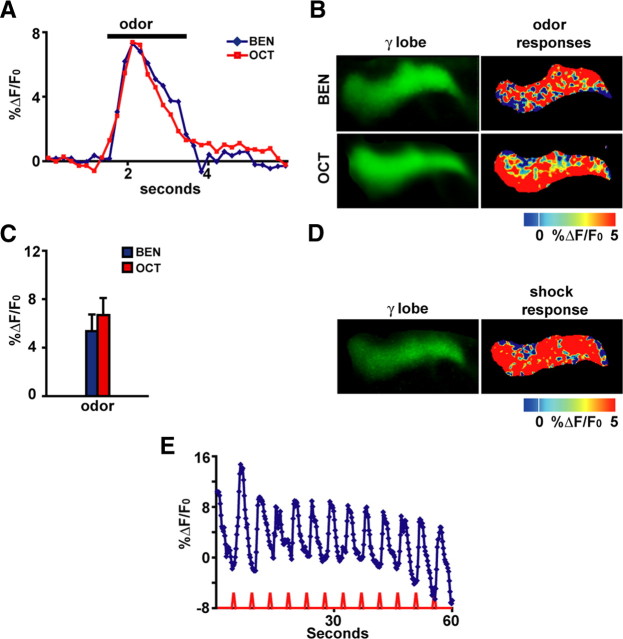
Figure 1.
The γ mushroom body neurons respond with calcium influx into their axons when odors or electric shock stimuli are delivered to Drosophila. A, Representative time course for the fluorescence response to the odor Oct or Ben in the axons of γ MB neurons. The response was calculated as the percentage increase in fluorescence over baseline (%ΔF/F0) as a function of time. For subsequent bar graphs, the %ΔF/F0 was calculated as the percentage difference between the maximum average intensity over five successive imaging frames during the 3 s odor application and the average intensity over five successive frames just before odor application. Representative images are shown (n = 6–7). B, Images of the basal fluorescence of Uas–G-CaMP expressed with 1471–Gal4 in the axons of γ MB neurons (left column). The change in fluorescence (%ΔF/F0), calculated as the percentage change in fluorescence (ΔF) relative to baseline (F0) that occurs after exposure to Ben or Oct, is illustrated as a false color image to the right of each panel showing the basal fluorescence. Each pseudocolor image shown here and in other figures is a single-frame snapshot of the response during stimulation. Because the spatial response pattern fluctuates between frames during the stimulation on a pixel-by-pixel basis, the group data (C) better represent the average peak response across the flies that were imaged. Representative images are shown (n = 6–7). C, The amplitude of the response to odor from group data for the axons of γ MB neurons (n = 6–7) is illustrated. The ratio ΔF/F0 was typically between 6 and 7% and proved to be statistically significant (p ≤ 0.0277, Wilcoxon's matched-pairs tests) compared with zero for both odors. Error bars are the SEM. D, Images of the basal fluorescence of Uas–G-CaMP expressed with 1471–Gal4 in the axon branches of γ MB neurons (left). The response (%ΔF/F0) of the axon branches to 90 V electric shock pulses is illustrated as a false color image in the right. Representative images are shown (n = 6–7). E, Calcium influx into the axons of γ MB neurons that occurs with 90 V, 1.25 s electric shock pulses every 5 s. The traces represent the average %ΔF/F0 across the region of interest in the axons of γ MB neurons. An obvious calcium response was observed, with each shock pulse riding on top of a decaying background attributable to bleaching over a 60 s scanning period.
Stimulation of the flies with the odors Oct or Ben elicited significant, reproducible and transient increases in calcium content in axons of the γ MB neurons (Fig. 1A–C). Figure 1A illustrates the transient response that is concurrent with the odor presentation. Figure 1B illustrates a false color representation of the percentage change in fluorescence that occurs after odor stimulation in the axons of the γ MB neurons. The group data show that the change in fluorescence is measurable and reproducible with amplitudes typically between 6 and 7% and statistically significant (Wilcoxon's matched-pairs test) for both odors compared with zero (Fig. 1C). Similarly, application to the fly's abdomen of electric shock pulses of the same intensity, duration, and frequency as those used for behavioral conditioning (Roman and Davis, 2001) produced significant calcium increases in the axons of γ MB neurons (Fig. 1D,E). These data therefore show that γ MB neurons respond with calcium increases as detected by fluorescence changes in G-CaMP to odor or electric shock stimuli delivered to the fly.
The γ MB neurons form a long-term memory trace
Flies carrying one copy of the calcium reporter G-CaMP plus a copy of 1471–Gal4, a Gal4 driver that promotes expression in the γ MB neurons, were trained to associate an odor with electric shock using three experimental protocols (Fig. 2A): (1) a single cycle conditioning protocol (1× forward), (2) a massed conditioning protocol of five consecutive single conditioning cycles (5× massed forward), and (3) a spaced conditioning protocol consisting of five single conditioning cycles with a 15 min intertrial interval (5× spaced forward). Each conditioning protocol was likewise performed in a backward manner for control groups, wherein the CS+ odor was presented after the onset of the electric shock stimuli. Backward conditioning protocols generally fail to produce the robust excitatory behavioral conditioning that occurs with forward conditioning (Rescorla, 1988). In addition, each of the two odorant used, Oct and Ben, were used as the CS+ in one set of experiments and as the CS− in the other. Each group of flies that received olfactory conditioning was partnered with a corresponding group of naive flies that were passed through the same manipulations except that they did not receive the associative conditioning of odor with electric shock stimuli. The performance of the flies attributable to associative conditioning with the CS+ odor was then computed as a ΔPI (see Materials and Methods) by subtracting the performance of each naive group from its corresponding conditioned group (Yu et al., 2006).
Figure 2.
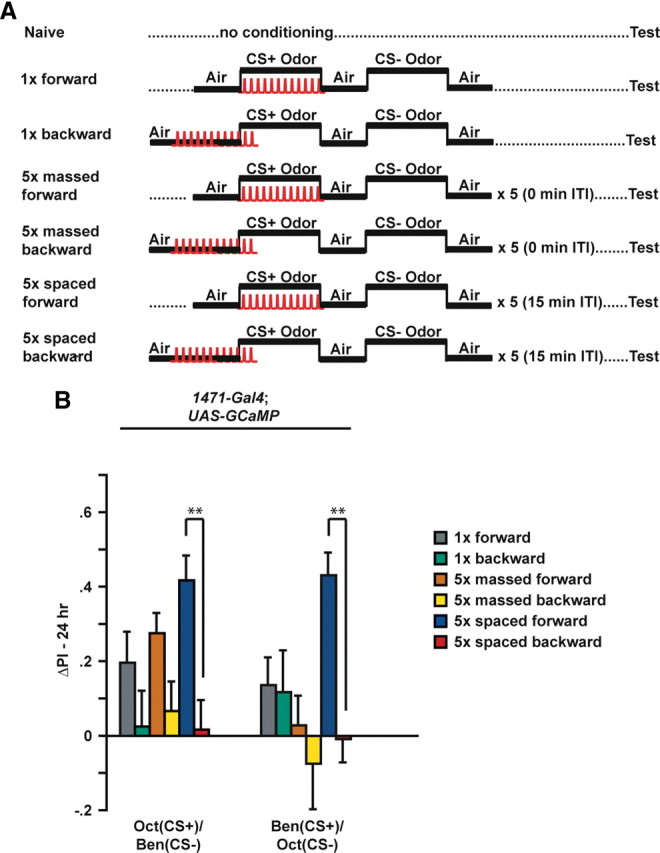
Conditioning protocols: spaced conditioning produces robust 24 h long-term memory. A, Diagram illustrating the conditioning protocols that were used for these experiments. Flies carried one copy of 1471–Gal4 and one copy of Uas–G-CaMP. Naive flies were carried through the same procedures as the conditioned animals except that they were not exposed to odor and electric shock. For 1× training, flies received forward or backward conditioning (45 s offset) with 1 min exposure to the CS+ odor with 12 electric shock pulses (90 V), followed by 1 min exposure to the CS− odor without electric shock. The CS− odor was applied after a 30 s exposure to fresh air. For massed and spaced conditioning, the 1× training protocol was performed a total of five times with either a 0 or 15 min intertrial interval (ITI), respectively. Flies were transferred to a T-maze at 24 h after conditioning and tested for behavioral memory. Some flies were separated before behavioral testing and analyzed for cellular memory by functional imaging. B, The performance gains of flies carrying 1471–Gal4 and Uas–G-CaMP that were trained to associate Oct or Ben as the CS+ are shown. The ΔPI was computed by subtracting the scores of each naive group from the corresponding conditioned group. In all cases, the scores of naive animals were not statistically significant (Wilcoxon's matched-pairs tests) from zero (p ≥ 0.4953, n = 10–12). For Oct (CS+) versus Ben (CS−) with 1471–Gal4; Uas–G-CaMP flies, 5× spaced forward training had a significant effect when compared with the ΔPI scores for all the backward trained groups (Kruskal–Wallis statistic of 18.041, p = 0.0029; Mann–Whitney pairwise comparisons, p ≤ 0.0001; n = 10–18 for all groups). None of the ΔPI scores of the backward trained groups were statistically significant (Wilcoxon's matched-pairs tests) from zero (p ≥ 0.1258; n = 10–12 for all groups). For Ben (CS+) versus Oct (CS−), 5× spaced forward training had a significant effect when compared with the ΔPI scores for all of the backward trained groups (Kruskal–Wallis statistic of 28.105, p < 0.0001; Mann–Whitney pairwise comparisons, p ≤ 0.0001), as well as 1× forward and 5× massed forward groups (p ≤ 0.0009, Mann–Whitney pairwise comparisons, n = 10–18 for all groups). None of the ΔPI scores of the backward trained groups were statistically significant (Wilcoxon's matched-pairs tests) from zero (p ≥ 0.1466; n = 10–12 for all groups). In all behavioral experiments, the ΔPIs were subjected to nonparametric tests, i.e., a Mann–Whitney U test for comparing two independent samples, Wilcoxon's matched-pairs test to test single performance indices against zero, and Kruskal–Wallis test for multiple comparisons with genotype as the main effect. Error bars are the SEM. **p < 0.01.
Flies conditioned using the spaced forward conditioning protocol exhibited a significantly higher level of associative memory when tested at 24 h after conditioning than flies conditioned using any of the backward conditioning protocols (Fig. 2B). This was observed for flies trained with either Oct or Ben as the CS+ odor. Flies conditioned using the 1× forward conditioning protocol and Oct as the CS+ exhibited associative memory indistinguishable from flies conditioned using the 5× massed forward conditioning protocol. Previous experiments have shown a significant difference in 24 h performance of single- and mass-trained flies if 10 conditioning cycles are used (Tully et al., 1994). Unexpectedly, 1471–Gal4; Uas–G-CaMP flies receiving 5× massed forward conditioning using Ben as the CS+ and Oct as the CS− failed to exhibit memory at 24 h. Whether this odor-specific effect is attributable to genetic background or the expression of G-CaMP in the neurons defined by 1471–Gal4 is unknown, although it is inconsequential to our results identifying a late-phase, long-term memory trace in the γ neurons after 5× spaced forward conditioning. In all cases, the corresponding backward conditioned group failed to show any significant performance gains. These data are consistent with previous studies that established these conditioning protocols and indicate that spaced forward conditioning induces robust 24 h memory (Tully et al., 1994), although it should be noted that our experiments used 5× conditioning protocols in contrast to the 10× protocols performed in previous experiments (Tully et al., 1994).
Following the various conditioning protocols (Fig. 2A), flies from each group were removed before behavioral testing at 24 h and functionally imaged for calcium responses to odor. We observed that all of the groups save one exhibited CS+ odor responses in the γ MB neurons that were not quantitatively different from those observed typically for naive flies (ΔF/F that is in the 6–7% range as shown in Fig. 1C) and that the responses to the CS+ odor were indistinguishable from the CS− odor (Fig. 3A,C). This held true for the 1× (forward and backward), 5× massed (forward and backward), and 5× spaced backward groups. For the 5× spaced forward group, we detected odor-elicited calcium response nearly twice as large as any of the other groups (Fig. 3A–C), and this increase was specific to the CS+ odor compared with the CS− odors (Fig. 3A,C,D). Therefore, spaced forward conditioning induces cellular changes that lead to an increased calcium influx into axons of the γ MB neurons in response to the CS+ odor when measured at 24 h after conditioning.
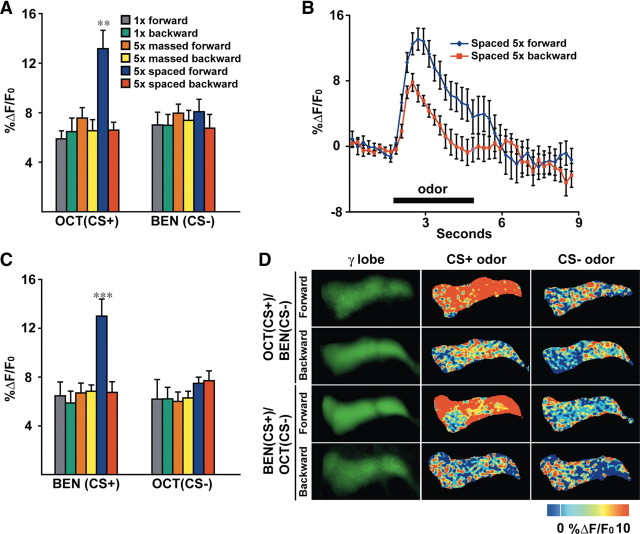
Figure 3.
A long-term memory trace forms in the axons of the γ MB neurons after spaced forward conditioning. A, Response to the CS+ and CS− odors 24 h after conditioning. A significant increase in %ΔF/F0 was detected in the axons of γ MB neurons using Oct as the test stimulus after 5× spaced forward conditioning with Oct as the CS+ and Ben as the CS− compared with any other group (Kruskal–Wallis statistic of 25.561, p = 0.0009; Mann–Whitney pairwise comparisons, p ≤ 0.011). The response magnitudes for all other groups (1× forward and backward, 5× massed forward and backward, and 5× spaced backward) were similar to each other and naive animals presented with odor (see Fig. 1C) (Kruskal–Wallis statistic of 2.557, p = 0.7679; Mann–Whitney pairwise comparisons, p ≥ 0.1069). No significant differences in the %ΔF/F0 response to the CS− (Ben) were detected among any of the conditioned groups (Kruskal–Wallis statistic of 3.11, p = 0.6831; Mann–Whitney pairwise comparisons, p ≥ 0.1926; n = 12–20 for all groups). Error bars are the SEM. **p < 0.01. B, Group time course for the response to the CS+ of Oct in the axons of γ MB neurons after spaced forward conditioning compared with spaced backward conditioning. The graph was made using the data from the same flies used for the bar graph in A. Error bars are the SEM. C, Calcium responses in the axons of γ MB neurons in animals conditioned with Ben as the CS+ and Oct as the CS−. A significant increase in %ΔF/F0 was detected in the axons of γ MB neurons using Ben as the test stimulus after 5× spaced forward conditioning with Ben as the CS+ and Oct as the CS− compared with any other group (Kruskal–Wallis statistic of 18.02, p = 0.0029; Mann–Whitney pairwise comparisons, p ≤ 0.0045). The response magnitudes for all other groups (1× forward and backward, 5× massed forward and backward, and 5× spaced backward) were similar to each other and naive animals presented with odor (see Fig. 1C) (Kruskal–Wallis statistic of 3.799, p = 0.5787; Mann–Whitney pairwise comparisons, p ≥ 0.3284). No significant differences in the %ΔF/F0 response to the CS− (Oct) were detected among any of the conditioned groups (Kruskal–Wallis statistic of 7.599, p = 0.1798; Mann–Whitney pairwise comparisons, p ≥ 0.0914), except for the response between the 5× massed forward and 5× spaced forward (Mann–Whitney test, p = 0.0346). n = 8–11 for all groups. Error bars are the SEM. ***p < 0.0001. D, Images of the basal fluorescence of Uas–G-CaMP expressed with 1471–Gal4 in the axons of γ MB neurons (left column). The change in fluorescence (%ΔF/F0) that occurs after exposure to the CS+ or CS− odor is illustrated as a false color image (middle and right columns, respectively). A robust increase in calcium influx was detected in the γ axons after CS+ odor stimulation 24 h after 5× spaced forward conditioning for both odor combinations, whereas the calcium responses to the CS+ after spaced backward conditioning and to the CS− for both spaced forward and spaced backward conditioning was similar to the odor responses of naive animals.
The γ MB cellular memory trace forms between 9 and 18 h and persists until 48 h after conditioning
To delimit the time of γ MB trace formation, we assayed flies carrying both 1471–Gal4 and Uas–G-CaMP at various times after spaced forward conditioning. At 3 h after training, behavioral memory is robust for animals trained with either Oct or Ben as the CS+. The scores show a gradual decay for Oct as the CS+ with flies tested at 3 and 9 h showing significantly higher performance compared with flies tested at 24 h and beyond (Fig. 4A). For Ben as the CS+, flies tested at 3 h showed significantly higher scores compared with all of the other groups with the performance dropping abruptly by 9 h and remaining relatively constant until 48 h. This abrupt drop in performance is probably not attributable to a difference in how flies remember the two different odors (Oct vs Ben), because other groups of flies trained with Ben fail to show the abrupt performance drop (Fig. 4D). Performance was lowest at 96 h with the scores from flies trained with Oct as the CS+ being significantly lower compared with 3, 9, and 18 h, whereas for flies trained with Ben as the CS+, the 96 h scores were significantly lower than scores at 3, 9, and 24 h. In contrast, the cellular memory trace formed in response to the CS+ exhibits the opposite trend, such that at 3 and 9 h after training the response in the γ MB neurons was at the naive level for both Ben and Oct as the CS+ (Fig. 4B,C). An enhanced calcium response was observed starting at 18 h, and this persisted until at least 48 h after conditioning. The response dropped back to naive levels for flies tested at 96 h after conditioning. Calcium responses to the CS− were indistinguishable from naive responses for both odors tested.
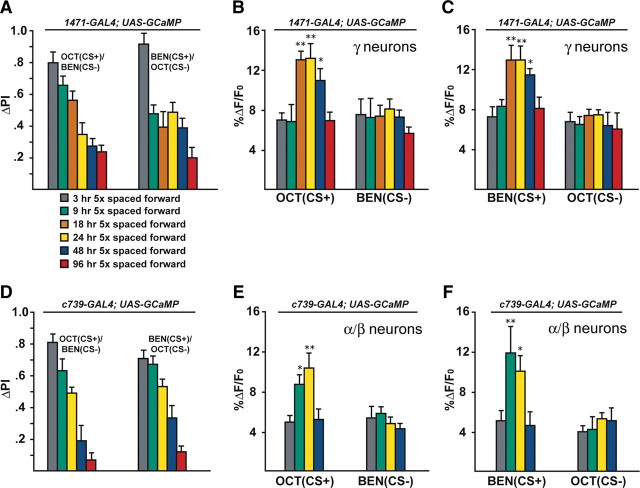
Figure 4.
The memory trace in the axons of γ MB neurons forms between 9 and 18 h and persists until 48 h after spaced forward conditioning. A, Time course for long-term memory of flies carrying 1471–Gal4 and Uas–G-CaMP. Memory after spaced forward conditioning decreases with time. Flies were trained using the 5× spaced forward conditioning protocol with Oct (CS+) versus Ben (CS−) and Ben (CS+) versus Oct (CS−) and tested 3, 9, 18, 24, 48, and 96 h later for behavioral memory (ΔPI). For Oct (CS+), the performance of the flies gradually decreased with time (Kruskal–Wallis statistic of 38.215, p < 0.0001), and flies tested at 3 and 9 h performed significantly better than flies tested at 24, 48, and 96 h (Mann–Whitney pairwise comparisons, p ≤ 0.0047). Flies tested at 18 h demonstrated significantly higher performance scores compared with flies tested at 48 and 96 h (Mann–Whitney pairwise comparisons, p ≤ 0.0179). For Ben (CS+), flies tested at 3 h performed significantly better than all other time points (Kruskal–Wallis statistic of 26.776, p < 0.0001; Mann–Whitney pairwise comparisons, p ≤ 0.0024). The PI scores for flies tested at the other time points were not significantly different from each other (Mann–Whitney pairwise comparisons, p ≥ 0.0865), except that the PI score for flies tested at 96 h was significantly different from 9 and 24 h (Mann–Whitney pairwise comparisons, p ≤ 0.0084; n = 10–12 for all groups). Error bars are the SEM. B, Time course for the cellular memory trace in the axons of the γ MB neurons with Oct (CS+). There was a significant increase in the measured calcium transients (%ΔF/F0) in response to the CS+ (Oct) at 18, 24, and 48 h after spaced forward conditioning compared with 3, 9, and 96 h (Kruskal–Wallis statistic of 22.504, p = 0.0004; Mann–Whitney pairwise comparisons, p ≤ 0.0233). There were no significant differences among the 18, 24, and 48 time points (Kruskal–Wallis statistic of 0.234, p = 0.8894; Mann–Whitney pairwise comparisons, p ≥ 0.0741) or the 3, 9, and 96 h time points (Kruskal–Wallis statistic of 0.611, p = 0.7368; Mann–Whitney pairwise comparisons, p ≥ 0.5078). There were no significant differences in response to the CS− (Ben) among any of the time points (Kruskal–Wallis statistic of 2.159, p = 0.8268; Mann–Whitney pairwise comparisons, p ≥ 0.0715; n = 8–20 for all groups). Error bars are the SEM. *p < 0.05, **p < 0.01. C, Time course for the cellular memory trace in the axons of the γ MB neurons with Ben (CS+). There was a significant increase in the measured calcium transients (%ΔF/F0) in response to the CS+ (Ben) in the axons of the γ MB neurons at 18, 24, and 48 h after spaced forward conditioning compared with 3, 9, and 96 h (Kruskal–Wallis statistic of 23.247, p = 0.0003; Mann–Whitney pairwise comparisons, p ≤ 0.0318) except for the response at 48 and 96 h (Mann–Whitney test, p = 0.0723). There were no significant differences among the18, 24, and 48 h time points (Kruskal–Wallis statistic of 1.394, p = 0.498; Mann–Whitney pairwise comparisons, p ≥ 0.3181) or the 3, 9, and 96 h time points (Kruskal–Wallis statistic of 0.479, p = 0.7870; Mann–Whitney pairwise comparisons, p ≥ 0.4328). There were no significant differences in response to the CS− (Ben) among any time point (Kruskal–Wallis statistic of 4.431, p = 0.4891; Mann–Whitney pairwise comparisons, p ≥ 0.1546; n = 8–15 for all groups). Error bars are the SEM. *p < 0.05, **p < 0.01. D, Time course of long-term memory for flies carrying c739–Gal4 and Uas–G-CaMP. Memory after spaced forward conditioning decreases with time. Flies were trained using the 5× spaced forward conditioning protocol with Oct (CS+) versus Ben (CS−) and Ben (CS+) versus Oct (CS−) and tested at different time points for behavioral memory (ΔPI). For Oct (CS+), the performance of the flies gradually decreased with time (Kruskal–Wallis statistic of 37.828, p < 0.0001), and all pairwise comparisons were significantly different from one another (Mann–Whitney test, p ≤ 0.0479) except for the 3 vs 9 h and 48 vs 96 h PI values (Mann–Whitney test, p ≥ 0.0647). Similarly, for Ben (CS+), the performance of the flies gradually decreased with time (Kruskal–Wallis statistic of 34.018, p < 0.0001), and all pairwise comparisons were significantly different from one another (Mann–Whitney test, p ≤ 0.0433) except for the performance at 9 h, which was not significantly different from 3 and 24 h (Mann–Whitney test, p ≥ 0.0865; n = 10–12 for all groups). Error bars are the SEM. E, Time course for the cellular memory trace in the α branch of the α/β MB neurons with Oct (CS+). There was significant increment in the measured calcium transients (%ΔF/F0) in response to Oct (CS+) at 9 and 24 h after spaced conditioning compared with 3 and 48 h (Kruskal–Wallis statistic of 16.312, p = 0.0010; Mann–Whitney pairwise comparisons, p ≤ 0.0138). There was no significant difference between the 9 and 24 h time points (Mann–Whitney pairwise comparisons, p = 0.3823) or the 3 and 48 h time points (Mann–Whitney pairwise comparisons, p = 0.902). In addition, there were no significant differences in response to the CS− among the different time points (Kruskal–Wallis statistic of 2.595, p = 0.4583; Mann–Whitney pairwise comparisons, p ≥ 0.0743). Data for 3, 9, and 24 h time points are reproduced from Yu et al. (2006). n = 9–12 for all groups. Error bars are the SEM. *p < 0.05, **p < 0.01. F, Time course for the cellular memory trace in the α branch of the α/β MB neurons with Ben (CS+). There was significant increase in the measured calcium transients (%ΔF/F0) in response to the CS+ (Ben) at 9 and 24 h after spaced conditioning training compared with 3 and 48 h (Kruskal–Wallis statistic of 14.378, p = 0.0024; Mann–Whitney pairwise comparisons, p ≤ 0.0128). There was no significant difference between the 9 and 24 h time point (Mann–Whitney test, p = 0.4469) or between the 3 and 48 h time points (Mann–Whitney test, p = 0.7913). In addition, there were no significant differences in response to the CS− among the different time points (Kruskal–Wallis statistic of 2.365, p = 0.5002; Mann–Whitney pairwise comparisons, p ≥ 0.1604). Data for the 24 h time point are reproduced from Yu et al. (2006). n = 7–12 for all groups. Error bars are the SEM. *p < 0.05, **p < 0.01.
The time window for detection of the γ MB neuron memory trace after spaced conditioning was different from that reported previously for the α branch-specific memory trace, which appears by 9 h after training with Oct as the CS+ (Yu et al., 2006). This suggested that two distinct long-term memory traces are formed in different sets of MB neurons after spaced forward conditioning. To confirm and extend the data indicating their distinction, we examined the α/β MB neuron memory trace using Oct and Ben as the CS+ and added additional time points for assay after training (18, 48, and 96 h). Flies carrying c739–Gal4, an enhancer detector element that drives expression from Uas transgenes specifically in the α/β MB neurons using anonymous enhancers in the vicinity of the hr39 gene (our unpublished data), were used to examine the effects on the α/β MB trace. After 5× spaced forward conditioning, the performance of flies carrying c739–Gal4 and Uas–G-CaMP showed a gradual decrease with time for both odors tested (Fig. 4D). For Oct (CS+), the performance of the flies gradually decreased across time, and flies tested at 3 h performed significantly better than flies tested at 24, 48, or 96 h. Flies tested at all other time points (9, 24, 48, and 96 h) demonstrated significantly different performance scores compared with one another except for the scores of flies tested at 48 versus 96 h. We observed the same gradual decrement in memory for flies trained using Ben as the CS+ with the flies tested at 3 h performing significantly better than flies tested at 24, 48, and 96 h. Flies tested at 9 h were not significantly different from flies tested at 24 h, but the performance score was significantly higher than flies tested at 48 and 96 h. When the trained c739–Gal4/Uas–G-CaMP flies were assayed using functional optical imaging, we discovered that the α/β MB neuron memory trace forms by 9 h after training and persists until at least 24 h but that it decays to baseline levels by 48 h after training (Fig. 4E,F). This was observed for both odors tested, and there was no significant difference in response to the CS− odor among the different time points. Thus, the combined data indicate that the γ MB neurons form a memory trace that is detected between 18 and 48 h after spaced forward conditioning, whereas the α/β MB neurons form a memory trace that is detected between 9 and 24 h after spaced forward conditioning. The distinct time windows for the existence of the two long-term memory traces suggest that there are mechanistic differences underlying their formation and their persistence or that there is a differential delay in the transit of the necessary information to spur the formation of the traces in the two sets of neurons.
Expression of a dCreb repressor molecule or a CaMKII hairpin RNA blocks the cellular memory trace that forms in the γ MB neurons
The transcription factor Creb has been identified as being important for the formation of long-term memory (Yin et al., 1994; Kandel, 2001; Perazzona et al., 2004; Yu et al., 2006). We showed previously that expression of a Uas–dCreb2-b transgene, which encodes a repressor isoform of dCreb2, in the α/β MB neurons blocks the formation of long-term behavioral memory (Yu et al., 2006). Behavioral experiments revealed that control flies carrying the dCreb2-b transgene without the Gal4 driver have 24 h memory after spaced forward conditioning comparable with wild-type flies. In contrast, flies additionally carrying the c739–Gal4 driver element are impaired in long-term memory but have wild-type levels of 3 min memory, confirming that expression of the repressor in the α/β neurons specifically impairs long-term memory measured at 24 h. Functional imaging experiments revealed that the expression of the dCreb2-b repressor using the c739–Gal4 driver abolished the branch-specific long-term memory trace that is normally observed with either Oct or Ben as the CS+ odor (Yu et al., 2006). A second molecule that has well described roles for mammalian and fly synaptic plasticity and memory is CaMKII (Lisman et al., 2002; Griffith et al., 2003; Elgersma et al., 2004; Griffith, 2004). Changes in CaMKII levels (Ashraf et al., 2006) and phosphorylation state (Mehren and Griffith, 2004) have been implicated in Drosophila synaptic plasticity.
To examine the requirement for these molecules in the MB neurons for behavioral plasticity and the memory traces that form in these neurons, we expressed the dCreb2-b repressor using the γ driver 1471–Gal4. We also expressed a CaMKII hairpin RNA that has been shown previously (Ashraf et al., 2006) to attenuate long-term behavior when expressed with a CaMKII–Gal4 driver, which promotes expression broadly throughout the brain. The expression of dCreb2-b and CaMKII hairpin RNA in neurons defined by the 1471–Gal4 driver severely attenuated or abolished long-term behavioral memory after spaced forward conditioning for both CS+ odors tested compared with control flies carrying only the Gal4 driver and Uas–G-CaMP (Fig. 5A). Functional imaging revealed a significant difference in the measured calcium trace for both CS+ odors 24 h after spaced forward conditioning between flies carrying only the expressed reporter (1471–Gal4; Uas–G-CaMP) and flies that carried the expressed reporter along with Uas–dCreb2-b or Uas–CaMKIIhpn (Fig. 5B,C). The response to the CS− was similar for the control flies and the dCreb2-b- and CaMKIIhpn-expressing flies. Thus, the expression of a dominant negative for the transcription factor Creb or a transgene that inhibits CaMKII expression in the γ MB neurons blocks the formation of the γ MB neuron memory trace in parallel with long-term behavioral memory as measured 24 h later.
When we expressed the CaMKII hairpin RNA in the α/β MB neurons using c739–Gal4, we observed a similar abolishment of behavioral long-term memory after spaced forward conditioning for both CS+ odors compared with control flies (Fig. 5D). In addition, there was a significant difference in the measured α branch memory trace in response to the CS+ odors 24 h after spaced forward conditioning between flies carrying only the expressed reporter (c739–Gal4; Uas–G-CaMP) and flies that carried the expressed reporter along with Uas–CaMKIIhpn (Fig. 5E,F). There was no significant difference in response to the CS− among the genotypes (Fig. 5E,F).
Performance at 24 h after spaced conditioning in Drosophila is attributable to at least two distinct components, a protein-synthesis-dependent component and a protein-synthesis-independent component (Tully et al., 1994), the latter also being known as anesthesia-resistant memory because of its resistance to cold anesthesia presented immediately after training. Massed conditioning generates the protein-synthesis-independent component of long-term memory but not the protein-synthesis-dependent component. To determine whether Creb and CaMKII are functionally important for protein-synthesis-independent long-term memory and further relate the γ MB neuron memory trace to the distinct long-term memory components, we used mass conditioning protocols to train flies expressing either the dCreb2-b repressor or the CaMKII hairpin RNA interference (RNAi) in the α/β MB or γ MB neurons. Flies expressing either transgene in either type of MB neuron exhibited 24 h memory that was not significantly different from controls (Fig. 5G,H). This indicates that protein-synthesis-independent memory does not require these biochemical functions, at least to the level perturbed by these disruptive reagents. Furthermore, these data provide additional evidence that the γ MB neuron memory trace is relevant only to protein-synthesis-dependent long-term memory, because the dCreb2-b repressor and the CaMKII hairpin disrupt behavioral performance after spaced conditioning in parallel with the memory trace.
The various genotypes were also tested for 3 min performance after single cycle conditioning to determine whether the transgenes impaired short-term memory. Flies expressing the dCreb2-b repressor or the CaMKII hairpin RNAi in combination with G-CaMP using the 1471–Gal4 or c739–Gal4 drivers exhibited short-term memory performance that was not significantly different from control w(CS10) flies (Fig. 5I). Thus, expression of the dCreb2-b repressor or the CaMKII hairpin RNAi in either the γ or α/β MB neurons specifically impairs long-term, protein-synthesis-dependent behavioral memory.
Discussion
Two different long-term memory traces form in different types of MB neurons
MBs play a central role in Drosophila learning and memory, and there are significant data indicating that the different classes of MB neurons are biochemically, developmentally, morphologically, and functionally distinct (Yang et al., 1995; Crittenden et al., 1998; Zars et al., 2000; McGuire et al., 2001, 2003; Akalal et al., 2006; Krashes et al., 2007). Here, we identify a cellular memory trace that forms in the γ MB neurons after spaced forward olfactory conditioning. This memory trace, registered as increased calcium influx to the CS+ odor after training, was not generated by other conditioning protocols, including spaced backward conditioning, single-cycle conditioning in both forward and backward configurations, and massed conditioning in both forward and backward configurations. Furthermore, we demonstrate that this long-term memory trace requires the normal activity of the transcription factor Creb and CaMKII in the γ MB neurons, like long-term behavioral memory. The parallel requirements of the γ MB cellular memory trace and long-term behavioral memory for spaced forward conditioning, normal Creb activity, and intact CaMKII signaling provide strong evidence that the γ MB memory trace is intimately tied to long-term behavioral memory.
The γ MB memory trace is similar in some respects to a recently reported long-term memory trace that forms in only one axonal branch of the α/β MB neurons. Like the γ MB neuron memory trace, the memory trace that forms in α/β MB neurons requires spaced forward conditioning, normal Creb activity (Yu et al., 2006), and, as shown here, intact signaling through CaMKII. The finding that Creb and CaMKII signaling in either the α/β or γ MB neurons is necessary for long-term memory shows that there is some overlap in the molecular mechanisms that underlie the formation of these long-term memory traces in these subsets of MB neurons. However, the memory traces are distinct in that the α branch-specific MB trace forms by 9 h and persists until 24 h after 5× spaced forward conditioning, whereas the γ memory trace forms by 18 h and persists up to 48 h after conditioning. Thus, the memory traces occur in distinct windows of time and are therefore described as a long-term memory trace (α/β) and a late-phase, long-term memory trace (γ).
The discovery of a role for the γ MB neurons in late-phase, long-term memory is surprising given the previous evidence ascribing a role for these neurons in short-term memory and the emerging evidence attributing long-term memory primarily to the α/β MB neurons. The short-term memory deficit of rutabaga (rut) mutants is partially rescued by the expression of a wild-type rut transgene with Gal4 drivers that express in the γ MB neurons (Zars et al., 2000; Akalal et al., 2006), indicating a requirement for rut gene function in these neurons for short-term memory. The neuroanatomical mapping of short-term memory formed from courtship conditioning has been attributed primarily to the γ MB neurons (Joiner and Griffith, 1999). Long-term memory is disrupted in α lobes absent (ala) flies that are missing the vertical lobes of the MBs and consequently the α axons of the α/β MB neurons (Pascual and Préat, 2001). A long-term memory trace also forms after 5× spaced forward conditioning in the α axons of the α/β MB neurons (Yu et al., 2006), consistent with a role of the α/β neurons in long-term memory processes. Finally, previous studies using Uas–Shibirets1 (Uas–Shits) combined with c739–Gal4 or 1471–Gal4 to functionally inactivate synaptic transmission using 30°C as the restrictive temperature before testing after spaced forward conditioning provided evidence that neurotransmission from the α/β neurons, but not γ neurons, is required for the full expression of 24 h behavioral memory (Isabel et al., 2004). However, we attempted to replicate these results using Uas–Shits1 and 1471–Gal4 but found that this combination produced locomotor and postural defects at restrictive temperatures of ≥32°C that were not observed with the α/β driver c739–Gal4 (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). This is presumably attributable to inactivating synaptic transmission from neurons that express 1471–Gal4 and that are required for normal posture and locomotor activity. Thus, the previous experiments performed to test the role for γ MB neuron synaptic transmission in long-term behavior may have failed to sufficiently inactivate these neurons to uncover their role in long-term behavioral memory. Furthermore, recent experiments have revealed that the function of orb2 is required in the γ MB neurons for long-term courtship conditioning (Keleman et al., 2007). The combined observations along with our new results suggest that both the α branch of the α/β MB neurons and the γ MB neurons are involved in long-term memory formation.
What is the significance of having two distinct, olfactory long-term memory traces occurring in distinct time windows that form in two different sets of MB neurons? The most attractive general explanation is that behavioral memory, which is robust initially after training and decays continuously over time, is not attributable to a single memory trace that decays over time in one type of neuron. Rather, behavioral memory is underlain by multiple, olfactory memory traces that form in many different types of neurons within the olfactory nervous system with each memory trace forming from network interactions and through molecular mechanisms intrinsic to each type of neuron. This would provide for differential kinetics in the formation and decay of individual cellular memory traces. The idea that different temporal forms of memory are stored in different sets of neurons is not new. The traditional view of mammalian long-term memory involves hippocampal-dependent consolidation of labile short-term memory into stable, longer-lasting memory stored in the neocortex (Squire and Alvarez, 1995; McGaugh, 2000; Wittenberg and Tsien, 2002; Squire et al., 2004; Wang et al., 2006). Testing whether the Drosophila long-term memory traces form in serial or parallel requires additional tools, including but not limited to, the generation of new gene promoter systems that are independent of the UAS–Gal4 system to allow for expression of a reporter for functional imaging in some neuron types while blocking synaptic transmission from other neurons types by expressing Uas–Shibire.
Creb and CaMKII
The results presented here along with those of Yu et al. (2006) reveal that both Creb and CaMKII activities are required in the α/β and the γ MB neurons for normal long-term behavioral memory. The specific role for Creb in long-term memory is not surprising, although the combined results indicate that Creb activity is required in at least these two types of MB neurons for normal long-term behavioral memory. Studies in Aplysia, Drosophila, and rodents indicate that Creb is important for the conversion of short-term to long-term memory (for review, see Frank and Greenberg, 1994; Silva et al., 1998; Mayford and Kandel, 1999). In Aplysia, Creb inhibitors can selectively block long-term facilitation but not short-term facilitation (Dash et al., 1990). In Drosophila, a repressor isoform of Drosophila Creb (dCreb2-b) has been shown to reduce the long-term performance of trained flies without affecting 3 min memory, as well as to abolish the long-term memory calcium trace normally observed in the α branch of MB α/β neurons (Yu et al., 2006). Reducing the level of Creb activity in mice also impairs certain forms of long-term memory (Bourtchuladze et al., 1994; Balschun et al., 2003). Data presented here indicate that disrupting Creb in the γ MB neurons impairs long-term behavioral memory but spares short-term memory.
It is surprising that CaMKII has a specific role in Drosophila long-term olfactory memory generated by classical conditioning. We demonstrate here that disruption of CaMKII signaling using a CaMKII hairpin RNA in either the α/β or the γ MB neurons specifically impairs long-term behavioral memory. A previous report (Ashraf et al., 2006) also demonstrated that broad expression of the CaMKII hairpin RNA in Drosophila using a CaMKII–Gal4 driver impairs long-term behavioral memory. However, early memory after courtship conditioning requires CaMKII activity in the antennal lobes (Joiner and Griffith, 1999), and this is enhanced after expression of a constitutively activated form of CaMKII (Mehren and Griffith, 2004). Furthermore, CaMKII has a well accepted role in the induction phase of LTP (for review, see Lisman et al., 2002; Colbran and Brown, 2004; Griffith, 2004). Additional studies are required to dissect the differential roles of CaMKII in various temporal forms of memory.
Using a combination of behavioral assays, functional imaging, and transgenic disruption experiments, our combined results indicate that there are parallel and partially overlapping temporal memory traces that form in distinct sets of MB neurons and may represent protein-synthesis-dependent, long-term behavioral memory and late-phase, long-term behavioral memory after spaced olfactory conditioning in Drosophila. These differentially timed long-term memory traces in the MB neurons along with the immediate and short-lived antennal lobe projection neuron memory trace (Yu et al., 2004) and the medium-term memory trace in the DPM neurons (Yu et al., 2005) lend support to the concept that behavioral memory is the sum of multiple cellular memory traces that form in discrete subpopulations of neurons.
Footnotes
This work was supported by National Institute of Neurological Disorders and Stroke Grants NS19904 and NS52352. D.-B.G.A., D.Y., and R.L.D. conceived and designed the experiments. D.-B.G.A. performed the behavioral experiments, and D.Y. performed the imaging experiments. They also analyzed their data. D.-B.G.A., D.Y., and R.L.D. wrote the paper.
The authors have declared that no competing interests exist.
References
- Akalal DB, Wilson CF, Zong L, Tanaka NK, Ito K, Davis RL. Roles for Drosophila mushroom body neurons in olfactory learning and memory. Learn Mem. 2006;13:659–668. doi: 10.1101/lm.221206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf SI, McLoon AL, Sclarsic SM, Kunes S. Synaptic protein synthesis associated with memory is regulated by the RISC pathway in Drosophila. Cell. 2006;124:191–205. doi: 10.1016/j.cell.2005.12.017. [DOI] [PubMed] [Google Scholar]
- Aso Y, Grübel K, Busch S, Friedrich AB, Siwanowicz I, Tanimoto H. The mushroom body of adult Drosophila characterized by Gal4 drivers. J Neurogenet. 2009;23:156–172. doi: 10.1080/01677060802471718. [DOI] [PubMed] [Google Scholar]
- Balschun D, Wolfer DP, Gass P, Mantamadiotis T, Welzl H, Schütz G, Frey JU, Lipp HP. Does cAMP response element-binding protein have a pivotal role in hippocampal synaptic plasticity and hippocampus-dependent memory? J Neurosci. 2003;23:6304–6314. doi: 10.1523/JNEUROSCI.23-15-06304.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck CD, Schroeder B, Davis RL. Learning performance of Drosophila after repeated conditioning trials with discrete stimuli. J Neurosci. 2000;20:2944–2953. doi: 10.1523/JNEUROSCI.20-08-02944.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- Colbran RJ, Brown AM. Calcium/calmodulin-dependent protein kinase II and synaptic plasticity. Curr Opin Neurobiol. 2004;14:318–327. doi: 10.1016/j.conb.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Crittenden JR, Skoulakis EM, Han KA, Kalderon D, Davis RL. Tripartite mushroom body architecture revealed by antigenic markers. Learn Mem. 1998;5:38–51. [PMC free article] [PubMed] [Google Scholar]
- Dash PK, Hochner B, Kandel ER. Injection of the cAMP-responsive element into the nucleus of Aplysia sensory neurons blocks long-term facilitation. Nature. 1990;345:718–721. doi: 10.1038/345718a0. [DOI] [PubMed] [Google Scholar]
- Davis RL. Olfactory memory formation in Drosophila: From molecular to systems neuroscience. Annu Rev Neurosci. 2005;28:275–302. doi: 10.1146/annurev.neuro.28.061604.135651. [DOI] [PubMed] [Google Scholar]
- Elgersma Y, Sweatt JD, Giese KP. Mouse genetic approaches to investigating calcium/calmodulin-dependent protein kinase II function in plasticity and cognition. J Neurosci. 2004;24:8410–8415. doi: 10.1523/JNEUROSCI.3622-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber T, Joerges J, Menzel R. Associative learning modifies neural representations of odor in the insect brain. Nat Neurosci. 1999;2:74–78. doi: 10.1038/4576. [DOI] [PubMed] [Google Scholar]
- Frank DA, Greenberg ME. CREB: a mediator of long-term memory from mollusks to mammals. Cell. 1994;79:5–8. doi: 10.1016/0092-8674(94)90394-8. [DOI] [PubMed] [Google Scholar]
- Griffith LC. Calcium/calmodulin-dependent protein kinase II: an unforgettable kinase. J Neurosci. 2004;24:8391–8393. doi: 10.1523/JNEUROSCI.2888-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith LC, Lu CS, Sun XX. CaMKII, an enzyme on the move: regulation of temporospatial localization. Mol Interv. 2003;3:386–403. doi: 10.1124/mi.3.7.386. [DOI] [PubMed] [Google Scholar]
- Isabel G, Pascual A, Preat T. Exclusive consolidated memory phases in Drosophila. Science. 2004;304:1024–1027. doi: 10.1126/science.1094932. [DOI] [PubMed] [Google Scholar]
- Joiner MA, Griffith LC. Mapping of the anatomical circuit of CaM kinase-dependent courtship conditioning in Drosophila. Learn Mem. 1999;6:177–192. [PMC free article] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Keleman K, Krüttner S, Alenius M, Dickson BJ. Function of the Drosophila CPEB protein Orb2 in long-term courtship memory. Nat Neurosci. 2007;10:1587–1593. doi: 10.1038/nn1996. [DOI] [PubMed] [Google Scholar]
- Krashes MJ, Keene AC, Leung B, Armstrong JD, Waddell S. Sequential use of mushroom body neuron subsets during Drosophila odor memory processing. Neuron. 2007;53:103–115. doi: 10.1016/j.neuron.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Liu X, Davis RL. The GABAergic anterior paired lateral neuron suppresses and is suppressed by olfactory learning. Nat Neurosci. 2009;12:53–59. doi: 10.1038/nn.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayford M, Kandel ER. Genetic approaches to memory storage. Trends Genet. 1999;15:463–470. doi: 10.1016/s0168-9525(99)01846-6. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory: a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- McGuire SE, Le PT, Davis RL. The role of Drosophila mushroom body signaling in olfactory memory. Science. 2001;293:1330–1333. doi: 10.1126/science.1062622. [DOI] [PubMed] [Google Scholar]
- McGuire SE, Le PT, Osborn AJ, Matsumoto K, Davis RL. Spatiotemporal rescue of memory dysfunction in Drosophila. Science. 2003;302:1765–1768. doi: 10.1126/science.1089035. [DOI] [PubMed] [Google Scholar]
- Mehren JE, Griffith LC. Calcium-independent calcium/calmodulin-dependent protein kinase II in the adult Drosophila CNS enhances the training of pheromonal cues. J Neurosci. 2004;24:10584–10593. doi: 10.1523/JNEUROSCI.3560-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual A, Préat T. Localization of long-term memory within the Drosophila mushroom body. Science. 2001;294:1115–1117. doi: 10.1126/science.1064200. [DOI] [PubMed] [Google Scholar]
- Perazzona B, Isabel G, Preat T, Davis RL. The role of cAMP response element-binding protein in Drosophila long-term memory. J Neurosci. 2004;24:8823–8828. doi: 10.1523/JNEUROSCI.4542-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA. Behavioral studies of Pavlovian conditioning. Annu Rev Neurosci. 1988;11:329–352. doi: 10.1146/annurev.ne.11.030188.001553. [DOI] [PubMed] [Google Scholar]
- Roman G, Davis RL. Molecular biology and anatomy of Drosophila olfactory associative learning. BioEssays. 2001;23:571–581. doi: 10.1002/bies.1083. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Kogan JH, Frankland PW, Kida S. CREB and memory. Annu Rev Neurosci. 1998;21:127–148. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- Squire LR, Alvarez P. Retrograde amnesia and memory consolidation: a neurobiological perspective. Curr Opin Neurobiol. 1995;5:169–177. doi: 10.1016/0959-4388(95)80023-9. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Tully T, Quinn WG. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol A. 1985;157:263–277. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- Tully T, Preat T, Boynton SC, Del Vecchio M. Genetic dissection of consolidated memory in Drosophila. Cell. 1994;79:35–47. doi: 10.1016/0092-8674(94)90398-0. [DOI] [PubMed] [Google Scholar]
- Wang H, Hu Y, Tsien JZ. Molecular and systems mechanisms of memory consolidation and storage. Prog Neurobiol. 2006;79:123–135. doi: 10.1016/j.pneurobio.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Wang JW, Wong AM, Flores J, Vosshall LB, Axel R. Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell. 2003;112:271–282. doi: 10.1016/s0092-8674(03)00004-7. [DOI] [PubMed] [Google Scholar]
- Wittenberg GM, Tsien JZ. An emerging molecular and cellular framework for memory processing by the hippocampus. Trends Neurosci. 2002;25:501–505. doi: 10.1016/s0166-2236(02)02231-2. [DOI] [PubMed] [Google Scholar]
- Yang MY, Armstrong JD, Vilinsky I, Strausfeld NJ, Kaiser K. Subdivision of the Drosophila mushroom bodies by enhancer-trap expression patterns. Neuron. 1995;15:45–54. doi: 10.1016/0896-6273(95)90063-2. [DOI] [PubMed] [Google Scholar]
- Yin JC, Wallach JS, Del Vecchio M, Wilder EL, Zhou H, Quinn WG, Tully T. Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila. Cell. 1994;79:49–58. doi: 10.1016/0092-8674(94)90399-9. [DOI] [PubMed] [Google Scholar]
- Yu D, Ponomarev A, Davis RL. Altered representation of the spatial code for odors after olfactory classical conditioning. Neuron. 2004;42:437–449. doi: 10.1016/s0896-6273(04)00217-x. [DOI] [PubMed] [Google Scholar]
- Yu D, Keene AC, Srivatsan A, Waddell S, Davis RL. Drosophila DPM neurons form a delayed and branch-specific memory trace after olfactory classical conditioning. Cell. 2005;123:945–957. doi: 10.1016/j.cell.2005.09.037. [DOI] [PubMed] [Google Scholar]
- Yu D, Akalal DB, Davis RL. Drosophila α/β mushroom body neurons form a branch-specific, long-term cellular memory trace after spaced olfactory conditioning. Neuron. 2006;52:845–855. doi: 10.1016/j.neuron.2006.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zars T, Fischer M, Schulz R, Heisenberg M. Localization of a short-term memory in Drosophila. Science. 2000;288:672–675. doi: 10.1126/science.288.5466.672. [DOI] [PubMed] [Google Scholar]