Abstract
Monoclonal antibodies of the immunoglobulin G (IgG) isotype have become a well-established therapeutic tool for the targeting of malignant cells in tumor patients. Despite tremendous success in the treatment of lymphoma and breast cancer, it has also become clear that we may not be able to further improve antibody therapy of cancer by simply generating more tumor-specific antibodies with a higher affinity. Instead, the work of many groups in the past years suggests that optimizing the recruitment of effector functions provided by the adaptive and innate immune systems via engineering of the IgG constant domain may hold great promise to achieve enhanced therapeutic activities. A major goal in cancer therapy would be to initiate adaptive immune responses to the patient’s tumor that would result in long-term protection against recurrence. The use of immunostimulatory antibodies shows great promise in stimulating adaptive immune responses. Surprisingly, recent studies also implicate an important role for the antibody constant domain in the activity of these molecules in vivo, opening up new possibilities to further improve the activity of immunomodulatory antibodies by Fc engineering.
Keywords: antibody, cancer therapy, Fc receptor, Fc engineering, inflammation
Introduction
The occasion for this review is motivated by our desire to recognize and celebrate the enormous contributions of Lloyd Old to the advancement of immunotherapeutic approaches for the treatment of cancer. It is fair to say that Lloyd was more than a champion of this field; he was, in reality, its father, advocating an immunological approach to cancer treatment in an era when chemotherapeutic and surgical approaches were the dominant, and in many cases the sole, avenue for treatment. Lloyd recognized the immense capacity of the immune system to act as the ultimate effector mechanism for the elimination of unwanted cells, be they microbial or neoplastic, based on his pioneering early studies on transplantation. Lloyd was an advocate for the concept of “targeting inflammation,” harnessing the capacity of the immune system to elicit potent pro-inflammatory and cytotoxic mediators, including the central mediator tumor necrosis factor (TNF), a molecule that he discovered based on its capacity to induce tumor necrosis. The challenge, as Lloyd saw it, was how to focus those pathways to a site of tumor implantation, thereby avoiding the systemic toxicity of uncontrolled inflammatory responses. Specificity was the key, and Lloyd appreciated the capacity of the adaptive immune response, through antibodies and T cells, to provide that function. His ideas were prescient, and the development of antibody- and T cell-mediated anti-tumor agents owes its existence to Lloyd’s ambitious goal. Lloyd’s fierce intellect and unrelenting commitment to the idea of immunotherapy of cancer inspired, cajoled, challenged, and engaged the many scientists who have contributed to make this field the new frontier of cancer biology. We dedicate this review to Lloyd’s memory, keenly aware of and profoundly saddened by the gap he has left in the field he created.
Looking back from our current perspective on the first successful use of mouse monoclonal antibodies in the therapy of lymphoma in humans 30 years ago, it is clear that we have gained significant insights into how tumor-specific antibodies can kill malignant cells in vivo and which molecular and cellular mechanisms are responsible for this potent cytotoxic activity (1, 2). From the outset, the goal of passive tumor immunotherapy by antibodies was to develop approaches to further improve their therapeutic activity (1, 3, 4). One of the first obstacles that had to be overcome was the immunogenicity of the mouse antibodies in humans which led to the first wave of antibody engineering aimed at eliminating mouse sequences and creating versions that would be compatible with the human immune system. By exchanging the mouse IgG constant domain with human Fc sequences, a generation of chimerized antibodies was introduced into the clinic, which resulted in a lower level of immunogenicity and paved the way for the broad application of this class of molecules in human cancer therapy (5–7). Subsequent efforts humanized the variable regions, as well, resulting in antibodies that retained minimal mouse sequences. Today, the introduction of transgenic mice expressing human antibody genes, the use of phage display techniques, and the direct cloning of antibodies from human B cells have overcome many of the initial issues with immunogenicity (8–10).
A second focus of antibody engineering was to increase the affinity for the target antigen, which was essential for the generation of high-affinity antibodies for target antigens that induced only low-affinity antibody responses during immunization. It became clear early on, however, that not every high-affinity antibody would be suitable for tumor immunotherapy. In lymphoma therapy, for example, despite the availability of many antibodies specific for CD19 and CD20, so far only CD20-specific antibodies of various specificities have turned out to have a high capacity to kill tumor cells efficiently in vivo. To increase the intrinsic cytotoxic activity of antibodies, coupling to toxins or radionuclides was employed to directly introduce the effector molecules to tumor tissue and thereby minimize systemic toxicity that resulted from the use of these toxic molecules alone. The most recent attempts to enhance the in vivo function of cytotoxic antibodies build on the understanding of how antibodies such as anti-CD20 mediate their clinical efficacy in patients, through the capacity of the antibody constant domain to recruit the potent effector functions of the innate immune system (2, 11).
The role of Fc effector function in the therapeutic efficacy of anti-tumor antibodies will be the focus of this review. In addition, we will comment on several recent studies suggesting that the antibody constant region may also be of major importance for the activity of immunomodulatory antibodies crucial for the initiation of adaptive anti-tumor immune responses.
Fc is key for IgG activity in vivo
As a general consideration, a tumor-specific antibody may kill a tumor cell by any or all of a variety of different pathways, involving both the variable domains and the constant region of the antibody. While previous in vitro data supported roles only for the variable region recognition function of the antibody in triggered tumor cell death, either by apoptosis or by depriving the cell of an essential growth factor, studies over the past decade have established the essential role of the constant region in the in vivo activity of an anti-tumor antibody (1, 2, 12, 13). These Fc-dependent functions, in principle, could include the initiation of the lytic complement pathway by the classical pathway or the recruitment and activation of innate immune effector cells via crosslinking of Fcγ receptors (FcγR) ubiquitously expressed on the surface of NK cells, monocytes, and macrophages. Although in vitro studies suggested that all of these pathways could be operative, the use of F(ab’)2 fragments of tumor-specific antibodies, antibodies modified in their Fc domain to abrogate either complement or FcR binding, and mouse strains deficient either in components of the complement pathway or individual FcγRs clearly established a dominant role for FcγR engagement in the in vivo activity of anti-tumor antibodies (14–20). In humans, there is evidence that complement activation may even reduce the NK cell-dependent cytotoxic activity of therapeutic antibodies such as rituximab (21–23). Consistent with this notion, response rates were increased and time to relapse was prolonged in rituximab-treated follicular lymphoma patients carrying a C1q allelic variant resulting in reduced levels of this complement component (24). In contrast, patients carrying allelic variants of the activating FcγRIIA and IIIA, conferring a higher affinity binding to the therapeutic antibody, responded better to therapy with CD20-, EGFR-, or HER2/neu-specific anti-tumor antibodies (25–30).
FcγRs are a family of molecules consisting of three activating (FcγRI, FcγRIII, and FcγRIV in mice; FcγRIA, FcγRIIA, and FcγRIIIA in humans) and one inhibitory (FcγRIIB) receptor (11,13, 31). On the majority of cell types one or more activating FcγR is coexpressed with the inhibitory FcγRIIB. By determining the affinity of the individual FcγRs for the different IgG subclasses and by using mouse strains deficient in individual FcγRs, it became clear that the varying in vivo activities of the different IgG subclasses (with IgG2a being the most active followed by IgG2b and IgG1 in mice) correlated with the ratio of the affinities with which the individual IgG subclass bound to certain activating and inhibitory FcγR (termed A/I ratio) (18, 31). Consistent with this notion, deletion of the inhibitory FcγRIIB enhanced therapeutic IgG activity in a subclass-specific manner in mice in vivo (16, 18). Thus mouse IgG1 anti-tumor antibodies, which had a very low anti-tumor activity due to a high affinity for FcγRIIB, gained a high cytolytic activity in mice deficient in the inhibitory FcγR (18). The effector cells involved in the anti-tumor activity based on these results suggested that besides NK cells, which selectively express one activating (FcγRIII in mice and FcγRIIIA in humans) but not the inhibitory FcγRIIB in mice and man, other innate immune effector cell populations, such as monocytes/ macrophages, are likely to contribute to IgG-mediated tumor cell killing in vivo.
Indeed, a recent study identified the subset of resident or nonclassical monocytes as major mediators for antibody-mediated killing of B cells in vivo (15). In mice and humans, this monocyte population expresses the broadest set of FcγRs, including all members of the canonical mouse FcγR family and human FcγRIA, FcγRIIA, FcγRIIB, and FcγRIIIA, respectively. Nonetheless, a recent study by Veeramani and colleagues demonstrated that NK cells become activated in lymphoma patients following rituximab infusion (32). Moreover, a study of Weng and Levy in follicular lymphoma patients carrying either the functionally intact FcγRIIB-232I allele or the defect FcγRIIB-232T allele did not show enhanced therapeutic activity of rituximab in the latter patient subgroup (30). However, in this study only two patients had the FcγRIIB-232T allele, making it hard to draw definitive conclusions. This study, however, together with many other studies in human lymphoma, colorectal cancer, and breast cancer patient cohorts, suggest that FcγRIIIA (and FcγRIIA) may be crucial for human IgG activity in vivo. These findings provide the basis for the concept that enhancing the interaction of the IgG constant domain, especially with low-affinity cellular FcγRIIA and FcγRIIIA, may translate into enhanced therapeutic activity (Figure 1). Indeed many pharmaceutical companies have generated Fc-engineered antibodies with enhanced binding to activating FcγRs, which are currently in various phases of clinical trials (1, 12).
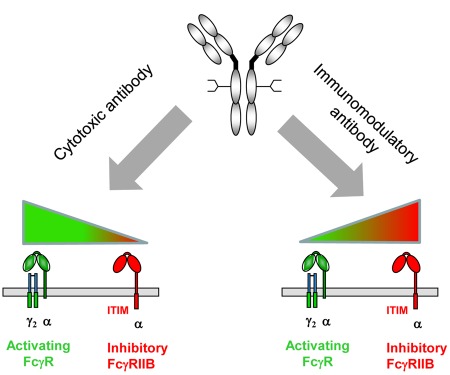
Figure 1.
Antibody engineering approaches to enhance cytotoxic and immunomodulatory therapeutic antibodies. Shown is the differential dependence of cytotoxic antibodies, such as rituximab (left panel) and immunomodulatory antibodies, such as anti-CD40 (right panel) on activating and inhibitory FcγRs for their activity in vivo. Based on this model, antibody engineering approaches to enhance cytotoxic antibody function should focus on increased binding to activating FcγRs, whereas the activity of immunomodulatory antibodies may be optimized by a selective binding to the inhibitory FcγRIIB.
Optimizing anti-tumor activity via engineering the Fc protein backbone
As indicated, most of the efforts [with the notable exception of ofatumumab, a CD20-specific antibody optimized for complement activation (33, 34)] to improve therapeutic IgG activity focus on enhancement of binding to activating FcγRs (1, 3). Several strategies have been employed to identify IgG variants with enhanced FcγR binding. Initially, Shields and colleagues have used an alanine scanning approach to identify an IgG1 triple mutant (S298A/E333A/L334A) with enhanced FcγRIIIA binding and antibody-dependent cell-mediated cytotoxicity (ADCC) activity (35). By using an in silico approach, Lazar and colleagues identified several additional IgG1 variants with strongly enhanced binding to FcγRIIIA (12). Among those, the S239D/I332E and S239D/I332E/A330L mutations showed the greatest increase in affinity for FcγRIIIA and a decrease in FcγRIIB binding. In contrast to the double mutation, the triple variant lost the capacity to activate the complement pathway, creating a useful tool to study the contribution of the complement pathway for IgG activity in vivo. Introduction of these mutations into antibodies such as alemtuzumab (CD52-specific), trastuzumab (HER2/neu-specific), rituximab (CD20-specific), and cetuximab (EGFR-specific) translated into greatly enhanced ADCC activity in vitro. Importantly, the S239D/I332E variant showed an enhanced capacity to deplete B cells in monkeys, providing convincing evidence that this approach might translate into enhanced therapeutic activity in humans (12).
By introducing an even greater set of mutations (L235V, F243L, R292P, Y300L, and P396L), Stavenhagen, and later Nordstrom and colleagues, were also able to generate IgG1 mutants with enhanced binding to FcγRIIIA that also translated into enhanced ADCC activity in transgenic mice expressing human FcγRIIIA in models of B cell malignancies and breast cancer (36, 37). Interestingly, even CD19-specific antibodies usually having a much lower cytolytic activity than CD20-specific antibodies in vivo could be turned into therapeutically active antibodies if they were Fc engineered to have increased affinity for activating FcγRs, suggesting that Fc engineering might enable the repertoire of therapeutic targets on tumor cells to be broadened (38, 39).
In addition to enhancing the binding to activating FcγRs, prolonging the in vivo half-life of antibodies was shown to translate into greater anti-tumor activity. IgG half-life is dependent on the neonatal Fc receptor FcRn, a member of the MHC class I superfamily, which is expressed on many cell types including endothelial cells and macrophages (40, 41). In contrast to the interaction of IgG with classical FcγRs, FcRn binds to the CH2/CH3 domain of IgG at an acidic pH ensuring that endocytosed IgG will not be destroyed in lysosomal compartments but shuttled back to the cell surface where it is released into the blood. Thus, increasing the affinity of IgG for FcRn should result in a longer half-life and therefore a greater likelihood to initiate anti-tumor responses. Indeed, Fc-engineered variants (M428L/N434S) of the VEGF-specific antibody bevacizumab and the EGFR-specific antibody cetuximab demonstrated enhanced half-life in monkeys and translated into better anti-tumor responses in mice (42).
Finally, the essential role of the IgG Fc-linked glycan in the binding interaction between the Fc and cellular FcγRs has limited production of therapeutic antibodies to mammalian cell lines to obtain proper glycosylation. Bacterial cell expression, by virtue of their lack of glycosylation, and yeast or insect cell production systems, which differ substantially in the complex sugar structures added to recombinantly expressed proteins, such as antibodies, are limited in their usefulness. Attempts to address these concerns have focused either on generating IgG Fc variants that retain FcR binding in the absence of Fc glycosylation or developing modified bacterial or yeast strains that are engineered to mimic human glycosylation patterns (43, 44).
Optimizing anti-tumor antibody activity via Fc glycoengineering
In addition to generating ADCC-enhanced therapeutic antibodies via introduction of amino acid modifications in the IgG backbone, engineering of the glycan moiety attached to each Fc fragment at the asparagine 297 (N297) residue provides the opportunity to modify the interaction of antibodies with FcγRs. While the essential role of this glycan domain for an intact IgG structure and its binding to FcγRs has been known for some time, it became evident only recently that certain sugars in this glycan moiety can dramatically alter IgG activity (45, 46). If terminal sialic acid residues are present at high levels, for example, IgG molecules have a reduced affinity for classical FcγRs and gain affinity for non-canonical glycosylation-dependent Fc receptors such as DC-SIGN (47–53). As these IgG variants additionally have an active anti-inflammatory activity, this may result in a reduction of therapeutic activity of tumor-specific antibodies and must be avoided. Other sugar moieties have also been shown to modify FcR binding interactions. The absence of branching fucose residues strongly enhances the interaction of IgG with activating FcγRIIIA, and consequently, ADCC. Recent evidence suggests that sugar moieties of both FcγRIIIA and the IgG Fc are involved in this high-affinity interaction (54, 55). There is convincing evidence that afucosylated tumor-specific antibodies translate into enhanced therapeutic activity in mouse models in vivo (18, 56). At present, several afucosylated next generation anti-tumor antibodies targeting antigens such as CD20 and CD19 in chronic lymphocytic leukemia, non-Hodgkin lymphoma, and other B cell malignancies, CD30 in Hodgkin lymphoma, EGFR in colorectal cancer, CCR4 in T cell leukemia, and the ganglioside GM2 in multiple myeloma are in different stages of clinical testing (1, 3, 57).
Bispecific antibodies to recruit effector cell populations
An alternative approach to use the specificity of antibodies to direct effector cell populations to tumor cells and specifically trigger effector molecules of choice is to generate bispecific antibodies (58). In these constructs, one arm of the molecule recognizes an antigen on the tumor cell (CD19, CD30, HER2/ neu), and the other arm a target structure on the effector cell. To achieve a higher avidity, bi-, tri-, or multivalent formats of these molecules have been generated. Using this strategy, it is possible to replace the low-affinity innate immune cell-recruiting function of the Fc fragment with an antibody variable region recognizing FcγRIIIA, for example (59–62). Whereas bispecific antibodies including FcγRIIIA-specific arms are only in early phases of preclinical and clinical testing, antibody formats using CD3-specific arms to recruit T cells have shown great success in clinical phase I and phase II trials (63). This so-called BiTE (bispecific T cell engager) format was used in non-Hodgkin lymphoma as a CD19 x CD3 fusion (blinatumomab) and a similar format used to treat ascites due to cancer growth in the peritoneal cavity as an EpCAM x CD3 fusion (catumaxomab) (64, 65). Given the success of this strategy, we will surely see more variants of the BiTE molecules in the near future.
Enhancement of immunomodulatory antibodies via Fc engineering
Although passive antibody therapy has demonstrated significant efficacy in the treatment of cancer, it clearly also has its limitations. Patients can become refractory to antibody therapy due to selection of tumor variants that have lost the target antigen or through the loss of the effector response. Ideally, a therapeutic approach that resulted in the induction of an adaptive immune response to the tumor, with the development of immunological memory against the tumor, is the long-term goal of tumor immunotherapy.
One such approach is to deliver tumor antigens to dendritic cells in vivo with the resulting antigen presentation and stimulation of cognate effector T cells. Among the approaches to accomplish this, for example, the tumor antigen can be coupled genetically to antibodies specific for cell surface receptors on dendritic cells (DCs) such as DEC-205 (66–68). As DCs need to be matured to initiate a productive immune response through the induction of costimulatory molecules, it is essential to coadminister antibodies crosslinking molecules such as CD40 on DCs. Interestingly, recent evidence suggests that the activity of these non-depleting immunomodulatory antibodies is also dependent on the IgG Fc fragment in vivo (69–72). In contrast to the essential role of activating FcγRs for cytotoxic antibodies, the inhibitory FcγRIIB seemed to be crucial for immunomodulatory IgG activity in vivo (69, 70). Thus, the proliferation of antigen-specific T cells induced by injection of different CD40-specific antibodies was abrogated in FcγRIIB-deficient mice. Of note, this effect may be independent of the induction of signaling pathways initiated by crosslinking of FcγRIIB, as signaling-deficient FcγRIIB variants were sufficient for these effects in vitro (70). One may speculate that FcγRIIB may provide a scaffold in vivo that is required to efficiently crosslink CD40 on DCs. In principle, this scaffold could be provided in cis (by FcγRIIB expressed on DCs) or in trans (by other cells including B cells, monocytes, macrophages, and neutrophils). Indeed, some studies have suggested that FcγRIIB on B cells may be involved in this process, but further studies will be required to understand this novel pathway in vivo in greater detail (70, 71).
Similar results were suggested for other members of the TNF receptor superfamily, such as CD95, where agonistic antibodies required the presence of FcγRIIB to induce liver inflammation and initiate apoptosis in murine lymphoma cells (71, 72). In general, this unexpected requirement of FcγRIIB opens up new possibilities for the enhancement of immunomodulatory IgG activity (Figure 1). Thus, IgG variants (such as the S267E and L328F mutants) have been identified that selectively enhance the binding to the inhibitory FcγR (73). Introduction of these mutations into the IgG backbone would be expected to further improve their activity and potentially make them safer, as unwanted co-crosslinking of activating FcγRs may result in the additional release of proinflammatory cytokines, which could be the reason for some of the side effects of this type of antibody therapy.
Conclusion
Taken together, research over the last decade has resulted in the identification of novel ADCC-enhanced Fc fragments, which have been incorporated into anti-tumor antibodies such as rituximab. These Fc-modified antibodies are currently in various phases of clinical studies and will provide important insights into the development of the next generation of antibody therapeutics. Fc engineering has revealed that novel therapeutic targets, such as CD19 in lymphoma, may become available if the interaction of the IgG Fc fragment with cellular FcγRs is enhanced (Figure 1).
Moreover, basic studies into the underlying mechanisms behind the activity of anti-tumor therapeutics, be they cytotoxic or immunomodulatory, has revealed how Fc engineering may enable us to increase therapeutic IgG activity. Thus, recent studies suggest that FcγRIIB on B cells induces internalization of CD20 bound by CD20-specific antibodies, resulting in reduced cell surface expression of the target antigen (74, 75). By generating IgG variants with reduced FcγRIIB binding, one may be able to circumvent this problem and additionally increase the activation of innate immune effector cells involved in anti-tumor ADCC reactions. The finding that immunomodulatory antibodies are dependent on the inhibitory FcγRIIB will enable us to use this class of antibodies in a hopefully much safer way and bring us one step closer to the induction of adaptive anti-tumor immune responses. Novel antibody formats including bispecific or multispecific molecules will similarly benefit from engineering approaches that result in enhanced in vivo potency derived from a basic understanding of the underlying pathways by which IgG antibodies, through their Fc domain, mediate their biological properties.
Acknowledgments
We apologize to all our colleagues whose important work could not be directly cited due to limitations of space. This work was funded by grants from the NIH (J.V.R.), the German Research Foundation (F.N.), and BayGene (F.N.).
Abbreviations
- BiTE
bispecific T cell engager;
- ADCC
antibody-dependent cell-mediated cytotoxicity
References
- 1.Lim SH, Beers SA, French RR, Johnson PW, Glennie MJ, Cragg MS. Anti-CD20 monoclonal antibodies: historical and future perspectives. Haematologica. 2010;95:135–143. doi: 10.3324/haematol.2008.001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nimmerjahn F, Ravetch JV. Antibodies, Fc receptors and cancer. Curr Opin Immunol. 2007;19:239–245. doi: 10.1016/j.coi.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Desjarlais JR, Lazar GA. Modulation of antibody effector function. Exp Cell Res. 2011;317:1278–1285. doi: 10.1016/j.yexcr.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 4.Robak T, Robak E. New anti-CD20 monoclonal antibodies for the treatment of B-cell lymphoid malignancies. BioDrugs. 2011;25:13–25. doi: 10.2165/11539590-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 5.Jefferis R. Antibody therapeutics: isotype and glycoform selection. Expert Opin Biol Ther. 2007;7:1401–1413. doi: 10.1517/14712598.7.9.1401. [DOI] [PubMed] [Google Scholar]
- 6.Reichert JM, Rosensweig CJ, Faden LB, Dewitz MC. Monoclonal antibody successes in the clinic. Nat Biotechnol. 2005;23:1073–1078. doi: 10.1038/nbt0905-1073. [DOI] [PubMed] [Google Scholar]
- 7.Carter PJ. Potent antibody therapeutics by design. Nat Rev Immunol. 2006;6:343–357. doi: 10.1038/nri1837. [DOI] [PubMed] [Google Scholar]
- 8.Lonberg N, Taylor LD, Harding FA, Trounstine M, Higgins KM, Schramm SR, Kuo CC, Mashayekh R, Wymore K, McCabe JG, Munoz-O’Regan D, O’Donnell SL, Lapachet ESG, Bengoechea T, Fishwild DM, Carmack CE, Kay RM, Huszar D. Antigen-specific human antibodies from mice comprising four distinct genetic modifications. Nature. 1994;368:856–859. doi: 10.1038/368856a0. [DOI] [PubMed] [Google Scholar]
- 9.Nelson AL, Dhimolea E, Reichert JM. Development trends for human monoclonal antibody therapeutics. Nat Rev Drug Discov. 2010;9:767–774. doi: 10.1038/nrd3229. [DOI] [PubMed] [Google Scholar]
- 10.Corti D, Sallusto F, Lanzavecchia A. High throughput cellular screens to interrogate the human T and B cell repertoires. Curr Opin Immunol. 2011;23:430–435. doi: 10.1016/j.coi.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Hogarth PM. Fc receptors are major mediators of antibody based inflammation in autoimmunity. Curr Opin Immunol. 2002;14:798–802. doi: 10.1016/s0952-7915(02)00409-0. [DOI] [PubMed] [Google Scholar]
- 12.Lazar GA, Dang W, Karki S, Vafa O, Peng JS, Hyun L, Chan C, Chung HS, Eivazi A, Yoder SC, Vielmetter J, Carmichael DF, Hayes RJ, Dahiyat BI. Engineered antibody Fc variants with enhanced effector function. Proc Natl Acad Sci U S A. 2006;103:4005–4010. doi: 10.1073/pnas.0508123103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takai T. Roles of Fc receptors in autoimmunity. Nat Rev Immunol. 2002;2:580–592. doi: 10.1038/nri856. [DOI] [PubMed] [Google Scholar]
- 14.Beers SA, Chan CH, James S, French RR, Attfield KE, Brennan CM, Ahuja A, Shlomchik MJ, Cragg MS, Glennie MJ. Type II (tositumomab) anti-CD20 monoclonal antibody out performs type I (rituximab-like) reagents in B-cell depletion regardless of complement activation. Blood. 2008;112:4170–4177. doi: 10.1182/blood-2008-08-172999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biburger M, Aschermann S, Schwab I, Lux A, Albert H, Danzer H, Woigk M, Dudziak D, Nimmerjahn F. Monocyte subsets responsible for immunoglobulin G-dependent effector functions in vivo. Immunity. 2011;35:932–944. doi: 10.1016/j.immuni.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 16.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 17.Hamaguchi Y, Xiu Y, Komura K, Nimmerjahn F, Tedder TF. Antibody isotype-specific engagement of Fc gamma receptors regulates B lymphocyte depletion during CD20 immunotherapy. J Exp Med. 2006;203:743–753. doi: 10.1084/jem.20052283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nimmerjahn F, Ravetch JV. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science. 2005;310:1510–1512. doi: 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
- 19.Otten MA, van der Bij GJ, Verbeek SJ, Nimmerjahn F, Ravetch JV, Beelen RH, van de Winkel JG, van Egmond M. Experimental antibody therapy of liver metastases reveals functional redundancy between Fc gammaRI and Fc gammaRIV. J Immunol. 2008;181:6829–6836. doi: 10.4049/jimmunol.181.10.6829. [DOI] [PubMed] [Google Scholar]
- 20.Uchida J, Hamaguchi Y, Oliver JA, Ravetch JV, Poe JC, Haas KM, Tedder TF. The innate mononuclear phagocyte network depletes B lymphocytes through Fc receptor-dependent mechanisms during anti-CD20 antibody immunotherapy. J Exp Med. 2004;199:1659–1669. doi: 10.1084/jem.20040119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang SY, Veeramani S, Racila E, Cagley J, Fritzinger DC, Vogel CW, St. John W, Weiner GJ. Depletion of the C3 component of complement enhances the ability of rituximab-coated target cells to activate human NK cells and improves the efficacy of monoclonal antibody therapy in an in vivo model. Blood. 2009;114:5322–5330. doi: 10.1182/blood-2009-01-200469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang SY, Weiner G. Complement and cellular cytotoxicity in antibody therapy of cancer. Expert Opin Biol Ther. 2008;8:759–768. doi: 10.1517/14712598.8.6.759. [DOI] [PubMed] [Google Scholar]
- 23.Wang SY, Racila E, Taylor RP, Weiner GJ. NK-cell activation and antibody-dependent cellular cytotoxicity induced by rituximab-coated target cells is inhibited by the C3b component of complement. Blood. 2008;111:1456–1463. doi: 10.1182/blood-2007-02-074716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Racila E, Link BK, Weng WK, Witzig TE, Ansell S, Maurer MJ, Huang J, Dahle C, Halwani A, Levy R, Weiner GJ. A polymorphism in the complement component C1qA correlates with prolonged response following rituximab therapy of follicular lymphoma. Clin Cancer Res. 2008;14:6697–6703. doi: 10.1158/1078-0432.CCR-08-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, Watier H. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99:754–758. doi: 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]
- 26.Musolino A, Naldi N, Bortesi B, Pezzuolo D, Capelletti M, Missale G, Laccabue D, Zerbini A, Camisa R, Bisagni G, Neri TM, Ardizzoni A. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol. 2008;26:1789–1796. doi: 10.1200/JCO.2007.14.8957. [DOI] [PubMed] [Google Scholar]
- 27.Weng WK, Czerwinski D, Timmerman J, Hsu FJ, Levy R. Clinical outcome of lymphoma patients after idiotype vaccination is correlated with humoral immune response and immunoglobulin G Fc receptor genotype. J Clin Oncol. 2004;22:4717–4724. doi: 10.1200/JCO.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol. 2003;21:3940–3947. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 29.Bibeau F, Lopez-Crapez E, Di Fiore F, Thezenas S, Ychou M, Blanchard F, Lamy A, Penault-Llorca F, Frébourg T, Michel P, Sabourin JC, Boissière-Michot F. Impact of Fc{gamma}RIIa-Fc{gamma}RIIIa polymorphisms and KRAS mutations on the clinical outcome of patients with metastatic colorectal cancer treated with cetuximab plus irinotecan. J Clin Oncol. 2009;27:1122–1129. doi: 10.1200/JCO.2008.18.0463. [DOI] [PubMed] [Google Scholar]
- 30.Weng WK, Levy R. Genetic polymorphism of the inhibitory IgG Fc receptor FcgammaRIIb is not associated with clinical outcome in patients with follicular lymphoma treated with rituximab. Leuk Lymphoma. 2009;50:723–727. doi: 10.1080/10428190902829441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nimmerjahn F, Ravetch JV. Fcgamma receptors: old friends and new family members. Immunity. 2006;24:19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 32.Veeramani S, Wang SY, Dahle C, Blackwell S, Jacobus L, Knutson T, Button A, Link BK, Weiner GJ. Rituximab infusion induces NK activation in lymphoma patients with the high-affinity CD16 polymorphism. Blood. 2011;118:3347–3349. doi: 10.1182/blood-2011-05-351411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coiffier B, Lepretre S, Pedersen LM, Gadeberg O, Fredriksen H, van Oers MH, Wooldridge J, Kloczko J, Holowiecki J, Hellmann A, Walewski J, Flensburg M, Petersen J, Robak T. Safety and efficacy of ofatumumab, a fully human monoclonal anti-CD20 antibody, in patients with relapsed or refractory B-cell chronic lymphocytic leukemia: a phase 1–2 study. Blood. 2008;111:1094–1100. doi: 10.1182/blood-2007-09-111781. [DOI] [PubMed] [Google Scholar]
- 34.Taylor PC, Quattrocchi E, Mallett S, Kurrasch R, Petersen J, Chang DJ. Ofatumumab, a fully human anti-CD20 monoclonal antibody, in biological-naive, rheumatoid arthritis patients with an inadequate response to methotrexate: a randomised, double-blind, placebo-controlled clinical trial. Ann Rheum Dis. 2011;70:2119–2125. doi: 10.1136/ard.2011.151522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shields RL, Namenuk AK, Hong K, Meng YG, Rae J, Briggs J, Xie D, Lai J, Stadlen A, Li B, Fox JA, Presta LG. High resolution mapping of the binding site on human IgG1 for Fc gamma RI, Fc gamma RII, Fc gamma RIII, and FcRn and design of IgG1 variants with improved binding to the Fc gamma R. J Biol Chem. 2001;276:6591–6604. doi: 10.1074/jbc.M009483200. [DOI] [PubMed] [Google Scholar]
- 36.Nordstrom JL, Gorlatov S, Zhang W, Yang Y, Huang L, Burke S, Li H, Ciccarone V, Zhang T, Stavenhagen J, Koenig S, Stewart SJ, Mokore PA, Johnson S, Bonvini E. Anti-tumor activity and toxico-kinetics analysis of MGAH22, an anti-HER2 monoclonal antibody with enhanced Fcgamma receptor binding properties. Breast Cancer Res. 2011;13:R123. doi: 10.1186/bcr3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stavenhagen JB, Gorlatov S, Tuaillon N, Rankin CT, Li H, Burke S, Huang L, Vijh S, Johnson S, Bonvini E, Koenig S. Fc optimization of therapeutic antibodies enhances their ability to kill tumor cells in vitro and controls tumor expansion in vivo via low-affinity activating Fcgamma receptors. Cancer Res. 2007;67:8882–8890. doi: 10.1158/0008-5472.CAN-07-0696. [DOI] [PubMed] [Google Scholar]
- 38.Horton HM, Bernett MJ, Pong E, Peipp M, Karki S, Chu SY, Richards JO, Vostiar I, Joyce PF, Repp R, Desjarlais JR, Zhukovsky EA. Potent in vitro and in vivo activity of an Fc-engineered anti-CD19 monoclonal antibody against lymphoma and leukemia. Cancer Res. 2008;68:8049–8057. doi: 10.1158/0008-5472.CAN-08-2268. [DOI] [PubMed] [Google Scholar]
- 39.Zalevsky J, Leung IW, Karki S, Chu SY, Zhukovsky EA, Desjarlais JR, Carmichael DF, Lawrence CE. The impact of Fc engineering on an anti-CD19 antibody: increased Fcgamma receptor affinity enhances B-cell clearing in nonhuman primates. Blood. 2009;113:3735–3743. doi: 10.1182/blood-2008-10-182048. [DOI] [PubMed] [Google Scholar]
- 40.Ghetie V, Ward ES. Multiple roles for the major histocompatibility complex class I- related receptor FcRn. Annu Rev Immunol. 2000;18:739–766. doi: 10.1146/annurev.immunol.18.1.739. [DOI] [PubMed] [Google Scholar]
- 41.Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7:715–725. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- 42.Zalevsky J, Chamberlain AK, Horton HM, Karki S, Leung IW, Sproule TJ, Lazar GA, Roopenian DC, Desjarlais JR. Enhanced antibody half-life improves in vivo activity. Nat Biotechnol. 2010;28:157–159. doi: 10.1038/nbt.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sazinsky SL, Ott RG, Silver NW, Tidor B, Ravetch JV, Wittrup KD. Aglycosylated immunoglobulin G1 variants productively engage activating Fc receptors. Proc Natl Acad Sci U S A. 2008;105:20167–20172. doi: 10.1073/pnas.0809257105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Potgieter TI, Cukan M, Drummond JE, Houston-Cummings NR, Jiang Y, Li F, Lynaugh H, Mallem M, McKelvey TW, Mitchell T, Nylen A, Rittenhour A, Stadheim TA, Zha D, d’Anjou M. Production of monoclonal antibodies by glycoengineered Pichia pastoris. J Biotechnol. 2009;139:318–325. doi: 10.1016/j.jbiotec.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 45.Arnold JN, Wormald MR, Sim RB, Rudd PM, Dwek RA. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu Rev Immunol. 2007;25:21–50. doi: 10.1146/annurev.immunol.25.022106.141702. [DOI] [PubMed] [Google Scholar]
- 46.Lux A, Nimmerjahn F. Impact of differential glycosylation on IgG activity. Adv Exp Med Biol. 2011;780:113–124. doi: 10.1007/978-1-4419-5632-3_10. [DOI] [PubMed] [Google Scholar]
- 47.Schwab I, Biburger M, Kronke G, Schett G, Nimmerjahn F. IVIg-mediated amelioration of ITP in mice is dependent on sialic acid and SIGNR1. Eur J Immunol. 2012 doi: 10.1002/eji.201142260. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 48.Anthony RM, Kobayashi T, Wermeling F, Ravetch JV. Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway. Nature. 2011;475:110–113. doi: 10.1038/nature10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anthony RM, Nimmerjahn F. The role of differential IgG glycosylation in the interaction of antibodies with FcgammaRs in vivo. Curr Opin Organ Transplant. 2010 doi: 10.1097/MOT.0b013e328342538f. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 50.Anthony RM, Nimmerjahn F, Ashline DJ, Reinhold VN, Paulson JC, Ravetch JV. Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science. 2008;320:373–376. doi: 10.1126/science.1154315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anthony RM, Wermeling F, Karlsson MC, Ravetch JV. Identification of a receptor required for the anti-inflammatory activity of IVIG. Proc Natl Acad Sci U S A. 2008;105:19571–19578. doi: 10.1073/pnas.0810163105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313:670–673. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- 53.Scallon BJ, Tam SH, McCarthy SG, Cai AN, Raju TS. Higher levels of sialylated Fc glycans in immunoglobulin G molecules can adversely impact functionality. Mol Immunol. 2007;44:1524–1534. doi: 10.1016/j.molimm.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 54.Ferrara C, Stuart F, Sondermann P, Brunker P, Umaña P. The carbohydrate at FcgammaRIIIa Asn-162. An element required for high affinity binding to non-fucosylated IgG glycoforms. J Biol Chem. 2006;281:5032–5036. doi: 10.1074/jbc.M510171200. [DOI] [PubMed] [Google Scholar]
- 55.Ferrara C, Grau S, Jäger C, Sondermann P, Brunker P, Waldhauer I, Hennig M, Ruf A, Rufer AC, Stihle M, Umaña P, Benz J. Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcgammaRIII and antibodies lacking core fucose. Proc Natl Acad Sci U S A. 2011;108:12669–12674. doi: 10.1073/pnas.1108455108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mossner E, Brunker P, Moser S, Puntener U, Schmidt C, Herter S, Grau R, Gerdes C, Nopora A, van Puijenbroek E, Ferrara C, Sondermann P, Jäger C, Strein P, Fertig G, Friess T, Schüll C, Bauer S, Dal Porto J, Del Nagro C, Dabbagh K, Dyer MJ, Poppema S, Klein C, Umaña P. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood. 2010;115:4393–4402. doi: 10.1182/blood-2009-06-225979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paz-Ares LG, Gomez-Roca C, Delord JP, Cervantes A, Markman B, Corral J, Soria JC, Bergé Y, Roda D, Russell-Yarde F, Hollingsworth S, Baselga J, Umaña P, Manenti L, Tabernero J. Phase I pharmacokinetic and pharmacodynamic dose-escalation study of RG7160 (GA201), the first glycoengineered monoclonal antibody against the epidermal growth factor receptor, in patients with advanced solid tumors. J Clin Oncol. 2011;29:3783–3790. doi: 10.1200/JCO.2011.34.8888. [DOI] [PubMed] [Google Scholar]
- 58.Peipp M, van de Winkel JG, Valerius T. Molecular engineering to improve antibodies’ anti-lymphoma activity. Best Pract Res Clin Haematol. 2011;24:217–229. doi: 10.1016/j.beha.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 59.Kellner C, Bruenke J, Stieglmaier J, Schwemmlein M, Schwenkert M, Singer H, Mentz K, Peipp M, Lang P, Oduncu F, Stockmeyer B, Fey GH. A novel CD19-directed recombinant bispecific antibody derivative with enhanced immune effector functions for human leukemic cells. J Immunother. 2008;31:871–884. doi: 10.1097/CJI.0b013e318186c8b4. [DOI] [PubMed] [Google Scholar]
- 60.Ardnt MA, Krauss J, Kipriyanov SM, Pfreundschuh M, Little M. A bispecific diabody that mediates natural killer cell cytotoxicity against xenotransplantated human Hodgkin’s tumors. Blood. 1999;94:2562–2568. [PubMed] [Google Scholar]
- 61.Kipriyanov SM, Little M. Generation of recombinant antibodies. Mol Biotechnol. 1999;12:173–201. doi: 10.1385/MB:12:2:173. [DOI] [PubMed] [Google Scholar]
- 62.Kellner C, Bruenke J, Horner H, Schubert J, Schwenkert M, Mentz K, Barbin K, Stein C, Peipp M, Stockmeyer B, Fey GH. Heterodimeric bispecific antibody-derivatives against CD19 and CD16 induce effective antibody-dependent cellular cytotoxicity against B-lymphoid tumor cells. Cancer Lett. 2011;303:128–139. doi: 10.1016/j.canlet.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 63.Baeuerle PA, Kufer P, Bargou R. BiTE: Teaching antibodies to engage T-cells for cancer therapy. Curr Opin Mol Ther. 2009;11:22–30. [PubMed] [Google Scholar]
- 64.Seimetz D, Lindhofer H, Bokemeyer C. Development and approval of the trifunctional antibody catumaxomab (anti-EpCAM x anti-CD3) as a targeted cancer immunotherapy. Cancer Treat Rev. 2010;36:458–467. doi: 10.1016/j.ctrv.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 65.Bargou R, Leo E, Zugmaier G, Klinger M, Goebeler M, Knop S, Noppeney R, Viardot A, Hess G, Schuler M, Einsele H, Brandl C, Wolf A, Kirchinger P, Klappers P, Schmidt M, Riethmüller G, Reinhardt C, Baeuerle PA, Kufer P. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321:974–977. doi: 10.1126/science.1158545. [DOI] [PubMed] [Google Scholar]
- 66.Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, Cheong C, Liu K, Lee HW, Park CG, Steinman RM, Nussenzweig MC. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 67.Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, Ravetch JV, Steinman RM, Nussenzweig MC. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–779. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Steinman RM, Hawiger D, Liu K, Bonifaz L, Bonnyay D, Mahnke K, Iyoda T, Ravetch J, Dhodapkar M, Inaba K, Nussenzweig M. Dendritic cell function in vivo during the steady state: a role in peripheral tolerance. Ann N Y Acad Sci. 2003;987:15–25. doi: 10.1111/j.1749-6632.2003.tb06029.x. [DOI] [PubMed] [Google Scholar]
- 69.Li F, Ravetch JV. Inhibitory Fcgamma receptor engagement drives adjuvant and anti-tumor activities of agonistic CD40 antibodies. Science. 2011;333:1030–1034. doi: 10.1126/science.1206954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.White AL, Chan HT, Roghanian A, French RR, Mockridge CI, Tutt AL, Dixon SV, Ajona D, Verbeek JS, Al-Shamkhani A, Cragg MS, Beers SA, Glennie MJ. Interaction with FcgammaRIIB is critical for the agonistic activity of anti-CD40 monoclonal antibody. J Immunol. 2011;187:1754–1763. doi: 10.4049/jimmunol.1101135. [DOI] [PubMed] [Google Scholar]
- 71.Wilson NS, Yang B, Yang A, Loeser S, Marsters S, Lawrence D, Li Y, Pitti R, Totpal K, Yee S, Ross S, Vernes JM, Lu Y, Adams C, Offringa R, Kelley B, Hymowitz S, Daniel D, Meng G, Ashkenazi A. An Fcgamma receptor-dependent mechanism drives antibody-mediated target-receptor signaling in cancer cells. Cancer Cell. 2011;19:101–113. doi: 10.1016/j.ccr.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 72.Xu Y, Szalai AJ, Zhou T, Zinn KR, Chaudhuri TR, Li X, Koopman WJ, Kimberly RP. Fc gamma Rs modulate cytotoxicity of anti-Fas antibodies: implications for agonistic antibody-based therapeutics. J Immunol. 2003;171:562–568. doi: 10.4049/jimmunol.171.2.562. [DOI] [PubMed] [Google Scholar]
- 73.Horton HM, Chu SY, Ortiz EC, Pong E, Cemerski S, Leung IW, Jacob N, Zalevsky J, Desjarlais JR, Stohl W, Szymkowski DE. Antibody-mediated coengagement of FcgammaRIIb and B cell receptor complex suppresses humoral immunity in systemic lupus erythematosus. J Immunol. 2011;186:4223–4233. doi: 10.4049/jimmunol.1003412. [DOI] [PubMed] [Google Scholar]
- 74.Beers SA, French RR, Chan HT, Lim SH, Jarrett TC, Vidal RM, Wijayaweera SS, Dixon SV, Kim H, Cox KL, Kerr JP, Johnston DA, Johnson PW, Verbeek JS, Glennie MJ, Cragg MS. Antigenic modulation limits the efficacy of anti-CD20 antibodies: implications for antibody selection. Blood. 2010;115:5191–5201. doi: 10.1182/blood-2010-01-263533. [DOI] [PubMed] [Google Scholar]
- 75.Lim SH, Vaughan AT, Ashton-Key M, Williams EL, Dixon SV, Chan HT, Beers SA, French RR, Cox KL, Davies AJ, Potter KN, Mockridge CI, Oscier DG, Johnson PW, Cragg MS, Glennie MJ. Fc gamma receptor IIb on target B cells promotes rituximab internalization and reduces clinical efficacy. Blood. 2011;118:2530–2540. doi: 10.1182/blood-2011-01-330357. [DOI] [PubMed] [Google Scholar]