The first time I met Lloyd Old was in late 1976, several weeks after I had started a fellowship at Memorial Sloan-Kettering Cancer Center (MSKCC) in New York City. With the adventure of experiencing life in New York City as my major goal, I had come to MSKCC without clearly defined plans, only with a vague idea to study immunology in the context of the pathogenesis and therapy of cancer. The idea to employ immunology for the diagnosis and treatment of cancer was not widespread at that time in clinical medicine when density-gradient isolation of peripheral blood mononuclear cells by Ficoll-Hypaque was just introduced and T lymphocytes were defined by forming rosettes with sheep erythrocytes. As an M.D., I wanted to study human tumor immunology and, to this end, the lab of Bob Good, who at that time was president of MSKCC, seemed to be the appropriate place. Meanwhile, the lab of MSKCC’s vice president Lloyd Old had the reputation to be not very profitable for foreign fellows because it was well known that a publication (without which you would not dare return to Europe) with Dr. Old would take years and was not achievable without the sacrifice of late hours and work during the weekends. While the latter did not deter me, it was the fact that Dr. Old’s reputation was based on his excellent work on tumor immunology in the mouse that made me hesitate when Herbert Oettgen invited me to join Dr. Old’s group. I eventually accepted when Dr. Oettgen told me that I could work on a project on human tumor immunology without any “mouse work.”
Dr. Old, together with Tom Carey and Toshitada Takahashi, had just published their Proceedings of the National Academy of Sciences paper in which they described for the first time antibody reactivity in the serum of a patient with melanoma that recognized a tumor-specific antigen on the surface of cultured melanoma cells. This was the first convincing evidence that a serological immune response existed in patients with malignant tumors against antigens expressed on the surface of the tumor cells (1). A second paper with Hiroshi Shiku as the first author was under way, where additional surface antigens on human malignant melanoma cells were described (2). The specificity of the observed reactions was characterized by absorption studies with a wide range of malignant and benign cell lines. “Autologous typing”—the immunological characterization of a tumor by means of its reactivity with antibodies in the serum of the autologous patient, i.e., the patient from which the tumor under study was derived—was thus born. These two papers signified a new era in human tumor immunology and called for expansion. We all knew that melanoma was special with respect to the immunobiological relationship between the tumor and the tumor-bearing patient because there was no other malignancy associated with such a prevalence of autoimmune phenomena and a comparatively high rate of spontaneous regression. With the contagious enthusiasm that Dr. Old spread, however, we refused to acknowledge that specific anti-tumor reactions would be much harder to demonstrate in other human tumors; on the contrary, we were convinced to find tumor-specific immunoreactions in many (and secretly hoping in all) patients with cancer.
Yet, the expansion of autologous typing to other types of human tumors was limited by the fact that we needed tumor cell lines that grew permanently in vitro from the patients who were to be studied for tumor-specific antibodies in their blood. Fresh tumor biopsies did not provide enough cells for the primary testing and were completely insufficient for the exhaustive absorption studies necessary to determine the specificity of an observed antibody response. Thus, the establishment of a tumor cell line was the prerequisite and the bottleneck to embarking on autologous typing of a patient’s tumor. Dr. Old inaugurated a tumor procurement system with surgeons at Memorial Hospital and with the surgical and neurosurgical departments of several other New York City hospitals. We accepted any tumor specimen that we could acquire, but we had to learn fast that, apart from melanoma—at a time when no growth factors or other cytokines had been identified—only renal cancer and malignant brain tumors yielded permanent cell lines in vitro at a rate high enough to start a tumor type-specific effort at autologous typing.
Under the supervision of Dr. Shiku, Ryuzo Ueda and I (they used to call us the “serology twins”) did everything in the lab together. I do not know when we decided that my subject should be the autologous typing of brain tumors (3), and Dr. Ueda’s project, the autologous typing of kidney cancers. Maybe it was when it became clear that the kidney cancer project was lengthier and Dr. Ueda not as eager to return home as I was. We worked hard to make our “babies,” the fresh tumor samples, survive and grow in cell culture and hardly dared to ask for a vacation, which was socially problematic anyway with my Japanese co-workers and Dr. Old, who—if my memory does not deceive me—did not have a single vacation during the two years that I spent in his lab.
When Dr. Old showed up in the lab, it was usually in the evening. We always wondered whether this was due to his busy schedule during normal working hours or because of his curiosity. He was always well informed on the state of every project pursued in the lab and knew when new results would be ready. He enjoyed most when he was present at the very moment the results of our experiments came out. When the results were not as expected (which was the case with most of our experiments), Dr. Old comforted us with his standard comment on negative or disappointing results: “There are no negative results, there are only interesting results” or alternatively, “These results are important because they teach us a lot.” Positive results or those that met our expectations were simply “just fascinating.”
The specificity of the tumor-associated antigens that we detected by autologous typing was determined serologically by absorption of the respective patient’s sera with a broad spectrum of cells and cell lines of benign and malignant origin. These absorption studies often revealed that seemingly tumor-specific reactivities were indeed unspecific, thus destroying many hopes and confirming a dictum of Dr. Old with respect to cancer research in general: “Immunology is the king, serology the queen, and absorption the mistress.”
In my first paper with Dr. Old (3), we described three classes of tumor-associated antigens: individually tumor-specific (class I) antigens, which were found only on the tumor cells of the patient whose serum had an antibody reactivity to this antigen; tumor-type specific (class II) antigens, shared by tumors of the same or similar histological origin; and tumor-associated antigens, the expression of which was not restricted to malignant cells (class III antigens). A logical next step after the serological was the biochemical characterization of the serologically defined antigens. It should be kept in mind that the titers of the patients’ serum reactivities against the autologous tumor cell lines were generally low, even though we had developed probably the most sensitive assays to detect antibodies bound to the surface of cultured tumor cells. Of the four assays that indicated human antibodies bound to the cell surface by forming a rosette of specifically prepared erythrocytes around the tumor cell [antibody mixed hemadsorption assay (MHA), immune adherence assay, anti-C3 MHA, and protein A assay], the anti-C3 MHA showed highest sensitivity. The C3-MHA assay had a propensity to detect IgM antibodies because complement binding activity was a prerequisite for an antibody to be detected by this assay. However, even with this highly sensitive assay, the titers of the antibodies rarely exceeded 1:500, rendering any attempt to further characterize the antigen a formidable task, and in the few cases where successful, the biochemical characterization remained quite vague (4). Besides the characterization of the antigens detected by autologous typing, the demonstration of a T cell reactivity against the respective antigens proved to be very difficult at a time when the mechanisms of the presentation of antigen-derived peptides in the context of MHC by antigen presenting cells to T cells were still unknown (5).
I left the lab in 1978, when the technology to develop monoclonal antibodies against defined antigens became available (6). This technology promised easy and fast success in tumor immunology. As had many other labs at that time, Dr. Old’s tuned in, as did I with my group after returning to Germany. Even though my group had successfully established monoclonal antibodies against several types of human malignancies (7), some of which proved to be useful for the imaging of human tumors in vivo (8); had pioneered the therapeutic use of bispecific antibodies in a preclinical model (9); and had succeeded in performing the first-in-human clinical trial with bispecific antibodies (10), where we observed surprising and unexpected anti-tumor activity, I deplored the fact that monoclonal antibodies derived from the mouse could not extend our knowledge on the immunological tumor-host relationship in human cancer patients. During that period I met Dr. Old only rarely, but whenever I did, I frankly confessed my doubts about the future and clinical value of tumor immunology, and I always returned home refilled by him with new enthusiasm and full of new ideas.
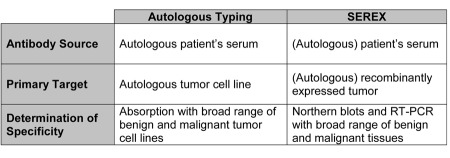
It was in the late 1980s that I bore the idea of applying the new technologies of molecular biology to readdress the old question of the immunological relationship between tumor and host in patients with cancer. Recombinant expression cloning was hip and offered a possibility to reexamine the old question of specific interactions between antibodies in the serum of a patient against antigens expressed by her/his own (autologous) tumor. The basic idea was to monoclonalize the antigen rather than the antibody in an approach that later became known as SEREX, an acronym that stands for “serological identification of antigens by recombinant expression cloning.” To establish a cDNA derived from a patient’s tumor, less fresh tissue was necessary than for the establishment of a tumor cell line for autologous typing. As for autologous typing, in the early days of SEREX we strictly adhered to the principle of restricting our analysis to autologous reactions in order to exclude allogeneic reactions. This fear, though deeply implanted into our thinking by Dr. Old, was not justified because we hardly ever detected a serological response to allogeneic HLA alleles during our SEREX analysis of a broad spectrum of tumors. Instead of cell lines permanently established and growing in cell culture, clones derived from tumor cDNA libraries and expressed in E. coli were used for the screening of antibodies reacting with tumor-derived antigens. A major problem that we encountered when we screened tumor-derived cDNA clones expressed in E. coli and probed them with antibodies in the serum of the autologous patients was a strong background reactivity, which made it very difficult to distinguish the true from the false positive clones. It took us a long time to overcome this problem, but as a former student of Dr. Old, I solved it by absorption of the patients’ sera not only with non-transfected E. coli, but also with transfected E. coli, indicating that the spectrum of “background” antibodies in human serum included not only antibodies against E. coli (which was expected), but also antibodies against phage-derived antigens (something we had not expected). Despite these modifications, the comparison of autologous typing and SEREX (Table 1) makes it evident that SEREX is a daughter of autologous typing and that, without autologous typing, SEREX never would have been born. As Dr. Old put it, it took only the prepared mind that chance had to meet to design SEREX, and I was lucky enough of having my mind prepared by my scientific education in Dr. Old’s lab.
Table 1.
Comparison of autologous typing and SEREX.
After we had overcome the technical problems of what was later called SEREX, we were astonished by the plethora of tumor-derived antigens detected by antibodies in the sera of cancer patients. But this was not the only advantage of SEREX; it also allowed for the direct sequencing and identification of the gene coding for the respective tumor-associated antigen, obviating the need for tedious biochemical analyses. Finally, for the determination of an observed anti-tumor reactivity, the absorption studies from the era of autologous typing were replaced first by Northern blot analyses, and later by RT-PCR. Despite these modifications, SEREX was a direct descendant of autologous typing, and had I not met Dr. Old in the time of autologous typing, I hardly would have had the background to design the SEREX approach for the discovery of human tumor antigens. Since then, SEREX has also been employed in other fields of immunology, and SEREX per se or modifications thereof are still the screening method of choice whenever antigen-antibody interactions are to be demonstrated, but both the antigen and the antibody are unknown.
When we had identified by SEREX the first tumor-associated antigens in melanoma, renal cancer, and glioma (exactly the three types of tumors autologous typing had started with), as well as in Hodgkin disease, I reported our results to Dr. Old. To my surprise, Dr. Old met these long hoped-for results not with unrestricted enthusiasm, but rather treated them for some time with reserve. Perhaps this reserve was due to the fact that our observation—that most, if not all, human tumors elicit multiple antibody responses against antigens expressed by the autologous tumor in patients with cancer—was so contrary to current opinion and common belief at the time and so much unlike what we and other tumor immunologists all over had been experiencing for decades. While a handful of tumor antigens had been defined before the advent of SEREX, our finding probably seemed to be just too unbelievable to Dr. Old, who had spent half of his life searching for and finding very few antigens to be associated with or even specific for human tumors. It was not until we demonstrated that antibodies with specificity to MAGE and tyrosinase, two melanoma antigens that had just been shown to elicit specific T cell responses, that Dr. Old started to believe in SEREX, and we searched for the “baby’s” name. As we came to understand that our serological approach combined with recombinant expression cloning would change the world of human tumor immunology, it became clear that serology should be the queen no longer, but rather the king; hence, we created “SEREX” for “serologia rex” (serology as the king in tumor immunology). The official name (serological identification of antigens by recombinant expression cloning) was provided as an explanation for SEREX only later.
However, convincing Dr. Old was just the first hurdle. Our finding of frequent antibody reactions against antigens derived from the autologous tumor met the same disbelief when we submitted our first manuscript on SEREX to several high-ranking journals. In the end, we were glad and grateful that Dr. Old, as a member of the National Academy of Sciences, communicated our manuscript to Proceedings of the National Academy of Sciences, where it appeared in December 1995 (11).
Once convinced by SEREX, Dr. Old wanted to thoroughly plough the entire field of human tumor antigens and aimed at defining the whole spectrum of tumor-associated antigens expressed by human tumors. In one of the first “-omics” approaches in medicine, Dr. Old organized a world-wide SEREX collaborative in order to define what he called the “tumor immunome.”
In order to achieve this goal, it was necessary to disseminate the methodology and techniques of SEREX to other investigators. As I had never seen an academic teacher who was so liberal with his support of his pupils, even after they had left him, it was without hesitation that I brought SEREX to Dr. Old’s group, with the feeling of a little pride, but mostly with thankfulness of being able to give back to my scientific teacher only a small part of what he had given me throughout my entire scientific career. Yao-Tseng Chen and Matt Scanlan were the first to come to our lab at Saarland University in Homburg to learn the techniques necessary to perform SEREX, and after they returned to New York, SEREX spread from there to all over the world. Within five years, the most clinically relevant antigens had been discovered and characterized. When this was achieved, I was skeptical that additional efforts would be likely to discover more antigens with clinical relevance, i.e., antigens that are expressed frequently and in a broad spectrum of tumors. Nevertheless, Dr. Old went on to employ a wide range of modifications of SEREX in addition to molecular-based methodologies like representational difference analysis, massively parallel signature sequencing, and in silico analyses. It was not until these investigations were completed that Dr. Old’s curiosity was satisfied as much as this could ever be the case. With more than 2,000 human tumor antigens listed in the SEREX data bank, including more than 150 genes coding for the clinically attractive group of Cancer/Testis (CT) antigens, the definition of the tumor immunome is as close to complete as it can reasonably be. Even if a few human tumor antigens still await discovery, both tumor immunologists and clinical immunotherapists now have the choice to select the appropriate antigen from a large armamentarium of promising targets.
Dr. Old’s view on the future of tumor vaccines was always much more optimistic than mine, and this difference of opinion was the point of origin for many fruitful discussions that often crossed the borders from science to philosophy. While I still am not convinced that vaccination with molecularly defined tumor antigens (which have now been available for more than 20 years) has saved the life of a single patient with cancer to date, I agree with Dr. Old that we have still to learn “how to immunize in order to successfully vaccinate.”
I am grateful that I had the chance to share 35 years of tumor immunology with Dr. Old—35 years during which the molecular basis of tumor immunology was built. This molecular basis has liberated tumor immunology from the odor of subjectivity and poor reproducibility and has established tumor immunology as a scientifically accepted and equitable partner for the conquest of cancer. That tumor immunology has gained this high reputation is the great merit of Dr. Old. I am also grateful for having met an outstanding personality with a caring and compassionate soul who always eased not only scientific, but also personal troubles of those who asked his advice. All those who had the privilege of his acquaintanceship will remember him as the great scholar and scientist, as the perfect gentleman, and as a friend who was always there for us when we needed him, at any time and in any place in the world.
References
- 1.Carey TE, Takahashi T, Resnick LA, Oettgen HF, Old LJ. Cell surface antigens of human malignant melanoma: mixed hemadsorption assays for humoral immunity to cultured autologous melanoma cells. Proc Natl Acad Sci U S A. 1976;73:3278–3282. doi: 10.1073/pnas.73.9.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shiku H, Takahashi T, Oettgen HF. Cell surface antigens of human malignant melanoma. II. Serological typing with immune adherence assays and definition of two new surface antigens. J Exp Med. 1976;144:873–881. doi: 10.1084/jem.144.4.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pfreundschuh M, Shiku H, Takahashi T, Ueda R, Ransohoff J, Oettgen HF, Old LJ. Serological analysis of cell surface antigens of malignant human brain tumors. Proc Natl Acad Sci U S A. 1978;75:5122–5126. doi: 10.1073/pnas.75.10.5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carey TE, Lloyd KO, Takahashi T, Travassos LR, Old LJ. AU cell-surface antigen of human malignant melanoma: solubilization and partial characterization. Proc Natl Acad Sci U S A. 1979;76:2898–2902. doi: 10.1073/pnas.76.6.2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knuth A, Danowski B, Oettgen HF, Old LJ. T-cell-mediated cytotoxicity against autologous malignant melanoma: analysis with interleukin 2-dependent T-cell cultures. Proc Natl Acad Sci U S A. 1984;81:3511–3515. doi: 10.1073/pnas.81.11.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 7.Pfreundschuh M, Mommertz E, Meissner M, Feller AC, Hassa R, Krueger GR, Diehl V. Hodgkin and Reed-Sternberg cell associated monoclonal antibodies HRS-1 and HRS-2 react with activated cells of lymphoid and monocytoid origin. Anticancer Res. 1988;8:217–224. [PubMed] [Google Scholar]
- 8.Carde P, Da Costa L, Manil L, Pfreundschuh M, Lumbroso J-D, Saccavini J-C, Caillou B, Ricard M, Boudet F, Hayat M, Diehl V, Parmentier C. Immunoscintigraphy of Hodgkin’s disease: in vivo use of radiolabelled monoclonal antibodies derived from Hodgkin cell lines. Eur J Cancer. 1990;26:474–479. doi: 10.1016/0277-5379(90)90019-p. [DOI] [PubMed] [Google Scholar]
- 9.Renner C, Jung W, Sahin U, Denfield R, Pohl C, Trumper L, Hartmann F, Diehl V, van Lier R, Pfreundschuh M. Cure of xenografted human tumors by bispecific monoclonal antibodies and human T cells. Science. 1994;264:833–835. doi: 10.1126/science.8171337. [DOI] [PubMed] [Google Scholar]
- 10.Hartmann F, Renner C, Jung W, Sahin U, Pfreundschuh M. Treatment of Hodgkin’s disease with bispecific antibodies. Ann Oncol. 1996;7(Suppl 4):143–146. doi: 10.1093/annonc/7.suppl_4.s143. [DOI] [PubMed] [Google Scholar]
- 11.Sahin U, Türeci Ö, Schmitt H, Cochlovius B, Johannes T, Schmits R, Stenner F, Luo G, Schobert I, Pfreundschuh M. Human neoplasms elicit multiple specific immune responses in the autologous host. Proc Natl Acad Sci U S A. 1995;92:11810–11813. doi: 10.1073/pnas.92.25.11810. [DOI] [PMC free article] [PubMed] [Google Scholar]